Submitted:
01 October 2024
Posted:
02 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Background
1.2. Objectives
2. Methods
2.1. Eligibility Criteria
2.1.1. Study Characteristics
- Copeptin measurement: We selected studies that measure baseline copeptin levels, and studies that assessed copeptin levels after hypertonic saline infusion or arginine stimulation. The protocol aims to achieve a target serum sodium level of approximately 150 mmol/L, corresponding to a serum osmolality of approximately 300 mOsm/kg [6,10].
- Water Deprivation Test: We considered all studies that utilized a standardized water deprivation protocol, which involves depriving the patient of fluid for up to eight hours or until a 3% loss in body weight is achieved. During the test, plasma osmolality was measured at regular intervals to ensure adequate increase in endogenous vasopressin release. The urine volume and osmolality were monitored throughout the test. Following the fluid deprivation test, the patient was administered arginine desmopressin, a synthetic form of vasopressin, and urine volume and osmolality were measured to assess the response to exogenous vasopressin [1,5,6].
- Other relevant alternative diagnostic tests for patients presenting with diabetes insipidus related symptoms.
- For copeptin measurement, studies must include specific determination of baseline copeptin levels. Another test that will be assessed is represented by the copeptin levels after intravenous infusion of 3% NaCl solution, as well as the arginine stimulation test, or other copeptin stimulation methods. These mechanisms aim to achieve a target serum sodium level of approximately 150 mmol/L, corresponding to a serum osmolality of approximately 300 mOsm/kg.
- Additionally, studies comparing copeptin with other traditional diagnostic methods such as the water deprivation test were considered. This test is usually extended for 8 hours, and is followed by arginine desmopressin administration, in order to assess the urinary response, or ADH. To be included, the study should have recorded serum sodium levels ranging from 145 mmol/L to 150 mmol/L and urinary osmolality ranging from 300 to 1200 mOsm/kg.
2.1.2. Report Characteristics
2.2. Information Sources
2.3. Search Strategy
2.4. Study Selection
2.5. Data Collection Process
2.6. Definitions for Data Extraction
2.7. Risk of Bias and Applicability
2.8. Principal Diagnostic Accuracy Measures
2.9. Data Handling for Synthesis of Results
- Standardization of Target Conditions: Definitions of conditions such as CDI, NDI, and PP were standardized using clinical criteria across studies.
- Harmonization of threshold: Different thresholds for test positivity were harmonized, and subgroup analyses were performed according to different diagnostic methods. The use of Hierarchical Summary Receiver Operating Characteristic (HSROC) modeling further allowed us to account for and understand these threshold effects, providing a more nuanced analysis of diagnostic accuracy across studies.
- Indeterminate results: Clear definitions and sensitivity analyses were used for copeptin levels within a borderline range, and statistical adaptations were performed for undefined values when necessary.
- Meta-Analysis techniques: were carried out using fixed-effects models applied for the assessment of measures of diagnostic accuracy because the studies showed low heterogeneity. Heterogeneity was assessed using the I² statistic, Cochran’s Q test and Tau-squared (τ²) test. Statistical analyses were performed following standard meta-analysis protocols, to ensure robust and reliable estimates, using forest plots to visually compare diagnostic accuracy. Furthermore, we used the HSROC model to assess threshold variability and to obtain a deeper insight into diagnostic accuracy across studies [7].
2.10. Additional Analyses
3. Results
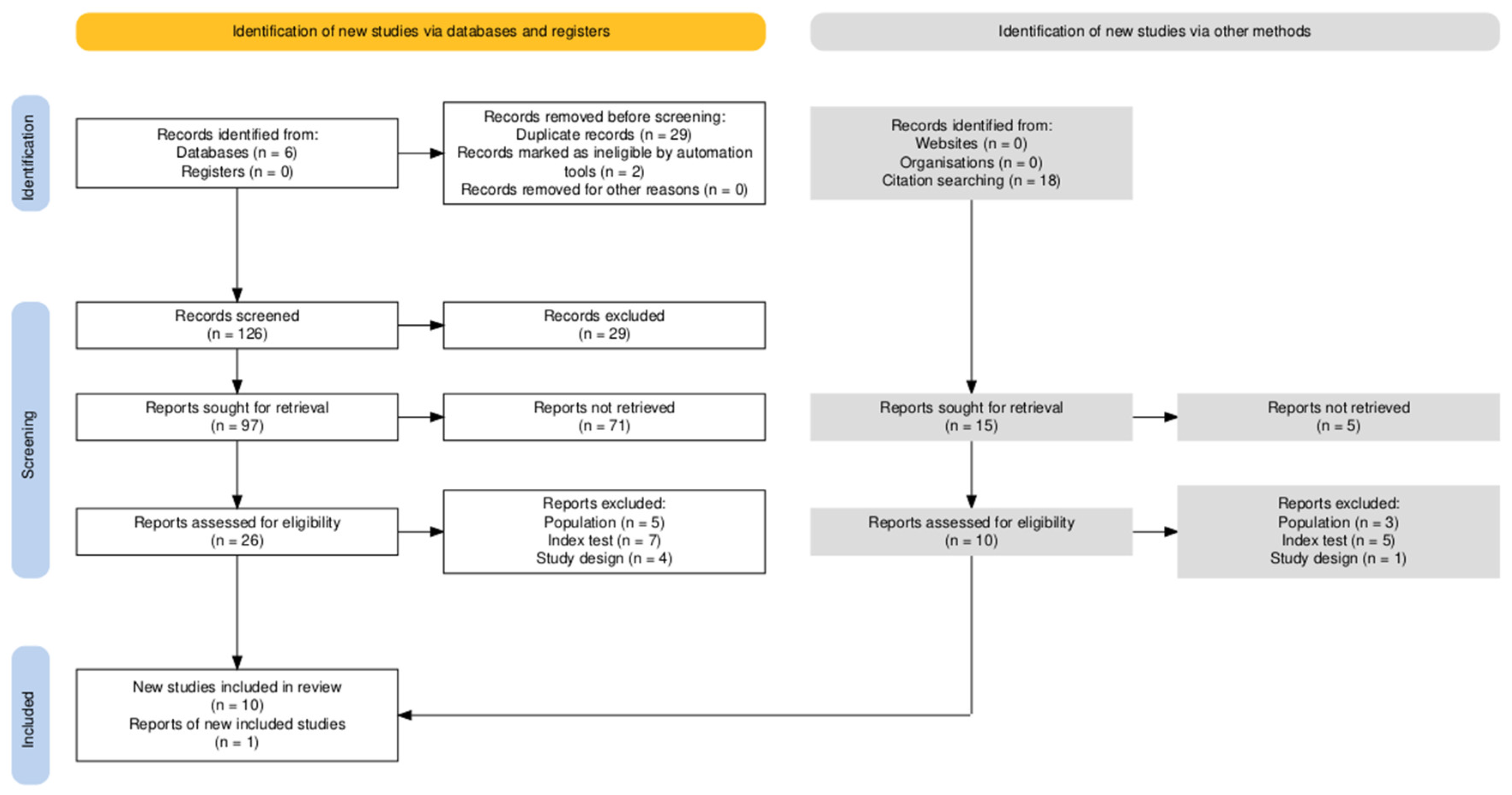
3.1. Study Selection
- Population:
- Index Test:
- Target condition:
3.2. Study Characteristics
3.3. Quality Assessment and Publication Bias
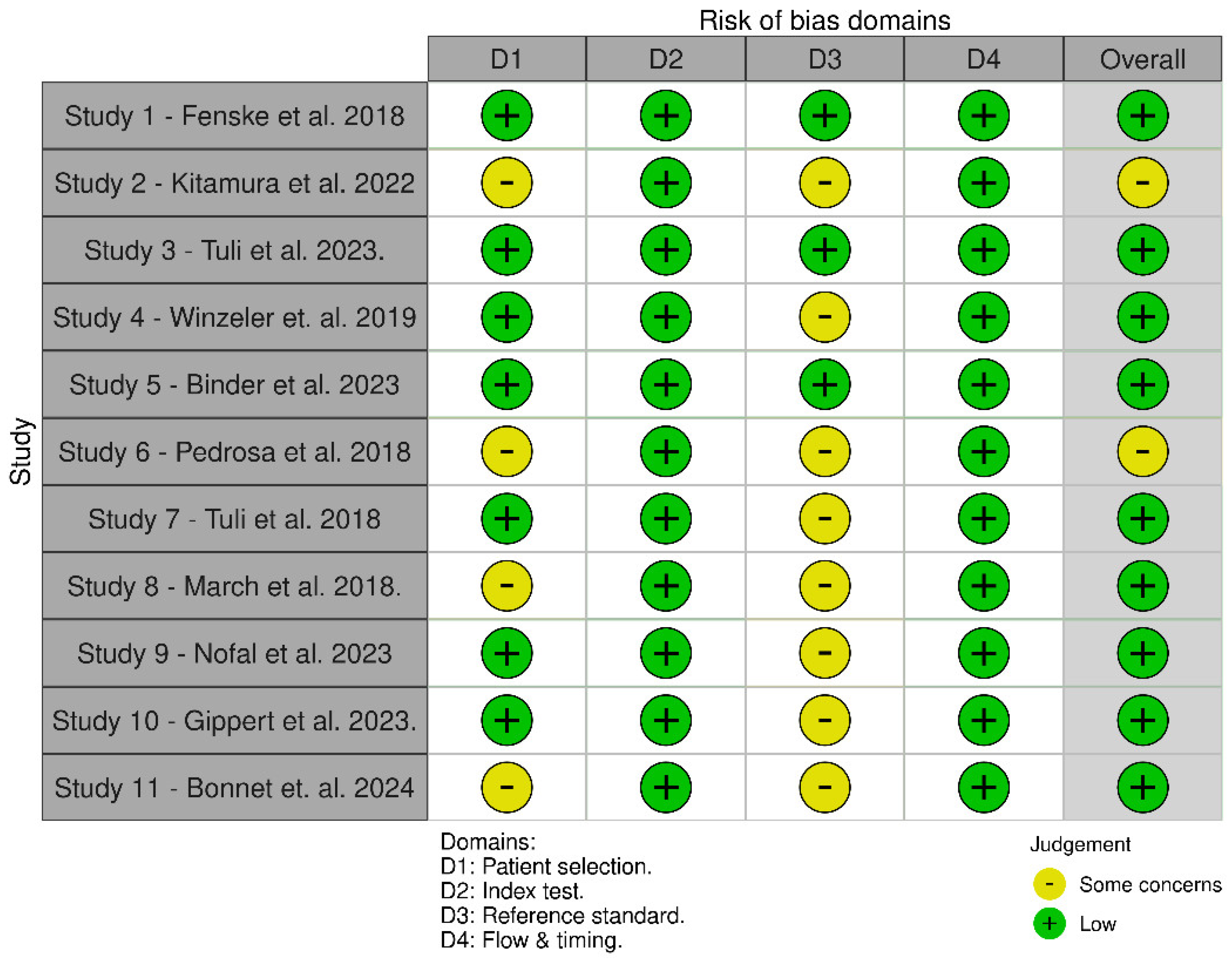
3.3.1. QUADAS-2 Was Used to Assess the Quality of the Diagnostic Accuracy Studies. It Evaluates the Risk of Bias and Applicability Concerns in Four Key Domains. Detailed Assessment is Available in Appendix C
3.4. Results of Individual Studies
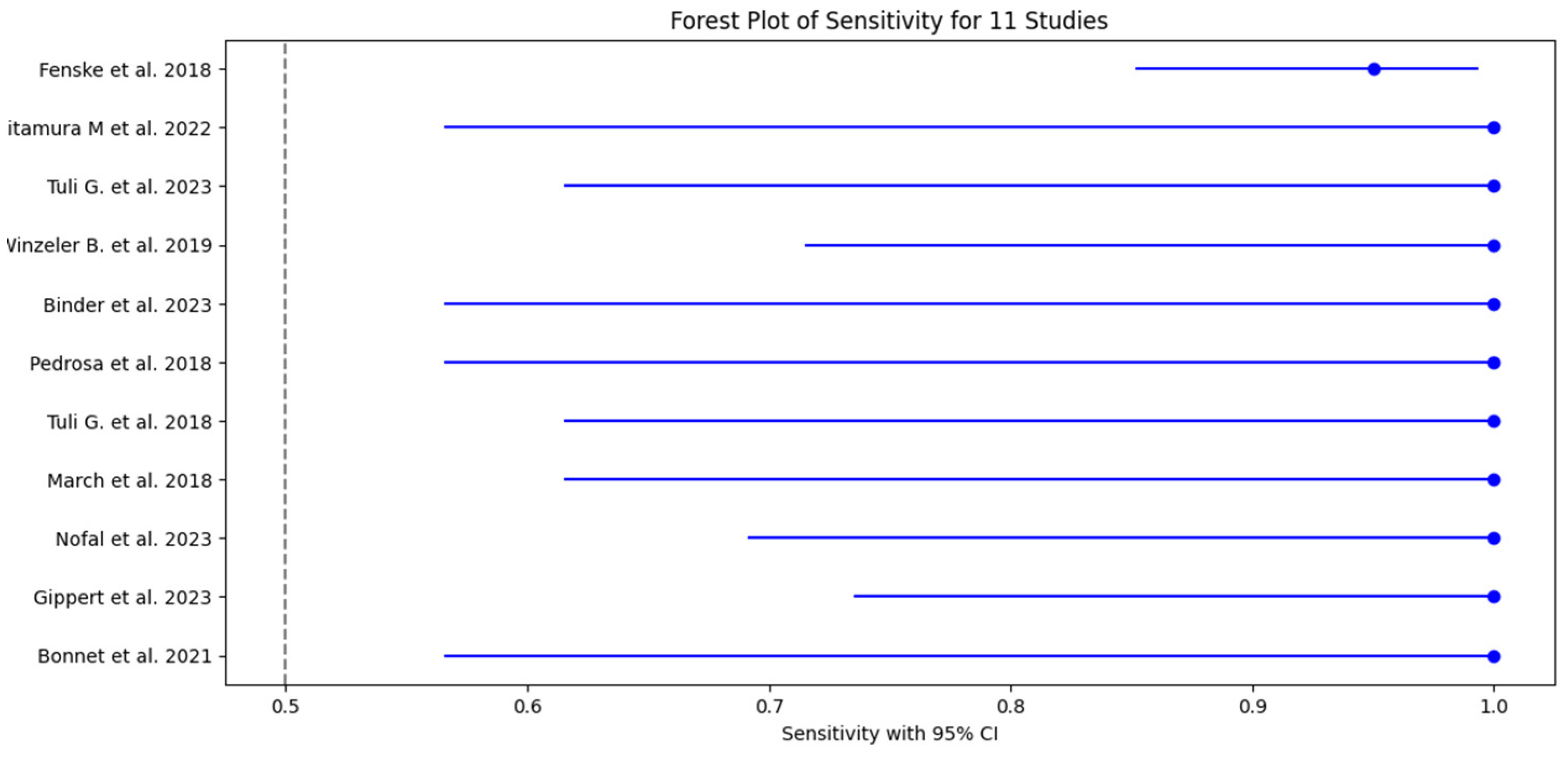
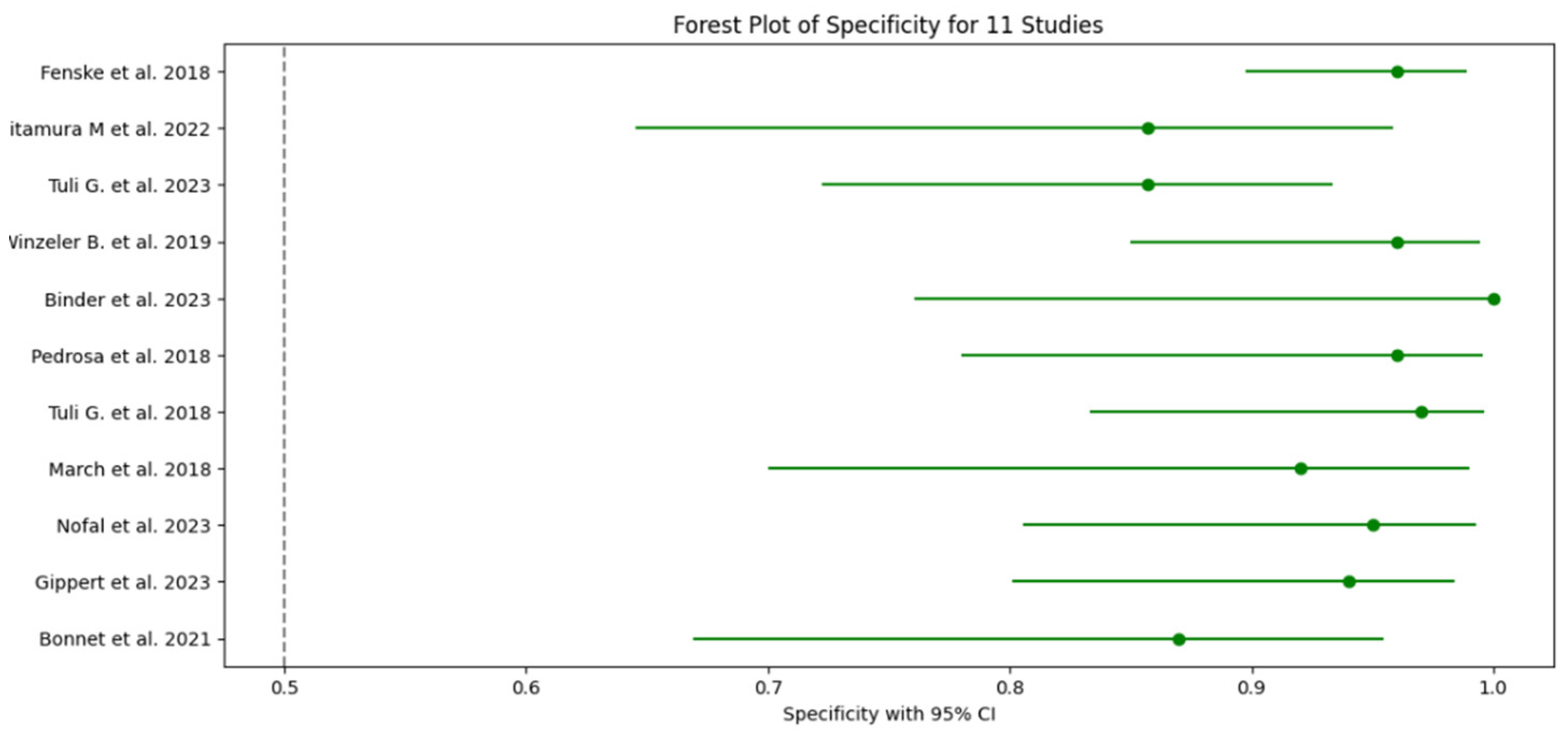
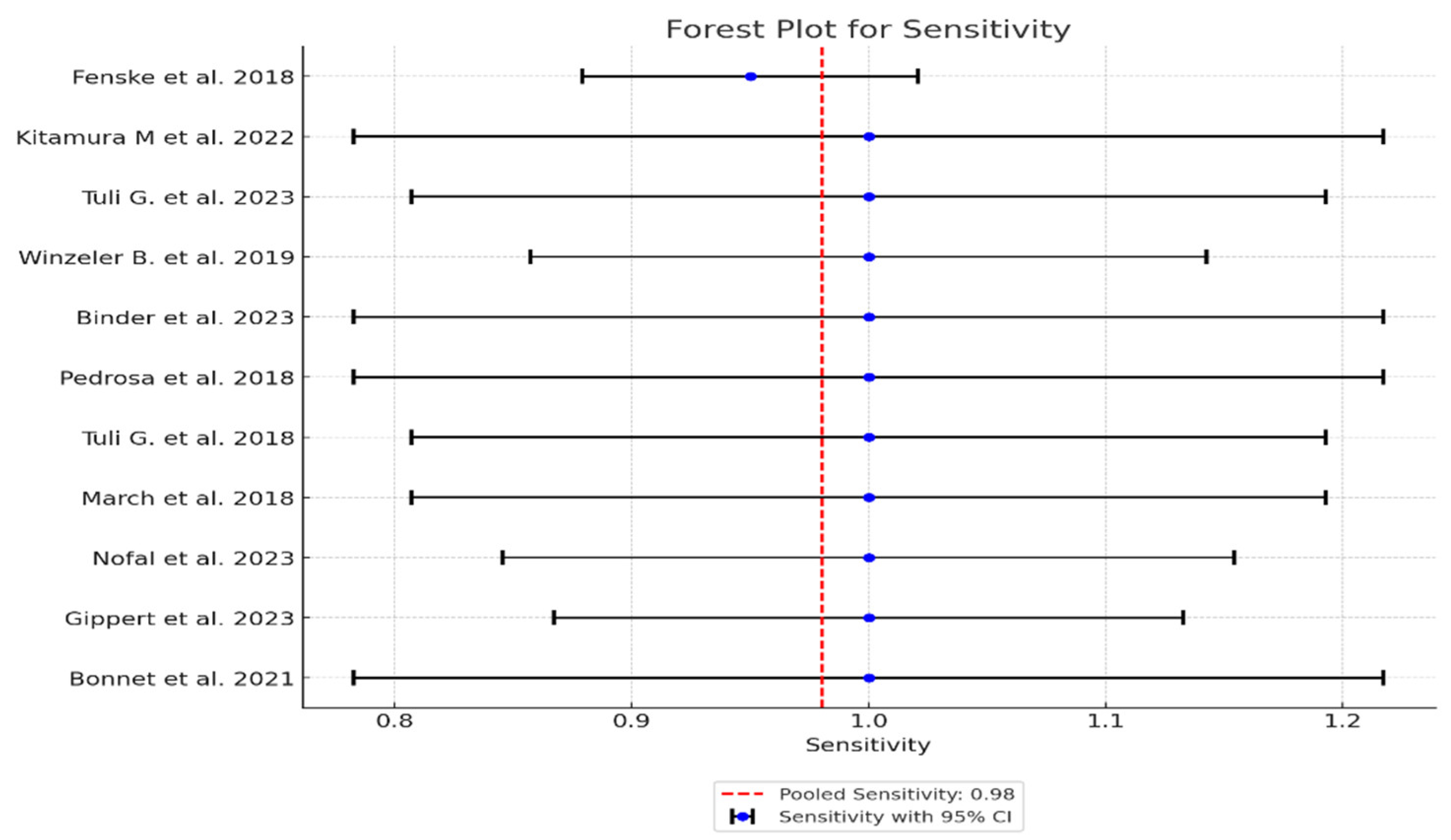
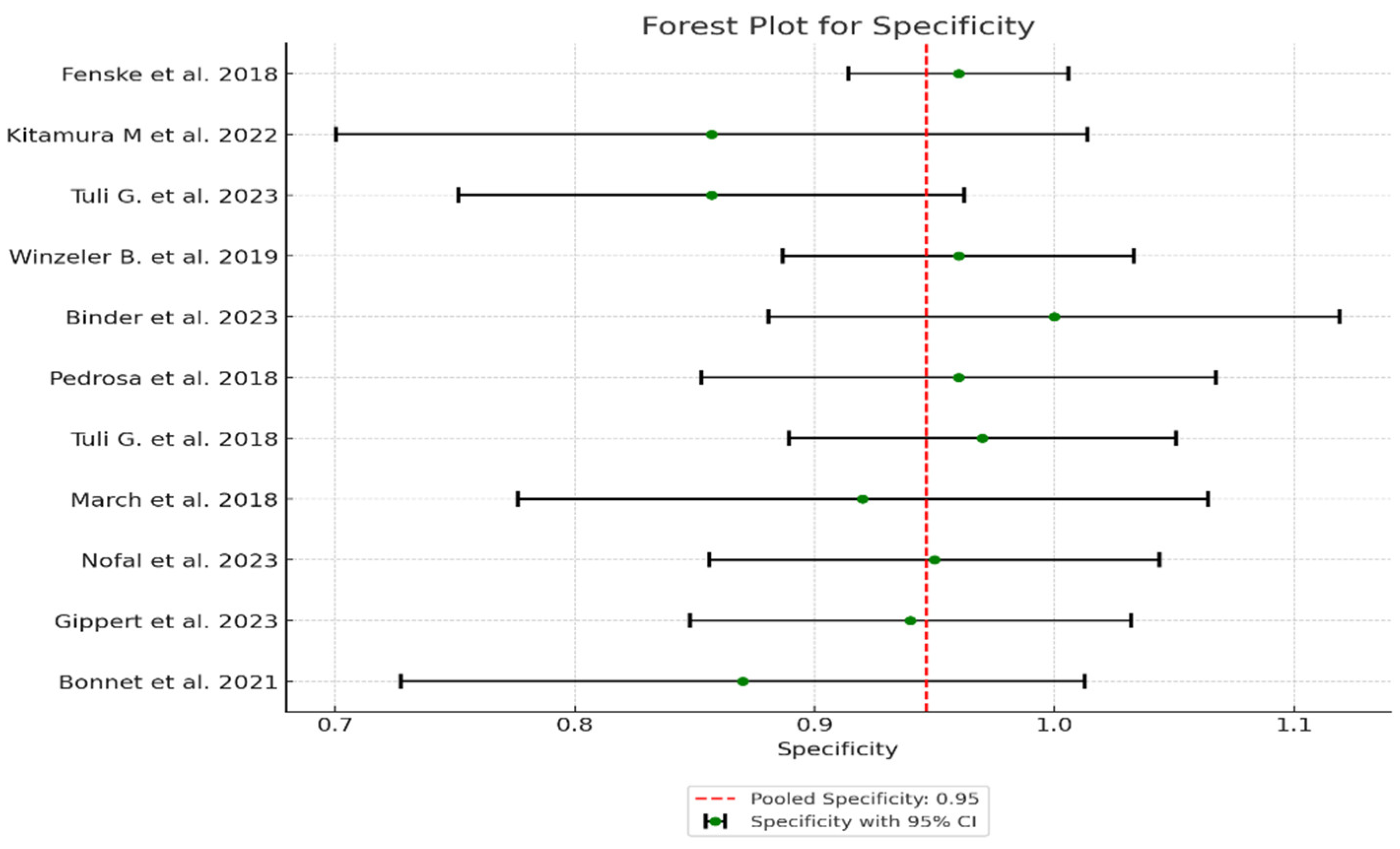
3.5. Test Accuracy and Variability
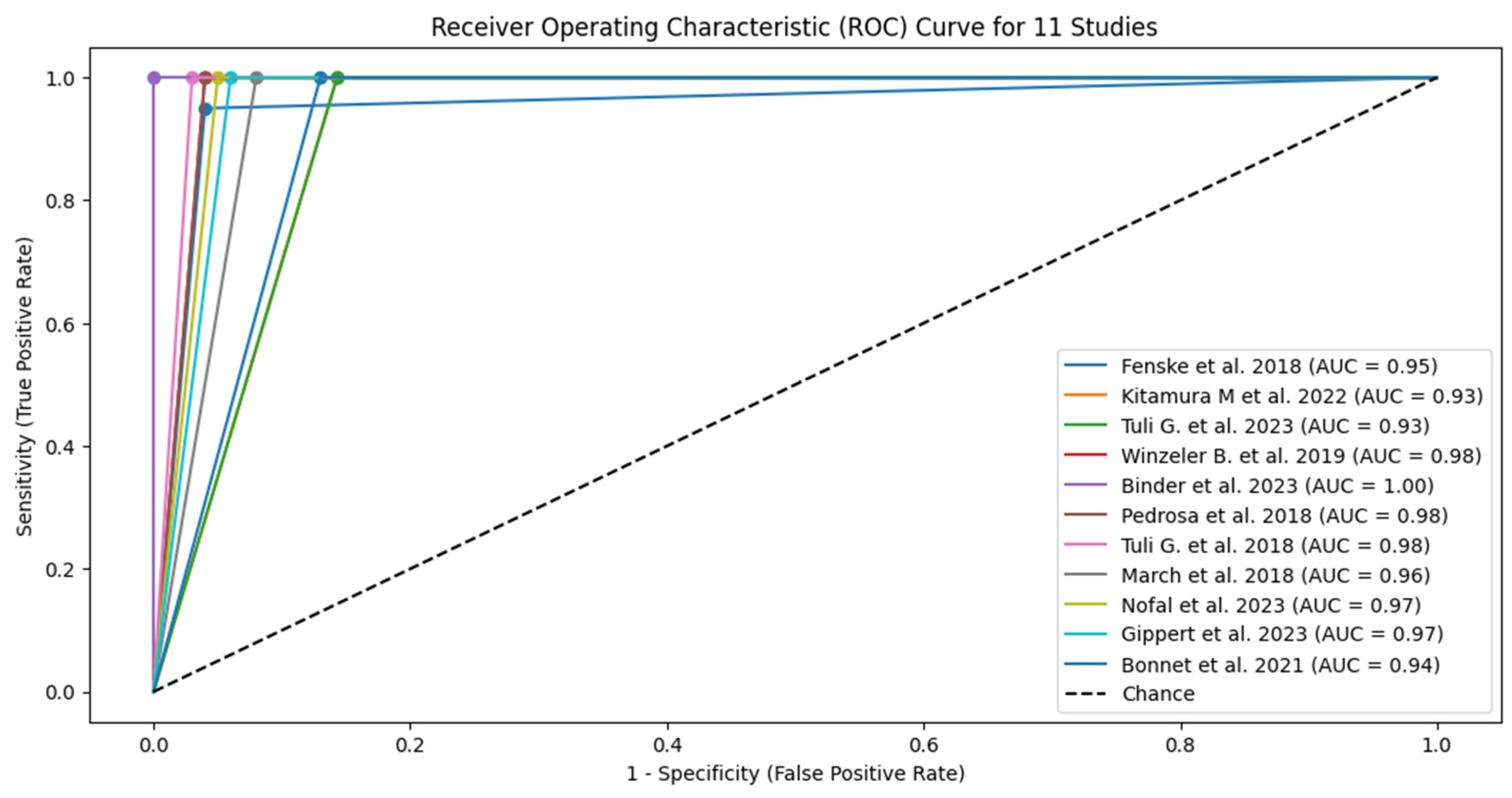
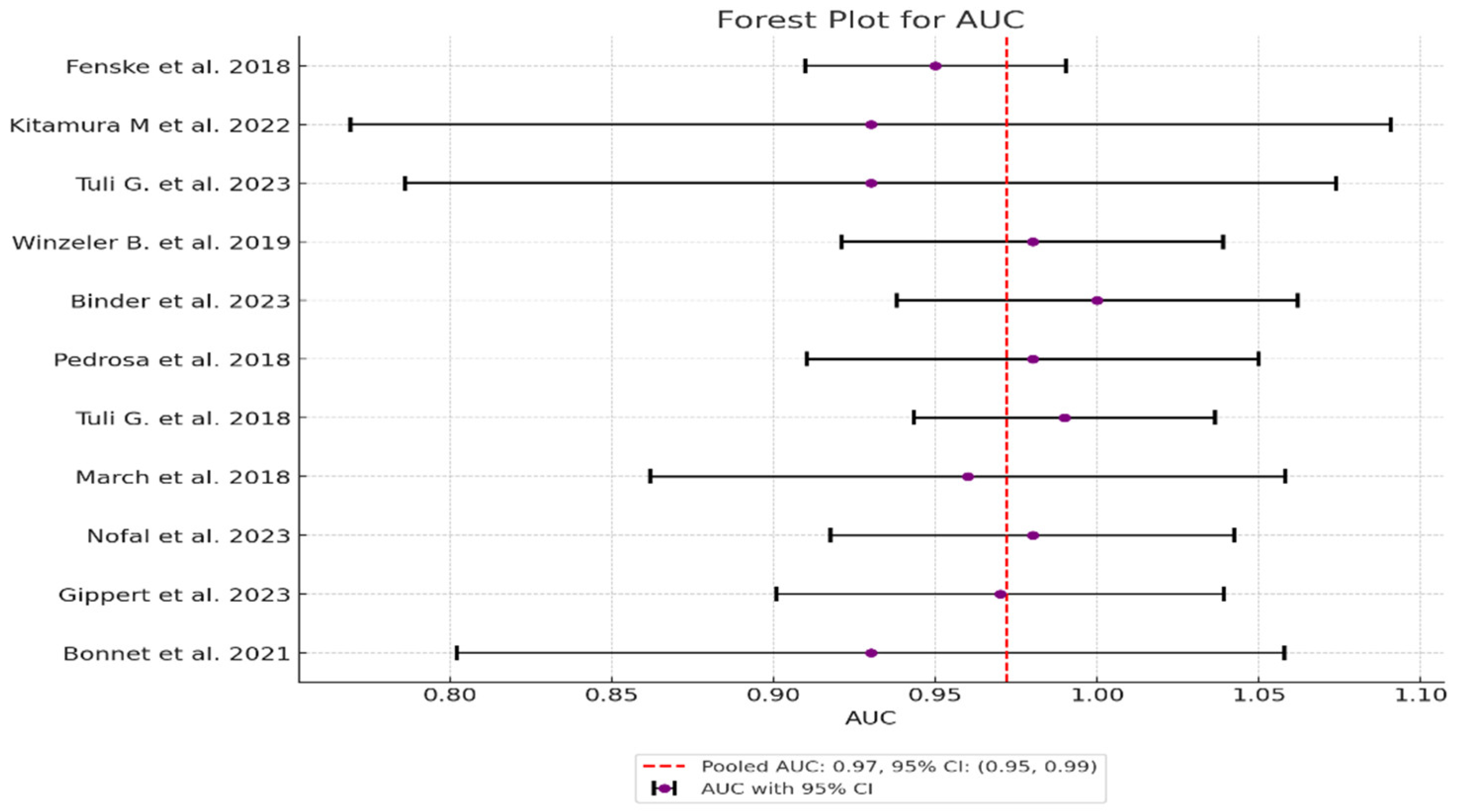
3.6. Meta-Analysis
3.6.1. HSROC Model Results
3.6.2. Assessment of Heterogeneity
3.6.3. Publication Bias Assessment
3.6.4. Sensitivity Analysis Based on Risk of Bias
3.6.5. Sensitivity Analysis after Excluding Adult-Inclusive Studies
3.7. Sensitivity of Sub-Group Analysis for Main Diagnostic Methods
4. Discussion
4.1. Principal Findings
4.2. Comparison with Previous Studies
4.3. Clinical Implications
4.4. Limitations
5. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Table A1 - Search Strategy
| Database | Search String | Limits |
| PubMed | ((“Polyuria”[Mesh] OR “Polydipsia”[Mesh] OR “Central Diabetes Insipidus”[Mesh] OR “Primary Polydipsia”[Mesh] OR “Nephrogenic Diabetes Insipidus”[Mesh]) AND (“Arginine Vasopressin”[Mesh] OR “C-Terminal Provasopressin”[Mesh] OR “Water Deprivation Test”[Mesh] OR “Desmopressin”[Mesh] OR “Copeptin”[Mesh] OR “Baseline Copeptin” OR “Copeptin Stimulation” OR “Copeptin Test” OR “Saline Infusion Test” OR “Arginine Stimulation”) AND (“Child”[Mesh] OR “Adolescent”[Mesh]) AND (“Diagnostic Accuracy” OR “Sensitivity” OR “Specificity” OR “ROC” OR “AUC” OR “Predictive Value”)) | Publication date from 2018 to 2024, Humans, Children and Adolescents |
| Cochrane Library | ((Polyuria OR Polydipsia OR “Central Diabetes Insipidus” OR “Primary Polydipsia” OR “Nephrogenic Diabetes Insipidus”) AND (“Arginine Vasopressin” OR “C-Terminal Provasopressin” OR “Water Deprivation Test” OR “Desmopressin” OR “Copeptin” OR “Baseline Copeptin” OR “Copeptin Stimulation” OR “Copeptin Test” OR “Saline Infusion Test” OR “Arginine Stimulation”) AND (Child OR Adolescent) AND (“Diagnostic Accuracy” OR “Sensitivity” OR “Specificity” OR “ROC” OR “AUC” OR “Predictive Value”)) | Publication date from 2018 to 2024, Trials, Reviews |
| Web of Science | (TS=(Polyuria OR Polydipsia OR “Central Diabetes Insipidus” OR “Primary Polydipsia” OR “Nephrogenic Diabetes Insipidus”) AND TS=(“Arginine Vasopressin” OR “C-Terminal Provasopressin” OR “Water Deprivation Test” OR “Desmopressin” OR “Copeptin” OR “Baseline Copeptin” OR “Copeptin Stimulation” OR “Copeptin Test” OR “Saline Infusion Test” OR “Arginine Stimulation”) AND TS=(Child OR Adolescent) AND TS=(“Diagnostic Accuracy” OR “Sensitivity” OR “Specificity” OR “ROC” OR “AUC” OR “Predictive Value”)) | Timespan: 2018-2024, Indexes: SCI-EXPANDED, SSCI, A&HCI, ESCI |
| ScienceDirect | Search String 1: (Polyuria OR Polydipsia OR “Central Diabetes Insipidus” OR “Primary Polydipsia”) AND (Copeptin OR “Arginine Vasopressin’‘ OR ‘‘Water deprivation test’‘) AND (Child OR Adolescent)Search String 2: (“Water Deprivation Test” OR Desmopressin OR “Copeptin Stimulation” OR “Saline Infusion Test”) AND (“Central Diabetes Insipidus” OR ‘‘Nephrogenic Diabetes Insipidus’‘ OR “Primary Polydipsia”) AND (Child OR Adolescent) | Date: 2018-2024, Article type: Research Articles |
| Scopus | TITLE-ABS-KEY(Polyuria OR Polydipsia OR “Central Diabetes Insipidus” OR “Primary Polydipsia” OR “Nephrogenic Diabetes Insipidus”) AND TITLE-ABS-KEY(“Arginine Vasopressin” OR “C-Terminal Provasopressin” OR “Water Deprivation Test” OR “Desmopressin” OR “Copeptin” OR “Baseline Copeptin” OR “Copeptin Stimulation” OR “Copeptin Test” OR “Saline Infusion Test” OR “Arginine Stimulation”) AND TITLE-ABS-KEY(Child OR Adolescent) AND TITLE-ABS-KEY(“Diagnostic Accuracy” OR “Sensitivity” OR “Specificity” OR “ROC” OR “AUC” OR “Predictive Value”) | Limits: Date: 2018-2024, Document type: Article; Humans; Child |
| Google Scholar | (Polyuria OR Polydipsia OR “Central Diabetes Insipidus” OR ‘’ Nephrogenic Diabetes Insipidus’’ OR “Primary Polydipsia”) AND (Copeptin OR “Arginine Vasopressin” OR “Water deprivation test”) AND (Child OR Adolescent) AND (Diagnosis OR “Diagnostic Accuracy” OR Sensitivity OR Specificity OR “ROC Curve”) -treatment -meta-analysis -case-report | Date: 2018-2024 |
Appendix B. Independent Read and Assessment of Quality Using QUADAS-2
- Independent Assessment: In each review, the two reviewers independently assessed the risk of bias and applicability using the QUADAS-2 tool. Any disagreement was resolved through discussion or consultation with a third reviewer to reach an agreement.
- Data Extraction Forms: The data extraction forms were piloted in a few studies to determine consistency and clarity. The extracted data included items on study design, patient demographics, details of the index test and reference standard, and measures of diagnostic accuracy. This allowed data extraction in all studies reviewed for the overview to be systematic and comprehensive.
- QUADAS-2 tool domains: The QUADAS-2 tool was run against these studies with respect to four key domains:
- Patient Selection: The review examined how participants were selected and the appropriateness of the selection criteria in projecting the possibility of selection bias. Common sources of bias were observed to originate from the retrospective study designs and small populations.
- Index Test: The focus was on the diagnostic test being studied, carried out and interpreted; in particular, whether investigators applied the reference standard blind. There are various methods of copeptin measurement and stimulation protocols across studies.
- Reference Standard: This domain examines the validity and applicability of the reference standard used to classify the subjects regarding the target condition. The reliability of standards such as WDT and plasma arginine-vasopressin analysis was generally well supported, although some studies varied in their application. In some cases, the reference standard was based on expert opinion without additional objective measures that could introduce some subjectivity, or was based on clinical judgment without blinding, which could also induce bias.
- Flow and Timing. In the methodology assessment it was examined whether investigations had been carried out with appropriate timing between the index test and the reference standard. Retrospective study designs often yield variability in timing, whereas prospective designs are very rare. Timing was usually adequate across studies; however, because of the variability in timing between the index test and the reference standard in retrospective studies, the results of some of these studies may not be generalized to clinical practice.
- Quality Assessment Findings: Results relating to the risk of bias and concerns relating to the appraisal of applicability are summarized in one structured table (Appendix C), indicating areas of potential bias and issues with respect to generalizability, as well as graphic representation using “traffic light” plots (Figure 1) of the domain-level judgements for each individual study, created using the ROBVIS tool.
- Sensitivity Analysis: A sensitivity analysis was performed to assess the contribution of studies with medium risk of bias in the overall results. The exclusion of studies with noticeable bias did not materially alter the overall findings, thus supporting the fact that the conclusions of this review are robust despite the identified risks of bias.
- Applicability Issues: Patient selection: The studies differed in age range, with most targeting the pediatric population. Nevertheless, some of the studies included adults and pediatric patients; therefore, generalization of the results to a pediatric population may be diminished.
- Index test conduct: Possible differences in the performance characteristics of copeptin measurement may bear on the modes of stimulation that affect its generalizability. Thus, standardization of test procedures is suggested for better comparability in further studies.
- Reference Standard: In general, the test protocols were consistent and reliable. The use of expert judgement without blinding is likely to influence study comparability. Future studies should strive toward greater consistency in applying reference standards to enhance the reliability of the pooled analyses.
- Flow and Timing: While generally appropriate, the retrospective design of some studies probably introduced potential variability in the timing of index tests and reference standards, which may affect the applicability of these findings to routine clinical practice. For this reason, future studies should establish the timing in a way that delineates consistency so that the results are more reliable and applicable.
Appendix C. Table C1 - QUADAS-2 Assessment Summary.
| Study | Patient Selection (Risk of Bias) | Index Test (Risk of Bias) | Reference Standard (Risk of Bias) | Flow and Timing (Risk of Bias) | Overall Summary of Bias | Observations |
|
Low (some concerns on applicability) | Low | Low (minor subjectivity) | Low | Low overall risk | The study involved patients from tertiary centers, which may not represent the general population. |
|
Moderate (retrospective, single-center) | Low to Moderate (potential blinding issues) | Moderate (expert consensus) | Low | Moderate overall risk | Retrospective design and single-center setting increase the selection bias; possible lack of blinding. |
|
Low | Low | Low | Low | Low overall risk | Prospective design with a well-defined patient population, reducing bias. |
|
Low (concerns about applicability to pediatric) | Low | Low to Moderate (subjectivity) | Low | Low overall risk | The study is focused on adults, limiting applicability to pediatric populations. |
|
Low | Low | Low | Low | Low overall risk | Clear protocol and prospective design contribute to low bias across domains. |
|
Moderate (retrospective, single-center) | Low | Moderate (subjectivity) | Low | Moderate overall risk | Retrospective design and reliance on expert opinion without blinding introduce moderate bias. |
|
Low | Low | Moderate (subjectivity) | Low | Low overall risk | Prospective study with rigorous methods, though expert consensus introduces some subjectivity. |
|
Low to Moderate (single-center setting) | Low | Moderate (subjectivity) | Low | Low to Moderate overall risk | Single-center design may affect generalizability, and expert consensus introduces subjectivity. |
|
Low | Low | Moderate (subjectivity) | Low | Low overall risk | Multicenter design reduces selection bias, but expert consensus may introduce some subjectivity. |
|
Low | Low | Moderate (subjectivity) | Low | Low overall risk | Consistent methods across centers, though reliance on expert consensus introduces moderate bias. |
|
Low to Moderate (single-center setting) | Low | Moderate (subjectivity) | Low | Low to Moderate overall risk | Single-center design could limit generalizability; expert consensus introduces moderate bias. |
References
- Gubbi S, H.-S. F. K. C. et al. Diagnostic Testing for Diabetes Insipidus. [Updated 2022 Nov 28]. . Endotext. https://www.ncbi.nlm.nih.gov/books/NBK537591/ (accessed 2024-03-28).
- Yoshimura, M.; Conway-Campbell, B.; Ueta, Y. Arginine Vasopressin: Direct and Indirect Action on Metabolism. Peptides (N.Y.) 2021, 142, 170555. [Google Scholar] [CrossRef] [PubMed]
- Fenske, W.; Quinkler, M.; Lorenz, D.; Zopf, K.; Haagen, U.; Papassotiriou, J.; Pfeiffer, A.F.H.; Fassnacht, M.; Störk, S.; Allolio, B. Copeptin in the Differential Diagnosis of the Polydipsia-Polyuria Syndrome—Revisiting the Direct and Indirect Water Deprivation Tests. J Clin Endocrinol Metab 2011, 96, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Fenske, W.; Refardt, J.; Chifu, I.; Schnyder, I.; Winzeler, B.; Drummond, J.; Ribeiro-Oliveira, A.; Drescher, T.; Bilz, S.; Vogt, D.R.; Malzahn, U.; Kroiss, M.; Christ, E.; Henzen, C.; Fischli, S.; Tönjes, A.; Mueller, B.; Schopohl, J.; Flitsch, J.; Brabant, G.; Fassnacht, M.; Christ-Crain, M. A Copeptin-Based Approach in the Diagnosis of Diabetes Insipidus. New England Journal of Medicine 2018, 379, 428–439. [Google Scholar] [CrossRef]
- Driano, J.E.; Lteif, A.N.; Creo, A.L. Vasopressin-Dependent Disorders: What Is New in Children? Pediatrics 2021, 147. [Google Scholar] [CrossRef]
- Mu, D.; Ma, Y.; Cheng, J.; Qiu, L.; Chen, S.; Cheng, X. Diagnostic Accuracy of Copeptin in the Differential Diagnosis of Patients With Diabetes Insipidus: A Systematic Review and Meta-Analysis. Endocrine Practice 2023, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Deeks JJ; Bossuyt PM; Leeflang MM, T.; akwoingi Y. Chapter PDFs of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (v2.0) | Cochrane Training. https://training.cochrane.org/handbook-diagnostic-test-accuracy/current (accessed 2024-08-02).
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; Hunt, H.A.; Hyde, C.J.; Korevaar, D.A.; Leeflang, M.M.G.; Macaskill, P.; Reitsma, J.B.; Rodin, R.; Rutjes, A.W.S.; Salameh, J.P.; Stevens, A.; Takwoingi, Y.; Tonelli, M.; Weeks, L.; Whiting, P.; Willis, B.H. Preferred Reporting Items for a Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Studies The PRISMA-DTA Statement. JAMA - Journal of the American Medical Association 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Salameh, J.-P.; Bossuyt, P.M.; McGrath, T.A.; Thombs, B.D.; Hyde, C.J.; Macaskill, P.; Deeks, J.J.; Leeflang, M.; Korevaar, D.A.; Whiting, P.; Takwoingi, Y.; Reitsma, J.B.; Cohen, J.F.; Frank, R.A.; Hunt, H.A.; Hooft, L.; Rutjes, A.W.S.; Willis, B.H.; Gatsonis, C.; Levis, B.; Moher, D.; McInnes, M.D.F. Preferred Reporting Items for Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Studies (PRISMA-DTA): Explanation, Elaboration, and Checklist. BMJ 2020, m2632. [Google Scholar] [CrossRef]
- Fenske, W.; Refardt, J.; Chifu, I.; Schnyder, I.; Winzeler, B.; Drummond, J.; Ribeiro-Oliveira, A.; Drescher, T.; Bilz, S.; Vogt, D.R.; Malzahn, U.; Kroiss, M.; Christ, E.; Henzen, C.; Fischli, S.; Tönjes, A.; Mueller, B.; Schopohl, J.; Flitsch, J.; Brabant, G.; Fassnacht, M.; Christ-Crain, M. A Copeptin-Based Approach in the Diagnosis of Diabetes Insipidus. New England Journal of Medicine 2018, 379, 428–439. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; Chou, R.; Glanville, J.; Grimshaw, J.M.; Hróbjartsson, A.; Lalu, M.M.; Li, T.; Loder, E.W.; Mayo-Wilson, E.; McDonald, S.; McGuinness, L.A.; Stewart, L.A.; Thomas, J.; Tricco, A.C.; Welch, V.A.; Whiting, P.; Moher, D. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, n71. [Google Scholar] [CrossRef]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; Hunt, H.A.; Hyde, C.J.; Korevaar, D.A.; Leeflang, M.M.G.; Macaskill, P.; Reitsma, J.B.; Rodin, R.; Rutjes, A.W.S.; Salameh, J.-P.; Stevens, A.; Takwoingi, Y.; Tonelli, M.; Weeks, L.; Whiting, P.; Willis, B.H. Preferred Reporting Items for a Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Studies. JAMA 2018, 319, 388. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; Blunt, H.; Brigham, T.; Chang, S.; Clark, J.; Conway, A.; Couban, R.; de Kock, S.; Farrah, K.; Fehrmann, P.; Foster, M.; Fowler, S.A.; Glanville, J.; Harris, E.; Hoffecker, L.; Isojarvi, J.; Kaunelis, D.; Ket, H.; Levay, P.; Lyon, J.; McGowan, J.; Murad, M.H.; Nicholson, J.; Pannabecker, V.; Paynter, R.; Pinotti, R.; Ross-White, A.; Sampson, M.; Shields, T.; Stevens, A.; Sutton, A.; Weinfurter, E.; Wright, K.; Young, S. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst Rev 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Fenske, W.; Kühn, F.; Frech, N.; Arici, B.; Rutishauser, J.; Kopp, P.; Allolio, B.; Stettler, C.; Müller, B.; Katan, M.; Christ-Crain, M. Diagnostic Accuracy of Copeptin in the Differential Diagnosis of the Polyuria-Polydipsia Syndrome: A Prospective Multicenter Study. J Clin Endocrinol Metab 2015, 100, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020 : An R Package and Shiny App for Producing PRISMA 2020-compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Systematic Reviews 2022, 18. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Nishioka, J.; Matsumoto, T.; Umino, S.; Kawano, A.; Saiki, R.; Tanaka, Y.; Yatsuga, S. Estimate Incidence and Predictive Factors of Pediatric Central Diabetes Insipidus in a Single-Institute Study. Endocrine and Metabolic Science 2022, 7–8, 100119. [Google Scholar] [CrossRef]
- Tuli, G.; Munarin, J.; De Sanctis, L. The Diagnostic Role of Arginine-Stimulated Copeptin in the Differential Diagnosis of Polyuria-Polydipsia Syndrome (PPS) in Pediatric Age. Endocrine 2023, 84, 677–682. [Google Scholar] [CrossRef]
- Winzeler, B.; Cesana-Nigro, N.; Refardt, J.; Vogt, D.R.; Imber, C.; Morin, B.; Popovic, M.; Steinmetz, M.; Sailer, C.O.; Szinnai, G.; Chifu, I.; Fassnacht, M.; Christ-Crain, M. Arginine-Stimulated Copeptin Measurements in the Differential Diagnosis of Diabetes Insipidus: A Prospective Diagnostic Study. The Lancet 2019, 394, 587–595. [Google Scholar] [CrossRef]
- Binder, G.; Weber, K.; Peter, A.; Schweizer, R. Arginine-stimulated Copeptin in Children and Adolescents. Clin Endocrinol (Oxf) 2023, 98, 548–553. [Google Scholar] [CrossRef]
- Pedrosa, W.; Drummond, J.B.; Soares, B.S.; Ribeiro-Oliveira, A. A Combined Outpatient and Inpatient Overnight Water Deprivation Test Is Effective and Safe in Diagnosing Patients with Polyuria-Polydipsia Syndrome. Endocrine Practice 2018, 24, 963–972. [Google Scholar] [CrossRef]
- Tuli, G.; Tessaris, D.; Einaudi, S.; Matarazzo, P.; De Sanctis, L. Copeptin Role in Polyuria-polydipsia Syndrome Differential Diagnosis and Reference Range in Paediatric Age. Clin Endocrinol (Oxf) 2018, 88, 873–879. [Google Scholar] [CrossRef]
- March, C.A.; Sastry, S.; McPhaul, M.J.; Wheeler, S.E.; Garibaldi, L. Copeptin Stimulation by Combined Intravenous Arginine and Oral LevoDopa/Carbidopa in Healthy Short Children and Children with the Polyuria-Polydipsia Syndrome. Horm Res Paediatr 2024, 1–11. [Google Scholar] [CrossRef]
- Al Nofal, A.; Hanna, C.; Lteif, A.N.; Pittock, S.T.; Schwartz, J.D.; Brumbaugh, J.E.; Creo, A.L. Copeptin Levels in Hospitalized Infants and Children with Suspected Vasopressin-Dependent Disorders: A Case Series. Journal of Pediatric Endocrinology and Metabolism 2023, 36, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Gippert, S.; Brune, M.; Dirksen, R.L.; Choukair, D.; Bettendorf, M. Arginine-Stimulated Copeptin-Based Diagnosis of Central Diabetes Insipidus in Children and Adolescents. Horm Res Paediatr 2024, 97, 270–278. [Google Scholar] [CrossRef]
- Bonnet, L.; Marquant, E.; Fromonot, J.; Hamouda, I.; Berbis, J.; Godefroy, A.; Vierge, M.; Tsimaratos, M.; Reynaud, R. Copeptin Assays in Children for the Differential Diagnosis of Polyuria-polydipsia Syndrome and Reference Levels in Hospitalized Children. Clin Endocrinol (Oxf) 2022, 96, 47–53. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandri-Silva, C.; Carpenter, M.; Ayoob, R.; Barcia, J.; Chishti, A.; Constantinescu, A.; Dell, K.M.; Goodwin, J.; Hashmat, S.; Iragorri, S.; Kaspar, C.; Mason, S.; Misurac, J.M.; Muff-Luett, M.; Sethna, C.; Shah, S.; Weng, P.; Greenbaum, L.A.; Mahan, J.D. Diagnosis, Treatment, and Outcomes in Children With Congenital Nephrogenic Diabetes Insipidus: A Pediatric Nephrology Research Consortium Study. Front Pediatr 2020, 7. [Google Scholar] [CrossRef]
- Loredana Petrea, C.; Ciortea, D.-A.; Miulescu, M.; Candussi, I.-L.; Chirila, S.I.; Isabela, G.; Răut, V. (; Berghes, S.-E.; Râs, M.C.; Berbece, S.I. A New Case of Paediatric Systemic Lupus Erythematosus with Onset after SARS-CoV-2 and Epstein-Barr Infection-A Case Report and Literature Review. 2024. [CrossRef]
- Serbis, A.; Rallis, D.; Giapros, V.; Galli-Tsinopoulou, A.; Siomou, E. Wolfram Syndrome 1: A Pediatrician’s and Pediatric Endocrinologist’s Perspective. Int J Mol Sci 2023, 24, 3690. [Google Scholar] [CrossRef] [PubMed]
- Sagna, Y.; Courtillot, C.; Drabo, J.Y.; Tazi, A.; Donadieu, J.; Idbaih, A.; Cohen, F.; Amoura, Z.; Haroche, J.; Touraine, P. Endocrine Manifestations in a Cohort of 63 Adulthood and Childhood Onset Patients with Langerhans Cell Histiocytosis. Eur J Endocrinol 2019, 181, 275–285. [Google Scholar] [CrossRef]
- Claude, F.; Ubertini, G.; Szinnai, G. Endocrine Disorders in Children with Brain Tumors: At Diagnosis, after Surgery, Radiotherapy and Chemotherapy. Children (Basel) 2022, 9. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Gaisl, O. Diabetes Insipidus. Presse Medicale. Elsevier Masson s.r.l. December 1, 2021. [CrossRef]
- Gionis, D.; Ilias, I.; Moustaki, M.; Mantzos, E.; Papadatos, I.; Koutras, D.A.; Mastorakos, G. Hypothalamic-Pituitary-Adrenal Axis and Interleukin-6 Activity in Children with Head Trauma and Syndrome of Inappropriate Secretion of Antidiuretic Hormone. J Pediatr Endocrinol Metab 2003, 16, 49–54. [Google Scholar] [CrossRef]
- Bockenhauer, D.; van’t Hoff, W.; Dattani, M.; Lehnhardt, A.; Subtirelu, M.; Hildebrandt, F.; Bichet, D.G. Secondary Nephrogenic Diabetes Insipidus as a Complication of Inherited Renal Diseases. Nephron Physiol 2010, 116, p23–p29. [Google Scholar] [CrossRef]
- Nakra, N.A.; Blumberg, D.A.; Herrera-Guerra, A.; Lakshminrusimha, S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children (Basel) 2020, 7. [Google Scholar] [CrossRef]
- Saleh, N.Y.; Aboelghar, H.M.; Garib, M.I.; Rizk, M.S.; Mahmoud, A.A. Pediatric Sepsis Diagnostic and Prognostic Biomarkers: Pancreatic Stone Protein, Copeptin, and Apolipoprotein A-V. Pediatr Res 2023, 94, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Mills, T.; Trivedi, A.; Tremoulet, A.H.; Hershey, D.; Burns, J.C. Hyponatremia in Patients With Multisystem Inflammatory Syndrome in Children. Pediatr Infect Dis J 2021, 40, e344–e346. [Google Scholar] [CrossRef] [PubMed]
- Choong, K. Vasopressin in Pediatric Critical Care. J Pediatr Intensive Care 2016, 5, 182–188. [Google Scholar] [CrossRef] [PubMed]
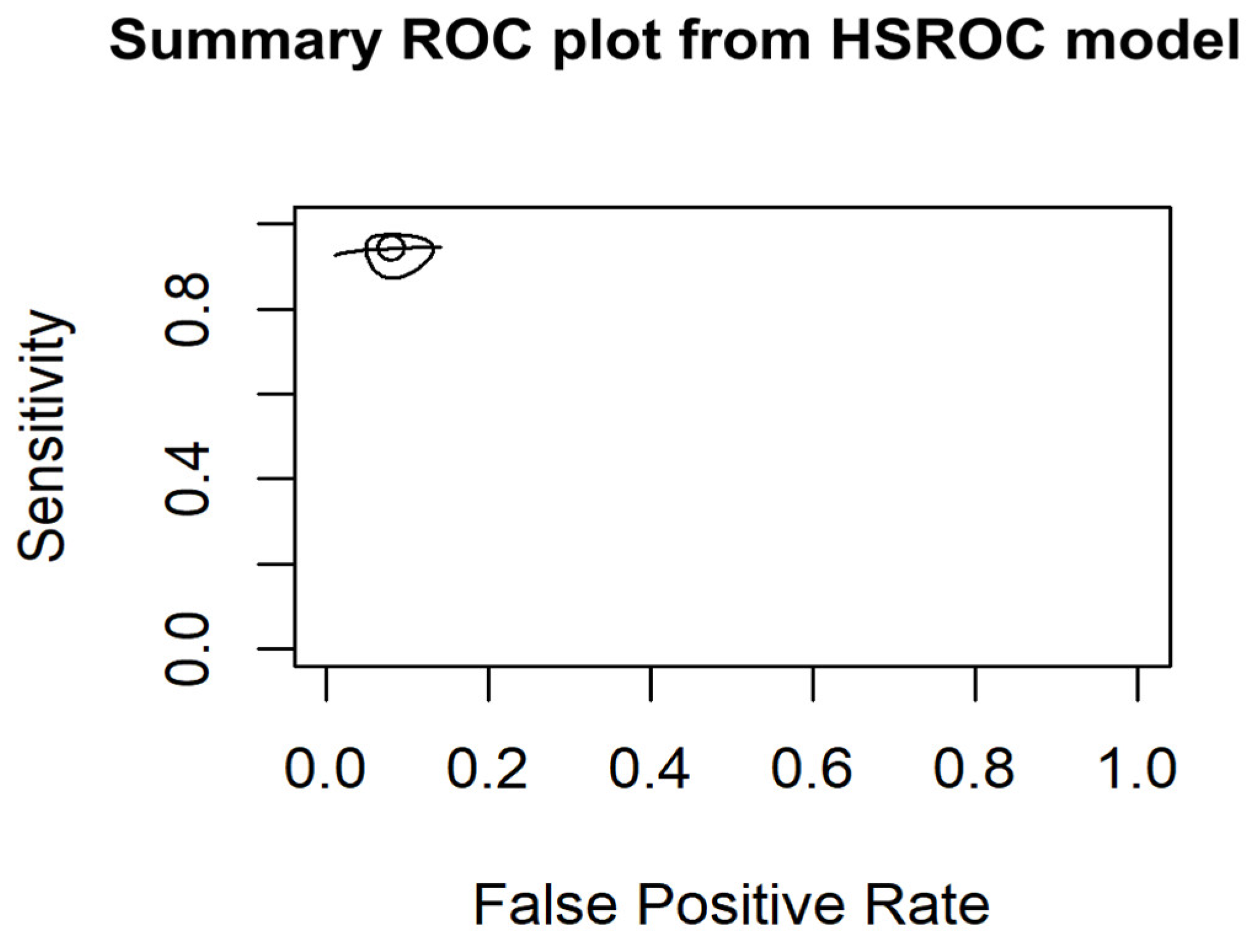

| Study | Citation | Participants | Clinical Setting | Study Design | Target Condition | Index Test | Reference Standard | Sample Size | Funding Sources |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Fenske et al., 2018 [10] | 156 patients aged 16 years or older | 11 tertiary medical centers in Switzerland, Germany, | Prospective multicenter study | Hypotonic polyuria | Copeptin measurement after water deprivation and hypertonic saline tests. | Final reference diagnosis by expert consensus. clinical diagnosis based on established criteria for central diabetes insipidus (CDI) and primary polydipsia (PP). | 156 participants | Supported by Swiss National Foundation, University Hospital Basel, Federal Ministry of Education and Research, Germany, Deutsche Forschungsgemeinschaft, Thermo Fisher Scientific |
| 2 | Kitamura M, et al. 2022 [16] | 27 patients polyuria and/or polydipsia (5 with central diabetes insipidus, 5 with primary polydipsia, 1 with nocturnal enuresis, and 16 with type 1 diabetes mellitus excluded due to hyperglycemia). | Department of Pediatrics and Child Health, Kurume University School of Medicine, Japan. | Retrospective chart review | Central diabetes insipidus (CDI), primary polydipsia (PP), nocturnal enuresis | Hypertonic saline test, urine gravity in the morning | Copeptin levels, urine volume over 24 hours, urinary osmolality, plasma osmolality | 27 participants | Not specified |
| 3 | Tuli, G., Munarin, J., De Sanctis, L. (2023) [17] | Children with PPS including 7 with primary polyuria (PP), 6 with central diabetes insipidus (CDI), and 50 control subjects. | Department of Pediatric Endocrinology, Regina Margherita Children Hospital. | Prospective study | Central diabetes insipidus (CDI), primary polyuria (PP). | Arginine-stimulated copeptin test | Water deprivation test (WDT) | 63 participants (13 patients with PPS, 50 controls). | Not specified. |
| 4 | Winzeler B., et. al. 2019 [18] | Development cohort: 52 patients (12 with complete DI, 9 with partial DI, 31 with PP), 20 healthy adults, and 42 child controls; Validation cohort: 46 patients (12 with complete DI, 7 with partial DI, 27 with PP) and 30 healthy adult controls. | Multi-center study involving University Hospital Basel and five other centers in Switzerland and Germany | Prospective diagnostic study | Complete and partial diabetes insipidus, primary polydipsia. | Arginine-stimulated copeptin measurements | diagnostic accuracy of copeptin levels | Development cohort: 114 participants; Validation cohort: 76 participants (after exclusions). | Swiss National Science Foundation and University Hospital Basel. |
| 5 | Binder, G., Weber, K., Peter, A., & Schweizer, R. (2023) [19] | 72 children and adolescents tested for growth hormone deficiency, including 4 with confirmed central diabetes insipidus (CDI). | University Children’s Hospital Tübingen, Germany | Monocentric retrospective analysis | Central diabetes insipidus (CDI). | Arginine-stimulated copeptin test. | Water deprivation test, serum osmolalityarginine-stimulation test and clinical evaluation | 72 participants (68 non-CDI, 4 with CDI) | Not specified. |
| 6 | Pedrosa et al., 2018 [20] | 52 patients aged 12–77 years | Hermes Pardini Endocrine Testing Center, Brazil | Retrospective analysis | Polyuria-Polydipsia Syndrome (PPS) | Hypertonic saline infusion followed by copeptin measurement | Plasma AVP levels | 52 participants | Supported by FAPEMIG and CNPq, JBD received a PhD grant from CAPES |
| 7 | Tuli, G., Munarin, J., De Sanctis, L. 2018 [21] | 80 children (53 control subjects, 12 hypopituitary children, and 15 children with PPS). | Pediatric Endocrinology Department. | Prospective observational study | Nephrogenic diabetes insipidus (NDI), complete central diabetes insipidus (CDI), and primary polydipsia (PP). | Copeptin levels after hypertonic saline infusion test and Water deprivation test | Plasma arginine-vasopressin (AVP) analysis | 80 participants | Not specified |
| 8 | March, C. A., Sastry, S., McPhaul, M. J., Wheeler, S. E., & Garibaldi, L. (2024). [22] | 47 healthy short children (controls), 10 children with primary polydipsia, and 10 children with AVP deficiency. | Pediatric endocrinology settings | Prospective interventional study. | Polyuria-polydipsia syndrome (PPS), arginine vasopressin (AVP) deficiency, primary polydipsia. | Arginine + LevoDopa/Carbidopa stimulation test (ALD-ST) for copeptin measurement. | Copeptin levels measured at baseline and after stimulation. | 67 participants (47 controls, 10 with primary polydipsia, 10 with AVP deficiency). | Not specified |
| 9 | Al Nofal, A., et. Al. (2024). [23] | 29 critically ill patients, including 6 infants, with hyper- or hypo-natremia. | Single-center study conducted in a hospital setting. | Retrospective case series. | Central diabetes insipidus (CDI), syndrome of inappropriate antidiuresis (SIAD). | Copeptin levels after hypertonic saline test. | Diagnostic thresholds for CDI and SIAD. | 29 patients (38% post-neurosurgical procedures). | Not specified |
| 10 | Gippert, S., Brune, M., Dirksen, R. L., Choukair, D., Bettendorf, M. (2024) [24] | 69 patients (32 with central diabetes insipidus [CDI], 32 matched controls, and 5 with primary polydipsia [PP]). | Single-center study in a pediatric endocrinology department. | Retrospective study | Central diabetes insipidus (CDI), primary polydipsia (PP) | Copeptin measurement following water deprivation test. | Comprehensive clinical and diagnostic characteristics, water deprivation test, and hypertonic saline infusion. | 69 participants | Not specified |
| 11 | Bonnet, L., et. al. 2024 [25] | 353 children aged 2 months to 18 years | Pediatric endocrinology and nephrology departments in France. | Single-center retrospective descriptive study | Central diabetes insipidus (CDI), primary polydipsia (PP), and other related conditions | Copeptin levels measured after arginine stimulation | Comparison with traditional diagnostic criteria for CDI and PP. | 353 participants, with 16 diagnosed with CDI and 18 with PP. | Funded by the University of Pittsburgh Clinical and Translational Science Institute Clinical and Translational Science Scholars Program (NIH/NCATS 1 KL2 TR001856). |
| Study | TP* | FN* | TN* | FP* | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR- (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Fenske et al. 2018 | 56 | 3 | 82 | 3 | 0.95 (95% CI: 85.15% - 99.36%) | 0.96 (96% CI: 89.73% - 98.89%) | 0.949 CI: (0.902-0.996) | 0.965 CI: (0.926-1.000) | 23.75 CI: (6.91-81.57) | 0.052 CI: (0.011-0.241) | 0.95 CI: (0.88-0.98) |
| 2 Kitamura et al. 2022 | 5 | 0 | 19 | 3 | 1 (100% CI: 56.56%-100%) | 0.857 (85.7% CI: 64.54% - 95.85%) | 0.625 CI: (0.396-0.854) | 1.000 CI: (0.829-1.000) | 7.00 CI: (2.55-19.24) | 0.000 CI: (0.00-0.00) | 0.93 CI: (0.85-0.99) |
| 3 Tuli G. et al. 2023 | 6 | 0 | 49 | 8 | 1 (100% CI: 61.51% -100%) | 0.857 (85.7% CI: 72.25% - 93.37%) | 0.429 CI: (0.237-0.621) | 1.000 CI: (0.928-1.000) | 7.00 CI: (2.55-19.24) | 0.000 CI: (0.00-0.00) | 0.93 CI: (0.83-0.98) |
| 4 Winzeler et al. 2019 | 11 | 0 | 54 | 2 | 1 (100% CI – 71.51% - 100%) | 0.96 (96% CI: 84.98% -99.46%) | 0.846 CI: (0.709-0.983) | 1.000 CI: (0.932-1.000) | 25.00 CI: (6.32-98.89) | 0.000 CI: (0.00-0.00) | 0.98 CI: (0.92-1.00) |
| 5 Binder et al. 2023 | 4 | 0 | 17 | 0 | 1 (100% CI: 56.56%-100%) | 1 (100% CI: 76.03% - 100%) | 1.000 CI: (0.783-1.000) | 1.000 CI: (0.803-1.000) | N/A (perfect tets) | N/A (perfect tets) | 1.00 (perfect test) CI: (0.90-1.00) |
| 6 Pedrosa et al. 2018 | 8 | 0 | 26 | 1 | 1 (100% CI: 56.56% - 1000%) | 0.96 (96% CI: 77.97% -99.57%) | 0.889 CI: (0.679-1.000) | 1.000 CI: (0.867-1.000) | 25.00 CI: (3.66-171.14) | 0.000 CI: (0.00-0.00) | 0.98 CI: (0.92-1.00) |
| 7 Tuli et al. 2018 | 9 | 0 | 36 | 1 | 1 (100% CI: 61.51% - 100%) | 0.97 (97% CI: 83.34% - 99.63%) | 0.900 CI: (0.742-1.000) | 1.000 CI: (0.889-1.000) | 33.00 CI: (4.72-230.84) | 0.000 CI: (0.00-0.00) | 0.99 CI: (0.92-1.00) |
| 8 March et al. 2018 | 8 | 0 | 22 | 2 | 1 (100% CI: 61.51% - 100%) | 0.92 (92% CI: 69.99% - 98.98%) | 0.800 CI: (0.543-1.000) | 1.000 CI: (0.824-1.000) | 12.50 CI: (3.46-45.13) | 0.000 CI: (0.00-0.00) | 0.96 CI: (0.88-0.99) |
| 9 Nofal et al. 2023 | 10 | 0 | 29 | 1 | 1 (100% CI: 69.15% - 100%) | 0.95 (95% CI: 80.53% -99.29%) | 0.909 CI: (0.725-1.000) | 1.000 CI: (0.868-1.000) | 20.00 CI: (4.65-86.08) | 0.000 CI: (0.00-0.00) | 0.98 CI: (0.90-0.99) |
| 10 Gippert et al. 2023 | 12 | 0 | 36 | 2 | 1 (100% CI: 73.54% - 100%) | 0.94 (94% CI: 80.09% - 98.40%) | 0.857 CI: (0.689-1.000) | 1.000 CI: (0.917-1.000) | 16.67 CI: (5.15-53.89) | 0.000 CI: (0.00-0.00) | 0.97 CI: (0.89-0.99) |
| 11 Bonnet et al. 2021 | 8 | 0 | 20 | 3 | 1 (100% CI: 56.56% - 100%) | 0.87 (87% CI: 66.89% - 95.45%) | 0.727 CI: (0.476-0.978) | 1.000 CI: (0.823-1.000) | 7.69 CI: (2.86-20.64) | 0.000 CI: (0.00-0.00) | 0.93 CI: (0.82-0.97) |
| Study | Sensitivity | Specificity | AUC (95% CI) | Sensitivity Variance | Specificity Variance | AUC Variance | Weight (Sensitivity) | Weight (Specificity) | Weight (AUC) |
|---|---|---|---|---|---|---|---|---|---|
| 1 Fenske et al. 2018 | 0.95 | 0.96 | 0.95 | 0.0013 | 0.000548 | 0.000423 | 769.23 | 1824.82 | 2364.07 |
| 2 Kitamura et al. 2022 | 1.00 | 0.857 | 0.93 | 0.0123 | 0.0064 | 0.006735 | 81.30 | 156.25 | 148.48 |
| 3 Tuli G. et al. 2023 | 1.00 | 0.857 | 0.93 | 0.0097 | 0.0029 | 0.005389 | 103.09 | 344.83 | 185.56 |
| 4 Winzeler et al. 2019 | 1.00 | 0.96 | 0.98 | 0.0053 | 0.0014 | 0.000904 | 188.68 | 714.29 | 1106.19 |
| 5 Binder et al. 2023 | 1.00 | 1.00 | 1.00 | 0.0123 | 0.0037 | 0.001 | 81.30 | 270.27 | 1000.0 |
| 6 Pedrosa et al. 2018 | 1.00 | 0.96 | 0.98 | 0.0123 | 0.0030 | 0.001271 | 81.30 | 333.33 | 786.78 |
| 7 Tuli et al. 2018 | 1.00 | 0.97 | 0.99 | 0.0097 | 0.0017 | 0.000565 | 103.09 | 588.24 | 1769.91 |
| 8 March et al. 2018 | 1.00 | 0.92 | 0.96 | 0.0097 | 0.0054 | 0.002507 | 103.09 | 185.19 | 398.88 |
| 9 Nofal et al. 2023 | 1.00 | 0.95 | 0.98 | 0.0062 | 0.0023 | 0.001015 | 161.29 | 434.78 | 985.22 |
| 10 Gippert et al. 2023 | 1.00 | 0.94 | 0.97 | 0.0046 | 0.0022 | 0.001247 | 217.39 | 454.55 | 801.92 |
| 11 Bonnet et al. 2021 | 1.00 | 0.87 | 0.93 | 0.0123 | 0.0053 | 0.004267 | 81.30 | 188.68 | 234.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





