Submitted:
09 September 2024
Posted:
10 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
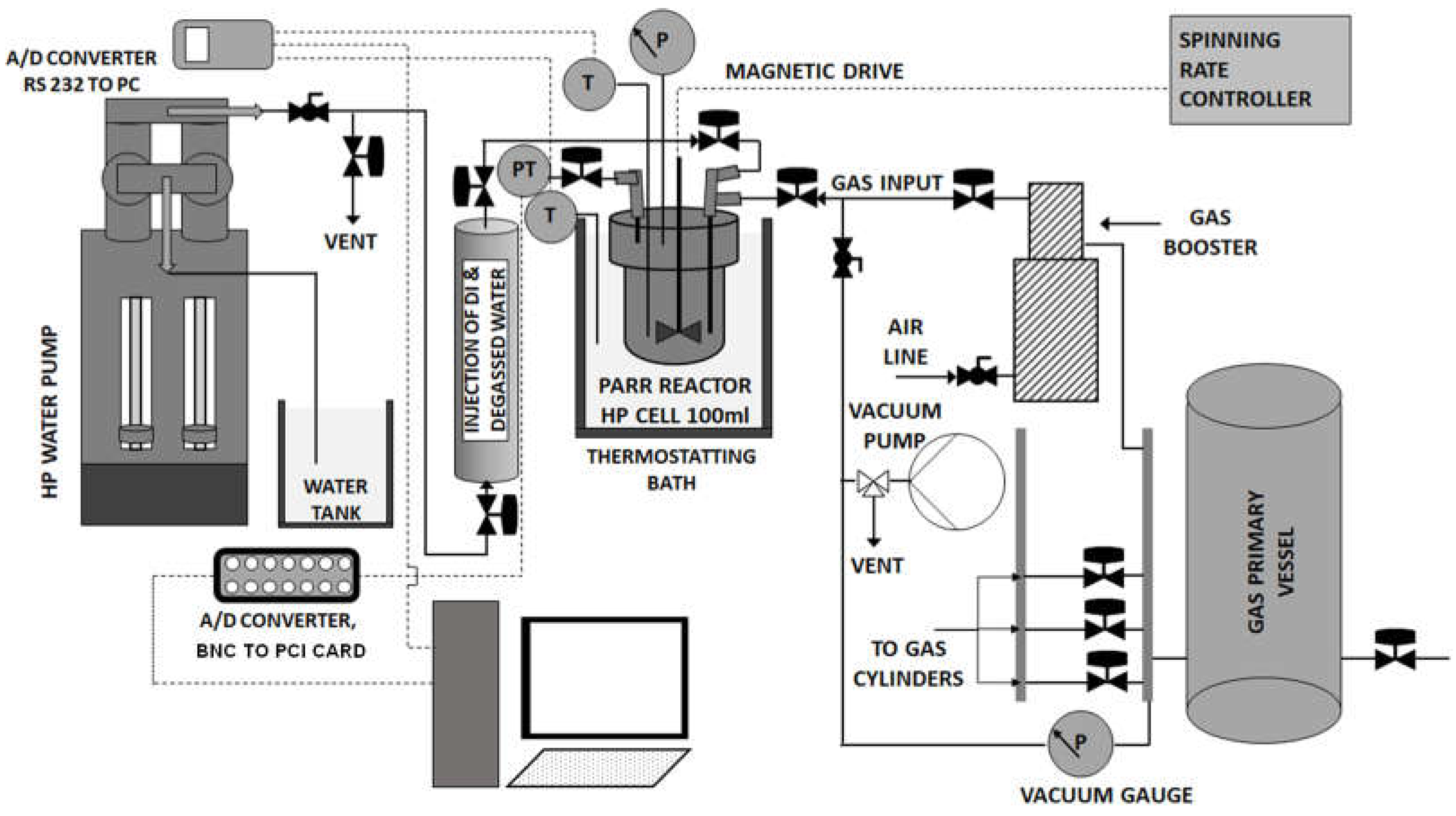
2.2. Experimental Setups
2.3. Gas Preparation Manifold
2.4. Description of the First PVT Apparatus
2.4.1. High Pressure PVT cell
2.4.2. Thermostated Air Cabinet
2.4.3. Monitoring and Data Acquisition
2.5. Description of the PVT Configuration Incorporating a Stirred Reactor
2.5.1. Stirred Reactor
2.5.2. Data Monitoring and Acquisition System
2.6. Water Supply Configuration
2.7. Experimental Procedure
3. Results
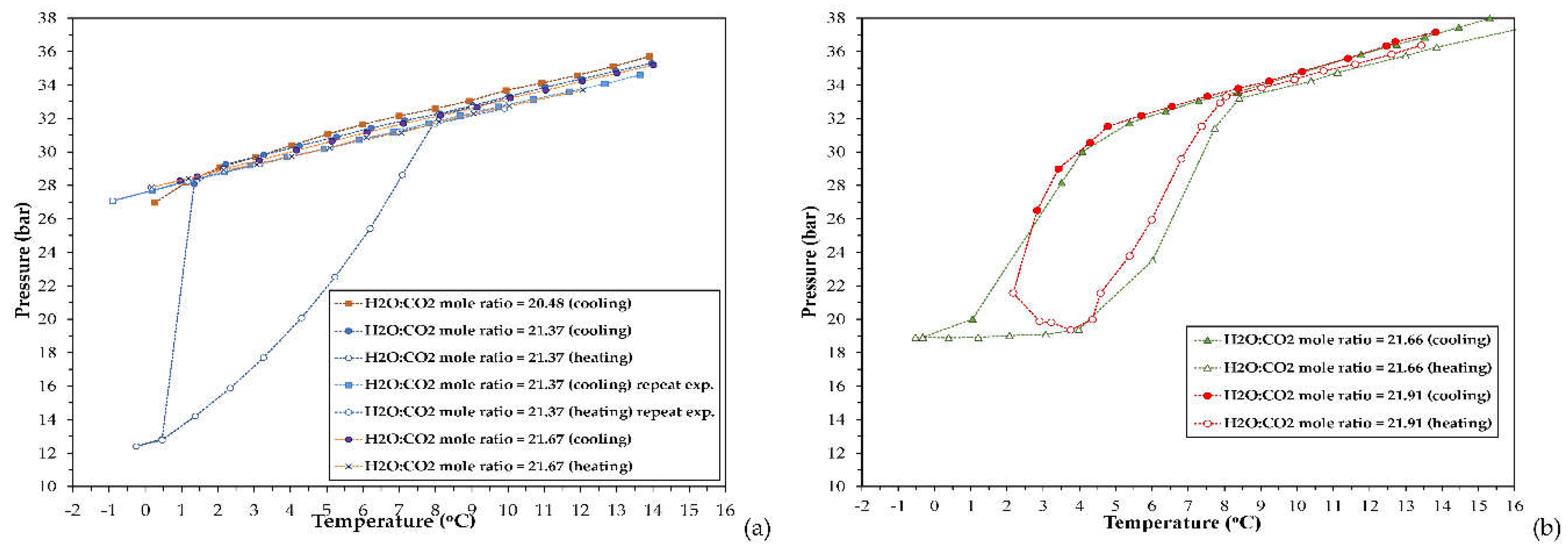
3.1. Experimental Pressure vs. Temperature Phase Diagrams
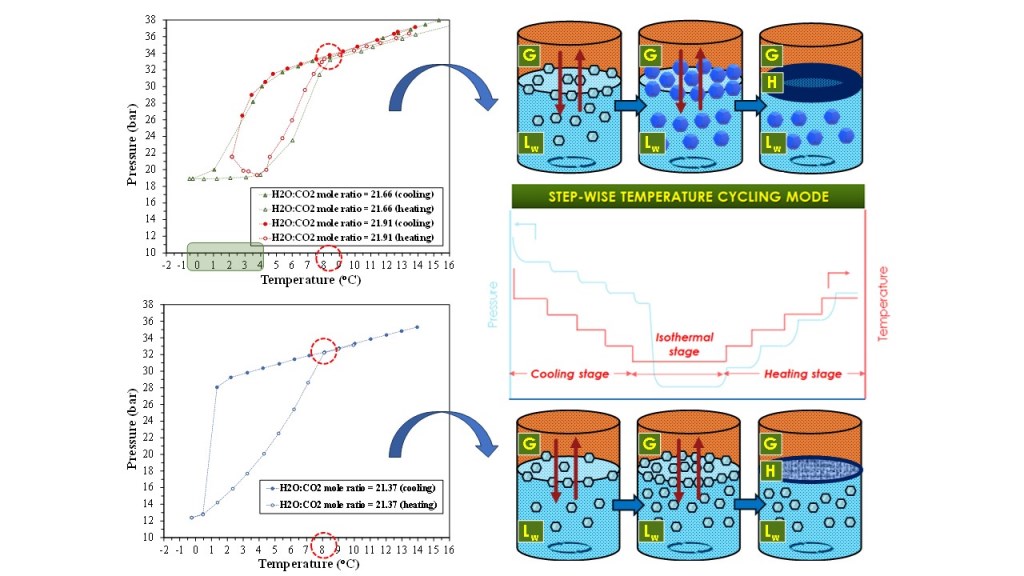
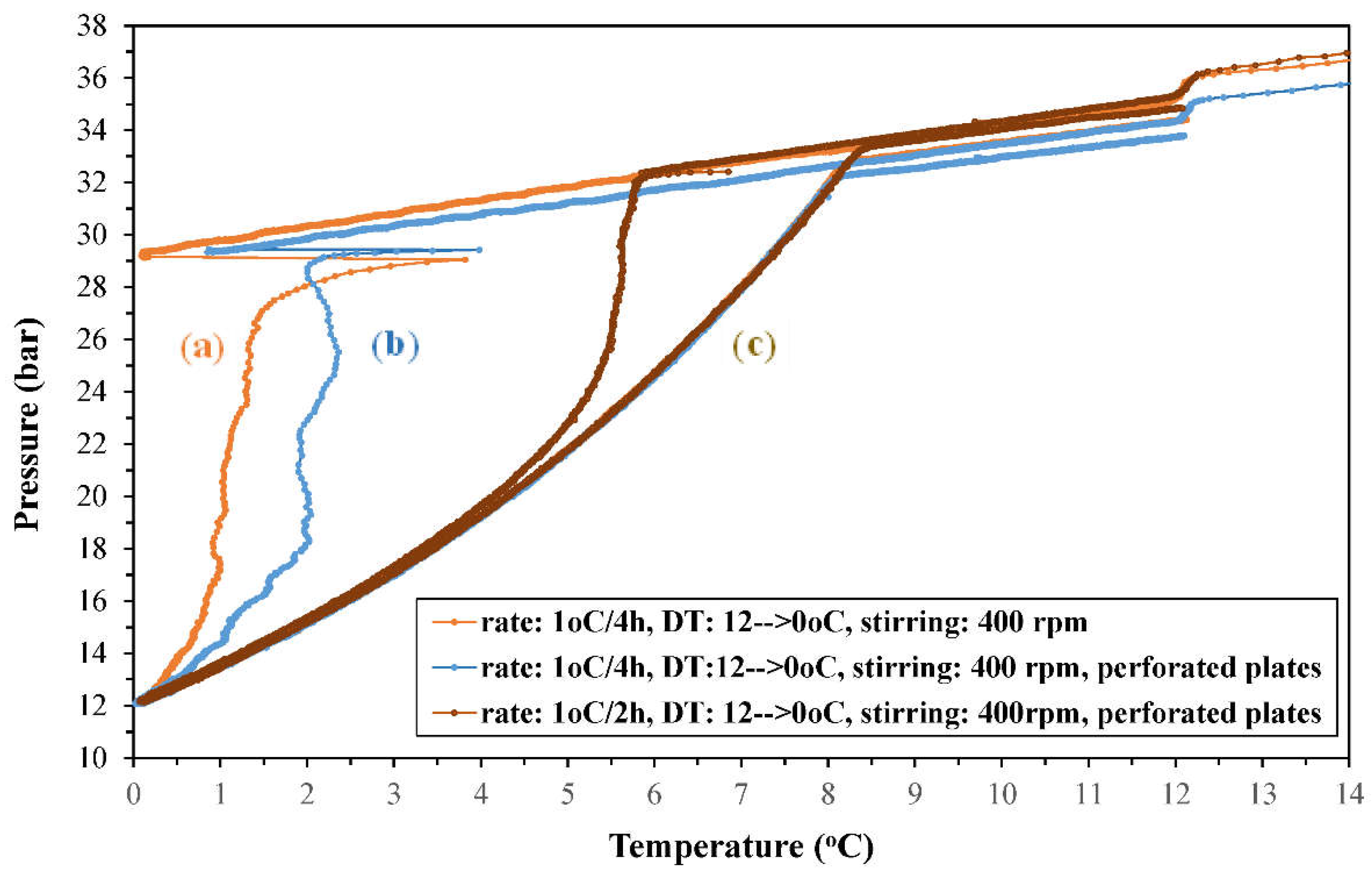
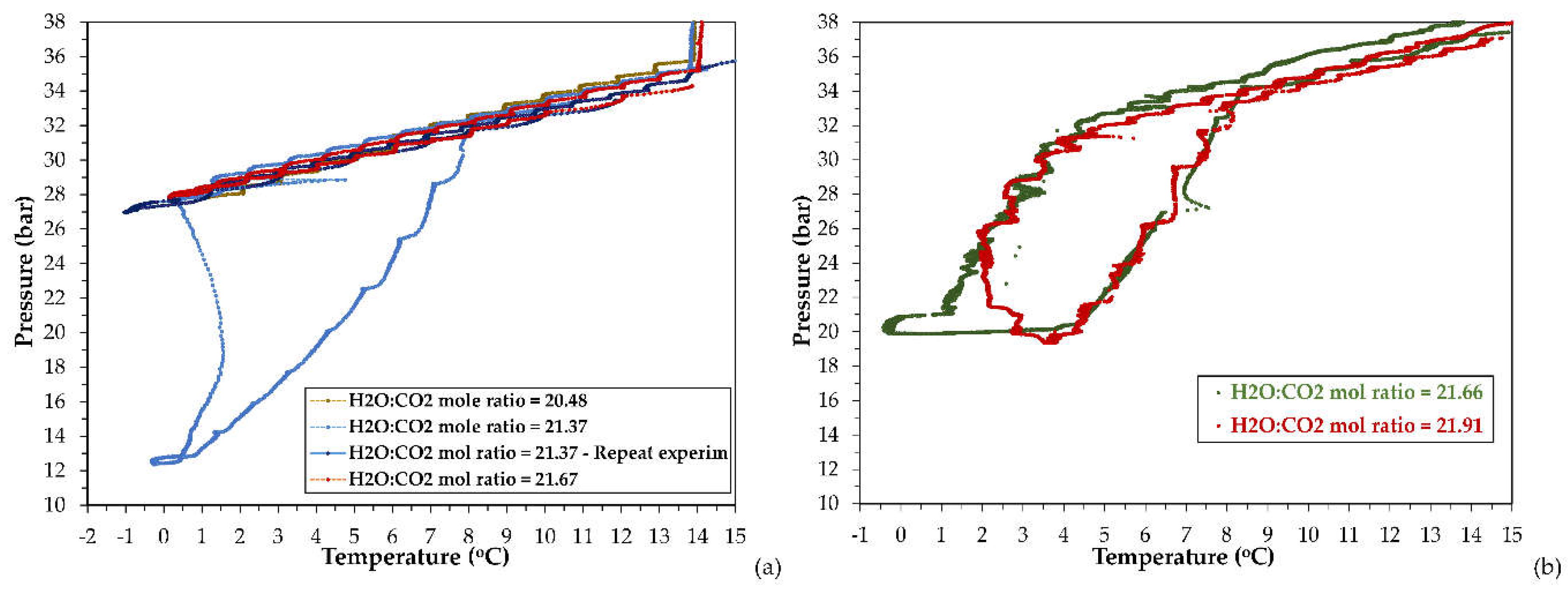
3.1.1. Experimental Runs Performed in Continuous Temperature Cycling Mode
3.1.2. Experimental Runs Performed in Incremental Temperature Cycling Mode
3.2. Correlation of the Pressure Evolution During the Cooling Stage with Crystallization Effects
3.3. Thermal Dissociation of the Hydrate Phase
3.4. Phase Transformations and Equilibrium at CO2 Hydrate Formation Conditions
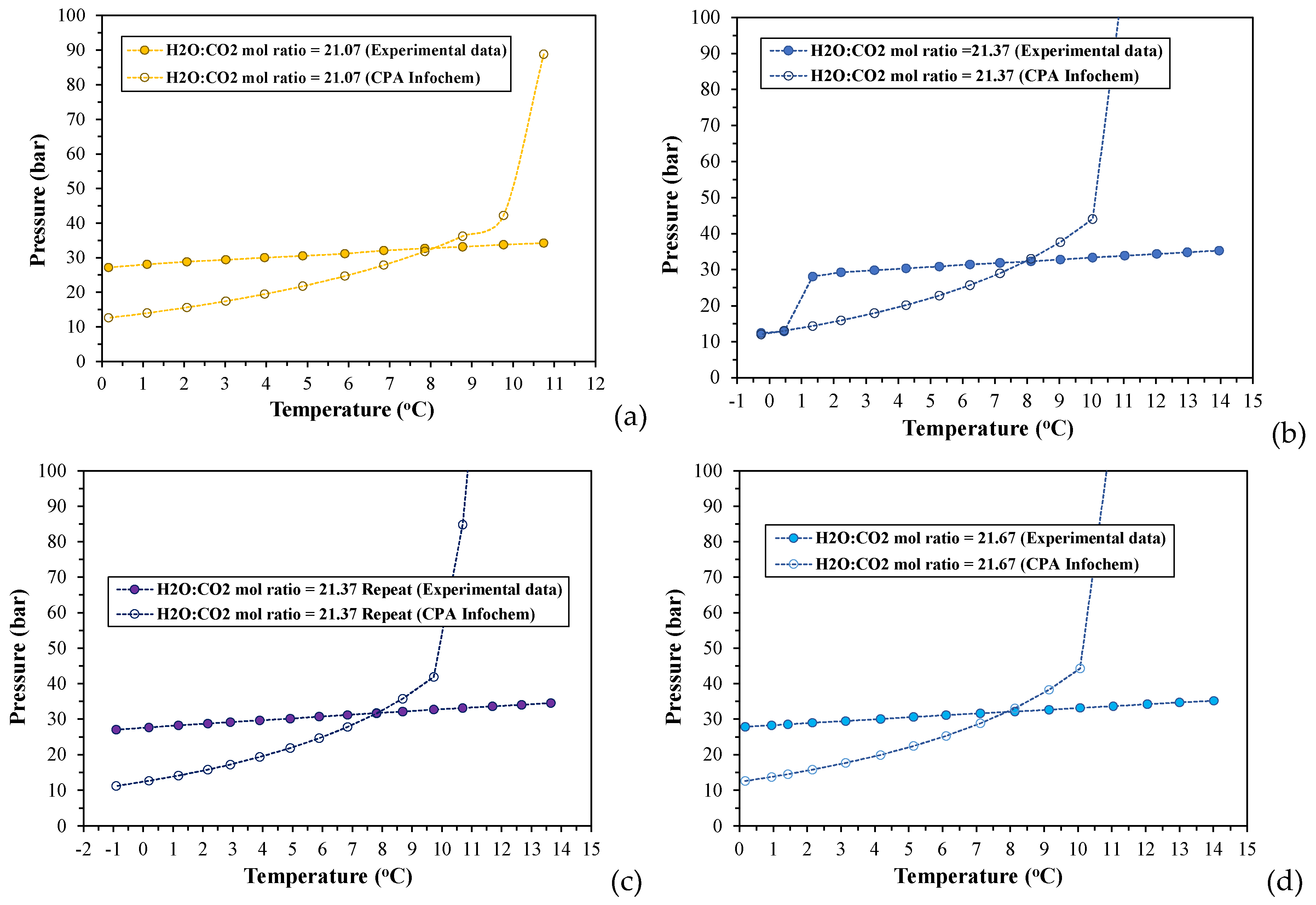
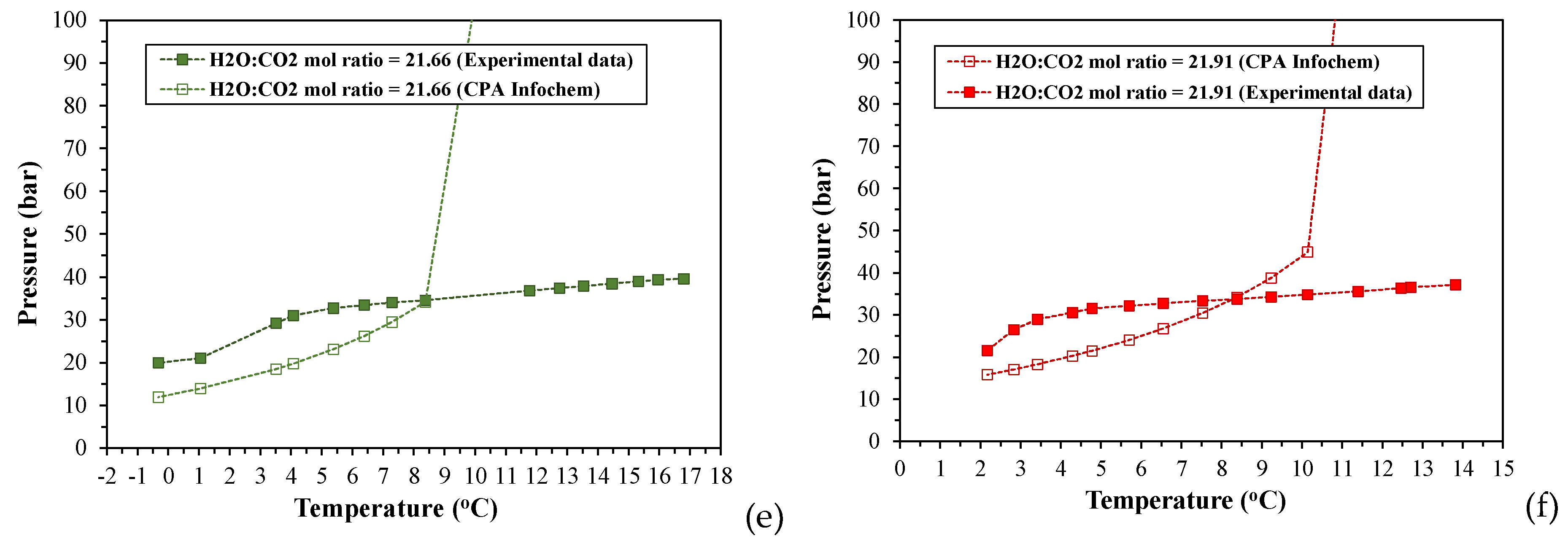
3.5. Comparison between Experimental and Computational Results on the Phase Behavior of The studied CO2-H2O Systems
3.5.1. Computational Results on the Incipient Conditions for CO2 Hydrate Formation
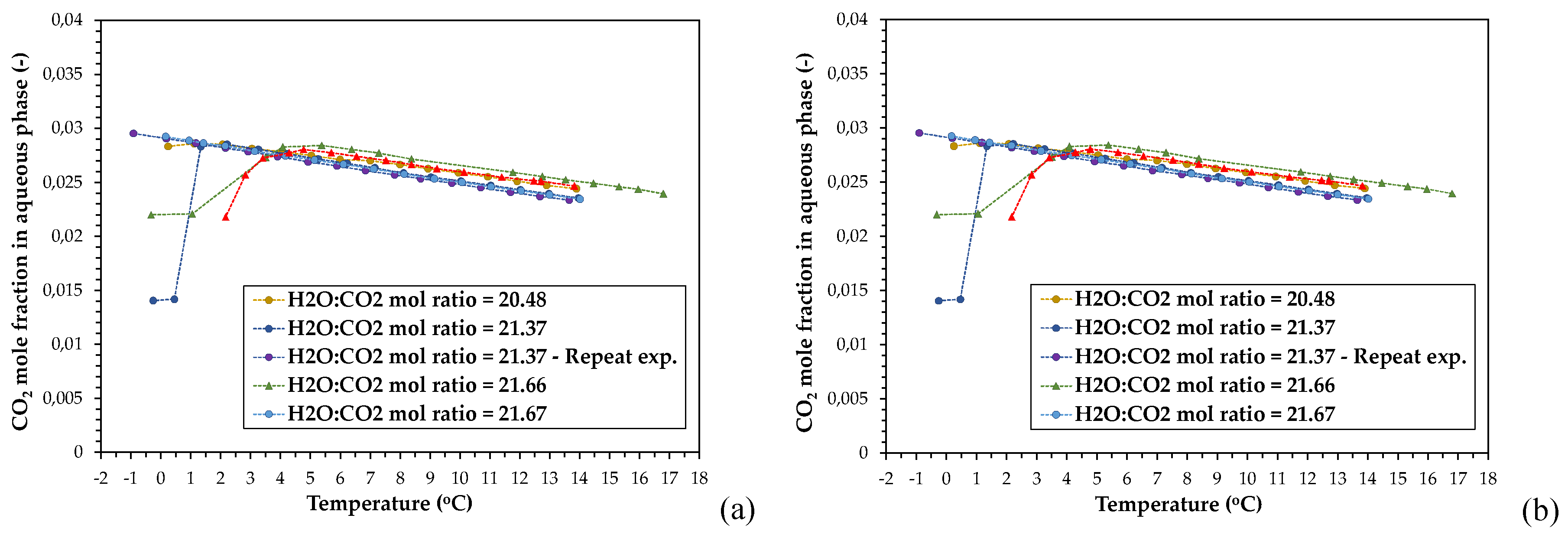
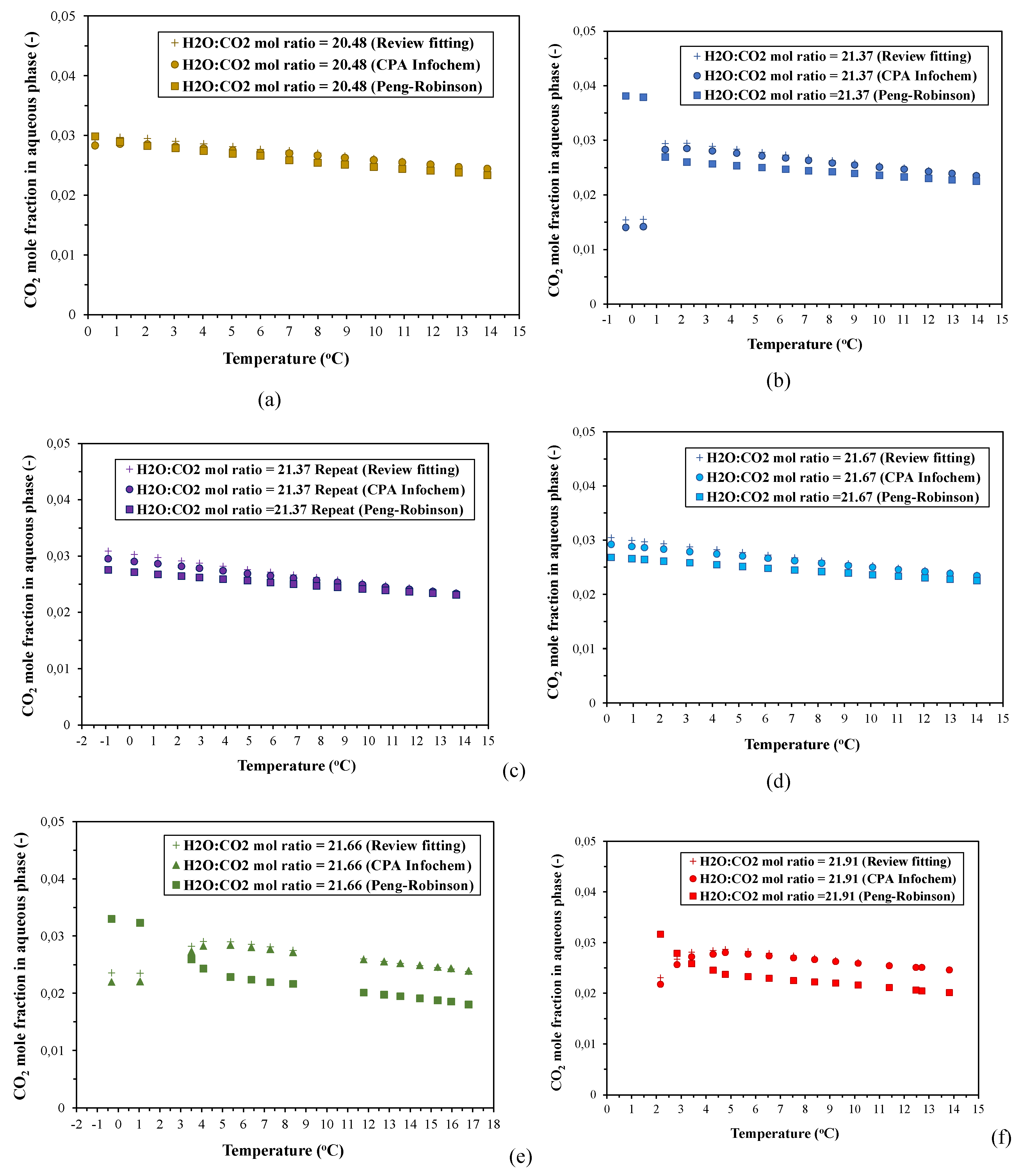
3.5.2. CO2 Solubility Calculations
3.5.2.1. Henry Coefficients at the Equilibrium Temperatures
3.5.2.2. Calculation of CO2 Dissolution in Water Using the Simulation Model and Comparison with the Calculation Results Derived from the Fitting Equation-Based Approach
3.5.2.3. Calculation of CO2 Dissolution in Water Using the Peng-Robinson EoS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.S.; Xu, C.G.; Zhang, Y. Ruan, X. K.; Li, G.; Wang, Y. Investigation into gas production from natural gas hydrate: A review. Appl. Energy 2016, 172, 286-322. [CrossRef]
- Chapoy, A.; Burgass, R.; Tohidi, B.; Alsiyabi, I. Hydrate and phase behavior modeling in CO2-rich pipelines. J. Chem. Eng. Data 2015, 60, 447-453. [CrossRef]
- Liu, W.; Hu, J.; Sun, F.; Sun, Z.; Li, X. A numerical study on the non-isothermal flow characteristics and hydrate risk of CO2 in buried transmission pipelines under the gas-phase transportation mode. Greenhouse Gas Sci. Technol. 2020, 10, 249-264. [CrossRef]
- Liu, B.; Liu, X.; Lu, C.; Godbole, A.; Michal, G.; Tieu, A.K. Multi-phase decompression modeling of CO2 pipelines. Greenhouse Gas Sci. Technol. 2017, 7, 665-679. [CrossRef]
- Wang, Z.; Zhao. Y.; Zhang, J.; Pan, S.; Yu, J.; Sun, B. Flow assurance during deepwater gas well testing: hydrate blockage prediction and prevention. J. Pet. Sci. Eng. 2018, 163, 211-216. [CrossRef]
- Xu, C.-G.; Li, X.-S. Research progress of hydrate-based CO2 separation and capture from gas mixtures. RSC Adv. 2014, 4, 18301-18316. [CrossRef]
- Li, A.; Wang, J.; Bao, B. High-efficiency CO2 capture and separation based on hydrate technology: A review. Greenhouse Gas Sci Technol. 2019, 9, 175-193. [CrossRef]
- Lee, Y.; Kim, H.; Lee, W.; Kang, D.W.; Lee, J.W.; Ahn, Y.-H. Thermodynamic and kinetic properties of CO2 hydrates and their applications in CO2 capture and separation. J. Environ. Chem. Eng. 2023, 11, 110933. [CrossRef]
- Cheng, Z.; Li, S.; Liu, Y.; Zhang, Y.; Ling, Z.; Yang, M.; Jiang, L.; Song, Y. Post-combustion CO2 capture and separation in flue gas based on hydrate technology: A review. Renew. Sust. Energ. Rev. 2022, 154, 111806. [CrossRef]
- Luling, L.; Shuanshi, F.; Qiuxiong, C.; Guang, Y.; Jinzhou, Z.; Na, W.; Yonggang, W. Experimental and modeling phase equilibria of gas hydrate systems for post-combustion CO2 capture. J. Taiwan Inst. Chem. Eng. 2019, 96, 35-44. [CrossRef]
- Babu, P.; Linga, P.; Kumar, R.; Englezos, P. A review of the hydrate based gas separation (HBGS) process for carbon dioxide pre-combustion capture. Energy 2015, 85, 261-279. [CrossRef]
- Gholinezhad, J.; Chapoy, A.; Tohidi, B. Separation and capture of carbon dioxide from CO2/H2 syngas mixture using semi-clathrate hydrates. Chem. Eng. Res. Des. 2011, 89, 1747-1751. [CrossRef]
- Hassan, M.H.A.; Sher, F.; Fareed, B.; Ali, U.; Zafar, A.; Bilal, M.; Iqbal, H.M.N. Sustainable hydrates for enhanced carbon dioxide capture from an integrated gasification combined cycle in a fixed bed reactor. Ind. Eng. Chem. Res. 2021, 60, 11346-11356. [CrossRef]
- Zheng, J.; Chong, Z.R.; M. Qureshi, M.F.; Linga, P. Carbon dioxide sequestration via gas hydrates: A potential pathway toward decarbonization. Energy Fuels 2020, 34, 10529-10546. [CrossRef]
- Tohidi, B.; Yang, J. Salehabadi, M.; Anderson, R.; Chapoy, A. CO2 hydrates could provide secondary safety factor in subsurface sequestration of CO2. Environ. Sci. Technol. 2010, 44, 1509-1514. [CrossRef]
- Teng, Y.; Zhang, D. Long-term viability of carbon sequestration in deep-sea sediments. Sci. Adv. 2018, 4, 1-8. [CrossRef]
- Liu, Y.; Wang, P.; Yang, M.; Zhao, Y.; Zhao, J.; Song, Y. CO2 sequestration in depleted methane hydrate sandy reservoirs. J. Nat. Gas Sci. Eng. 2018, 49, 428-434. [CrossRef]
- Koh, D.-Y.; Kang, H.; Lee, J.-W.; Park, Y.; Kim, S.-J.; Lee, J.; Lee, J.Y.; Lee, H. Energy-efficient natural gas hydrate production using gas exchange. Appl. Energy 2016, 162, 114-130. [CrossRef]
- Wilson, I.; Saini, S.; Sreenivasan, H.; Sahu, C.; Krishna, S.; Gupta, P. Review and perspectives of energy-efficient methane production from natural gas hydrate reservoirs using carbon dioxide exchange technology. Energy Fuels 2023, 37, 9841-9872. [CrossRef]
- Lim, J.; Choi, W.; Mok, J.; Seo, Y. Kinetic CO2 selectivity in clathrate-based CO2 capture for upgrading CO2-rich natural gas and biogas. Chem. Eng. J. 2019, 369, 686-693. [CrossRef]
- Beatrice Castellani, B.; Morini, E.; Bonamente, E.; Rossi, F. Experimental investigation and energy considerations on hydrate-based biogas upgrading with CO2 valorization. Biomass Bioenergy 2017, 105, 364-372. [CrossRef]
- Moghaddam, E.A.; Larsolle, A.; Tidåker, P.; Nordberg, A. Gas hydrates as a means for biogas and biomethane distribution. Front. Energy Res. 2021, 9, 568879. [CrossRef]
- Adisasmito, S.; Frank III, R.J.; Sloan Jr. E.D. Hydrates of carbon dioxide and methane mixtures. J. Chem. Eng, Data 1991, 36, 68-71. [CrossRef]
- Servio, P.; Englezos, P. Effect of temperature and pressure on the solubility of carbon dioxide in water in the presence of gas hydrate. Fluid Phase Equilib. 2001, 190, 127-134. [CrossRef]
- Martinez C.; Sandoval, J.F.; Ortiz, N.; Ovalle, S.; Beltran, J.G. Mechanisms, growth rates, and morphologies of gas hydrates of carbon dioxide, methane, and their mixtures. Methane 2022, 1, 2-23. [CrossRef]
- Ferrari, P.F.; Guembaroski, A.Z.; Neto, M.A.M.; Morales, R.E.M.; Sum, A.K. Experimental measurements and modelling of carbon dioxide hydrate phase equilibrium with and without ethanol. Fluid Phase Equilib. 2016, 413, 176-183. [CrossRef]
- Tariq, M.; Soromenho, M.R.C.; Rebelo, L.P.N.; Esperança, J.M.S.S. Insights into CO2 hydrates formation and dissociation at isochoric conditions using a rocking cell apparatus. Chem. Eng. Sci. 2022, 249, 117319. [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate hydrates of natural gases. 3rd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, 2008, pp. 113-188 and 189-256.
- Zou, X.; Zi, M.; Yang, C.; Liu, K.; Zhao, C.; Chen, D. High-throughput sapphire reaction system: A new experimental apparatus to evaluate hydrate kinetic inhibitors with high efficiency. J. Nat. Gas Sci. Eng. 2022, 104, 104687. [CrossRef]
- Freer, E.M.; Selim, M.S.; Sloan Jr., E.D. Methane hydrate film growth kinetics. Fluid Phase Equilib. 2001, 185, 65-75. [CrossRef]
- Saito, K.; Kishimoto, M.; Tanaka, R.; Ohmura, R. Crystal growth of clathrate hydrate at the interface between hydrocarbon gas mixture and liquid water. Cryst. Growth Des. 2010, 11, 295-301. [CrossRef]
- Servio, P.; Englezos, P. Morphology of methane and carbon dioxide hydrates formed from water droplets. AIChE J. 2003, 49, 269-276. [CrossRef]
- Ohmura, R.; Shigetomi, T.; Mori, Y.H. Formation, growth and dissociation of clathrate hydrate crystals in liquid water in contact with a hydrophobic hydrate-forming liquid. J. Cryst. Growth 1999, 196, 164-173. [CrossRef]
- Ueno, H.; Akiba, H.; Akatsu, S.; Ohmura, R. Crystal growth of clathrate hydrates formed with methane + carbon dioxide mixed gas at the gas/liquid interface and in liquid water. New J. Chem. 2015, 39, 8254-8262. [CrossRef]
- Pivezhani, F.; Roosta, H.; Dashti, A.; Mazloumi, S.H. Investigation of CO2 hydrate formation conditions for determining the optimum CO2 storage rate and energy: Modeling and experimental study. Energy 2016, 113, 215-226. [CrossRef]
- Hao, W.; Wang, J.; Fan, S.; Hao, W. Study on methane hydration process in a semi-continuous stirred tank reactor. Energy Convers. Manag. 2007, 48, 954-960. [CrossRef]
- Filarsky, F.; Hagelstein, M.; Schultz, H.J. Influence of different stirring setups on mass transport, gas hydrate formation, and scale transfer concepts for technical gas hydrate applications. Applied Research 2022, 2, 1-16. [CrossRef]
- Englezos, P.; Kalogerakis, N.; Dholobhai, P.D.; Bishnoi, P.R. Kinetics of formation of methane and ethane gas hydrates. Chem. Eng. Sci. 1987, 42, 2647-2658. [CrossRef]
- Kim, H.C.; Bishnoi, P.R.; Heidemann, R.A.; Rizvi, S.S.H. Kinetics of methane hydrate decomposition. Chem. Eng. Sci. 1987, 42, 1645-1653. [CrossRef]
- Windmeier, C.; Oellrich, L. Theoretical study of gas hydrate decomposition kinetics-model development. J. Phys. Chem. A 2013, 117, 10151-10161. [CrossRef]
- Windmeier, C.; Oellrich, L. Theoretical study of gas hydrate decomposition kinetics-model predictions, J. Phys. Chem. A 2013, 117, 12184-12195. [CrossRef]
- Clarke, M.; Bishnoi, P.R. Determination of the intrinsic rate of ethane gas hydrate decomposition. Chem. Eng. Sci. 2000, 55, 4869-4883. [CrossRef]
- Sean, W.Y.; Sato, T.; Yamasaki, A.; Kiyono, F. CFD and experimental study on methane hydrate dissociation Part I. Dissociation under water flow. AIChE J. 2007, 53, 262-274. [CrossRef]
- Hong, H.; Pooladi-Darvish, M.; Bishnoi, P.R. Analytical modelling of gas production from hydrates in porous media. J. Can. Pet. Technol. 2003, 42, 45–56. [CrossRef]
- Davies, S.R.; Selim, M.S.; Sloan, E.D.; Bollavaram, P.; Peters, D.J. Hydrate plug dissociation. AIChE J. 2006, 52, 4016-4027. [CrossRef]
- Chen, G.-J.; Guo, T.-M. Thermodynamic modeling of hydrate formation based on new concepts. Fluid Phase Equilib. 1996, 122, 43-65. [CrossRef]
- You, C.; Chen, Z.; Li, X.; Zhao, Q.; Feng, Y.; Wang, C. Benedict–Webb–Rubin–Starling Equation of State + hydrate thermodynamic theories: An enhanced prediction method for CO2 solubility and CO2 hydrate phase equilibrium in pure water/NaCl aqueous solution system. Energies 2024, 17, 2356. [CrossRef]
- Kontogeorgis, G.M.; Voutsas, E.C.; Yakoumis, I.V.; Tassios, D.P. An equation of state for associating fluids. Ind. Eng. Chem. Res. 1996, 35, 4310-4318. [CrossRef]
- Ballard, A.; Sloan, E. The next generation of hydrate prediction: an overview. J. Supramol. Chem. 2002, 2 385-392. [CrossRef]
- Klauda,J.B.; Sandler, S.I. Phase behavior of clathrate hydrates: a model for single and multiple gas component hydrates. Chem. Eng. Sci. 2003, 58, 27-41. [CrossRef]
- Kastanidis, P.; Romanos, G.E.; Michalis, V.K.; Economou, I.G.; Stubos, A.K.; Tsimpanogiannis, I.N. Development of a novel experimental apparatus for hydrate equilibrium measurements. Fluid Phase Equilib. 2016, 424, 152-161. [CrossRef]
- Maekawa, T. Equilibrium conditions for clathrate hydrates formed from carbon dioxide or ethane in the presence of aqueous solutions of 1,4-dioxane and 1,3-dioxolane. Fluid Phase Equilib. 2014, 384, 95-99. [CrossRef]
- Sun, Q.; Kang, Y.T. Review on CO2 hydrate formation/dissociation and its cold energy application. Renewable and Sustainable Energy Reviews 2016, 62, 478-494. [CrossRef]
- Mali, G.A.; Chapoy, A.; Tohidi, B. Investigation into the effect of subcooling on the kinetics of hydrate formation. J. Chem. Thermodynamics 2018, 117, 91-96. [CrossRef]
- Canale, V.; Fontana, A.; Siani, G.; Di Profio, P. Hydrate induction time with temperature steps: A novel method for the determination of kinetic parameters. Energy Fuels 2019, 33, 6113-6118. [CrossRef]
- Uchida, T.; Ebinuma, T.; Kawabata, J.; Narita, H. Microscopic observations of formation processes of clathrate-hydrate films at an interface between water and carbon dioxide. J. Cryst. Growth, 1999, 204, 348-356. [CrossRef]
- Uchida, T.; Ikeda, I.Y.; Takeya, S.; Ebinuma, T.; Nagao, J.; Narita, H. CO2 hydrate film formation at the boundary between CO2 and water: effects of temperature, pressure and additives on the formation rate. J. Cryst. Growth 2002, 237–239, 383-387. [CrossRef]
- Natarajan, V.; Bishnoi, P.R.; Kalogerakis, N. Induction phenomena in gas hydrate nucleation. Chem. Eng. Sci. 1994, 49, 2075-2087. [CrossRef]
- Mori, Y.H. Estimating the thickness of hydrate films from their lateral growth rates: application of a simplified heat transfer model. J. Cryst. Growth 2001, 223, 206-212. [CrossRef]
- Mori, Y.H.; Mochizuki, T. Mass transport across clathrate hydrate films – a capillary permeation model. Chem. Eng. Sci. 1997, 52, 3613-3616. [CrossRef]
- Sugaya, M., Mori, Y.H. Behavior of clathrate hydrate formation at the boundary of liquid water and a fluorocarbon in liquid or vapor state. Chem. Eng. Sci. 1996, 51, 3505-3517. [CrossRef]
- Li, X.; Wang, C.; Li, Q.; Fan, Q.; Chen, G.; Sun, C. Study on the growth kinetics and morphology of methane hydrate film in a porous glass microfluidic device. Energies 2021, 14, 6814. [CrossRef]
- Mori, Y.H.; Mochizuki, T. Modeling of simultaneous heat and mass transfer to/from and across a hydrate film. Ann. New York Acad. Sci. 2000, 912, 633-641. [CrossRef]
- Li, S.-L.; Sun, C.-Y.; Feng, X.-J.; Li, F.-G.; Chen, L.-T.; Chen, G.-J. Initial thickness measurements and insights into crystal growth of methane hydrate film. AIChE J. 2013, 59, 2145-2154. [CrossRef]
- Li, S.-L.; Sun, C.-Y.; Liu, B.; Li, Z.-Y.; Chen, G.-J.; Sum, A. K. New observations and insights into the morphology and growth kinetics of hydrate films. Sci. Rep. 2014, 4, 4129. [CrossRef]
- Carroll, J.J.; Slupsky, J.D.; Mather, A.E. The solubility of carbon dioxide in water at low pressure. J. Phys. Chem. Ref. Data 1991, 20, 1201-1209. [CrossRef]
- Saito, K.; Sum, A.K.; Ohmura, R. Correlation of hydrate-film growth rate at the guest/liquid-water interface to mass transfer resistance. Ind. Eng. Chem. Res. 2010, 49, 7102-7103. [CrossRef]
| Experiment No. |
Feed pressure (bara) |
Water amount (ml) |
H2O:CO2 mole ratio (MR) (-) |
Stirring rate (rpm) |
Temperature/ duration of 1st isothermal step (oC) / (hours) |
Temperature/ duration of 2nd isothermal step (oC) / (hours) |
Temperature alteration mode/heating stage(1) |
Inner total volume of the PVT cell (ml) |
| 1 | 32.09 | 60 | 20.48 | 400 | 13.90 / 12 | 0.25 / 24 | SW / - | 100 |
| 2 | 31.40 | 60 | 21.37 | 400 | 13.96 / 12 | -0.25 / 24 | SW / + | 100 |
| 3 | 31.40(2) | 60 | 21.37(2) | 400 | 13.65 / 12 | -0.90 / 24 | SW / + | 100 |
| 4 | 31.04 | 195 | 21.66 | 400 | 16.80 / 12 | -0.32 / 24 | SW / + | 325 |
| 5 | 31.39 | 60 | 21.67 | 900 | 14.01 / 12 | 0.17 / 24 | SW / + | 100 |
| 6 | 31.36 | 195 | 21.91 | 400 | 13.82 / 12 | 2.17 / 24 | SW / + | 325 |
| 7 | 33.14 | 60 | 20.09 | 400 | 12 / 2 | 0.1 / 24 | CM / + | 100 |
| 8 | 32.99(3) | 60 | 20.21(3) | 400 | 12 / 2 | 0.1 / 24 | CM / + | 100 |
| 9 | 32.75 | 60 | 20.28 | 400 | 12 / 2 | 0.1 / 48 | CM / + | 100 |
| 10 | 32.99(4) | 60 | 20.21(4) | 400 | 12 / 2 | 0.1 / 24 | CM / + | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





