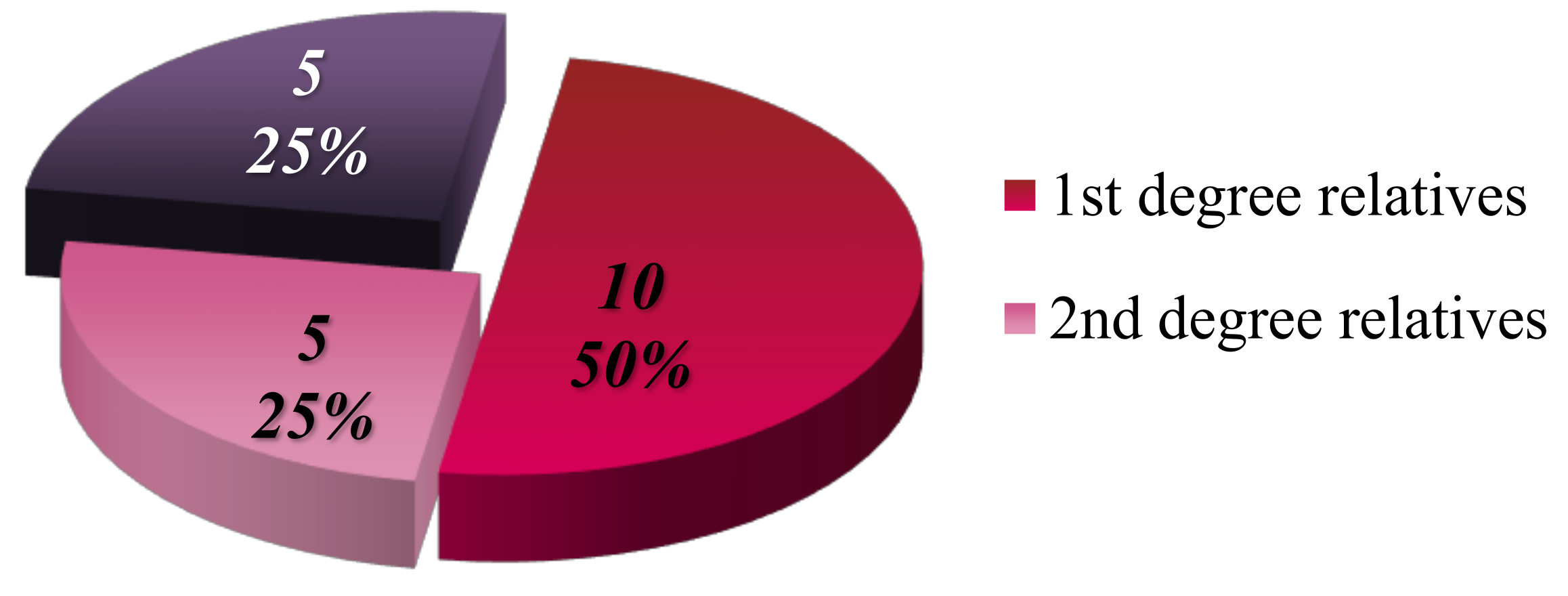Introduction
Positioned within the broad spectrum of lymphoproliferative disorders, malignant lymphomas are neoplasms recognized for their complexity and heterogeneity. They typically present with an insidious onset and are detected in the majority of cases worldwide only at advanced, terminal stages, when specific symptomatology or metastatic processes become evident. These malignancies are fundamentally distinguished by the presence of Reed-Sternberg (RS) cells, which originate from the pre-apoptotic germinal centers of B lymphocytes, losing their cellular identity and exhibiting multiple aberrations in signaling pathways.
Malignant lymphomas, as hematological neoplasms, are classified into two primary categories: HLs and NHLs. According to the most recent classifications established by the World Health Organization (WHO) in 2017, there are over 80 types of mature lymphoid neoplasms. HL accounts for approximately 30-40% of all malignant lymphomas, whereas NHLs occur in 60-70% of cases (Mihăescu et al., 2006; Oltean et al., 2009; De Leval and Jaffe, 2020).
Members of the WHO prefer to classify malignant lymphoid neoplasms into three categories: HLs, which are either nodular lymphocyte-predominant (5%) or classical (95%), the latter further subdivided into four histological types: mixed cellularity (15-30%), lymphocyte depletion (<1%), nodular sclerosis (60-80%), and lymphocyte-rich (5%). The second category includes B-cell NHLs , which account for 80-85% of cases and can be nodal, primarily extranodal, or predominantly disseminated. The third category encompasses T-cell or NK-cell NHLs (10%), which are primarily disseminated, nodal, or primarily extranodal or cutaneous. Neoplasms that exhibit characteristics overlapping classical HL (cHL) and diffuse large B-cell lymphoma (DLBCL) are extremely rare and fall under the spectrum of what is termed "grey zone lymphoma." These forms of lymphoma are notably more aggressive and are associated with a reduced survival rate among diagnosed patients (Jaffe et al., 2001; Oltean et al., 2009; Swerdlow et al., 2017; De Leval and Jaffe, 2020; EL-Sawalhy et al., 2021; Bosch-Schips et al., 2022).
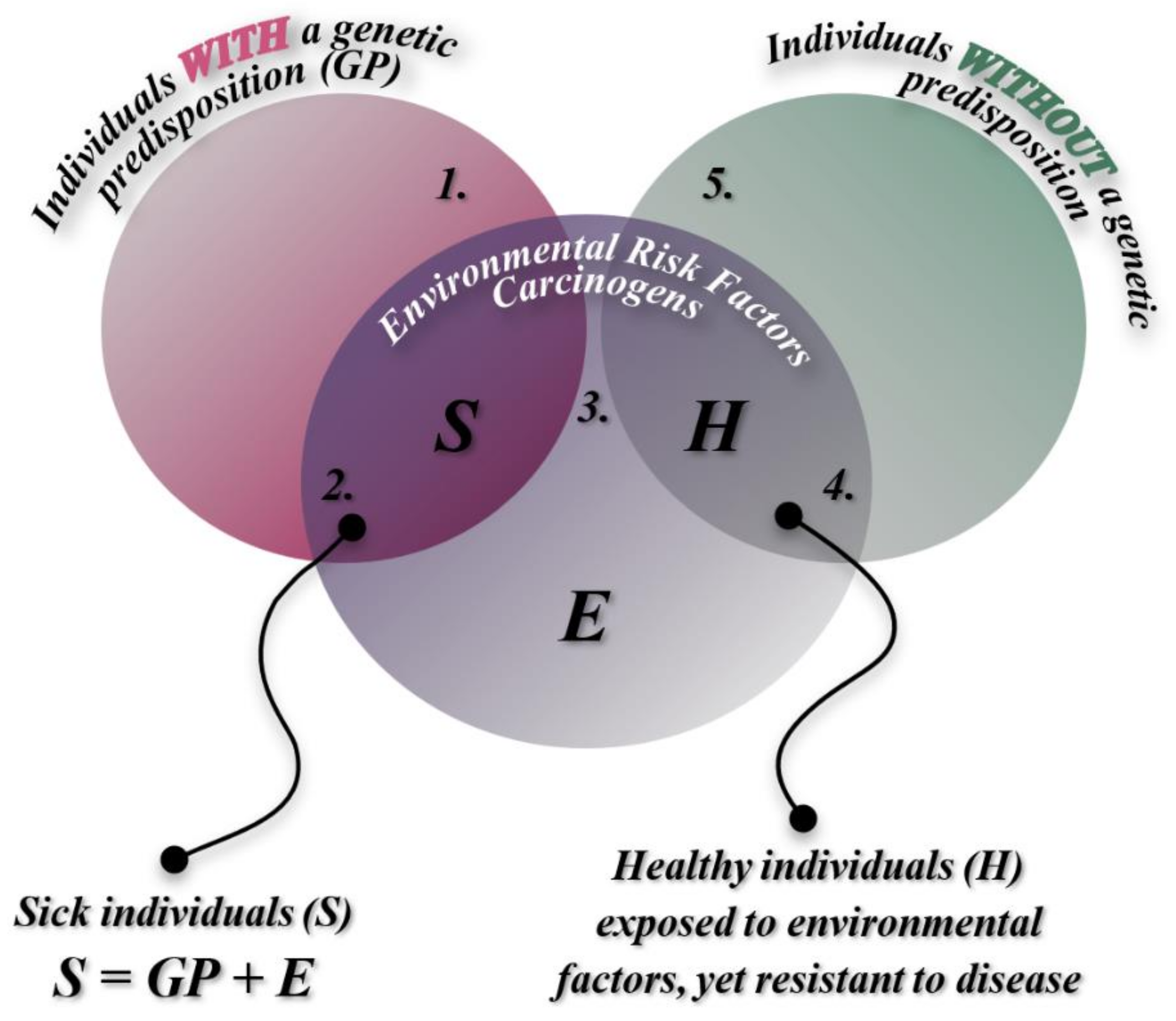
Classified as multifactorial diseases, malignant lymphomas are influenced by a wide range of genetic factors and non-genetic carcinogens. These conditions arise in humans through the interaction between environmental factors and heredity, or, in some cases, solely through the influence of one of these factors. Heredity can predispose an individual to a higher genetic risk of developing a specific disease during their lifetime. However, the transition from vulnerability to the onset of the disease itself occurs through the constant interaction of both major factor categories—only if environmental influences exceed a critical threshold that triggers the disease (
Figure 1).
This explains the preservation of intact health in individuals with genetic predispositions who are not exposed to environmental triggers, as well as those who come into contact with harmful factors but possess genetic resistance. Therefore, the disease emerges as the result of a complex and bidirectional interaction between genetic factors and carcinogenic environmental factors. Due to its multifactorial etiology, the onset and progression of the malignant process in lymphomas, like all multifactorial diseases, are primarily driven by the impact of environmental factors.
Affecting approximately one-third of the adult population, cancer remains one of the most common causes of mortality, accounting for 1 in 8 deaths, which corresponds to 12.5% (according to Covic, 2017, p. 547) and between 20-25% of total mortality (as per Gorduza, 2007, p. 365). The etiology of cancer is multifactorial, encompassing a range of causal factors, including exogenous and endogenous environmental agents (metabolic, immunological, endocrine), as well as individual factors such as age, sex, and genetic predispositions.
There are co-carcinogenic factors, consisting of genetic and environmental agents that act synergistically in the presence of an oncogenic virus, as well as intrinsic and extrinsic factors that contribute to the onset of malignant lymphomas.
Intrinsic factors include vitamins, chemical substances, dietary fats, hormonal, immunological, and genetic factors, while external factors encompass physical mutagens (such as UV radiation, X-rays, and radionuclides), chemical mutagens (pesticides, insecticides, tobacco, and pollutants), and biological agents (retroviruses and DNA viruses) (Israil, 2000).
Genetic factors involve a wide array of oncogenes, with the activation of these genes occurring through increased expression levels or the emergence of driver mutations, which add functions to inactive tumor suppressor genes, such as p53. This leads to the inhibition of apoptosis by permitting DNA damage and repair errors, which would otherwise trigger cell death. Additionally, a variety of chromosomal abnormalities, both balanced and unbalanced (such as isochromosomes, translocations, inversions, deletions), as well as numerical chromosomal alterations, also play a role in tumorigenesis.
Study Objective
The retrospective study aimed to provide conclusive statistical data and identify/observe both genetic and non-genetic factors involved in malignant lymphomas. The study includes patients admitted to the Hematology Clinic of "Sfântul Spiridon" Hospital in Iași over a 10-year period.
According to the "Medical Practice Guidelines" published by the Ministry of Health on February 23, 2021, the statistical incidence of malignant lymphomas in the European Union is 2.3 diagnosed patients per 100,000 inhabitants annually, with an annual mortality rate of 0.4 cases per 100,000 (Ministry of Health, 2021).
In 2018, it was estimated that there were 79,990 cases of HLs and 509,590 cases of NHLs globally—a 6.37-fold higher prevalence for NHL. Approximately 26,167 HL patients and 248,724 NHL patients died during this period (Bray et al., 2018; Baeker Bispo, Pinheiro, and Kobetz, 2020).
According to global statistics using data accumulated in 2020 from GLOBOCAN estimates by the International Agency for Research on Cancer, there has been an increase in the incidence of both types of malignant lymphomas, HL and NHL. However, while the mortality rate for NHLs has risen, the mortality rate for HLs has decreased. In 2020, 83,087 new cases of HL and 544,352 new cases of NHL were reported worldwide. Additionally, 23,376 patients with HLs and 259,793 with NHLs died globally in 2020 (Sung et al., 2021).
According to data published in 2022, a slight decrease in both the incidence and mortality of HL was observed, with 618 fewer new cases reported (82,469 newly diagnosed patients in 2022) and a reduction of 643 deaths compared to the previous year (22,733 deaths in 2022). This downward trend continues for NHLs only in terms of mortality, with 9,114 fewer deaths (250,679 deaths in 2022). However, the incidence of NHL increased, with 553,389 new cases, reflecting 9,037 more diagnoses in 2022 compared to 2020.
In Romania, as of January 19, 2022, according to the "National Cancer Control Plan," there are no comprehensive statistics regarding the total number of cases, incidence, mortality, or the rates of occurrence by age, gender, region, or contributing factors for malignant lymphomas. Data from the Romanian Society of Hematology over the past two years indicates 614 reported cases of HLs in Romania. Globocan’s 2020 report, however, lists 263 new diagnoses and 55 deaths annually, with an estimated 961 cases over a five-year period (5 cases per 100,000 inhabitants). For NHLs, no national Romanian institution, society, or organization has produced concrete statistics. However, Globocan’s 2020 data reports higher figures for both incidence (1,909 cases) and mortality (789 deaths) for NHL compared to HL. The five-year prevalence for NHL in Romania is estimated at 5,792 cases (30.11 cases per 100,000 inhabitants).
In Romania, trends in the incidence, prevalence, and mortality of malignant lymphomas appear to be fluctuating. A comparison of previously mentioned data with the global database published in 2022 reveals discrepancies: 318 diagnoses of HL and 105 deaths, versus 1,754 new cases of NHL and 749 deaths. Notably, male patients, who are often considered more susceptible, are affected similarly to their female counterparts. In the case of HL, the incidence among males is 53.45% (170 men), while the mortality rate is approximately 31.9% (65 men). Conversely, in NHL, the incidence is 49.7% (873 men) of new cases, and the mortality rate is 51% (382 men) of total deaths.
The decision to conduct a study on patients diagnosed with malignant lymphomas is motivated by both the need to generate conclusive regional statistics, given the lack of national data, and to explore the causes leading to these conditions. The general lack of public awareness about lymphoproliferative malignancies and the absence of preventive medical education contribute to late-stage diagnoses. Although current treatments are highly effective and can lead to long-term complete or partial remissions when administered in early stages, the avoidance of routine clinical and paraclinical examinations—whether by general practitioners or specialists—has a detrimental effect. This often results in a more challenging and prolonged treatment process, necessitating more intensive therapeutic regimens, sometimes involving a combination of chemotherapy and radiotherapy.
The COVID-19 pandemic caused by the SARS-CoV-2 virus has exacerbated the tendency to avoid medical consultations in recent years. Both patients already diagnosed with malignancies and those with undetected early-stage diseases have suffered. Immunocompromised individuals undergoing treatment have become particularly vulnerable to severe forms and complications of the virus. The complete lack of immune response capacity in such patients can be fatal. Their risk of exposure to asymptomatic carriers, contaminated surfaces, or high-aerosol environments (including the presence of Flügge droplets) has increased due to the necessity of hospital visits for routine checks, treatments, or surgeries.
I believe that conducting detailed studies on patients diagnosed with Hodgkin and NHLs, focusing on hereditary aspects through genealogical tree analysis, as well as on non-genetic carcinogenic factors to which individuals have been exposed, can lead to the development of accessible statistics and recommendations. These findings should be made public to raise awareness among the general population. In my view, the future of medicine is preventive and personalized, especially in the early detection of diseases, and research is progressively moving towards these goals.
Therefore, considering the aforementioned factors, the study was conducted by analyzing cohorts of patients diagnosed with malignant lymphomas within the Iași County region. It involved examining clinical and paraclinical aspects, genetic factors, and compiling statistics. The obtained data were compared with specialized publications to highlight factors influencing genetic predispositions and those that triggered individual malignant proliferation processes.
Materials and Methods
This retrospective study encompasses patients admitted to the Hematology Clinic of "Sfântul Spiridon" Hospital in Iași between January 1, 2010, and December 31, 2019, with the latter date marking the last recorded admission.
Patient selection was based on diagnostic data, focusing specifically on those identified with malignant lymphomas among the various hematologic neoplasms recorded in the hospital databases.
During the 10-year period, out of a total of 1,916 patients admitted with hematologic neoplasms, 392 were diagnosed with various forms of lymphoma. For the purpose of the genetic study, 84 cases demonstrating a genetic predisposition were selected, and their medical records were thoroughly analyzed.
Upon reviewing the medical records and concentrating on individuals with manifest genetic predispositions, it was found that 20 patients exhibited a higher genetic burden. The genetic transmission of these cases was as follows: 9 cases with maternal inheritance, 4 cases with paternal inheritance, and 7 cases where both parents were diagnosed with the same condition.
The relevant information for the cohort of patients with increased genetic burden has been concentrated and highlighted in the figures, with all personal and confidential data excluded. This information is categorized according to the following criteria: diagnosis, sex, age category, origin, comorbidities, genetic and environmental factors. The methodology involved an additional collaborative step with the hospital's IT department and statisticians to ensure data acquisition while maintaining patient confidentiality.
The relevant data for this study were graphically represented and included in tables to facilitate the visualization of observations based on the analyzed data.
The methodology encompassed the analysis of medical records, including additional investigation results, general clinical examinations, cancer patient records, analysis reports, admission and discharge notes, and medical letters (including diagnosis, anamnesis, clinical examination, laboratory and paraclinical tests, treatment). It also included justifications for various medical procedures (identification data, clinical data: diagnosis, previous and current investigations and treatments, warnings, and risks), medical referrals with the attending physician's proposals (records with: diagnosis, objective examination, admission date, clinical and paraclinical investigations, treatments, recovery plan, recovery prognosis, and proposals), inclusion criteria for specific treatments, justifications for continuing treatments, forms for verifying compliance with therapeutic protocol eligibility criteria, and monitoring records.
This process will ensure the collection of all necessary data for the characterization of the cohort and the development of statistics, as well as the analysis of determining factors in the onset of malignant lymphomas of genetic origin or environmental carcinogenic factors.
The stage of consulting databases and extracting information, with the consent of both patients and the medical team, is crucial. It represents the primary method for constructing the cohort upon which the presented research is based.
Results and Discussion
Over the 10-year period, 1916 patients with hematologic neoplastic disorders at various stages were admitted, with a diagnosis of malignant lymphoma established in 392 cases. Of these patients, 84 cases were selected for the study based on the presence of genetic predisposition, having relatives previously diagnosed with various forms of malignant lymphomas. Among these, 20 individuals with a higher genetic burden were analyzed in this study (9 cases with maternal transmission, 4 cases with paternal transmission, and 7 cases where both parents were diagnosed with the same condition).
The study underscores the significant position of malignant lymphomas within the broad spectrum of hematologic neoplasms, with a detection rate of 20.45% among patients in the clinic's records. Relevant data for the study were tabulated and graphically represented.
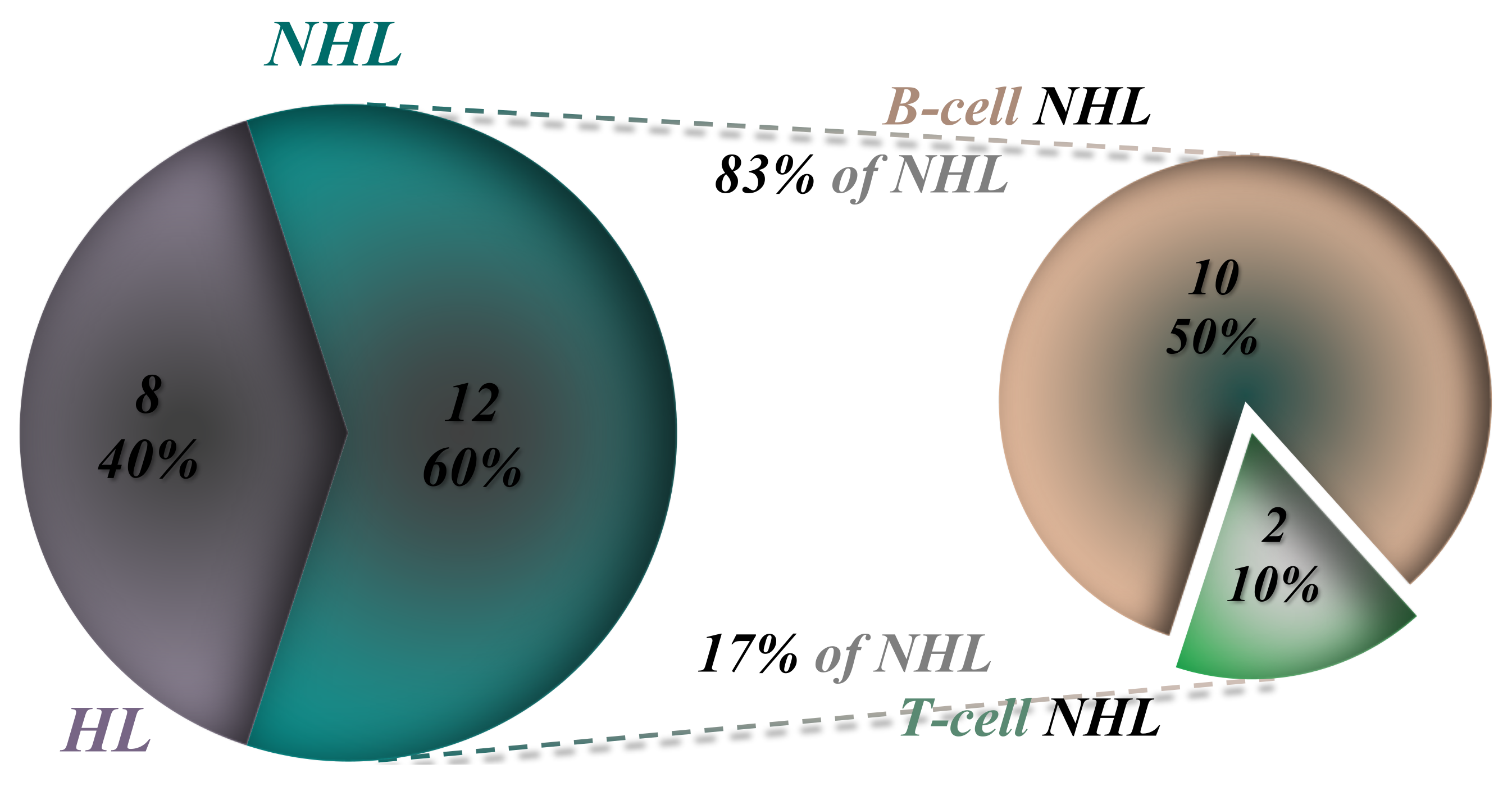
Analysis reveals that out of the 20 studied patients, 8 were diagnosed with HL and 12 with NHL, including 10 with B-cell NHL and 2 with T-cell NHL (Figure 3).
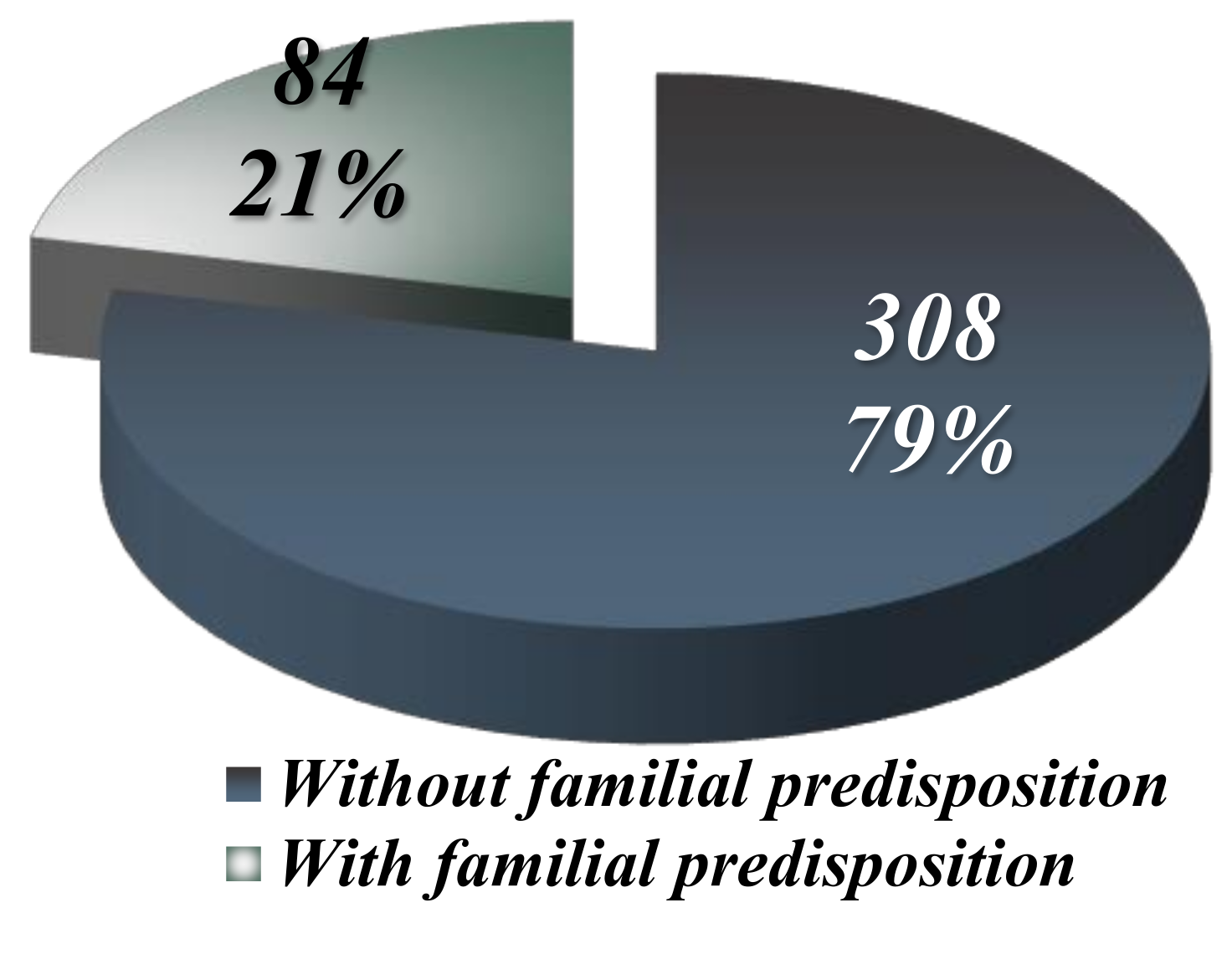
Percentage-wise, the 84 patients with manifest genetic predisposition out of 392 malignant lymphoma cases represent 21.42%. Thus, the 20 cases with increased genetic burden analyzed in this study constitute 5.1% of the total malignant lymphomas diagnosed (392 cases) over the ten-year period (2010-2019) and 23.8% of those with manifest genetic predisposition (84 cases), as illustrated in
Figure 2.
Figure 2.
The distribution of lymphomas diagnosed in patients with or without genetic predisposition.
Figure 2.
The distribution of lymphomas diagnosed in patients with or without genetic predisposition.
In examining the restricted cohort of 20 patients with genetic predisposition, NHL prevalence is observed to be 60-70%, with the majority being B-cell lymphomas (50% of the total), while HL accounts for approximately 30-40%, as highlighted in
Figure 3. Within the NHL category, B-cell lymphomas account for 83.3% of cases (10 out of 12 patients), whereas T-cell lymphomas are less prevalent at 16.6% (2 out of 12 patients), as illustrated in
Figure 3.
Figure 3.
The distribution of the 20 patients with a high genetic burden based on the type of lymphoma.
Figure 3.
The distribution of the 20 patients with a high genetic burden based on the type of lymphoma.
Sex Distribution
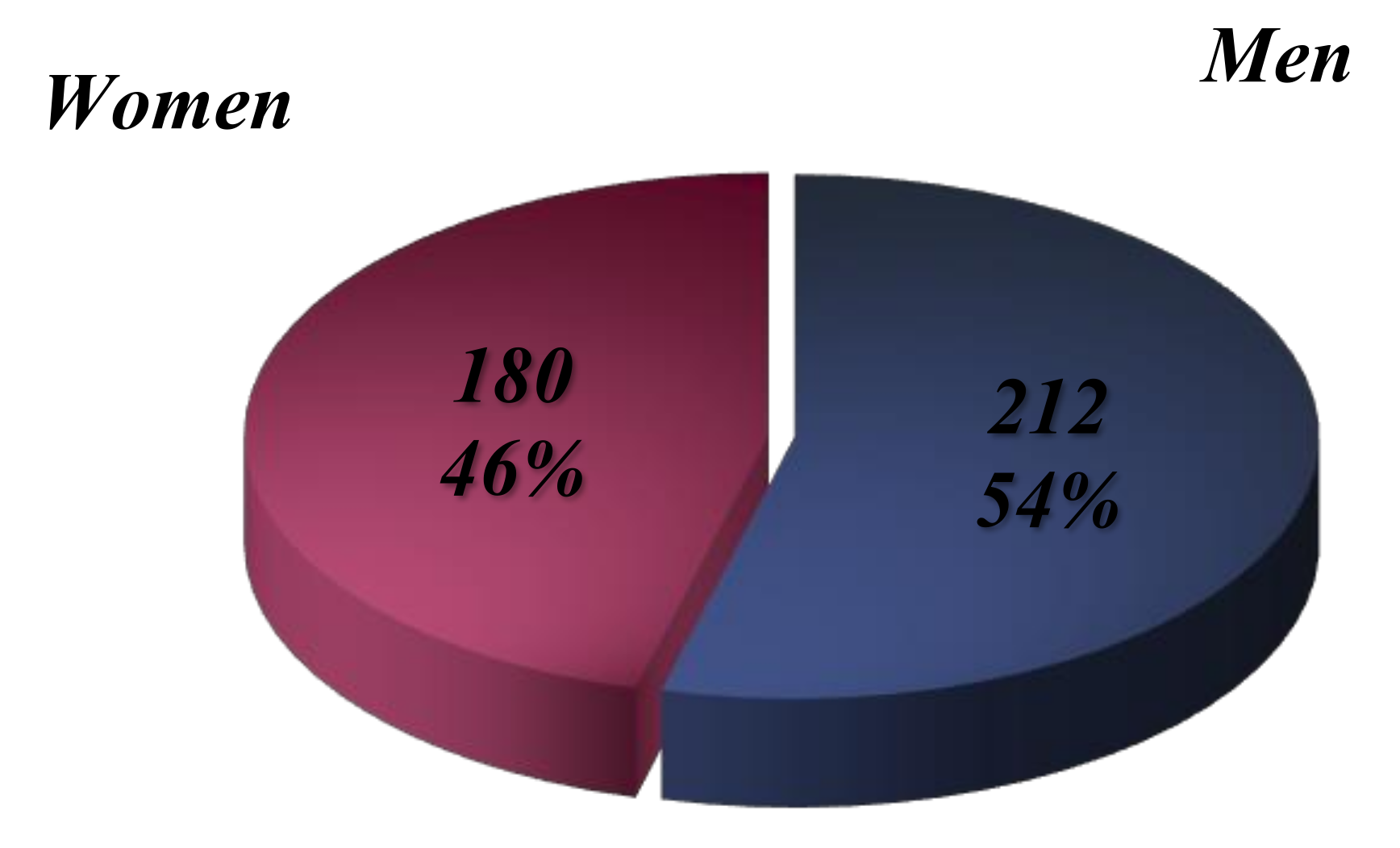
The analysis of medical records reveals that out of the 392 patients diagnosed with lymphoma at various stages, 180 are female (45.91%) and 212 are male (54.08%) (
Figure 4). This demonstrates a higher incidence in males, a trend supported by global statistics. The male sex is identified as a physiological risk factor, with a pronounced disparity in incidence rates favoring males, particularly evident in higher incidence rates.
Age Distribution
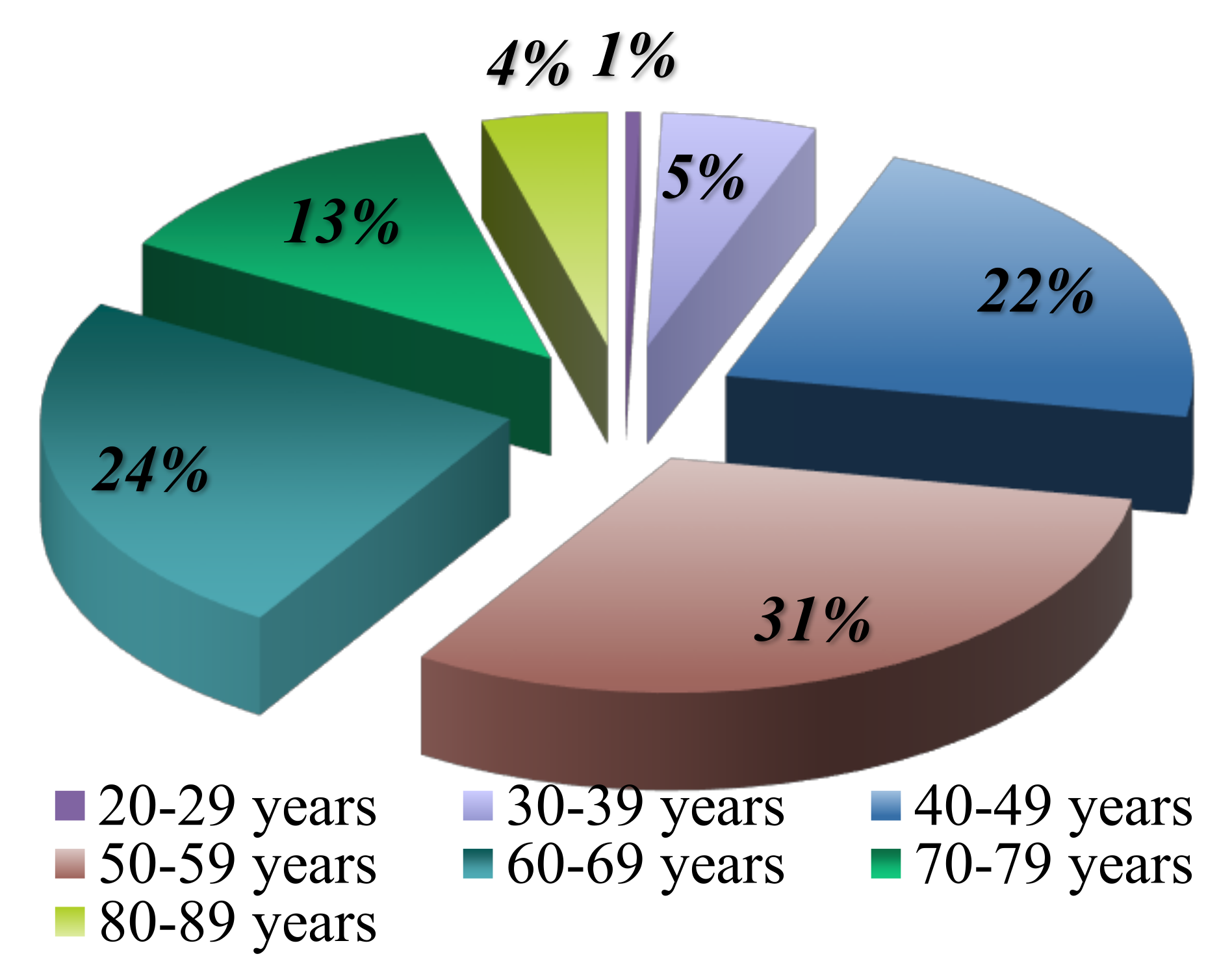
Age distribution is significantly related to risk factors. The majority of lymphoma cases are observed to debut in the sixth decade of life, with 31% of patients being between 50 and 59 years old at diagnosis. These results are consistent with the data available in the literature (
Figure 5,
Table 1).
The incidence of NHL increases with age, reaching peak values in individuals over 45-50 years old. In contrast, HL is more commonly discovered in younger individuals, with peak incidence rates occurring between the ages of 18 and 25, and also among adults with incidence peaks between 40-50 years or 60 years old.
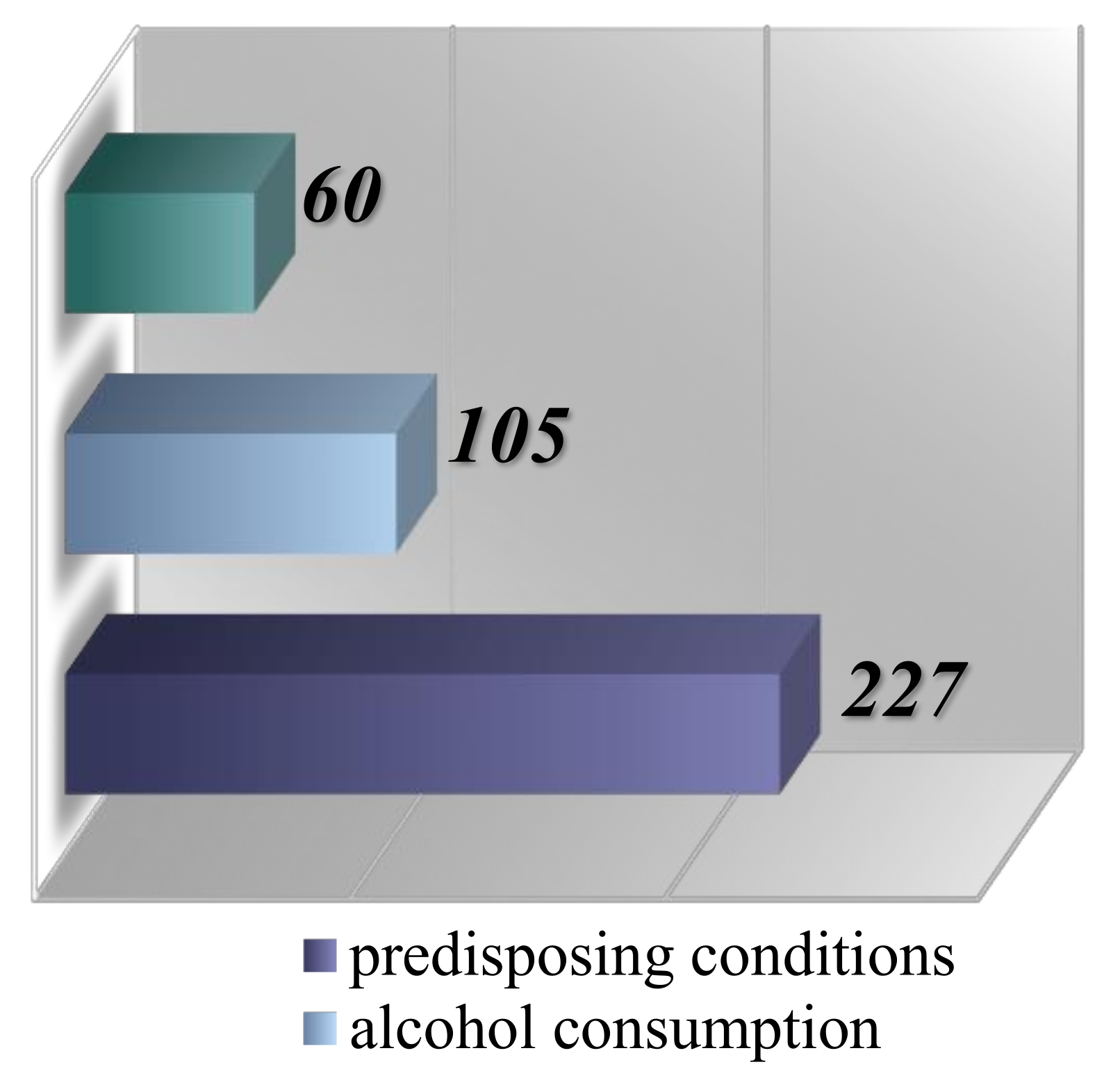
The analysis of pathological factors was conducted based on the increased risk associated with patients diagnosed with malignant lymphomas. Within the risk group, 227 individuals (57.9%) presented various predisposing conditions such as HIV, autoimmune diseases, diverse immunodeficiencies, and repeated infections with
Helicobacter pylori. Additionally, 105 patients (26.78%) had a history of alcohol consumption, identified as a cocarcinogen, and 60 cases (15.3%) were associated with pesticide exposure (
Figure 6).
In the etiopathogenesis of NHL , it is well-established that factors such as organic solvents, certain medications (including phenytoin and polychemotherapy), organophosphorus pesticides, and radiation (which includes radiotherapy-induced irradiation) play significant roles in triggering carcinogenic processes (Oltean, 1996; Mihăescu et al., 2006; Ioniță et al., 2015; Mihăilă, 2016).
The risk of developing NHL is significantly increased in individuals infected with Epstein-Barr Virus (EBV), Human T-cell Lymphotropic Virus Type 1 (HTLV-1), Human Herpesvirus Type 8 (HHV-8, also known as gammaherpesvirus), or Human Immunodeficiency Virus (HIV). Other risk factors include hepatitis C, various immunodeficiencies, or exposure to microorganisms such as Helicobacter pylori or Chlamydia psittaci, as well as exposure to ultraviolet (UV) or ionizing radiation. Furthermore, autoimmune conditions such as systemic lupus erythematosus, rheumatoid arthritis, celiac disease, psoriasis, and Sjögren’s syndrome, along with lifestyle factors like smoking, use of hair dye, or a diet high in proteins and fats, have been linked to the onset of NHL in recent years (Zhang et al., 2011; Bassig et al., 2012).
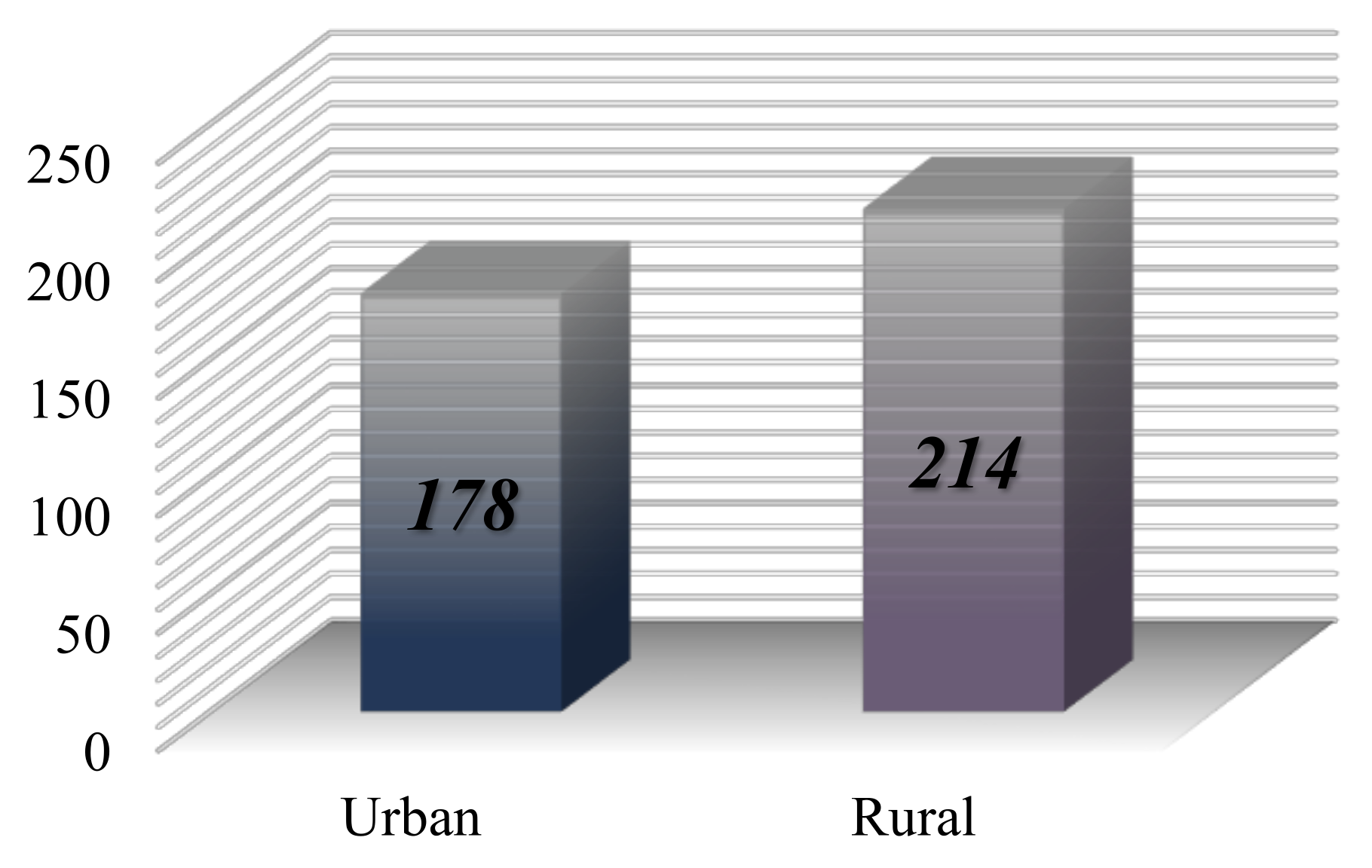
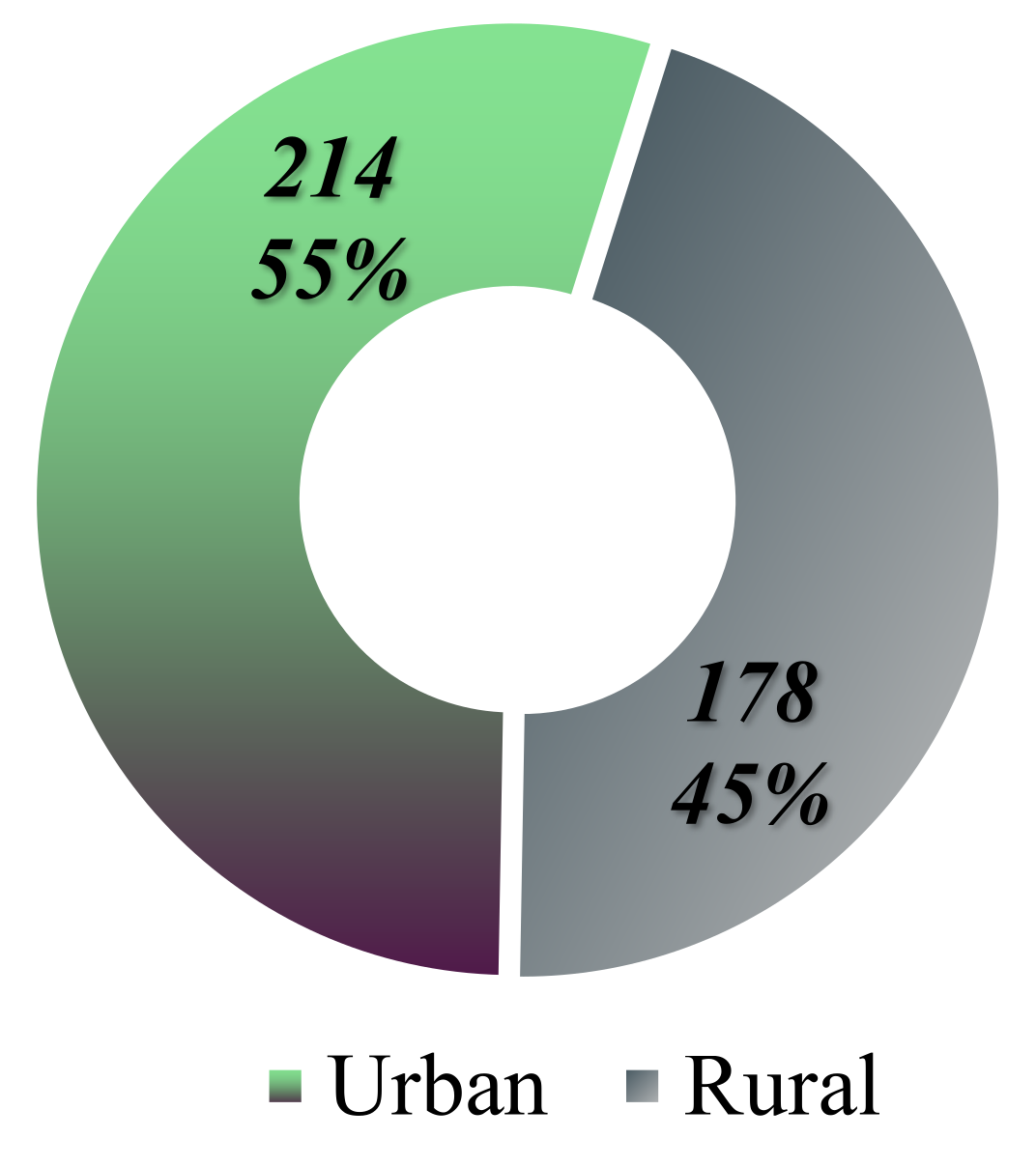
The distribution of cases based on the origin of the patients reveals that 214 individuals come from urban areas, while 178 are from rural settings (
Figure 7).
The study conducted in Iași indicates a predominance of malignant lymphoma diagnoses among individuals residing in urban areas, with a percentage of approximately 54.59%, equating to 214 patients. In contrast, 45.4% of the cases, or 178 patients, originate from rural areas (
Figure 8).
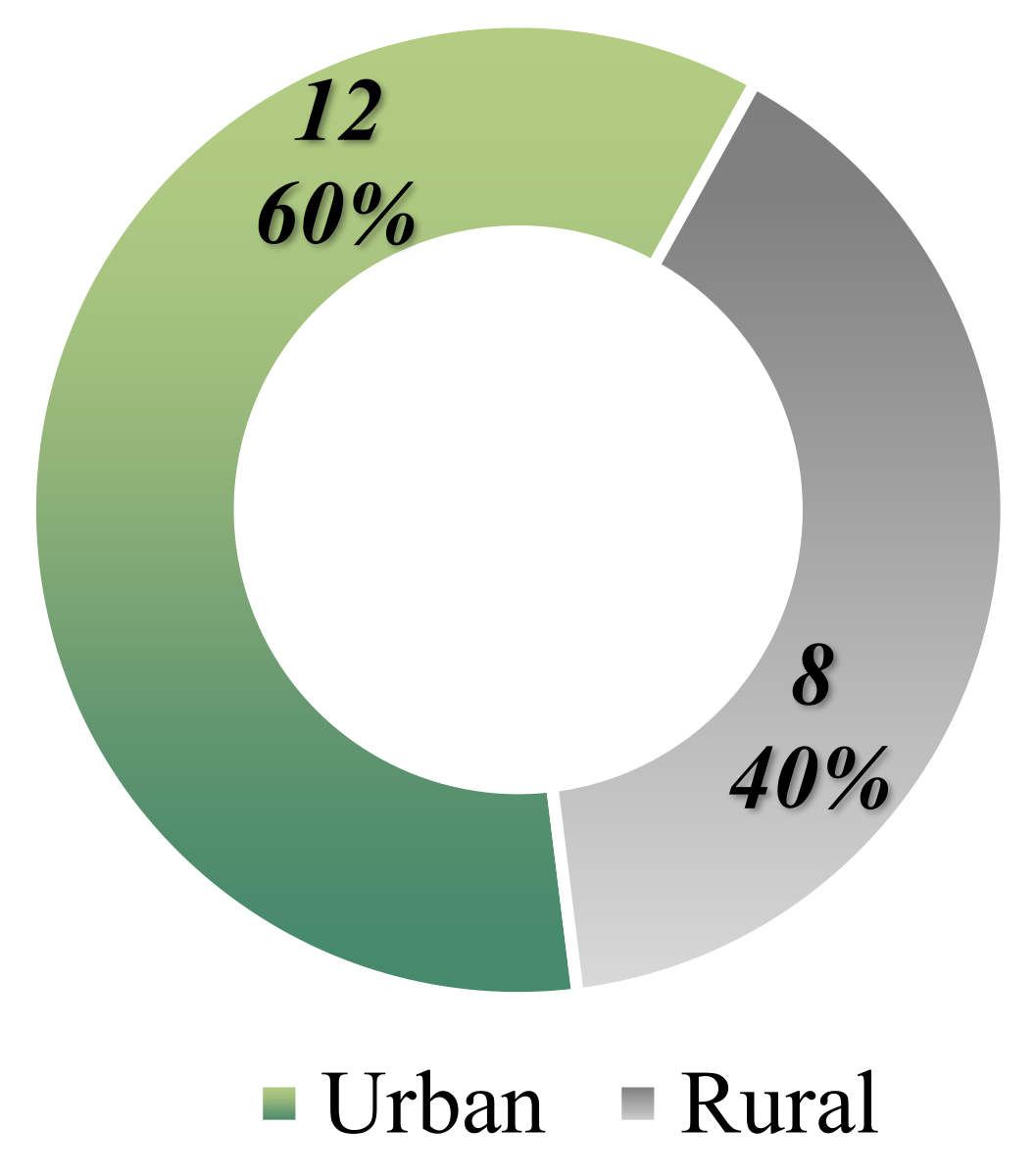
Among the 20 patients identified with increased genetic predisposition, 60% are from urban environments, with only 8 patients residing in rural areas (
Figure 9).
Familial malignancies were identified in 84 individuals, representing 21.42% of the total 392 cases of lymphomas (
Figure 10).
Focusing on the 20 patients with a high degree of genetic predisposition (defined as having at least one affected parent), it was determined that 50% of these patients have only first-degree relatives with a history of malignancy, 25% have only second-degree relatives affected, and the remaining 25% (5 cases) have both first- and second-degree relatives with malignancies (
Figure 11).
A critical objective of our study was to calculate the risk of recurrence. The genetic risk calculation was based on an empirical risk table (
Table 2), considering an approximate frequency of 1% and an estimated heritability of 60% (Smith, 1972).
Estimating the proportion of etiology attributed to genetic versus environmental and ecological factors is feasible, despite the challenge of directly measuring susceptibility to a particular disease, in this case HL or NHL. This challenge defines heritability. To evaluate the contribution of genetic determinants in the initiation of a condition or the emergence of a multifactorial trait, the heritability index (H²) can be calculated. The heritability coefficient is equal to the ratio of genetic variance (GV – additive genetic effects) to phenotypic variance (PV): H² = GV / PV. Understanding the heritability value, which represents the proportion of phenotypic variation due to genetic factors in a polygenic, multifactorial trait, is crucial for determining genetic susceptibility or predisposition to disease and for the prevention of multifactorial diseases such as malignant lymphomas with increased heritability (Tudose et al., 2000).
In the case of lymphomas, genetic factors are represented by multiple risk genes with minor yet additive effects, leading to a susceptibility to the disease. The number of these risk genes is highly variable. Multifactorial determinism involves a complex, continuous interaction between environmental and hereditary components, with a polygenic nature (the presence of multiple risk genes). The initiation of uncontrolled cell proliferation and the proliferation of cancerous clones can be triggered or exacerbated in susceptible individuals by exposure to certain environmental factors, leading to the transition to the actual disease and its specific symptomatology.
The achievement and surpassing of the risk threshold are closely interrelated with the number of mutant genes and allele variants that an individual inherits, whether maternally, paternally, or from both parental lines.
The evaluation of genetic risk in HL and NHL is conducted similarly to general risk assessments, following the values established in recurrence risk tables for multifactorial diseases. In cases where empirical risks are unattainable, the values specified in
Table 2 are utilized, which include previously established population frequency and heritability values. This table proves especially useful in analyzing families with multiple members affected by malignant hematological neoplasms. A critical point is that the table does not account for relatives diagnosed with HL or NHL beyond the second degree of relation (e.g., second, third, or fourth-degree relatives). Consequently, it is assumed that two second-degree relatives or multiple third or fourth-degree relatives with HL or NHL are equivalent to a single first-degree relative in terms of risk assessment. Therefore, if a family has one affected individual, whether in the first, second, third, or fourth degree of relation, the risk can be estimated using
Table 2.
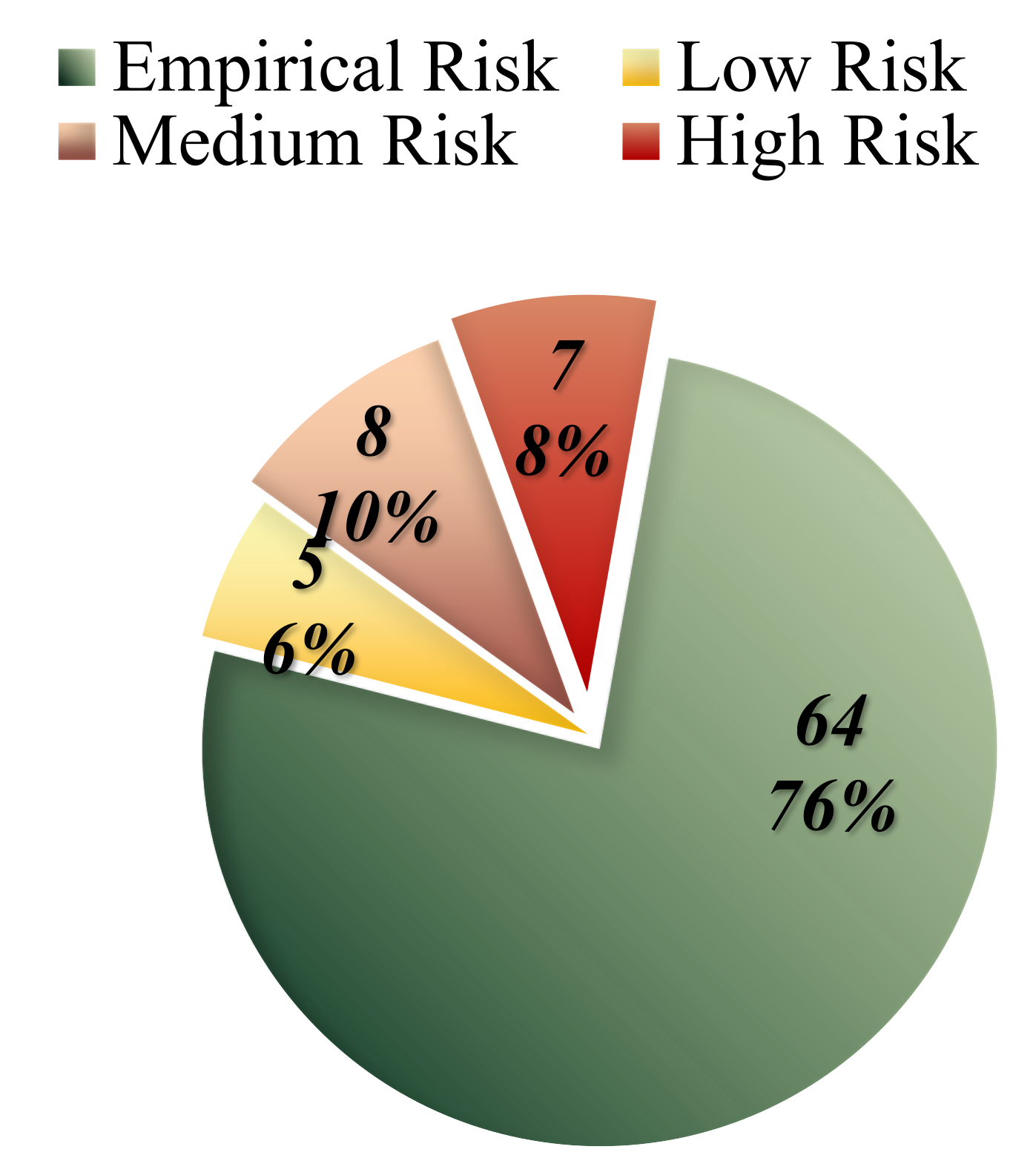
Based on this approach, the empirical risk of recurrence was estimated to be between 2% and 4% for the majority of cases studied with no affected relatives (64 out of 84 cases). However, in the seven familial cases (five of which are included in the high genetic load group) where two affected relatives were present, the risk ranged from 10% to 15%, indicating a high-risk level (
Table 3,
Figure 12).
The prophylactic activities involved providing genetic counseling, conducted according to the established standards of this complex and specialized medical practice. This process took into account the specific characteristics imposed by the educational background of the affected individuals, their ethical and religious beliefs, and the particularities associated with each type of malignant lymphoma.
Patients were advised that early detection of cancer is crucial. For individuals at increased risk, including their children and relatives, periodic screenings are essential. They were informed that if there is any suspicion, specific tests should be undertaken to detect the disease at its earliest stage. Early detection is aimed at increasing survival rates, improving treatment efficacy (since localized cancer is more effectively treated compared to aggressive, rapidly progressing forms detected in advanced stages, such as Stage III or IV, which have spread and metastasized to multiple regions of the body), and facilitating the therapeutic process. The variability in medication toxicity was also discussed, emphasizing that the degree of side effects and toxicity is generally lower with single-agent chemotherapy or localized radiotherapy compared to combination chemotherapy or radiotherapy involving extensive irradiation.
Conclusions
The analysis of the patient cohort highlights the necessity of studying environmental carcinogenic factors that may trigger inherited susceptibilities, as well as the importance of conducting genetic investigations and receiving expert recommendations.
The genetic study conducted on the cohort of patients at the Hematology Clinic of "Sfântul Spiridon" Hospital in Iași reveals that malignant HLs and NHLs hold a significant position among hematological neoplasms, accounting for 20.45% of the total cases. Although most individuals diagnosed do not have a familial history, resulting in an empiric recurrence risk estimated between 2% and 4%, predisposing diseases (such as rheumatoid arthritis, systemic lupus erythematosus, HIV, and EBV) and environmental carcinogenic factors, as well as the place of origin (urban environment), play a crucial role in the development of malignant lymphomas.
The study indicates that generally, older individuals (in their sixties), particularly those from urban environments and especially males, have a higher probability of being diagnosed with HL or NHL, and regular screenings are recommended for them.
Furthermore, this study underscores the importance of providing medical education to the general population. Most individuals tend to avoid consulting specialists or family doctors, often discovering these forms of cancer by chance. A small number of individuals in the studied cohort were diagnosed following routine check-ups, highlighting the need for increased awareness and proactive health management.
In the case of individuals with genetic predisposition, the study demonstrates a high risk of incidence for those with both parents affected or having a first-degree relative alongside a second-degree relative. It also underscores the importance of genetic counseling and screening at premarital, prenatal, and postnatal stages.
HLs and NHLs, classified within the broad spectrum of lymphoproliferative diseases, are noted for their complexity and heterogeneity, often presenting insidiously and being predominantly detectable at advanced, terminal stages, only when specific symptoms or metastasis become evident. Consequently, the study highlights the need for intensive research into the mechanisms of action and identification of individuals with heightened vulnerability. It also emphasizes the importance of public awareness about these conditions and the necessity of regular, preventive screenings.
Furthermore, while therapeutic interventions play a crucial role in curing the disease, the rates of mortality and partial remission remain a concern. The initial hypothesis suggests that conditions identified during a rapid progression or final stage exhibit greater resistance to recommended treatments. To address this, treatment efficacy is often enhanced through combinatory approaches (such as polychemotherapy and combining radiotherapy with chemotherapy) or increasing administered doses. However, intensifying treatment regimens proportionally increases the risk of toxicity and potential complications, adversely affecting the patient’s already challenging path to recovery.
Modern therapies employing pluripotent stem cells, CAR-T cells, or monoclonal antibodies show promising results, fostering hope for definitive eradication or the development of treatments that more rapidly and specifically target cancerous cells in malignant lymphomas. These advancements are actively studied to ensure patient safety and enhance efficacy, with the goal of making these treatments accessible to all affected individuals. Future medicine will likely integrate mixed immunotherapies, currently exploring combinations of lymphocytes with various anticancer agents, antibodies, immune-engaging cells, CARs, or checkpoint-modulating proteins.
In conclusion, with incidence rates continually rising, focusing research on uncovering the factors driving HLs and NHLs and the initiation of carcinogenesis processes, alongside the development of personalized modern therapies, is crucial in combating these trends effectively.
References
- Baeker Bispo, J.A., Pinheiro, P.S. and Kobetz, E.K. (2020) ‘Epidemiology and etiology of leukemia and lymphoma’, Cold Spring Harbor Perspectives in Medicine, 10(6). [CrossRef]
- Bassig, B.A. et al. (2012) ‘Current understanding of lifestyle and environmental factors and risk of non-Hodgkin lymphoma: An epidemiological update’, Journal of Cancer Epidemiology, 2012. [CrossRef]
- Bosch-Schips, J. et al. (2022a) ‘The Grey Zones of Classic Hodgkin Lymphoma’, Cancers, 14(3). [CrossRef]
- Bray, F. et al. (2018) ‘Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries’, CA: A Cancer Journal for Clinicians, 68(6), pp. 394–424. [CrossRef]
- Covic, M. (1976) Lucrări practice de biologie medicală. Iași.
- Covic, M. (1981) Curs de genetică medicală. Institutul de Medicină și Farmacie, Disciplina de Biologie si genetică medicală, Iași.
- Covic, M.; et al. (2017) Genetică Medicală. Editia a I. Iași: Polirom.
- Covic, M., Stefănescu, D. and Sandovici, I. (2004) Genetică medicală. Editura Polirom.
- De Leval, L. and Jaffe, E.S. (2020) ‘Lymphoma classification’, Cancer Journal (United States), 26(3), pp. 176–185. [CrossRef]
- EL-Sawalhy, E.; et al. (2021) ‘Grey Zone Lymphoma, Diagnostic and Therapeutic Challenges: A Rare Case Report’, American Journal of Medical Case Reports, 9(5), pp. 298–300. [CrossRef]
- Gorduza, E.V. (2007) Compendiu de genetică umană și medicală. Iași: Technopress.
- Ioniță, H.; et al. (2015) Hematologie Clinică. Timișoara: Editura Victor Babeș.
- Israil, A.M. (2000) Biologie moleculară. Prezent și perspective. București: HUMANITAS.
- Jaffe, E.S.; et al. (2001) ‘World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopopoietic and Lymphoid Tissues’, IARC Press:Lyon 2001 [Preprint].
- Mihăescu, Gr., Chifiriuc, C. and Dițu, L.-M. (2009) Imunobiologie. Editura Universității din București.
- Mihăescu, R.; et al. (2006) Bazele teoretice hematologice in laboratorul clinic - curs. Timișoara: LITO U.M.F. Timișoara.
- Mihăilă, R.-G. (2016) Noțiuni de hematologie clinică. Editura Universității „Lucian Blaga” din Sibiu.
- Ministerul Sanatatii (2021) Ghidul de practica medicala - aprobat prin ORDINUL nr. 219 din 23 februarie 2021, publicat în Monitorul Oficial, Partea I, nr. 189 din 25 februarie 2021.
- Oltean, G. (1996) Aspecte diagnostice si terapeutice in bolile hematologice. Editura Tipomur.
- Oltean, G.; et al. (2009) Curs de medicina interna - Bolile hematologice. Litografia U.M.F Targu-Mures.
- Smith, C. (1971). Recurrence Risks for Multifactorial Inheritance. American journal of human genetics, 23(6), 578–588.
- Sung, H.; et al. (2021) ‘Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries’, CA: A Cancer Journal for Clinicians, 71(3), pp. 209–249. Available at. [CrossRef]
- Swerdlow, S.H.; et al. (2017) ‘World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues’, IARC Press: Lyon, France, 4th ed.
- Tudose, C., Maniu, M. and Maniu, C. (2000) Genetică umană. Iași: Editura Corson.
- Zhang, Y.; et al. (2011) ‘Risk factors of non-Hodgkin’s lymphoma’, Expert Opin. Med. Diagn. - Informa UK, Ltd. ISSN 1753-0059, 5(6), pp. 539–550.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).