Submitted:
06 September 2024
Posted:
10 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Detrimental effects of abiotic stress on wheat crop
2.1. Wheat seed germination and growth under abiotic stress
2.2. ROS production under abiotic stress and their effects on wheat
2.3. RNS production under abiotic stress and their effects on wheat
2.4. Effects of frost and cold temperature on wheat
2.5. Effects of abiotic stress on physiological processes
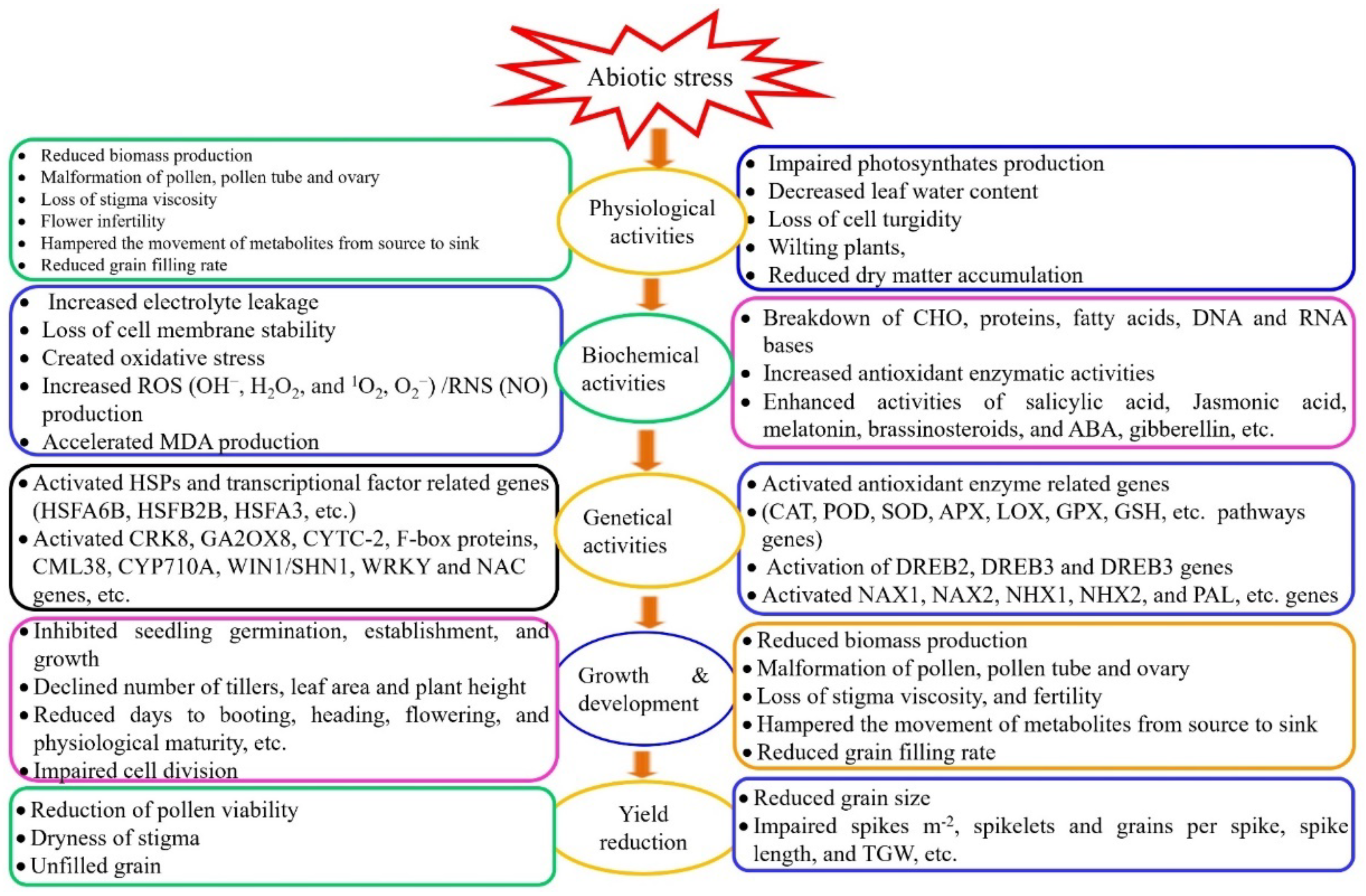
3. Approaches of abiotic stress management
3.1. Biochemical adaptation under abiotic oxidative stress
Glycine betaine (GB) application
Carbohydrates application
| Gene Name | Gene Symbol | Function | Reference |
| ECERIFERUM1 | TaCER1 | It is the CER1 enzyme that is responsible for the production of the very long-chain alkanes that are found in large concentrations during the late stage of wheat development. | Li et al. 2019 |
| Dehydration-responsive element binding protein 2 Dehydration-responsive element binding protein 3 Dehydration-responsive element binding protein 6 |
TaDREB2 TaDREB3 TaDREB6 |
DREBs are transcription factors, Transcriptional regulates involved in plant responses to drought, cold, and salt tolerance | Sadau et al. 2024 |
| Heat shock protein | TaHSP | Molecular chaperones protecting the proteome against environmental stresses; thermometry | Hill and Li 2022 |
| Heat shock transcription factor | TaHSF | Portion of signal transduction chains mediating the activating of genes responsive to both heat stress and other stresses | Charng et al. 2007 |
| Sodium/hydrogen antiporter Sodium/hydrogen exchanger 1 | TaNHX1 | K+/H+ antiporter is highly expressed in gourd cells. It is found in plasma membranes, tonoplast membranes, mitochondrial membranes, chloroplast membranes, and intercellular membranes | Athar et al. 2022 |
| Sodium/hydrogen antiporter | TaNHX2 | K+ and Na+/H+ antiporters are highly expressed in gourd cells, resulting in salt-tolerant stomatal conductance and turgor pressure | Yu et al. 2023 |
| NAX1 and NAX2 | - | Both genes regulate the exclusion of Na+ from plant leaves in saline soil by removing Na+ from the xylem | James et al. 2011 |
| Calmodulin | CML38 | Senses calcium levels and relays signals to calcium-sensitive enzymes, ion channels, and other proteins | Yang et al. 1996 |
| Proton-inorganic pyrophosphatase(AVP1) | TaAVP1 | ROS scavenging gene | Menadue et al. 2021 |
| Phenylalanine ammonia-lyase (PAL) | TaPAL | Polyphenol compounds biosynthesis like flavonoids, phenylpropanoids, and lignin in plants | Mamrutha et al. 2017 |
| SHINE1 | TaSHN1 | It controls plant wax biosynthesis | Bi et al. 2016 |
| NAC transcription factor 47 | TaNAC47 | NAC proteins regulate stress-related transcriptional reprogramming | Wang et al. 2015 |
| WRKY transcription factor 44 | TaWRKY44 | Proteins known as WRKY play important roles in the growth, development, and metabolic processes of plants as well as their responses to abiotic stresses. | Yu et al. 2023 |
| Glutathione peroxidase | TaGPX | GPX reduces hydrogen peroxide to water and oxygen and peroxide radicals to alcohols and oxygen. | Ursini and Maiorino 2012 |
| Mn-superoxide dismutase | TaMn-SOD | ROS (O2•–) scavenging gene | Vijayaraghavaredy et al. 2022 |
| Catalase | TaCAT | Antioxidant pathway-related, ROS (H2O2) scavenging gene | Caverzan et al. 2016 |
| Ascorbate peroxidase | TaAPX | APX enzymes catalyze the conversion of H2O2 to H2O using ascorbate as a specific electron donor | Li 2023 |
| Cu/Zn-superoxide dismutase | TaCu/Zn-SOD | ROS (O2•–) scavenging gene | Tyagi et al. 2017 |
| Iron-superoxide dismutase | TaFe-SOD | ROS (O2•–) scavenging gene | Himi et al. 2016 |
3.2. Controlling abiotic stress through upkeeping of antioxidant enzymatic pathways
Ascorbic acid (AA) and α-tocopherol
3.3. Exogenous application of phytohormones in mitigating the effects of abiotic stress
Salicylic acid (SA)
Abscisic acid (ABA)
Jasmonic acid (JA)
Gibberellic acid (GA)
Indole acetic acid (IAA)
Ethylene (ET)
Melatonin
Brassinosteroids (BRs)
3.4. Agronomic interventions
3.5. Molecular strategies for mitigating abiotic stress effects on wheat crop
3.5.1. Molecular approaches to heat stress management
3.5.2. Molecular approaches to salt stress management
Conclusions and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interests
References
- Abhinandan K, Skori L, Stanic M, Hickerson NMN, Jamshed M, Samuel MA (2018) Abiotic stress signaling in wheat-An inclusive overview of hormonal interactions during abiotic stress responses in wheat. Front Plant Sci 9:1-25. [CrossRef]
- Alabadi D, Oyama T, Yanovsky MJ, et al. (2001) Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293:880–883. [CrossRef]
- Alam MN, Bodruzzaman M, Hossain MM, Sadekuzzaman M (2014) Growth performance of spring wheat under heat stress conditions. Int J Agron Agric Res 4(6):91-103.
- Alam MN, Akhter MM, Hossain MM, Rokonuzzaman (2013c). Performance of different genotypes of wheat (Triticum aestivum L.) in heat stress conditions. Intl J Biosci 3(8):295-306. =: =. [CrossRef]
- Alam MN, Akhter MM, Hossain MM, Mahbubul SM (2013b) Phenological changes of different wheat genotypes (Triticum aestivum L.) in high temperature imposed by late seeding. J Biodivers Environ Sci 3(8):83-93.
- Alam MN, Mannaf MA, Sarker MAZ, Akhter MM (2013a) Effect of terminal high temperature imposed by late sowing on phenological traits of wheat (Triticum aestivum L.). Intl J Agron Agric Res 3(3):6-10.
- Alam MN, Wang Y, Chan Z (2018b) Physiological and biochemical analyses reveal drought tolerance in cool-season tall fescue (Festuca arundinacea) turf grass with the application of melatonin. Crop Past Sci 69(10):1041-1049. [CrossRef]
- Alam MN, Yang L, Yi X, Wang Q-F, Robin AHK (2021) Role of melatonin in inducing the physiological and biochemical processes associated with heat stress tolerance in tall fescue (Festuca arundinaceous). J Plant Growth Regul. [CrossRef]
- Alam MN, Zhang L, Yang L, Islam MR, Liu Y, Luo H, et al. (2018a) Transcriptomic profiling of tall fescue in response to heat stress and improved thermotolerance by melatonin and 24- epibrassinolide. BMC Genomics 19:224. [CrossRef]
- Alcazar R, Cuevas JC, Planas J, Zarza X, Bortolotti C, Carrasco P, et al. (2011) Integration of polyamines in the cold acclimation response. Plant Sci 180:31-38. [CrossRef]
- Aldesuquy HS, Ghanem HE (2015) Exogenous salicylic acid and trehalose ameliorate short-term drought stress in wheat cultivars by up-regulating membrane characteristics and antioxidant defense system. Plant Omics 2(2):1-10. [CrossRef]
- Aroca R, Porcel R, Ruiz-Lozano JM (2012) Regulation of root water uptake under abiotic stress conditions. J Exp Bot 63:43–57. [CrossRef]
- Arora P, Bhardwaj R, Kanwar MK (2012) Effect of 24-epibrassinolide on growth, protein content and antioxidative defense system of Brassica juncea L. subjected to cobalt ion toxicity. Acta Physiol Plant 34(5):2007-2017. [CrossRef]
- Astolfi S, Zuchi S (2013) Adequate S supply protects barley plants from adverse effects of salinity stress by increasing thiol contents. Acta Physiol Plant 35(1):175-181. [CrossRef]
- Atanga RA, Tankpa V (2021) Climate change, flood disaster risk and food security nexus in northern Ghana. Front Sustain Food Sys. 5. [CrossRef]
- Athar R, Zulfiqar, F, Moosa A, et al. (2022) Salt stress proteins in plants: An overview. Front Plant Sci 13. [CrossRef]
- Balouchi HR (2010) Screening wheat parents of mapping population for heat and drought tolerance, detection of wheat genetic variation. Int J Bio Life Sci 4:63–73.
- Bano A, Ullah F, Nosheen A (2012) Role of abscisic acid and drought stress on the activities of antioxidant enzymes in wheat. Plant, Soil Environ 58:181–185. [CrossRef]
- Barlow KM, Christy BP, O’Leary GJ, Riffkin PA, Nuttall JG (2015) Simulating the impact of extreme heat and frost events on wheat crop production: A review. Field Crops Res 171:109-119. [CrossRef]
- Begara-Morales JC, Chaki M, et al. (2018) Nitric oxide buffering and conditional nitric oxide release in stress response. J Exp Bot 69:3425–3438. [CrossRef]
- Kumar H, Chugh, V, Kumar M, et al. (2023) Investigating the impact of terminal heat stress on contrasting wheat cultivars: A comprehensive analysis of phenological, physiological, and biochemical traits. Front Plant Sci 14. [CrossRef]
- Bowne JB, Erwin TA, Juttner J, et al. (2012) Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol Plant 5:418–429.
- Budak H, Kantar M, Kurtoglu KY (2013) Drought tolerance in modern and wild wheat. Sci World J 548246. [CrossRef]
- Castro-Camba R, Sánchez C, Vidal N, Vielba JM (2022) Plant Development and Crop Yield: The Role of Gibberellins. Plants 11(19):2650. [CrossRef]
- Chauhan J, Prathibha M, Singh P, et al. (2023) Plant photosynthesis under abiotic stresses: Damages, adaptive, and signaling mechanisms. Plant Stress 10:100296. [CrossRef]
- Charng Y, Liu H, Liu N, Chi W, Wang C, Chang S, Wang T (2007) A heat-inducible transcription factor, hsfa2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol 143:251–262. [CrossRef]
- Caverzan A, Casassola A, Brammer SP (2016) Antioxidant responses of wheat plants under stress. Genet Mol Biol 39(1):1-6. [CrossRef]
- Chele KH, Tinte MM, Piater LA, Dubery IA, Tugizimana F (2021) Soil salinity, a serious environmental issue and plant responses: A metabolomics perspective. metabolites. 11(11):724. [CrossRef]
- Chen M, Chen J, Fang J, Guo Z, Lu S (2014) Down-regulation of S-adenosylmethionine decarboxylase genes results in reduced plant length, pollen viability, and abiotic stress tolerance. Plant Cell Tissue Organ Cult 116(3):311-322. [CrossRef]
- Chen Y, Guo Y, Guan P, et al. (2023) A wheat integrative regulatory network from large-scale complementary functional datasets enables trait-associated gene discovery for crop improvement. Mol Plant 16:393–414.
- Corpas FJ, Barroso JB (2014) Peroxynitrite (ONOO-) is endogenously produced in Arabidopsis peroxisomes and is overproduced under cadmium stress. Ann Bot 113(1):87-96. [CrossRef]
- Corpas FJ, Freschi L, Rodríguez-Ruiz M, et al (2018) Nitro-oxidative metabolism during fruit ripening. J Exp Bot 69:3449–3463. [CrossRef]
- Cromey MG, Wright DSC, Boddington HJ (1998) Effects of frost during grain filling on wheat yield and grain structure. New Zeal. J Crop Hortic Sci 26:279–290. [CrossRef]
- Dar MI, Naikoo MI, Rehman F, Naushin F, Khan FA (2016) Proline accumulation in plants: Roles in stress Emerging Omics Technologies; Springer: Berlin/Heidelberg, Germany, pp.155–166.
- Djanaguiraman M, Narayanan S, Erdayani E, Prasad PVV (2020) Effects of high-temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biol 20(1):268. [CrossRef]
- Dudziak K, Zapalska M, Börner A, et al. (2019) Analysis of wheat gene expression related to the oxidative stress response and signal transduction under short-term osmotic stress. Sci Rep 9:2743. [CrossRef]
- Fábián A, Sáfrán E, Szabó-Eitel G, Barnabás B, Jäger K (2019) Stigma Functionality and Fertility Are Reduced by Heat and Drought Co-stress in Wheat. Front. Plant Sci 10:2019. [CrossRef]
- Farooq M, Nawaz A, Chaudhry MAM, Indrasti R, Rehman A (2017) Improving resistance against terminal drought in bread wheat by exogenous application of proline and gamma-aminobutyric acid. J Agron Crop Sci 203:464–472. [CrossRef]
- Farouk S (2011) Ascorbic acid and α-tocopherol minimize salt-induced wheat leaf senescence. J Stress Physiol Biochem 7:58-79. https://doaj.org/article/769740fc01124310b03f58b085b089a8.
- Farshadfar E, Ghasempour H, Vaezi H (2008) Molecular aspects of drought tolerance in bread wheat (T. aestivum). Pakistan Journal of Biol Sci 11:118-122. [CrossRef]
- Fewson CA, Nicholas DJ (1960) Utilization of nitric oxide by microorganisms and higher plants. Nature 188:794-796. [CrossRef]
- Fokar M, Nguyen HT, Blum A (1998) Heat tolerance in spring wheat. I. Estimating cellular thermotolerance and its heritability. Euphytica 104(1):1-8. [CrossRef]
- Foyer CH, Noctor G (2003) Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes, and mitochondria. Physiol Plant 119:355–364. [CrossRef]
- Frederiks TM, Christopher JT, Borrell AK (2008) Low temperature adaption of wheat post head-emergence in northern Australia. In: (eds) Appels R, Eastwood R, Lagudah E, Langridge P, Mackay-Lynne M, The 11th International Wheat Genetics Symposium Proceedings. Sydney University Press.
- Frederiks TM, Christopher JT, Harvey GL, Sutherland MW, Borrell AK (2012) Current and emerging screening methods to identify post-head-emergence frost adaptation in wheat and barley. J Exp Bot 63:5405–5416. [CrossRef]
- Frederiks TM, Christopher JT, Sutherland MW, Borrell AK (2015) Post-head-emergence frost in wheat and barley: defining the problem, assessing the damage, and identifying resistance. J Exp Bot 66:3487–3498. [CrossRef]
- Frova C, Sarigorla M (1993) Quantitative expression of maize HSPs: genetic dissection and association with thermotolerance. Theor Appl Genet 86:213-220. [CrossRef]
- Fu SF, Wei JY, Chen HW, Liu YY, Lu HY, Chou JY (2015) Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal Behavior 10(8). [CrossRef]
- Fuller MP, Fuller AM, Kaniouras S, Christophers J, and Fredericks T (2007) The freezing characteristics of wheat at ear emergence. Eur J Agron 26: 435–441. [CrossRef]
- Ghobadi M, Shafiei Abnavi M, et al. (2012) Effect of Hormonal Priming (GA3) and Osmo-priming on Behavior of Seed Germination in Wheat (Triticum aestivum L.). J Agric Sci 4(9). [CrossRef]
- Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. [CrossRef]
- Guan P, Lu L, Jia L, et al. (2018) Global QTL Analysis identifies genomic regions on chromosomes 4A and 4B harboring stable loci for yield-related traits across different environments in wheat (Triticum aestivum L.). Front Plant Sci 9:529. [CrossRef]
- Gull A, Lone AA, Wani NUI (2019) Biotic and abiotic stresses in plants. In abiotic and biotic stress in plants; De Oliveira AB, Ed.; IntechOpen: London, UK.
- Gupta N, Sanjeev KT, Navtej SB (2014) Glycine betaine application modifies biochemical attributes of osmotic adjustment in drought-stressed wheat. Plant Growth Regul 72:221-228. [CrossRef]
- Gupta R, Chakrabarty SK (2013) Gibberellic acid in plant: still a mystery unresolved. Plant Signal Behav 8(9):e25504. [CrossRef]
- Gusta L, Benning N, Wu G, et al. (2009) Superoxide dismutase: an all-purpose gene for agri-biotechnology. Mol Breed 24:103–115. [CrossRef]
- Hameed A, Ahmed MZ, Hussain T, et al. (2021) Effects of salinity stress on chloroplast structure and function. Cells 10(8). [CrossRef]
- Hameed A, Iqbal N (2014) Chemo-priming with mannose, mannitol, and H2O2 mitigate drought stress in wheat. Cereal Res Commun 42:450. [CrossRef]
- Hasanuzzaman M, Nahar K, Alam MM, Fujita M (2012) Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust J Crop Sci 6:1314–1323.
- Hill CB, Li C (2022) Genetic improvement of heat stress tolerance in cereal crops. Agronomy 12(5):1205. [CrossRef]
- Hollander-Czytko H, Grabowski J, et al. (2005) Tocopherol content and activities of tyrosine aminotransferase and cystine lyase in Arabidopsis under stress conditions. J Plant Physiol 162:767–770. [CrossRef]
- Hong-Bo S, Xiao-Yan C, Li-Ye C, et al. (2006) Investigation on the relationship of proline with wheat anti-drought under soil water deficits. Colloids Surf B Biointerfaces. 53(1):113-9. [CrossRef]
- Hossain A, Islam MT, Islam MT (2020) Wheat (Triticum aestivum L.) in the rice-wheat systems of South Asia is influenced by terminal heat stress at late sown condition: A case in Bangladesh. In Plant Stress Physiology; IntechOpen: London, UK.
- Hsu YT and Kao CH (2004) Cadmium toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul 42:227-238. [CrossRef]
- Hu KD, Hu LY, Li YH, Zhang FQ, Zhang H, 2007. Protective roles of nitric oxide on germination and antioxidant metabolism in wheat seeds under copper stress. Plant Growth Regul 53:173-183. [CrossRef]
- Huseynova IM, Rustamova SM, Suleymanov SY, et al. (2016) Drought-induced changes in pho-to synthetic apparatus and antioxidant components of wheat (Triticum durum Desf.) varieties. Photosynth Res 130:215–223. [CrossRef]
- Ibrahim HA, Abdellatif YMR (2016) Effect of maltose and trehalose on growth, yield, and some biochemical components of wheat plant under water stress. Ann Agric Sci 61:267–274. [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change) (2014) The physical science basis: working group I contribution to the fifth assessment reports of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- Islam T, Croll D, Gladieux P, et al. (2016) Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol 14:84. [CrossRef]
- James RA, Blake C, Byrt CS, Munns R (2011) Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1;4 and HKT1;5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J Exp Bot 62(8):2939-47. [CrossRef]
- Kamran M, Shahbaz M, Ashraf M, Akram NA (2019) Alleviation of drought-induced adverse effects in spring wheat (Triticum aestivum L.) using proline as a pre-sowing seed treatment. Pak J Bot 41:621–632.
- Katiyar-Agarwal S, Agarwal M, Grover A (2003) Heat tolerant basmati rice engineered by over-expression of HSP101. Plant Mol Bio 51:677–686. [CrossRef]
- Keskin BC, Sarikaya AT, Yuksel B, Memon AR (2010) Abscisic acid regulated gene expression in bread wheat. Aust J Crop Sci 4:617–625.
- Keunen ELS, Darin P, Jaco V, Van Den Ende WIM, Cuypers ANN (2013) Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ 36:1242–1255. [CrossRef]
- Khan NA, Khan MIR (2014) The Ethylene: From senescence hormone to key player in plant metabolism. J Plant Biochem Physiol 2:e124. [CrossRef]
- Khorobrykh S, Havurinne V, Mattila H, Tyystjärvi E. Oxygen and ROS in photosynthesis. Plants (2020) 9(1):91. [CrossRef]
- Kim TH, Bohmer M, Hu H, Nishimura N, Schroeder J (2010) Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Ann Rev Plant Bio 61:561–591.
- Kingsbury R, Epstein E (1984) Selection for salt-resistant spring wheat1. Crop Sci 24(2):310-315. [CrossRef]
- Klepper LA (1975) Evolution of nitrogen oxide gases from herbicide treated plant tissues. WSSA Abstracts 184:70.
- Laspina NV, Groppa MD, Tomaro ML, Benavides MP (2005) Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci 169:323-330. [CrossRef]
- Li T, Sun Y, Liu T, Wu H, An P, Shui Z, et al. (2019) TaCER1- 1A is involved in cuticular wax alkane biosynthesis in hexaploid wheat and responds to plant abiotic stresses. Plant, Cell Environ. [CrossRef]
- Li S (2023) Novel insight into functions of ascorbate peroxidase in higher plants: More than a simple antioxidant enzyme. Redox Biol 64:102789. [CrossRef]
- Liao WB, Huang GB, Yu JH, Zhang ML (2012) Nitric oxide and hydrogen peroxide alleviate drought stress in marigold explants and promote its adventitious root development. Plant Physiol. Biochem 58:6-15. [CrossRef]
- Liu J, Qiu G, Liu C, Li H, Chen X, Fu Q, Lin Y, Guo B (2022) Salicylic acid, a multifaceted hormone, combats abiotic stresses in plants. Life (Basel) 12(6):886. [CrossRef]
- Liu Q, Nishibori N, Imai I, Hollibaugh JT (2016) Response of polyamine pools in marine phytoplankton to nutrient limitation and variation in temperature and salinity. Mar Ecol Prog Ser 544:93-105. [CrossRef]
- Ma Z, Hu L Jiang W (2024) Understanding AP2/ERF transcription factor responses and tolerance to various abiotic stresses in plants: a comprehensive review. Int J Mol Sci 25(2):893. [CrossRef]
- Menadue DJ, Riboni M, Baumann U, et al. (2021) Proton-pumping pyrophosphatase homeolog expression is a dynamic trait in bread wheat (Triticum aestivum). Plant Direct 5(10):e354. [CrossRef]
- Mamrutha HM, Kumar R, Kaur A, Kumar M (2017) Expressed sequence tags of PAL gene from Triticum aestivum L. cv. DPW. 621-650. https://www.ncbi.nlm.nih.gov/nuccore/JZ970255.
- Manghwar H, Hussain A, Ali Q, Liu F (2022) Brassinosteroids (BRs) Role in plant development and coping with different stresses. Int J Mol Sci 23(3). [CrossRef]
- Manohara K, Morajkar S, Shanbagh Y (2020) Genetic analysis of grain yield and its associated traits in diverse salt-tolerant rice genotypes under coastal salinity condition. J Cereal Res 12(3):290-296. [CrossRef]
- Marcellos H, Single WV (1984) Frost injury in wheat ears after ear emergence. Aust J Plant Physio l11:7-15. [CrossRef]
- Mason RE, Mondal S, Beecher FW, Pacheco A, Jampala B., et al. (2010) QTL associated with heat susceptibility index in wheat (Triticum aestivum L.) under short-term reproductive stage heat stress. Euphytica 174:423-436. [CrossRef]
- Mathur S, Allakhverdiev SI, Jajoo A (2011) Analysis of high temperature stress on the dynamics of antenna size and reducing side heterogeneity of Photosystem II in wheat leaves (Triticum aestivum). Biochim Biophys Acta Bioenerg 1807(1):22. [CrossRef]
- Mehta P, Jajoo A, Mathur S, Bharti S (2010) Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol Biochem 48(1):16-20. [CrossRef]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498. [CrossRef]
- Miura K, Furumoto T (2013) Cold signaling and cold response in plants. Int J Mol Sci 14(3):5312-5337. [CrossRef]
- Moller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Bio 58:459–481. [CrossRef]
- Mukherjee A, Wang S-YS, Promchote P (2019) Examination of the climate factors that reduced wheat yield in Northwest India during the 2000s. Water 11:343. [CrossRef]
- Munns R, Tester M (2008) Mechanism of salinity tolerance. Ann Rev Plant Biol 59:651-681. [CrossRef]
- Murakami T, Matsuba S, Funatsuki H, et al. (2004) Overexpression of a small heat shock protein, sHSP17.7 confers both heat tolerance and UV-B resistance to rice plants. Mol Breed 13:165–175. [CrossRef]
- Mwadzingeni L, Hussein S, Tsilo TJ (2017) Variance components and heritability of yield and yield components of wheat under under drought-stressed and non-stressed conditions. Aust J Crop Sci 11(11): 1425-1430. [CrossRef]
- Narayana Y, Lalonde S, Saini HS (1991) Water-stress induced ethylene production in wheat. A fact or artifact? Plant Physiol 96:406–410. [CrossRef]
- Nezhadahmadi A, Prodhan ZH, Faruq G (2013) Drought tolerance in wheat. Sci World J 2–7. [CrossRef]
- Nakamichi N, Kiba T, Kamioka, M, et al. (2012) Transcriptional repressor PRR5 directly regulates clock-output pathways. Proc Natl Acad Sci U.S.A. 109:17123–17128. [CrossRef]
- Nover L, Bharti K, Döring P, et al. (2001) Arabidopsis and the heat stress transcription factor world: how many heat stress transcription factors do we need? Cell Stress. 6(3):177-89. [CrossRef]
- Ottaviano E, Sari-Gorla M, Pe E, Frova C (1991) Molecular markers (RFLPs and HSPs) for the genetic dissection of thermotolerance in maize. Theor Appl Genet 81:713–719. [CrossRef]
- Paliwal R, Röder MS, Kumar U, Srivastava JP, Joshi AK (2012) QTL Mapping of terminal heat tolerance in hexaploid wheat (T. aestivum L.). Theor Appl Genet 125:561-575. [CrossRef]
- Pinto S, Chapman SC, McIntyre CL, Shorter R. Reynolds M (2008) QTL for canopy temperature response related to yield in both heat and drought environments. In: (eds) Appels R, Eastwood R, Lagudah E, Langridge P, Mackay M. et al. (eds) Proceedings of the 11th International Wheat Genetics Symposium, 24–28 August. Brisbane, Australia. Sydney University Press, Sydney. Available at: http://ses. library.usyd.edu.au/bitstream/2123/3351/1/ P172.pdf.
- Procházková D, Sumaira J, Wilhelmová N, et al. (2014) Reactive nitrogen species and the role of no in abiotic stress. In: (eds) Ahmad P, Rasool S, Emerging technologies and management of crop stress tolerance, Academic Press, pp.249-266.
- Qiu Z, Guo J, Zhu A, Zhang L, Zhang M (2014) Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress Ecotox Environ Safety 104:202–208. [CrossRef]
- Radi R (2013) Peroxynitrite, a stealthy biological oxidant. J Biol Chem 288(37):26464-26472. [CrossRef]
- Rath JR, Pandey J, Yadav RM, et al. (2022) Temperature-induced reversible changes in photosynthesis efficiency and organization of thylakoid membranes from pea (Pisum sativum). Plant Physiol Biochem 185:144 154. [CrossRef]
- Raza A, Razzaq A, Mehmood SS, et al. (2019) Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 8:34. [CrossRef]
- Reguera M, Peleg Z, Blumwald E (2012) Targeting metabolic pathways for genetic engineering abiotic stress-tolerance in crops. Biochim Biophy Acta 1819:186–194. [CrossRef]
- Rawat R, Takahashi N, Hsu PY, et al. (2011) REVEILLE8 and PSEUDO-REPONSE REGULATOR5 form a negative feedback loop within the Arabidopsis circadian clock. PLoS Genet. 7:e1001350. [CrossRef]
- Röder M, Thornley P, Campbell G, Bows-Larkin A (2014) Emissions associated with meeting the future global wheat demand: a case study of UK production under climate change constraints. Environ Sci Policy 39: 13–24. [CrossRef]
- Roeber VM, Bajaj I, Rohde M, Schelling T, Cortleven A (2021) Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 44:645–664. [CrossRef]
- Rodríguez-Rosales MP, Gálvez FJ, Huertas R, et al. (2009) Plant NHX cation/proton antiporters. Plant Signal Behav 4(4):265-276. [CrossRef]
- Rosa M, Hilal M, Juan AG, Fernando EP (2009) Low-temperature effect on enzyme activities involved in sucrose–starch partitioning in salt-stressed and salt-acclimated cotyledons of quinoa (Chenopodium quinoa Willd.) seedlings. Plant Physiol Biochem 47:300–307. [CrossRef]
- Rugnone ML, Faigon Soverna A, Sanchez SE, et al. (2013) LNK genes integrate light and clock signaling networks at the core of the Arabidopsis oscillator. Proc Natl Acad Sci USA. 110:12120–12125. [CrossRef]
- Sabagh EL, Islam A, Skalicky MS, et al. (2021) Salinity stress in wheat (Triticum aestivum L.) in the changing climate: Adaptation and Management Strategies. Front Agron 3. [CrossRef]
- Sadau SB, Liu Z, Ninkuu V, Guan L, Sun X (2024) DREB transcription factors are crucial regulators of abiotic stress responses in Gossypium spp. Plant Stress 11:100350. [CrossRef]
- Sah SK, Reddy KR, Li J (2016) Abscisic acid and abiotic stress tolerance in crop plants. Front Plant Sci 7:571. [CrossRef]
- Salgado KCPC, Von Pinho EVR, Guimaraes CT, et al. (2008) Mapping of quantitative trait locus associated with maize tolerance to high seed drying temperature. Genet Mol Res 7:1319–1326. [CrossRef]
- Sanchez A, Shin J, Davis SJ (2011) Abiotic stress and the plant circadian clock. Plant Signal. Beha. 6:223–231. [CrossRef]
- Sharma S, Garg O (1983) Comparative study of osmotic and salt stress effects on nitrate assimilation in wheat. Curr Agric 7:36-40.
- Shrivastava P, Kumar R (2015) Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22(2):123-131. [CrossRef]
- Singh C (1988) Modern techniques of raising field crops, 4th ed.; Oxford and IBH publishing Co. Pvt. Ltd.: New Delhi, India, p.46.
- Singh AK, Sharma L, Mallick N (2004) Antioxidative role of nitric oxide on copper toxicity to a chlorophycean alga, Chlorella. Ecotoxicol Environ Saf 59(2):223-7. [CrossRef]
- Serrano-Bueno G, Sanchez de Medina Hernandez V, and Valverde F (2021) Photoperiodic signaling and senescence, an ancient solution to a modern problem? Front. Plant Sci 12:634393. [CrossRef]
- Singh HP, Batish DR, Kaur G, Arora K, Kohli RK (2008) Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots. Environ Exp Bot 63(1-3):158-167. [CrossRef]
- Srivalli B, Chinnusami V, Renu KC (2003) Antioxidant defense in response to abiotic stresses in plants. J Plant Bio 30:121–139.
- Sun C, Liu L, Zhou W, Lingli Lu, Jin C, Lin X (2017) Aluminum induces distinct changes in the metabolism of reactive oxygen and nitrogen species in the roots of two wheat genotypes with different aluminum resistance. J Agric Food Chem, 65 (43): 9419–9427. [CrossRef]
- Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol Plant 126:45–51. [CrossRef]
- Swamy BP, Kaladhar K, Ashok RG, Viraktamath BC, Sarla N (2014) Mapping and introgression QTLs for yield and related traits in two backcross populations derived from O. sativa cv Swarna and two accessions of O. nivara. J Genet 93:643–654. [CrossRef]
- Tan J, Zhao H, Hong J, Han, Y, Li H, Zhao W (2008) Effects of exogenous nitric oxide on photosynthesis, antioxidant capacity and proline accumulation in wheat seedlings subjected to osmotic stress. World J Agric Sci 4:307-313.
- Tian F, Gong J, Zhang J, Zhang M, Wang G, Li A, Wang W (2013) Enhanced stability of thylakoid membrane proteins and antioxidant competence contribute to drought stress resistance in the tasg1 wheat stay-green mutant. J Exp Bot 64(6):1509-20. [CrossRef]
- Turk H, Erdal S, Genisel M, Atici O, Yavuz Demir Y, Yanmis D (2014) The regulatory effect of melatonin on physiological, biochemical, and molecular parameters in cold-stressed wheat seedlings. Plant Growth Regul 74:139-152. [CrossRef]
- Tyagi S, Sharma S, Taneja M, Kumar R, Sembi JK, Upadhyay SK (2017) Superoxide dismutases in bread wheat (Triticum aestivum L.): Comprehensive characterization and expression analysis during development and, biotic and abiotic stresses. Agri Gene 6:1-13. [CrossRef]
- ul Haq S, Khan A, Ali M, et al. (2019) Heat Shock Proteins: Dynamic biomolecules to counter plant biotic and abiotic stresses. Int J Mol Sci 20(21):5321. [CrossRef]
- Umbreen S, Lubega J, Cui B, Pan Q, Jiang J, Loake G (2018) Specificity in nitric oxide signaling. J Exp Bot 69:3439–3448. [CrossRef]
- Ursini F, Maiorino M (2012) Glutathione Peroxidases. In: Encyclopedia of Biological Chemistry,399-404. [CrossRef]
- Valluru R, Link J, Claupein W (2012) Consequences of early chilling stress in two Triticum species: plastic responses and adaptive significance. Plant Biol 14:641–651. [CrossRef]
- Vijayaraghavaredy P, Lekshmy SV, Struik PC, et al. (2022) Production and scavenging of reactive oxygen species confer to differential sensitivity of rice and wheat to drought stress. Crop Environ, 1(1):15-23. [CrossRef]
- Waadt R, Hsu PK, Schroeder JI (2015) Abscisic acid and other plant hormones: Methods to visualize distribution and signaling. Bioessays 37(12):1338-49. [CrossRef]
- Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: An overview. Environ Exp Bot 61:199–223. [CrossRef]
- Wang X, Li Q, Xie J, Huang M, Cai J, Zhou Q, et al. (2020) Abscisic acid and jasmonic acid are involved in drought priming-induced tolerance to drought in wheat. [CrossRef]
- Wang X, Zeng J, Li Y, Rong X, Sun J, Sun T, et al. (2015) Expression of TaWRKY44, a wheat WRKY gene, in transgenic tobacco confers multiple abiotic stress tolerances. Front Plant Sci 6:615. [CrossRef]
- Wang, Y. and Frei, M. (2011) Stressed Food—The Impact of Abiotic Environmental Stresses on Crop Quality. Agriculture, Ecosystems & Environment, 141, 271-286. [CrossRef]
- Wardlaw IF, Dawson IA, Munibi P, Fewster R (1989) The tolerance of wheat to high temperatures during reproductive growth. I. Survey procedures and general response patterns. Aust J Agric Res 40:1–13. [CrossRef]
- Whaley JM, Kirby EJM, et al. (2004) Frost damage to winter wheat in the UK: the effect of plant population density. Eur J Agron 21:105–115. [CrossRef]
- Xu W, Jia L, Shi W, Liang J, Zhou F, Li Q, Zhang J (2013) Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol 197:139–150. [CrossRef]
- Yadav S, Modi P, Dave A, Vijapura A, Patel D, Patel M (2020) Effect of abiotic stress on crops. In: (eds) Hasanuzzaman M, Minhoto MC, Filho T, Fujita M, Nogueira TAR Sustainable Crop Production, IntechOpen. [CrossRef]
- Yan L, Li S, Riaz M, Jiang C (2021) Proline metabolism and biosynthesis behave differently in response to boron deficiency and toxicity in Brassica napus. Plant Physiol Biochem 167:529-540. [CrossRef]
- Yang T, Segal G, Abbo S. Feldman M, Fromm H (1996) Characterization of the calmodulin gene family in wheat: structure, chromosomal location, and evolutionary aspects. Mol Gen Genet 252:684–694. [CrossRef]
- Yu Y, Song T, Wang Y, et al. (2023) The wheat WRKY transcription factor TaWRKY1-2D confers drought resistance in transgenic Arabidopsis and wheat (Triticum aestivum L.). Int J Biol Macromol 226:1203–1217. [CrossRef]
- Yue R, Zhou BO, Shimada IS, Zhao Z, Morrison SJ (2016) Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in the adult bone marrow. Cell Stem Cell 18(6):782-796. [CrossRef]
- Zhao C, Liu B, Piao S, Wang X, Lobell DB, Huang Y, et al. (2017) Temperature increase reduces global yields of major crops in four independent estimates. Proc Natl Acad Sci USA, 114:9326-9331. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





