1. Introduction
Acute ST-segment elevation myocardial infarction (STEMI) stands as a formidable medical emergency, demanding swift intervention and comprehensive management. Despite advancements in acute care, long-term prognosis remains a challenge, particularly in patients with underlying metabolic disorders [
1,
2]. Among these, prediabetes emerges as a critical yet often underappreciated factor influencing survival outcomes [
3,
4].
Prediabetes, defined by impaired fasting glucose or impaired glucose tolerance, represents an intermediate metabolic state with blood glucose levels elevated above normal but not high enough to be classified as diabetes. This condition is alarmingly prevalent, with significant implications for cardiovascular health [
5]. The pathophysiological mechanisms linking prediabetes to adverse cardiac events are multifaceted, involving insulin resistance, subclinical inflammation, endothelial dysfunction, and accelerated atherogenesis [
6].
Current literature highlights a compelling connection between prediabetes and increased cardiovascular risk, comparable to that observed in overt diabetes [
7]. Patients with prediabetes are at a higher risk of progressing to diabetes and experiencing cardiovascular events, including STEMI. Importantly, evidence suggests that prediabetic individuals who survive a STEMI face a significantly elevated long-term-risk of mortality and recurrent cardiac events compared to normoglycemic patients [
8].
The prognostic importance of prediabetes in the context of STEMI is underscored by several key studies. Research has demonstrated that even modest elevations in glycemic indices, such as hemoglobin A1c (HbA1c), are associated with worse outcomes post-STEMI. This highlights the insidious impact of prediabetes on myocardial recovery and long-term survival, necessitating early identification and aggressive management of this at-risk population [
9].
In clinical practice, the challenge lies in recognizing prediabetes as a potent risk factor and integrating it into risk stratification models for STEMI patients. Traditional risk assessments often overlook the subtle yet significant influence of prediabetic states, potentially leading to suboptimal management strategies [
10].
Incorporating glycemic parameters such as HbA1c into predictive models may enhance the precision of risk stratification, enabling tailored therapeutic approaches that address the unique metabolic vulnerabilities of prediabetic patients. Therefore, our study aims to bridge this gap by providing robust evidence on the impact of prediabetes on survival following STEMI. By analyzing a large cohort and employing rigorous statistical methods, we seek to elucidate the prognostic significance of prediabetes. This endeavor not only advances our understanding of the interplay between glycemic status and cardiovascular outcomes but also paves the way for personalized intervention strategies designed to improve long-term survival in STEMI patients.
introduction should briefly place the study in a broad context and highlight why it is important. It should define the purpose of the work and its significance. The current state of the research field should be carefully reviewed and key publications cited. Please highlight controversial and diverging hypotheses when necessary. Finally, briefly mention the main aim of the work and highlight the principal conclusions. As far as possible, please keep the introduction comprehensible to scientists outside your particular field of research. References should be numbered in order of appearance and indicated by a numeral or numerals in square brackets—e.g., [
1] or [
2,
3], or [
4,
5,
6]. See the end of the document for further details on references.
2. Materials and Methods
2.1. Study Population
For this retrospective study, all patients (n = 964) presenting with STEMI at a single large tertiary center in Salzburg, Austria, between January 1, 2018, and December 31, 2020, were screened. Eligible participants were STEMI patients aged 18 years or older, with documented cardiovascular risk factors and available baseline HbA1c values obtained during their initial hospitalization for STEMI (n = 725). Patients lacking HbA1c or with conservative treatment (without receiving a percutaneous coronary intervention) were excluded from the study. The primary endpoint was overall mortality in this study cohort over a time period of three years. A calculation performed to determine the sample size (use of G*Power 3.1 – test family: t-test; statistical test: means; type of power analysis: a priori) provided an optimal sample size of 105 patients per study group using an effect size d of 0.5, an alpha error of 0.05, a power (1 minus beta error) of 0.95, and an allocation ratio of 1.
2.2. Ethics Declaration
The study received ethical approval from the State of Salzburg Ethics Commission (EK-Nr. 1038/2021). All data handling complied with the Declaration of Helsinki principles and Good Clinical Practice (ICH-GCP) guidelines.
2.3. Data Extraction
Data were obtained from the ORBIS electronic medical records system (Agfa Healthcare, Version 08043301.04110DACHL) and the medical archiving system (Krankengeschichtsarchiv System, Uniklinikum Salzburg, Softworx by Andreas Schwab TM, 2008) at the University Clinic Salzburg (Austria). Patient information, including charts and reports from admissions, discharges, and laboratory results during the STEMI hospitalization, were extracted. The data were then pseudo-anonymized and entered into an Excel database.
2.4. Laboratory-Chemical Examinations
All laboratory values were analyzed at the University Institute for Medical-Chemical Laboratory Diagnostics at University Hospital Salzburg. HbA1c levels were measured using high-performance liquid chromatography (HPLC), a standard and precise method for quantifying percentage distribution of glycosylated hemoglobin. The intra- and inter-assay coefficients of variation for all kits were found to be less than 10%. This method allows for accurate assessment of average blood glucose levels over the preceding 2-3 months. The Friedewald formula was used to calculate plasma low-density lipoprotein cholesterol (LDL-C) concentration when triglyceride levels were below 275 mg/dL. For higher triglyceride levels, LDL particle number was directly measured using the c702 module of the Roche Cobas® 8000 analyzer (Roche Diagnostics, Mannheim, Germany) following the manufacturer's instructions for LDL-C measurement.
2.5. Clinical Criteria of STEMI
STEMI was diagnosed based on guidelines set by the European Society of Cardiology (ESC) [
11].
2.6. Categorization of Glycemic Status
Patients were categorized into three groups based on their glycemic status using a two-step approach:
Step 1: Medical History Review
Initially, a comprehensive medical history review (anamnesis) was conducted for each patient to determine if they had a prior diagnosis of diabetes and how it was managed (diet only, oral medication, or insulin). If a patient was identified as having pre-existing diabetes, they were categorized as diabetics regardless of their current HbA1c levels.
Step 2: HbA1c Measurement
For patients without a prior diabetes diagnosis, HbA1c levels were measured and used to classify them according to the following criteria:
Non-diabetic: HbA1c < 5.7%
Prediabetic: HbA1c 5.7% - 6.4%
Diabetic (new diagnosis): HbA1c ≥ 6.5%
Therefore, the diagnosis of non-diabetic and prediabetic patients was based solely on the measured HbA1c at the time of the STEMI event, whereas patients with a prior diagnosis of diabetes were categorized based on their medical history, independent of their current HbA1c levels [
12].
2.7. Comorbidity Identification
Obesity, CKD, heart failure, hypertension, dyslipidemia were defined and evaluated according to current clinical guidelines [
13,
14,
15,
16]. The conventional CKD-EPI formula was used to estimate the glomerular filtration rate (eGFR).
2.8. Statistical Analyses
Statistical analyses and data visualization were performed using SPSS Version 25 (IBM SPSS Statistics, Armonk, New York, NY, USA). The Kolmogorov–Smirnov–Lilliefors test was employed to evaluate the normality of variable distributions. Metrics exhibiting normal distribution were reported as mean ± standard deviation (SD) and analyzed using an unpaired Student’s t-test. For metrics not following a normal distribution, data were presented as median and interquartile range (IQR), with statistical comparisons made using the Mann–Whitney U test. Categorical variables were expressed as frequencies and percentages, with comparisons conducted using the chi-square test. Kaplan-Meier survival curves and corresponding log-rank tests were performed for both short- and long-term survival. Further analyses included calculating the area under the receiver operating characteristic (AUROC) curves for the studied HbA1c values in relation to short-term (up to 6 months) and long-term survival (up to 3 years). This also involved determining cut-off values with corresponding sensitivity, specificity, and the Youden Index (YI). In this study, we utilized various p-values to compare survival rates among different glycemic groups. The p_general value represents the overall comparison among all three groups (non-diabetic, prediabetic, and diabetic). The p* value denotes the comparison between non-diabetic and prediabetic patients. The p** value signifies the comparison between non-diabetic and diabetic patients, while the p*** value represents the comparison between prediabetic and diabetic patients. A p-value of ≤ 0.05 was determined as statistical significance.
3. Results
3.1. Study Population—No Diabetes, Prediabetes & Diabetes
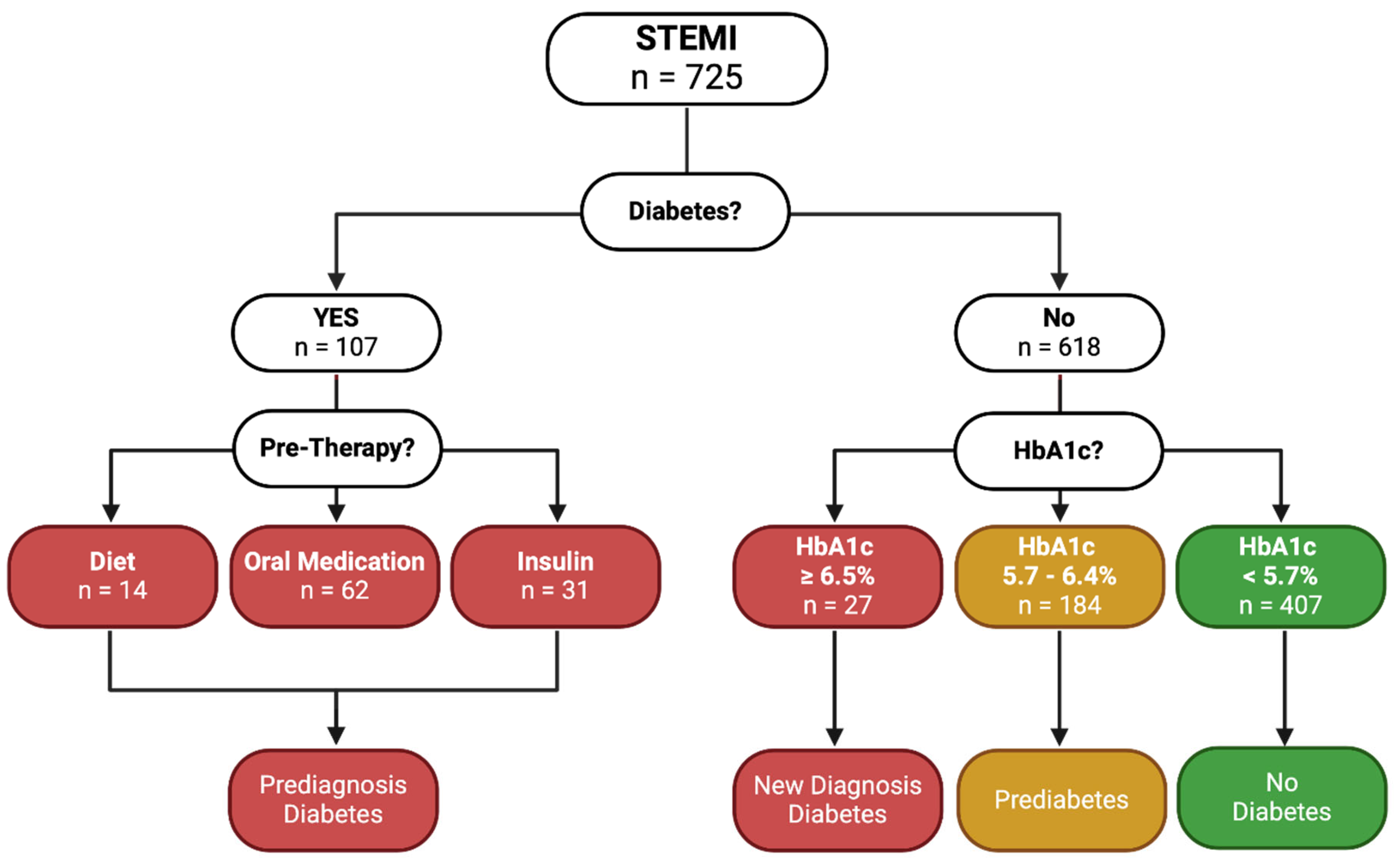
The study population comprised 725 patients presenting with STEMI at a single, large tertiary care center in Salzburg, Austria. These patients were systematically categorized based on their diabetes status. Initially, we screened for a history of diabetes. Of the total, 107 patients (14.8%) had a prior diabetes diagnosis. We reviewed their medical history to determine the nature of their diabetes management, identifying whether they controlled their condition through diet (n=14, 13.1%), oral medication (n=62, 57.9%), or insulin ± oral medication (n=31, 29.0%). Patients falling into these categories were classified as having pre-diagnosed diabetes, regardless of their HbA1c levels.
For the remaining 618 patients (85.2%) without a known history of diabetes, we assessed their HbA1c levels to determine their glycemic status at the time of the STEMI event. Based on the HbA1c values, patients were categorized into three groups. Those with HbA1c levels below 5.7% (n=407, 65.9%) were classified as non-diabetic, those with HbA1c levels between 5.7% and 6.4% (n=184, 29.8%) were considered prediabetic, and those with HbA1c levels equal to or greater than 6.5% (n=27, 4.4%) were identified as having newly diagnosed diabetes. This detailed categorization is visualized in
Figure 1.
3.2. Baseline Characteristics
In this study, we analyzed the baseline characteristics (
Table 1) of the three groups: no diabetes, prediabetes, and diabetes. Demographic analysis revealed no significant gender differences among the groups. However, age distribution indicated that non-diabetic patients were generally younger, with a higher percentage under 50 years compared to their prediabetic and diabetic counterparts, with significant differences between non-diabetic and diabetic patients (p** < 0.001), and non-diabetic and prediabetic patients (p* = 0.045). The comparison between prediabetic and diabetic patients also showed significant differences (p*** = 0.013).
Cardiovascular risk factors showed notable variations. Arterial hypertension was more prevalent in the diabetic group (82.8%) compared to non-diabetic (64.1%, p** < 0.001) and prediabetic (67.4%, p* = 0.034) patients, with a significant difference also observed between prediabetic and diabetic patients (p*** = 0.021). Dyslipidemia was most common in the prediabetic group (78.3%), and smoking rates were relatively similar across all groups, though slightly lower in the diabetic group (55.2%).
Body Mass Index (BMI) analysis highlighted significant differences, with diabetic patients having higher BMI values. The prevalence of obesity (BMI ≥ 30 kg/m²) was particularly pronounced in the diabetic group, with 27.6% having a BMI in the 30.0-34.9 kg/m² range and 9.7% in the 35.0-39.9 kg/m² range.
Regarding STEMI characteristics, diabetic patients exhibited a higher incidence of 3-vessel disease (44.0%) compared to non-diabetic (30.7%, p** < 0.001) and prediabetic (25.0%, p* = 0.015) patients. Cardiogenic shock was also more common in the diabetic group (20.9%). Laboratory values further illustrated significant variations, particularly in glycemic control markers. Lipid profiles indicated lower HDL and higher triglyceride levels in diabetic patients.
3.3. Kaplan-Meier Curves—Non Diabetes, Prediabetes & Diabetes
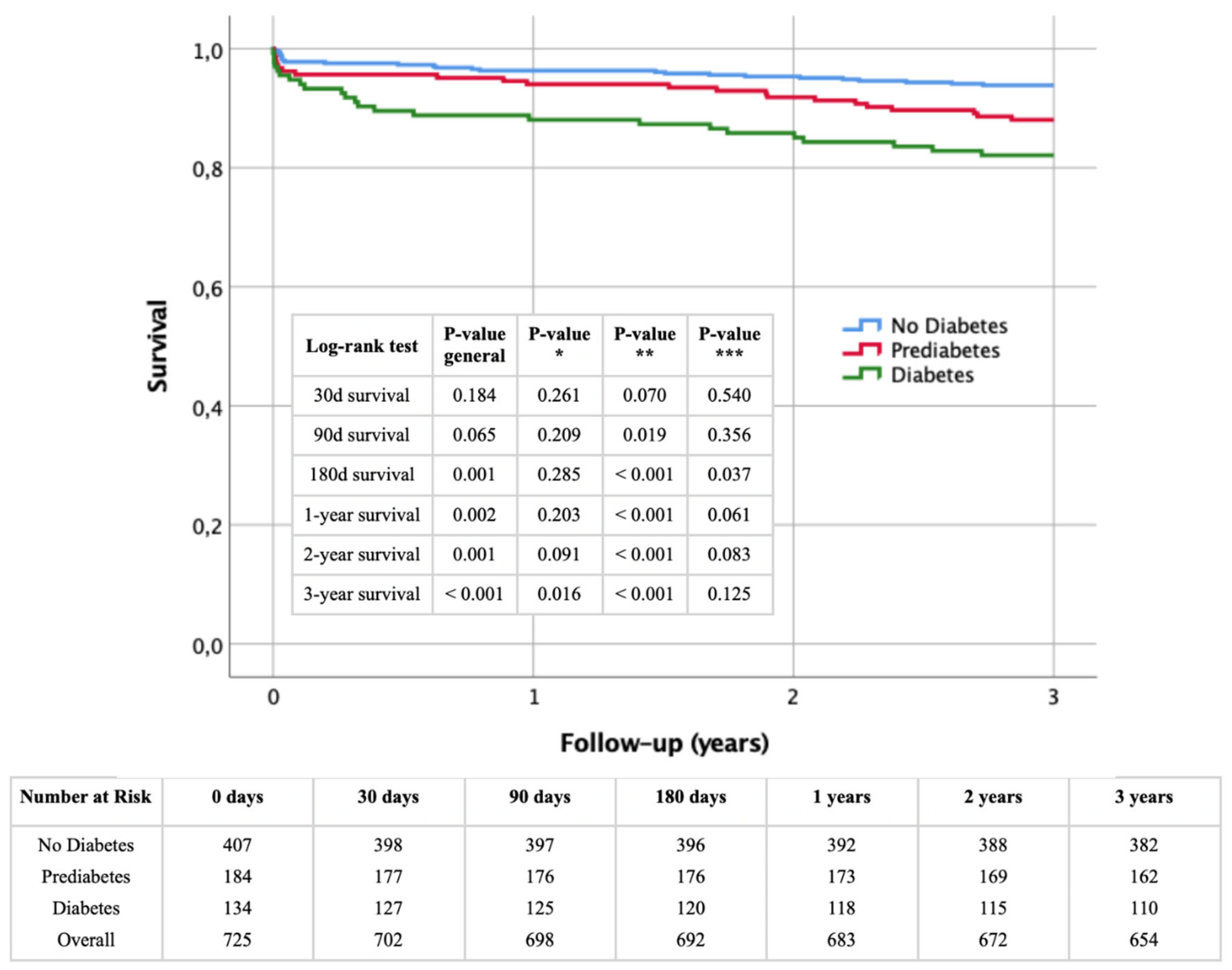
Figure 2 presents Kaplan-Meier survival curves for the study population stratified by glycemic status (non-diabetic, prediabetic, and diabetic) over a three-year period following STEMI. Survival rates were analyzed at 30 days, 90 days, 180 days, 1 year, 2 years, and 3 years.
At 30 days, survival rates did not significantly differ between the groups (log-rank p = 0.184). By 90 days, survival rates showed a trend towards significance (log-rank p = 0.065), with a significant difference between non-diabetic and diabetic patients (p** = 0.019). At 180 days, differences became more pronounced (log-rank p = 0.001), with significant disparities between non-diabetic and diabetic (p** < 0.001) and prediabetic and diabetic patients (p*** = 0.037).
One-year survival analysis showed significant differences (log-rank p = 0.002), mainly between non-diabetic and diabetic patients (p** < 0.001), with prediabetic vs. diabetic comparisons nearing significance (p*** = 0.061). At two years, significant differences persisted (log-rank p = 0.001), with notable differences between non-diabetic and diabetic (p** < 0.001), and prediabetic and diabetic patients (p*** = 0.083).
At three years, survival rates diverged significantly (log-rank p < 0.001), highlighting the impact of diabetes on long-term survival. The non-diabetic vs. diabetic (p** < 0.001) and prediabetic vs. diabetic (p*** = 0.125) comparisons underscored the risk. The non-diabetic vs. prediabetic comparison also became significant (p* = 0.016), indicating substantial long-term risks for prediabetic patients.
3.4. AUROC Analysis of HbA1c for Predicting Mortality
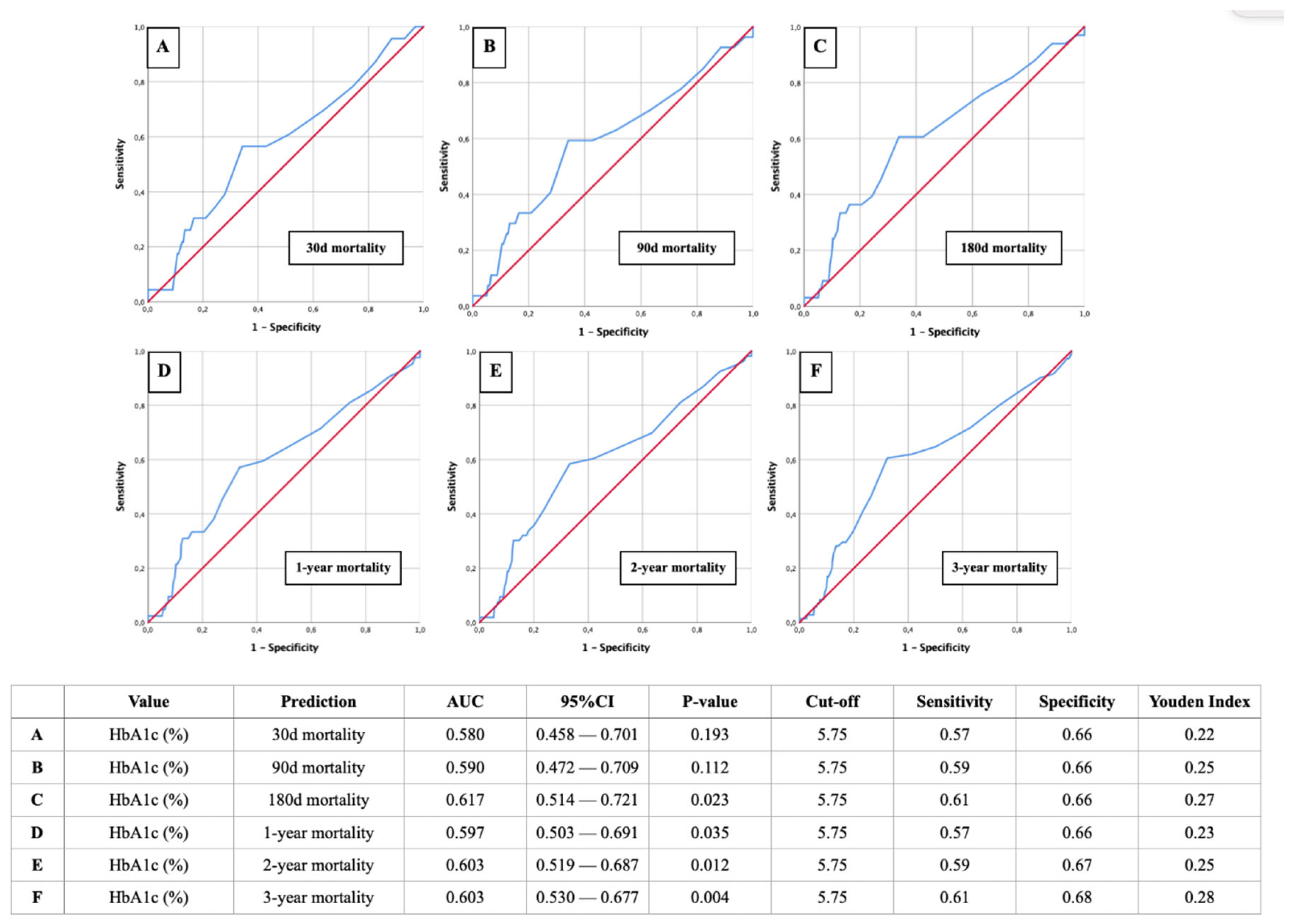
Figure 3 illustrates the AUROC analysis for HbA1c as a predictor of mortality at various time intervals: 30 days, 90 days, 180 days, 1 year, 2 years, and 3 years post-STEMI. The analysis shows that HbA1c has a moderate predictive power for mortality, with AUROC values improving slightly over longer follow-up periods. A HbA1c cut-off value of 5.75% and therefore prediabetic glycemic status was consistently identified as relevant across all time frames, demonstrating sensitivities between 57-61% and specificities between 66-68%. This highlights the utility of HbA1c in identifying patients at increased risk of mortality following STEMI.
3.5. Study Population—Non Diabetes, Prediabetes & Diabetes with Further Cardiovascular Risk Factors
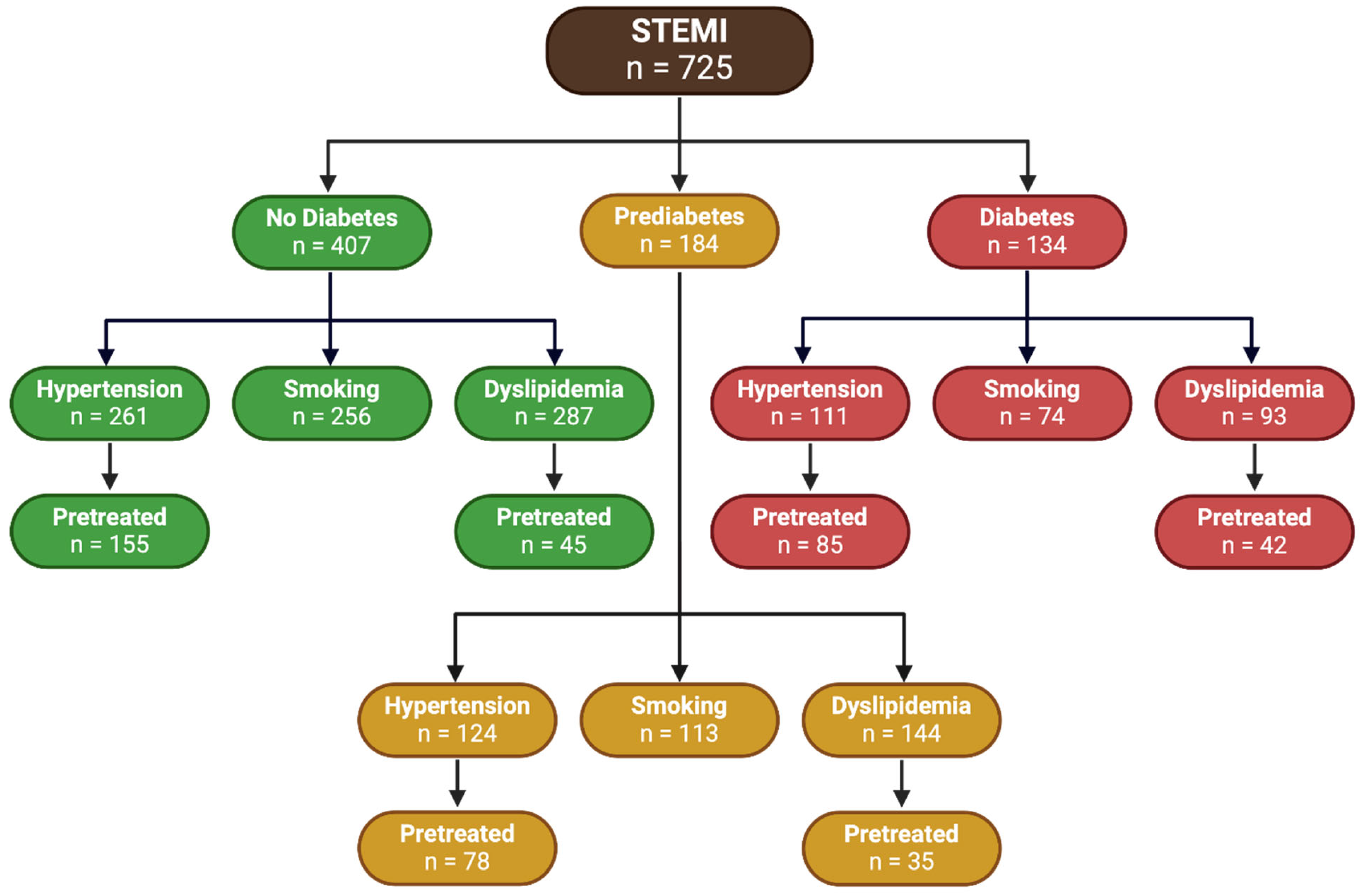
Figure 4 presents a comprehensive flowchart that illustrates the distribution of 725 patients diagnosed with STEMI, categorized by their glycemic status and associated comorbid conditions.
Among the total STEMI patients classified as having no diabetes, 64.1% (n = 261) suffered from hypertension, with 59.4% (n = 155) of these hypertensive patients receiving pretreatment. Additionally, 62.9% (n = 256) were smokers. Furthermore, 70.5% (n = 287) of non-diabetic patients had dyslipidemia, and 15.7% (n = 45) of these dyslipidemic patients were pretreated.
Within the prediabetic subgroup, 67.4% (n = 124) exhibited hypertension, and 62.9% (n = 78) of those hypertensive patients had been pretreated. With respect to smoking, 61.4% (n = 113) of prediabetic patients were smokers. Regarding dyslipidemia, 78.3% (n = 144) of prediabetic patients had this condition, and 24.3% (n = 35) were pretreated.
The diabetic group included 82.8% (n = 111) patients with hypertension, with 76.6% (n = 85) of these hypertensive patients being pretreated. Additionally, 54.8% (n = 74) were smokers. Regarding dyslipidemia, 69.4% (n = 93) of diabetic patients were affected, with 45.2% (n = 42) being pretreated.
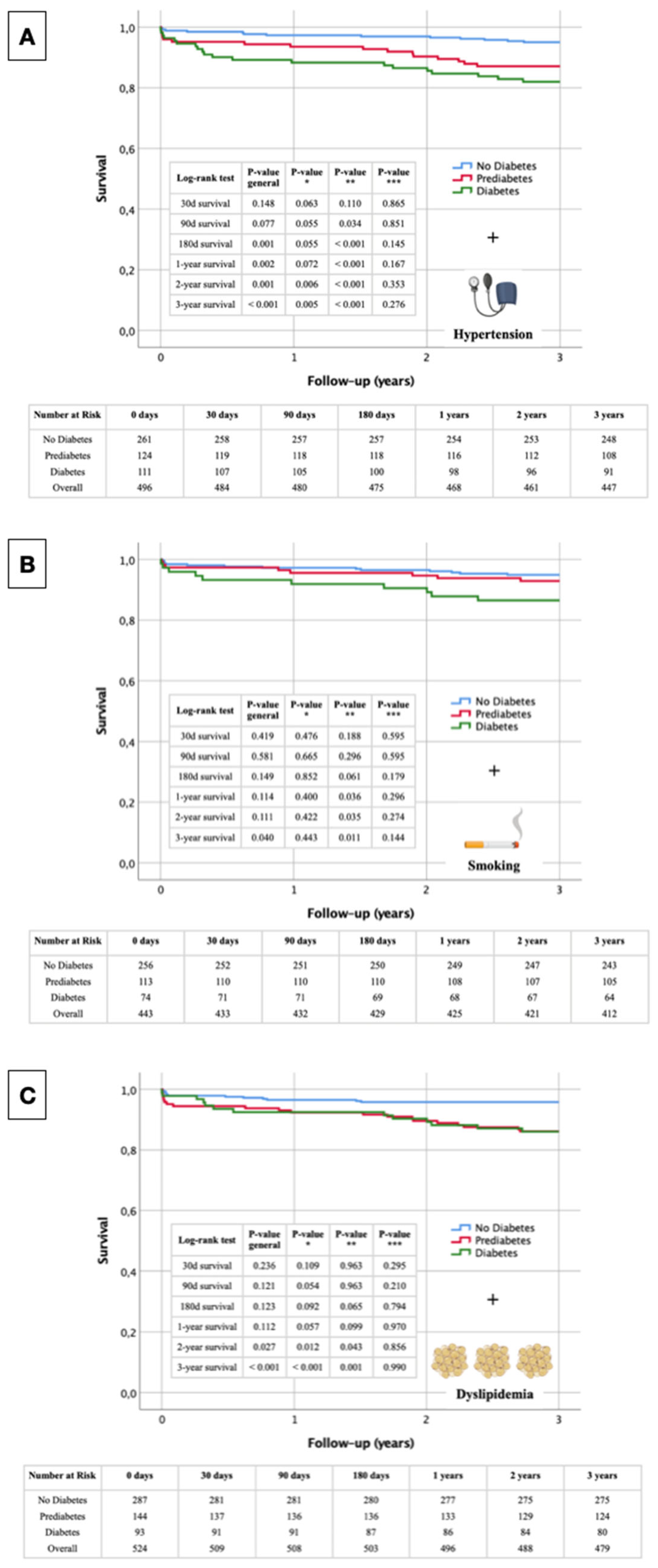
3.6. Kaplan-Meier Curves—Non Diabetes, Prediabetes & Diabetes with Further Cardiovascular Risk Factors
The following Kaplan-Meier survival curves presented in
Figure 5 illustrate the overall survival of STEMI patients, stratified by glycemic status (No Diabetes, Prediabetes, and Diabetes) in conjunction with the presence of arterial hypertension (
Figure 5A), smoking (
Figure 5B) and dyslipidemia (
Figure 5C). Key survival probabilities with respective p-values at specific time points (30 days, 90 days, 180 days, 1 year, 2 years, and 3 years) highlight the significance of differences in survival among the groups. Each figure also includes a tabular overview of the numbers at risk at these specific time points, providing a comprehensive view of the patient population throughout the study period.
3.6.1. Glycemic Status + Arterial Hypertension
The Kaplan-Meier survival curve in
Figure 5A shows significant differences in survival rates among non-diabetic, prediabetic, and diabetic STEMI patients over three years, with arterial hypertension as a stratifying factor. At 30 days, survival differences were not significant overall (p_general = 0.148), though a trend towards lower survival in diabetic patients was observed (p* = 0.063). By 90 days, differences approached significance (p_general = 0.077), with significantly lower survival in diabetic patients compared to non-diabetic patients (p** = 0.034). At 180 days, significant differences were evident (p_general = 0.001), particularly between non-diabetic and diabetic patients (p** < 0.001). One-year survival analysis revealed significant disparities (p_general = 0.002), with diabetic patients showing worse outcomes (p** < 0.001). By two years, the differences remained significant (p_general = 0.001), with poorer survival in diabetic patients compared to non-diabetic (p** < 0.001) and prediabetic patients (p* = 0.006). At three years, the survival disparities were highly significant (p_general < 0.001), with diabetic patients continuing to show the lowest survival rates (p** < 0.001) and significant differences observed between non-diabetic and prediabetic patients (p* = 0.005).
3.6.2. Glycemic Status + Smoking
The Kaplan-Meier survival curves for STEMI patients show significant differences based on glycemic status and smoking over three years (
Figure 5B). At 30 days, no significant survival differences were observed (p_general = 0.419). By 90 days, survival differences remained non-significant overall (p_general = 0.581). At 180 days, differences approached significance (p_general = 0.149), with a notable trend between non-diabetic and diabetic patients (p** = 0.061). One-year analysis showed significant differences, with diabetic patients having worse survival compared to non-diabetic patients (p** = 0.036). Two-year survival differences remained significant between non-diabetic and diabetic patients (p** = 0.035), with overall differences nearing significance (p_general = 0.111). By three years, significant survival disparities were observed (p_general = 0.040), especially between non-diabetic and diabetic patients (p** = 0.011).
3.6.3. Glycemic Status + Dyslipidemia
The Kaplan-Meier survival curves for STEMI patients, stratified by glycemic status and dyslipidemia, reveal significant differences in survival rates over three years (
Figure 5C). At 30 days, survival differences were not significant (p_general = 0.236), with non-significant comparisons between the different groups. By 90 days, overall survival differences remained non-significant (p_general = 0.121), though the difference between non-diabetic and prediabetic patients approached significance (p* = 0.054). At 180 days, survival differences approached significance overall (p_general = 0.123), with again a notable trend between non-diabetic and diabetic patients (p** = 0.065). One-year survival analysis indicated a trend towards significant differences (p_general = 0.112), particularly between non-diabetic and prediabetic patients (p* = 0.057). By two years, significant survival differences emerged overall (p_general = 0.027), with significant differences between non-diabetic and prediabetic patients (p* = 0.012) and non-diabetic and diabetic patients (p** = 0.043). At three years, survival disparities were highly significant (p_general < 0.001), especially between non-diabetic and prediabetic patients (p* < 0.001) and non-diabetic and diabetic patients (p** = 0.001).
4. Discussion
4.1. The Overlooked Risk in Prediabetic Patients
This study highlights a critical and often overlooked aspect of cardiovascular care on long-term survival following acute STEMI: the substantial risk faced by prediabetic patients, a group that is frequently underdiagnosed and undertreated. Our findings reveal that prediabetic individuals presenting with STEMI exhibit survival outcomes that are markedly worse than those of their non-diabetic counterparts and, in some cases, approach the poor outcomes observed in diabetic patients. This raises an urgent question for the medical community: are we doing enough for prediabetic patients?
4.2. Baseline Characteristics and Cardiovascular Risk
The baseline characteristics of our cohort underscore the increasing prevalence of dysglycemia among patients with cardiovascular disease. Prediabetic patients, while not yet reaching the glycemic thresholds for a diabetes diagnosis, exhibited significant cardiovascular risk factors such as arterial hypertension and dyslipidemia [
17] and are medically underserved [
18]. These risk factors were present at levels intermediate between those observed in non-diabetic and diabetic patients, suggesting a continuum of risk that starts well before the onset of overt diabetes [
19,
20].
4.3. Kaplan-Meier Survival Analysis: A Wake-Up Call
The Kaplan-Meier survival curves vividly illustrate the neglected plight of prediabetic patients. While the immediate post-STEMI survival (30 days) was comparable across all groups as also described in Tian et al. [
21], the divergence in survival rates became pronounced with longer follow-ups. By 90 days and 180 days, prediabetic patients began to show significantly worse survival outcomes, and this trend continued to worsen over one, two, and three years. By the three-year mark, prediabetic patients faced a survival disadvantage nearly as severe as that faced by diabetic patients, particularly when considering the compounded effects of other cardiovascular risk factors such as arterial hypertension and dyslipidemia. These findings are consistent with those of Xu et al. [
17,
22], who also reported that prediabetes was associated with worse long-term outcomes in young patients ≤ 45 years with acute coronary syndrome. Another point worth discussing is the proportion of STEMI patients with complete revascularization.. Unfortunately, It is not possible to assess the contribution of this factor to the survival rate of prediabetic patients compared to diabetic patients.
4.4. Better Management of Diabetes and Cardiovascular Risk Factors (Before or after STEMI?)
The study also highlights the importance of better management of diabetes, particularly concerning additional cardiovascular risk factors such as hypertension and dyslipidemia, as risk-based ASCVD prevention deficits were observed in patients with diabetes and pre-diabetes at time of presentation for STEMI. Effective treatment of diabetes, including optimal control of blood pressure and lipid levels, has been shown to significantly reduce cardiovascular events and improve survival outcomes [
17,
23]. This comprehensive approach to managing diabetes could serve as a model for treating prediabetic patients, addressing not only glycemic control but also the broader spectrum of cardiovascular risk factors. By adopting a holistic approach that addresses these additional risk factors, healthcare providers can significantly enhance patient outcomes and reduce the burden of cardiovascular disease in both prediabetic and diabetic populations.
4.5. The Imperative for Change: Treating Prediabetes
Our findings compel a re-evaluation of current clinical practices and treatment guidelines. The data clearly indicate that prediabetic patients are not merely in a transitional state but are at a critical juncture where early intervention could significantly alter their cardiovascular outcomes. Currently, the standard of care predominantly focuses on the treatment and management of diabetes, with prediabetic patients often receiving minimal intervention. Indeed the ESC Guidelines for the Management of Hyperlipidemia for example recognized diabetes mellitus I and II patients as those at high or very high risk for fatal 10 year CVD depending on the constellation of further risk factors including hypertension, hyperlipidemia, smoking status, and duration of diabetes, however, prediabetes is not yet found in higher risk categories [
24,
25]. This oversight places prediabetic patients at a disproportionate risk, as evidenced by their survival rates in this study. Similar concerns were highlighted by Kim et al. [
26], who found that prediabetic patients with STEMI and multivessel disease had worse clinical outcomes compared to non-diabetic patients, underscoring the need for more aggressive management in this population.
4.6. A Call to Action: Revolutionizing Prediabetes Management
The evidence presented necessitates a paradigm shift in how we approach prediabetes. It is imperative that we extend rigorous cardiovascular risk management strategies to prediabetic patients, similar to those employed for diabetic patients [
27]. This includes more aggressive monitoring, lifestyle interventions, and potentially pharmacological treatments aimed at controlling glycemic levels, blood pressure, and lipid profiles. The integration of prediabetic management into routine cardiovascular care could be transformative, while there are controversial findings regarding inconclusive impact of the agents, which are widely used in prediabetes/Diabetes mellitus Typ II, i.e. SGLT2 inhibitors, on clinical course of STEMI [
28]. Proactive treatment of prediabetes has the potential to prevent the progression to diabetes and mitigate the associated cardiovascular risks [
29]. The establishment of comprehensive prediabetes management programs could reduce the incidence of STEMI and improve long-term survival outcomes for this vulnerable population.
5. Limitation
This study has several limitations that must be considered when interpreting the results. Firstly, the single-center retrospective design may limit the generalizability of our findings to other populations and healthcare settings. Secondly, the classification of glycemic status was based on a single HbA1c measurement, which may not capture fluctuations in glycemic control over time and the lack of clamp test to identify the patients with ischemia-induced insulin resistance. Also fasting status was not captured at time of STEMI. Furthermore, potential confounding factors, such as variations in treatment regimens and patient adherence to medication and lifestyle modifications, were not addressed during the follow-up period. Yet, we did not evaluated the impact of several confounding factors, such as atrial fibrillation, severity of atherosclerosis, LVH, heart failure and its phenotypes, which had been previously defined as powerful predictors for all-cause and CV mortality in STEMI, on the risk of the STEMI-related outcomes. Thirdly, the follow-up period, although extending to three years, may still be insufficient to fully capture the long-term impact of prediabetes on survival outcomes post-STEMI. Future multi-center studies with longer follow-up periods and more comprehensive assessments of glycemic control and cardiovascular risk factors are warranted to validate and extend our findings for post-STEMI patients as well as other patient populations in secondary and primary cardiovascular prevention.
6. Conclusions
In conclusion, this study underscores the urgent need to redefine our approach to cardiovascular risk management by including prediabetic patients as a priority group. The significant survival disparities revealed through Kaplan-Meier analysis highlight the critical need for early intervention in prediabetic individuals with STEMI. By treating prediabetes with the same seriousness as diabetes, we can improve short- and long-term patient outcomes, reduce healthcare costs, and potentially prevent the progression to more severe forms of dysglycemia and cardiovascular disease. The time for change is now—our clinical practices must evolve to ensure that prediabetic STEMI patients are no longer the "stepchildren" of cardiovascular care but are recognized and treated as a high-risk group deserving of comprehensive management and attention.
Author Contributions
Conceptualization E.B., K.K.; Statistical analysis E.B.; Writing—original draft preparation E.B., K.K.; Writing—review and editing M.L., M.H., U.C.H.; Data collection E.B., K.K.; Implementation of interventions C.S., E.P., M.B., W.W., M.H., U.C.H.; Project administration M.L., U.C.H., K.K.; Language correction K.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the State of Salzburg Ethics Commission (EK-Nr. 1038/2021
Informed Consent Statement
Patient consent was waived due to retrospective nature of the study design.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
Graphical abstract, Figure 1 and Figure 4 were created with BioRender.com. Selected artwork shown in Figure 5 was also used from BioRender.com.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
References
- Elendu C, Amaechi DC, Elendu TC, Omeludike EK, Alakwe-Ojimba CE, Obidigbo B, et al. Comprehensive review of ST-segment elevation myocardial infarction: Understanding pathophysiology, diagnostic strategies, and current treatment approaches. Medicine (Baltimore). 2023;102:e35687. [CrossRef]
- Gao J, Wang Y, Yang YN, WU XY, Cui Y, Zou ZH, et al. Impact of Metabolic Syndrome and Its Components on Clinical Severity and Long-Term Prognosis in Patients With Premature Myocardial Infarction. Front Endocrinol (Lausanne). 2022;13:920470. [CrossRef]
- Chatzianagnostou K, Guiducci L, Paradossi U, De Caterina AR, Mazzone A, Berti S, et al. The Role of Prediabetes as a Predictive Factor for the Outcomes in Patients with STEMI. Which Is the Right Range of Glycated Hemoglobin to Adopt in This Setting? Applied Sciences. 2021; 11:5518.
- Mata Marín LA, Schmucker J, Fach A, Osteresch R, Rühle S, Garstka D, et al. Prevalence and clinical characteristics of prediabetes and diabetes mellitus in young patients with ST-segment elevation myocardial infarction. Clin Res Cardiol. 2021;110:1647-1658.
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J Diabetes. 2015;6:296-303. [CrossRef]
- Brannick B, Dagogo-Jack S. Prediabetes and Cardiovascular Disease: Pathophysiology and Interventions for Prevention and Risk Reduction. Endocrinol Metab Clin North Am. 2018;47:33-50.
- Mutie PM, Pomares-Millan H, Atabaki-Pasdar N, Jordan N, Adams R, Daly NL, et al. An investigation of causal relationships between prediabetes and vascular complications [published correction appears in Nat Commun. 2021;12:202.]. Nat Commun. 2020;11:4592.
- Mando R, Waheed M, Michel A, Karabon P, Halalau A. Prediabetes as a risk factor for major adverse cardiovascular events. Ann Med. 2021;53:2090-2098. [CrossRef]
- Zhang J, Du Y, Hu C, et al. Elevated Glycated Albumin in Serum Is Associated with Adverse Cardiac Outcomes in Patients with Acute Coronary Syndrome Who Underwent Revascularization Therapy. J Atheroscler Thromb. 2022;29(4):482-491. [CrossRef]
- Alvarez S, Coffey R, Mathias PM, Algotar AM. Prediabetes. In: StatPearls. Treasure Island (FL): StatPearls; Publishing: July, 2023.
- Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC Guidelines for the management of acute coronary syndromes [published correction appears in Eur Heart J. 2024 Apr 1;45(13):1145]. Eur Heart J. 2023;44:3720-3826.
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S17-S38.
- Lameire NH, Levin A, Kellum JA, Cheung M, Jadoul M, Winkelmayer WC, et al. Harmonizing acute and chronic kidney disease definition and classification: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2021;100(3):516-526. [CrossRef]
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure [published correction appears in Eur Heart J. 2021 Dec 21;42(48):4901.Eur Heart J. 2021;42(36):3599-3726.
- Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G,Januszewicz A, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA) [published correction appears in J Hypertens. 2024 Jan 1;42(1):194.J Hypertens. 2023;41(12):1874-2071.
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L,et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk [published correction appears in Eur Heart J. 2020 Nov 21;41(44):4255.Eur Heart J. 2020;41(1):111-188.
- Kalyani RR, Everett BM, Perreault L, Michos ED. Heart Disease and Diabetes. In: Lawrence JM, Casagrande SS, Herman WH, Wexler DJ, Cefalu WT, eds. Diabetes in America. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); Publishing: December, 2023.
- Ostrominski JW, Vaduganathan M, Girish MP, Gupta P, Hendrickson MJ, Qamar A, et al. Missed Opportunities for Screening and Management of Dysglycemia among Patients Presenting with Acute Myocardial Infarction in North India: The Prospective NORIN STEMI Registry. Glob Heart. 2022;17:54. [CrossRef]
- Nanavaty D, Green R, Sanghvi A, Sinha R, Singh S, Mishra T, et al. Prediabetes is an incremental risk factor for adverse cardiac events: A nationwide analysis. Atheroscler Plus. 2023;54:22-26. [CrossRef]
- Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370:m2297. [CrossRef]
- Tian L, Zhu J, Liu L, Liang Y, Li J, Yang Y. Prediabetes and short-term outcomes in nondiabetic patients after acute ST-elevation myocardial infarction. Cardiology. 2014;127(1):55-61. [CrossRef]
- Xu R, Wang C, Lang J, Wu J, Hu Y, Wang T, et al. Prediabetes is Associated with Worse Long-Term Outcomes in Young Patients with Acute Coronary Syndrome. Diabetes Metab Syndr Obes. 2023;16:3213-3222. [CrossRef]
- Joseph JJ, Deedwania P, Acharya T, Aguilar D, Bhatt DL, Chyun DA, et al. Comprehensive Management of Cardiovascular Risk Factors for Adults With Type 2 Diabetes: A Scientific Statement From the American Heart Association. Circulation. 2022;145:e722-e759. [CrossRef]
- Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37:2999-3058. [CrossRef]
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk [published correction appears in Eur Heart J. 2020 Nov 21;41(44):4255. Eur Heart J. 2020;41:111-188.
- Kim YH, Her AY, Jeong MH, Kim BK, Hong SJ, Kim S, et al. Two-Year Clinical Outcomes Between Prediabetic and Diabetic Patients With STEMI and Multivessel Disease Who Underwent Successful PCI Using Drug-Eluting Stents. Angiology. 2021;72(1):50-61.
- Færch K, Vistisen D, Johansen NB, Jørgensen ME. Cardiovascular risk stratification and management in pre-diabetes. Curr Diab Rep. 2014;14(6):493. [CrossRef]
- von Lewinski D, Kolesnik E, Tripolt NJ, Pferschy PN,Bendedikt M, Wallner M, et al. Empagliflozin in acute myocardial infarction: the EMMY trial. Eur Heart J. 2022;43(41):4421-4432. [CrossRef]
- Lizarzaburu-Robles JC, Herman WH, Garro-Mendiola A, Galdón Sanz-Pastor A, Lorenzo O. Prediabetes and Cardiometabolic Risk: The Need for Improved Diagnostic Strategies and Treatment to Prevent Diabetes and Cardiovascular Disease. Biomedicines. 2024; 12:363. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).