Submitted:
09 September 2024
Posted:
10 September 2024
You are already at the latest version
Abstract
Keywords:
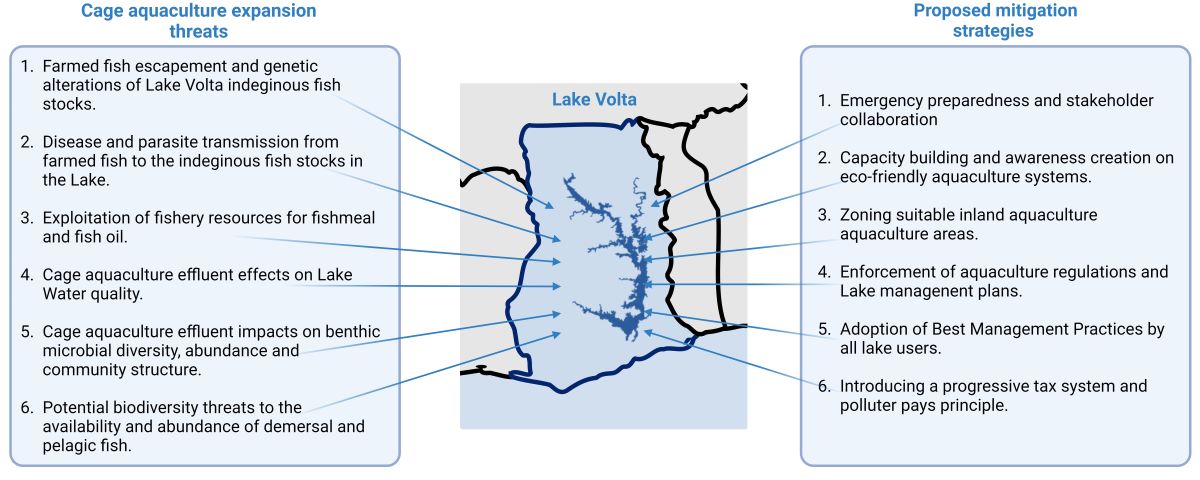
1. A Global Perspective on Cage Aquaculture and Biodiversity
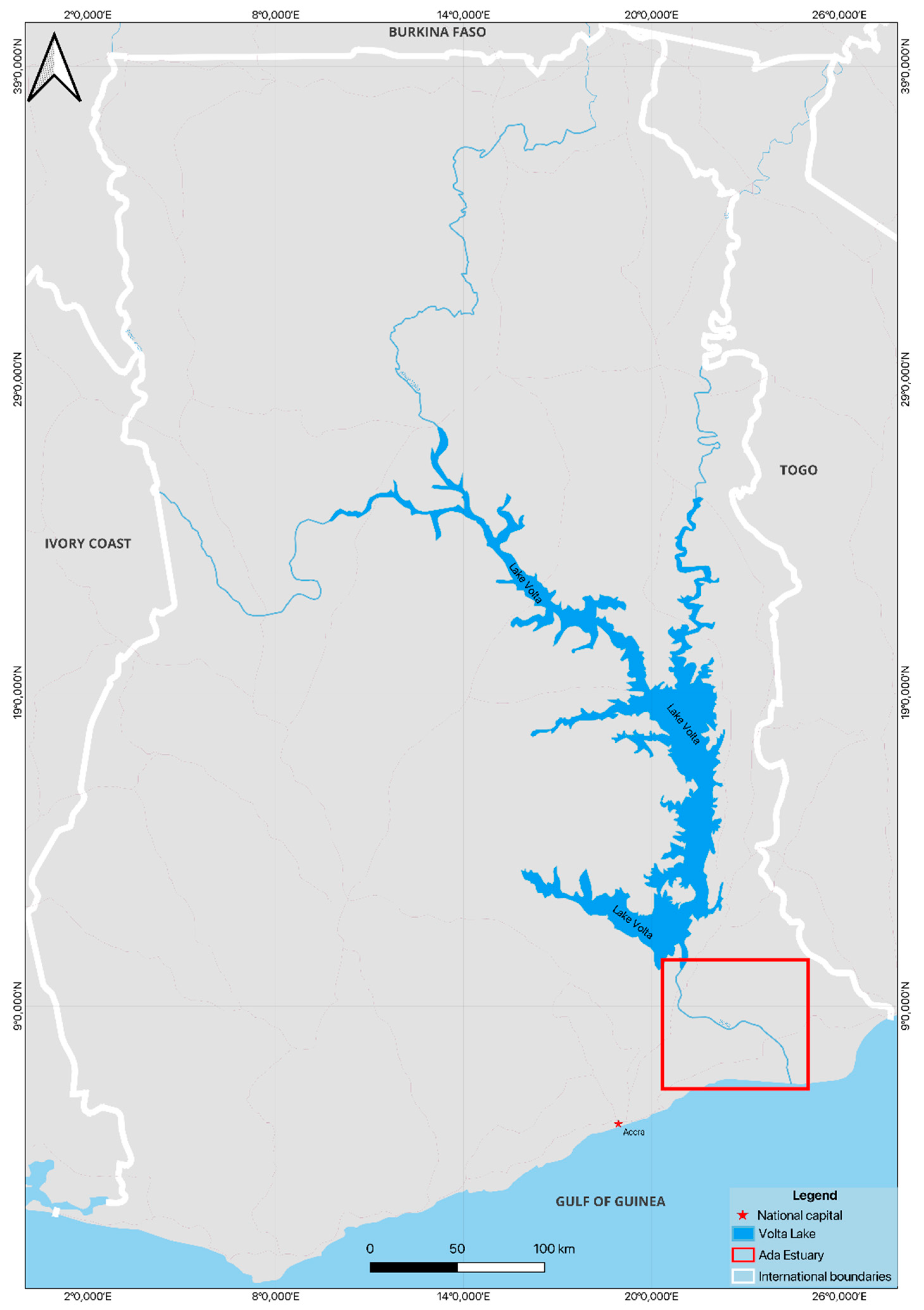
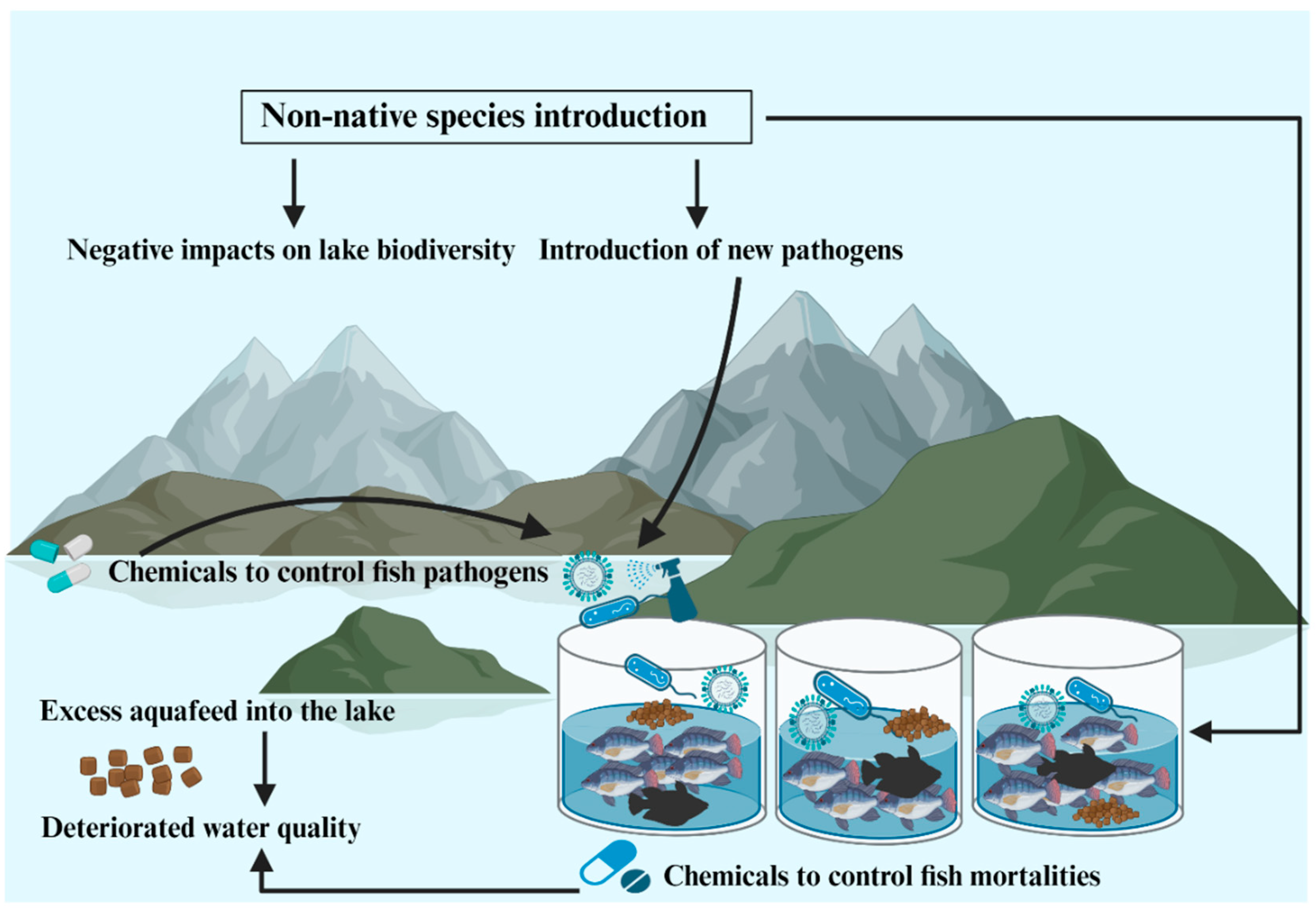
2. Negative Impacts of Lake Volta Cage Aquaculture in Ghana
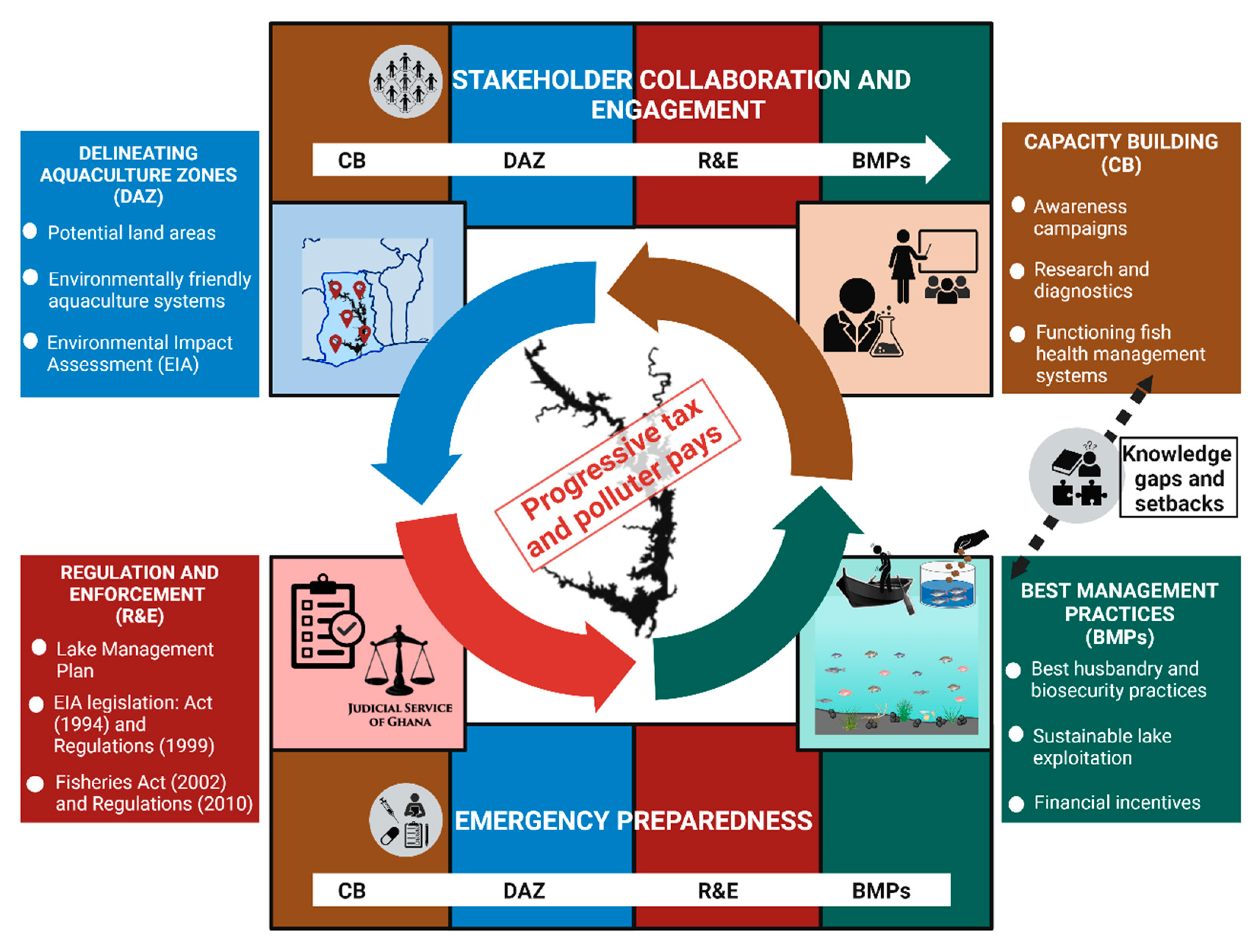
3. Transitioning from Lake Volta Cage Aquaculture to Land-Based Fish Production
3.1. Capacity Building (CB)
3.2. Delineating Aquaculture Zones (DAZ)
3.3. Regulation and Enforcement (R&E)
3.4. Adopting Best Management Practices (BMPs)
3.5. Emergency Preparedness and Stakeholder Collaboration & Engagement
3.6. Progressive Tax System and Polluter Pays Principle
4. Government’s Role in Transitioning from Open-Water to Land-Based Aquaculture
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
References
- Nadarajah, S.; Flaaten, O. Global aquaculture growth and institutional quality. Marine Policy. 2017, 84, 142–151. [Google Scholar] [CrossRef]
- FAO. Towards Blue Transformation. The State of World Fisheries and Aquaculture (SOFIA). Rome: Food and Agriculture Organization, 2022. p. 266. [CrossRef]
- Araujo, G.S.; da Silva, J.W.A.; Cotas, J.; Pereira, L. Fish Farming Techniques: Current Situation and Trends. Journal of Marine Science and Engineering. 2022, 10, 1598. [Google Scholar] [CrossRef]
- Etuk, E.A.; Ogban, G.O.; Idiong, C.I. A comparative economic analysis of aquaculture production systems in Southern Agricultural Zone of Cross River State, Nigeria. African Journal of Agricultural Research. 2021, 17, 104–111. [Google Scholar] [CrossRef]
- Losordo, T.M.; Ray, L.E.; DeLong, D.P. Flow-through and recirculating systems. Developments in Aquaculture and Fisheries Science. 2004, 545–560. [Google Scholar] [CrossRef]
- Swann, L. A Basic Overview of Aquaculture History. Technical Bulletin Series. 1992, Iowa State University, Ames, Iowa.
- Kumar, V.; Karnatak, G. Engineering consideration for cage aquaculture. IOSR Journal of Engineering (IOSRJEN). 2014. [CrossRef]
- Mwebaza-Ndawula, L.; Vincent, K.; Magezi, G.; Naluwayiro, J.; Gandhi-Pabire, W.; Henry, O. Effects of cage fish culture on water quality and selected biological communities in northern Lake Victoria, Uganda. Uganda Journal of Agricultural Sciences. 2013, 14, 61–75. [Google Scholar]
- Huguenin, J.E. The design, operations and economics of cage culture systems. Aquacultural Engineering. 1997, 16, 167–203. [Google Scholar] [CrossRef]
- Osei, L.K.; Asmah, R.; Aikins, S.; Karikari, A.Y. Effects of Fish Cage Culture on Water and Sediment Quality in the Gorge Area of Lake Volta in Ghana: A Case Study of Lee Fish Cage Farm. Ghana J. Sci. 2019, 60, 1–16. [Google Scholar] [CrossRef]
- Otu, M.K.; Bureau, D.P.; Podemski, C.L. Freshwater Cage Aquaculture: Ecosystems Impacts from Dissolved and Particulate Waste Phosphorus. Fisheries and Oceans Canada. Doc. 2017, 2017/059. v+ 55 p. Available online: https://waves-vagues.dfo-mpo.gc.ca/library-bibliotheque/40643761.pdf (accessed on 30 April 2024).
- Price, C.; Black, K.D.; Hargrave, B.T.; Morris Jr., J. A. Marine cage culture and the environment: effects on water quality and primary production. Aquaculture Environment Interactions. 2015, 6, 151–174. [Google Scholar] [CrossRef]
- Diana, J.S. Aquaculture Production and Biodiversity Conservation. BioScience. 2009, 59, 27–38. [Google Scholar] [CrossRef]
- Watts, J.; Conklin, D.E. A review of the literature on the environmental impacts of marine fish cage culture. UNICIENCIA. 1998, Pp. 143-155.
- Glover, K.A.; Pertoldi, C.; Besnier, F.; Wennevik, V.; Kent, M.; Skaala, Ø. Atlantic salmon populations invaded by farmed escapees: quantifying genetic introgression with a Bayesian approach and SNPs. BMC Genetics. 2013, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.L.; Williams, S.L.; Strong, D.R. Aquaculture- A Gateway for exotic species. Science. 2001, 294, 1655–1656. [Google Scholar] [CrossRef] [PubMed]
- Stokesbury, M.J.W.; Lacroix, G.L.; Price, E.L.; Knox, D.; Dadswell, M.J. Identification of scale analysis of farmed Atlantic salmon juveniles in southwestern New Brunswick rivers. Transactions of the American Fisheries Society. 2001, 130, 815–822. [Google Scholar] [CrossRef]
- Silva, S.S.D.; Nguyen, T.T.T.; Turchini, G.M.; Amarasinghe, U.S.; Abery, N.W. Alien Species in Aquaculture and Biodiversity: A Paradox in Food Production. AMBIO: A Journal of the Human Environment. 2009, 38, 24–28. [Google Scholar] [CrossRef]
- Goldburg, R.; Naylor, R. Future seascapes, fishing, and fish farming. Frontiers in Ecology and Environment. 2005, 31, 21–28. [Google Scholar] [CrossRef]
- Hulata, G. Genetic manipulations in aquaculture: a review of stock improvement by classical and modern technologies. Genetica. 2001, 111, 155–73. [Google Scholar] [CrossRef]
- Hixon, M.A.; Green, S.J.; Albins, M.A.; Akins, J.L.; Morris, J.A. Jr. Lionfish: a major marine invasion. Mar Ecol Prog Ser. 2016, 558, 161–165. [Google Scholar] [CrossRef]
- Abd Hamid, M.; Md Sah, A.S.R.; Idris, I.; Mohd Nor, S.A.; Mansor, M. Impacts of Tilapia Aquaculture on Native Fish Diversity at an Ecologically Important Reservoir. PeerJ. 2023, 11, e15986. [Google Scholar] [CrossRef]
- Kour, R.; Bhatia, S.; Sharma, K.K. Nile Tilapia (Oreochromis niloticus) as a successful biological invader in Jammu (J&K) and its impacts on native ecosystem. International Journal of Interdisciplinary and Multidisciplinary Studies (IJIMS). 2014, 1, 1–5. [Google Scholar]
- Canonico, G.C.; Arthington, A.; McCrary, J.K.; Thieme, M.L. The effects of introduced tilapias on native biodiversity. Aquatic Conservation: Marine and Freshwater Ecosystems. 2005, 15, 463–483. [Google Scholar] [CrossRef]
- Krkosek, M.; Lewis, M.A.; Morton, A.; Frazer, L.N.; Volpe, J.P. Epizootics of wild fish induced by farm fish. Proceedings of the National Academy of Sciences. 2006, 133, 15506–15510. [Google Scholar] [CrossRef] [PubMed]
- Krkosek, M.; Lewis, M.A.; Volpe, J.P.; Morton, A. Fish farms and sea lice infestations of wild juvenile salmon in Broughton Archipelago -a rebuttal to Brooks (2005). Fisheries Science. 14, 1–11. [CrossRef]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and food safety in aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.E.M.; Schreier, H.J.; Lanska, L.; Hale, M.S. The Rising Tide of Antimicrobial Resistance in Aquaculture: Sources, Sinks and Solutions. Marine drugs. 2017, 15, 158. [Google Scholar] [CrossRef]
- Erkinharju, T.; Dalmo, R.A.; Hansen, M.; Seternes, T. Cleaner fish in aquaculture: review on diseases and vaccination. Reviews in Aquaculture. 2021, 13, 189–237. [Google Scholar] [CrossRef]
- Haugland, G.T.; Olsen, A.B.; Ronneseth, A.; Andersen, L. Lumpfish (Cyclopterus lumpus L.) develop amoebic gill disease (AGD) after experimental challenge with Paramoeba perurans and can transfer amoebae to Atlantic salmon (Salmo salar L.). Aquaculture 2017, 478, 48–55. [Google Scholar] [CrossRef]
- VKM; Rimstad, E.; Basic, D.; Gulla, S.; Hjeltnes, B.; Mortensen. S. Risk assessment of fish health associated with the use of cleaner fish in aquaculture. Opinion of the Panel on Animal Health and Welfare of the Norwegian Scientific Committee for Food and Environment. VKM report 2017, 2017:32, ISBN: 978-82-8259-289-5, ISSN: 2535-4019. Norwegian Scientific Committee for Food and Environment (VKM), Oslo, Norway.
- Newton, R.W.; Maiolo, S.; Malcorps, W.; Little, D.C. Life Cycle Inventories of marine ingredients. Aquaculture. 2023, 565. [Google Scholar] [CrossRef]
- Einarsson, M.I.; Jokumsen, A.; Bæk, A.M.; Jacobsen, C.; Pedersen, S.A.; Samuelsen, T.A.; Pálsson, J.; Eliasen, O.; Flesland, O. Nordic Centre of Excellence Network in Fishmeal and Fish oil. 2019, 1-124. [CrossRef]
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature. 2000, 405, 1017–1024. [Google Scholar] [CrossRef]
- IFFO. Key facts. IFFO—The Marine Ingredients Organization. 2024. (Accessed June 18, 2024) Available online: https://www.iffo.com/key-facts.
- IFFO. By-product. In: Marine Ingredients Organization. 2021. (Accessed June 18, 2024) Available online: www.iffo.com/product.
- EUMOFA—European Market Observatory for Fisheries and Aquaculture Products. Fishmeal and fish oil—Production and Trade Flows in the EU, 2021; ISBN 978-92-76-28913-5. [CrossRef]
- Kok, B.; Malcorps, W.; Tlusty, M.F.; Eltholth, M.M.; Auchterlonie, N.A.; Little, D.C.; Harmsen, R.; Newton, R.W.; Davies, S.J. Fish as feed: Using economic allocation to quantify the Fish In: Fish Out ratio of major fed aquaculture species. Aquaculture. 2020, 528. [Google Scholar] [CrossRef]
- Olsen, Y. Resources for fish feed in future mariculture. Aquaculture Environment Interactions. 2011, 1, 187–200. [Google Scholar] [CrossRef]
- Chuenpagdee, R.; Degnbol, P.; Bavinck, M.; Jentoft, S.; Johnson, D.; Pullin, R.; Williams, S. Challenge and concerns in capture fisheries and aquaculture. In: Fish for life. Interactive governance for fisheries. MARE Publications Series, Amsterdam. [CrossRef]
- Garno, Y.S.; Riyadi, A.; Iskandar, I.; Kendarto, D.R.; Sachoemar, S.I.; Susanto, J.P.; Widodo, L.; Suwedi, N.; Prayogo, T.; Dewa, R.P.; et al. The Impact of Aquaculture in Floating Net Cages Exceeding the Carrying Capacity on Water Quality and Organic Matter Distribution: the Case of Batur Lake, Indonesia. Polish Journal of Environmental Studies. 2024, 33, 3651–3663. [Google Scholar] [CrossRef]
- Price, C.S; Morris, J.A.; Jr. Marine Cage Culture and the Environment: Twenty-first Century Science Informing a Sustainable Industry. NOAA Technical Memorandum NOS NCCOS. 2013, 164, 158. [Google Scholar]
- Doglioli, A.M.; Magaldi, M.G.; Vezzulli, L.; Tucci, S. Development of a numerical model to study the dispersion of wastes coming from a marine fish farm in the Ligurian Sea (Western Mediterranean). Aquaculture. 2004, 231, 215–235. [Google Scholar] [CrossRef]
- Gorlach-Lira, K.; Pacheco, C.; Carvalho, L.C.T.; Melo, J.H.N.; Crispim, M.C. The influence of fish culture in floating net cages on microbial indicators of water quality. Brazilian Journal of Biology. 2013, 73, 457–463. [Google Scholar] [CrossRef]
- Bissett, A.; Burke, C.; Cook, P.L.M.; Bowman, J.P. Bacterial community shifts in organically perturbed sediments. Environmental Microbiology 2007, 9, 46–60. [Google Scholar] [CrossRef]
- Felsing, M.; Glencross, B.; Telfer, T. Preliminary study on the effects of exclusion of wild fauna from aquaculture cages in a shallow marine environment. Aquaculture. 2005, 243, 159–174. [Google Scholar] [CrossRef]
- Holmer, M. Environmental issues of fish farming in offshore waters: perspectives, concerns and research needs. Aquaculture Environment Interactions. 2010, 1, 57–70. [Google Scholar] [CrossRef]
- Klaoudatos, S.D.; Klaoudatos, D.S.; Smith, J.; Bogdanos, K.; Papageorgiou, E. Assessment of site specific benthic impact of floating cage farming in the eastern Hios Island, Eastern Aegean Sea, Greece. Journal of Experimental Marine Biology and Ecology. 2006, 338, 96–111. [Google Scholar] [CrossRef]
- Soto, D.; Norambuena, F. Evaluation of salmon farming effects on marine systems in the inner seas of southern Chile: A large-scale mensurative experiment. Journal of Applied Ichthyology, 2004, 20, 493–501. [Google Scholar] [CrossRef]
- AL-Keriawy, H.H.A. Impact of Fish Farming in floating cages on zooplankton Community in Euphrates River, Iraq. IOP Conference Series: Earth and Environmental Science. 2021, 722. [CrossRef]
- Dias, J.D.; Takahashi, E.M.; Santana, N.F.; Bonecker, C.C. Impact of fish cage-culture on the community structure of zooplankton in a tropical reservoir. Iheringia. Série Zoologia. 2011, 101, 75–84. [Google Scholar] [CrossRef]
- Trushenski, J.; Flagg, T.; Kohler, C. Use of hatchery fish for conservation, restoration, and enhancement of fisheries. 2010, 261–293. In W. Hubert and M. Quist, editors. Inland fisheries management in North America, 3rd edition. American Fisheries Society, 8 February 5506. [Google Scholar]
- FAO. Contributing to food security and nutrition for all. The State of World Fisheries and Aquaculture (SOFIA). Rome: Food and Agriculture Organization, 2016, pp. 200. ISBN: 9789251093085.
- Machias, A.; Karakassis, I.; Labropoulou, M.; Somarakis, S.; Papadopoulou, K.N.; Papaconstantinou, C. Changes in wild fish assemblages after the establishment of a fish farming zone in an oligotrophic marine eco system. Estuarine, Coastal and Shelf Science. 2004, 60, 771–779. [Google Scholar] [CrossRef]
- Romakkaniemi, A.; Pera, I.; Karlsson, L.; Jutila, E.; Carlsson, V.; Pakarinen, T. Development of wild Atlantic salmon stocks in the rivers of the northern Baltic sea in response to management measures. ICES Journal of Marine Sciences. 2003, 60, 329–342. [Google Scholar] [CrossRef]
- Machias, A.; Karakassis, I.; Giannoulaki, M.; Papadopoulou, K.N.; Smith, C.J.; Somarakis, S. Response of demersal fish communities to the presence of fish farms. Marine Ecology Progress Series. 2005, 288, 241–250. [Google Scholar] [CrossRef]
- Asmah, R.; Karikari, A.Y.; Abban, E.K.; Ofori, J.K; Awity, L.K. Cage Fish Farming in the Volta Lake and the Lower Volta: Practices and Potential Impacts on Water Quality. Ghana J. Sci. 2014, 54, 33–47. [Google Scholar]
- Kassam, L. Aquaculture and food security, poverty alleviation and nutrition in Ghana: Case study prepared for the Aquaculture for Food Security, Poverty Alleviation and Nutrition project. WorldFish. 2014. [CrossRef]
- Frimpong, S.K; Adwani, A. The Challenges and Prospects of Fish Farming in Ghana: A Project Management Perspective. International Journal of ICT and Management. 2015, 3, 29–34. [Google Scholar]
- Asmah, R.; Falconer, L.; Telfer, T.C.; Karikari, A.Y.; Al Wahaibi, M.; Xia, I.F.; Handisyde, N.; Quansah, K.E.; Amoah, D.K.; Alshihhi, J.; Ross, L.G. Waterbody Scale Assessment Using Spatial Models to Identify Suitable Locations for Cage Aquaculture in Large Lake Systems: A Case Study in Volta Lake, Ghana. Aquaculture Research 2021, 52, 3854–3870. [Google Scholar] [CrossRef]
- Rurangwa, E.; Agyakwah, S. K.; Boon, H.; Bolman, B.C. Development of Aquaculture in Ghana: Analysis of the Fish Value Chain and Potential Business Cases; IMARES Wageningen UR: IJmuiden, 2015.
- Fisheries Commission. National fisheries and aquaculture policy. 2021. In: Zornu, J.; Oyih, M.; Binde, M.; Viglo, J.; Agbekpornu, H.; Nkansa, M.; Tavornpanich, S.; Norheim, K.; Brun, E.; Cudjoe, K.S. Stakeholder perspectives on the 2023 Ghana national aquaculture development plan: an integration within the ecosystem approach framework. Aquaculture, Fish and Fisheries. 2023, 3, 459–471. [Google Scholar] [CrossRef]
- MOFAD. Annual report—2022, Ministry of Fisheries and Aquaculture Development. 2023.
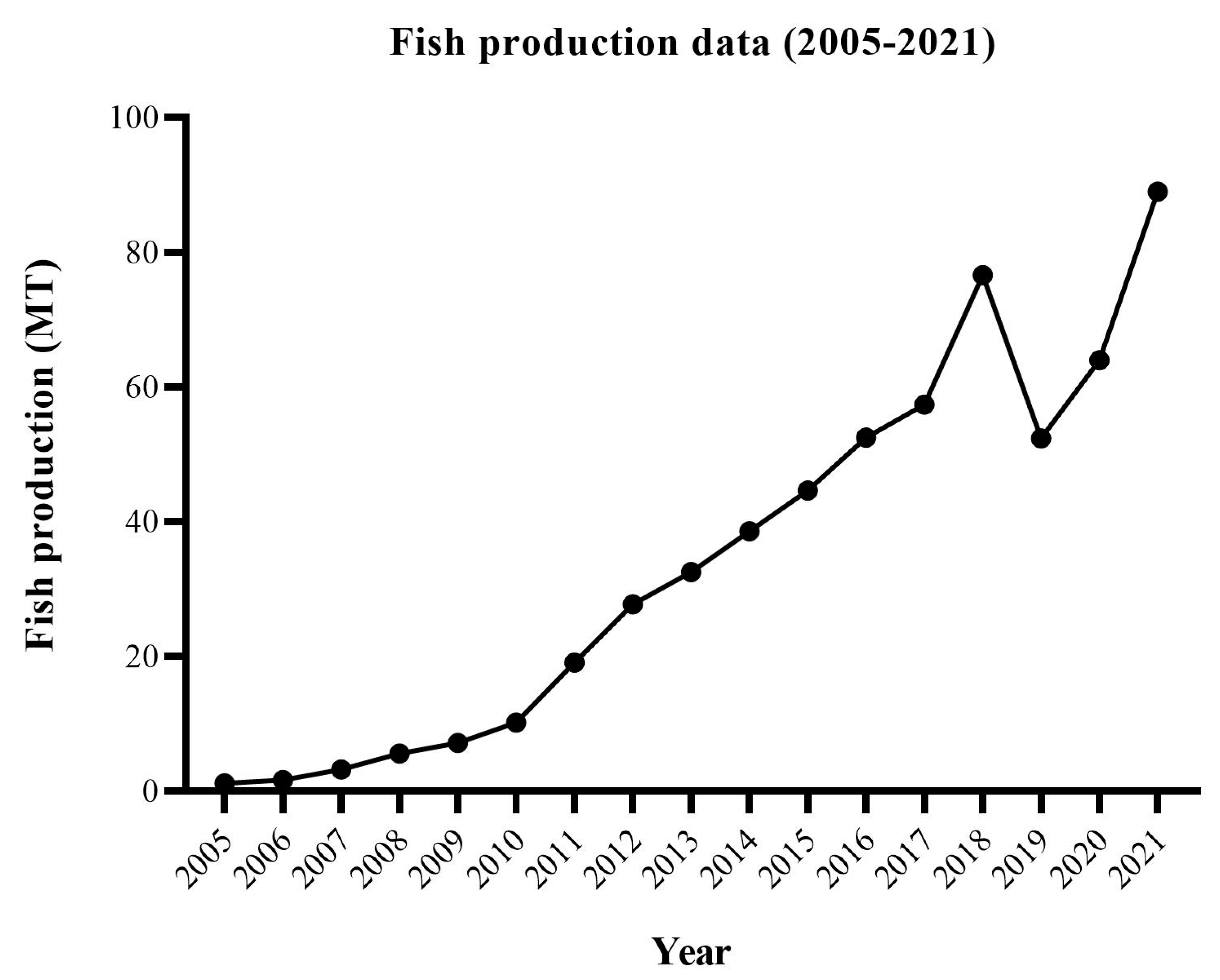
- World Bank. Aquaculture production in Ghana from 2008 to 2021 (in 1,000 metric tons). 2024. https://www.statista.com/statistics/1118781/aquaculture-production-in-ghana/ (Accessed March 10, 2024).
- MWRWH. National Water Policy. The Ministry of Water Resources, Works and Housing. 2007.
- Zornu, J.; Tavornpanich, S.; Brun, E.; van Zwieten, P.A.M.; van de Leemput, I.; Appenteng, P.; Anchirinah, J.; Cudjoe, K.S. Understanding tilapia mortalities and fish health management in Lake Volta: a systematic approach. Front. Sustain. Food Syst. 2023, 7, 1249898. [Google Scholar] [CrossRef]
- Ramírez-Paredes, J.G.; Paley, R.K.; Hunt, W.; Feist, S.W.; Stone, D.M.; Field, T.R.; Haydon, D.J.; Ziddah, P.A.; Nkansa, M.; Guilder, J.; et al. First detection of Infectious Spleen and Kidney Necrosis Virus (ISKNV) associated with massive mortalities in farmed tilapia in Africa. Transbound. Emerg. Dis. 2021, 68, 1550–1563. [Google Scholar] [CrossRef] [PubMed]
- Anane-Taabeah, G.; Frimpong, E.A.; Hallerman, E. Aquaculture-mediated invasion of the genetically improved farmed Tilapia (GIFT) into the lower Volta Basin of Ghana. Diversity. 2019, 11, 188. [Google Scholar] [CrossRef]
- Akpojotor, E. Development of Carrying Capacity Estimates for Zonation of Cage Aquaculture in Lake Volta, Ghana. 2015. [CrossRef]
- Akrasi, S.A. The assessment of suspended sediment inputs to Volta Lake. Lakes & Reservoirs: Research & Management. 2005, 10, 179–186. [Google Scholar] [CrossRef]
- Karikari, A.Y.; Asmah, R.; Anku, W.W.; Amisah, S.; Trevor, T.; Lindsay, R. Assessment of cage fish farm impacts on physico-chemical parameters of the Volta Lake in Ghana. Journal of Fisheries and Coastal Management. 2021, 3, 22–35. [Google Scholar] [CrossRef]
- Abban, K.E.; Agbenyega, O.; Asmah, R.; Afele, J.T.; Ofori, L.A.; Nimo, E. Assessment of social acceptance of caged fish culture for improvement and sustainability: A study on Volta Lake, Ghana. International Journal of Multidisciplinary Research and Growth Evaluation. 2022. [CrossRef]
- Duodu, S.; Ayiku, A.N.A.; Adelani, A.A.; Daah, D.A.; Amoako, E.K.; Jansen, M.D.; Cudjoe, K.S. Serotype distribution, virulence and antibiotic resistance of Streptococcus agalactiae isolated from cultured tilapia Oreochromis niloticus in Lake Volta, Ghana. Diseases of Aquatic Organisms. 2024, 158, 27–36. [Google Scholar] [CrossRef]
- Abarike, E.D.; Atuna, R.A.; Agyekum, S.; Akongyuure, D.N.; Alhassan, E.H. Isolation and Characterization of Aeromonas Jandaei from Nile Tilapia in Lake Volta, Ghana, and Its Response to Antibiotics and Herbal Extracts. Journal of aquatic animal health. 2022, 34, 140–148. [Google Scholar] [CrossRef]
- Appleyard, S.A.; Mather, P.B. Genetic characterization of cultured tilapia in Fiji using allozymes and random amplified polymorphic DNA. Asian Fisheries Science. 2002, 15. [Google Scholar] [CrossRef]
- Taabu-Munyaho, A.; Marshall, B.E.; Tomasson, T.; Marteinsdottir, G. Nile perch and the transformation of Lake Victoria. African Journal of Aquatic Science. 2016, 41, 127–142. [Google Scholar] [CrossRef]
- Kitchell, J.F. , Schindler, D.E., Ogutu-Ohwayo, R., Reinthal, P.N. The Nile Perch in Lake Victoria: interactions between predation and fisheries. Ecological Applications. 1997, 7, 653–664. [Google Scholar] [CrossRef]
- FAO. Yearbook of Fisheries Statistics. Rome, Italy. 2024. [CrossRef]
- Rao, D.; Perrino, E.S.; Barreras, E. The sustainability of tilapia fish farming in Ghana. Blue Kitabu Research Institute. 2012, 37–40. [Google Scholar]
- DFO. Responsible, realistic, and achievable: The Government of Canada announces transition from open-net pen salmon aquaculture in coastal British Columbia. Fisheries and Oceans Canada. 2024. Available online: https://www.canada.ca/en/fisheries-oceans/news/2024/06/responsible-realistic-and-achievable-the-government-of-canada-announces-transition-from-open-net-pen-salmon-aquaculture-in-coastal-british-columbia.html. (Accessed August 08, 2024).
- Garlock, T.; Asche, F.; Anderson, J.; Bjørndal, T.; Kumar, G.; Lorenzen, K.; Ropicki, A.; Smith, M. D.; Tveterås, R. A Global Blue Revolution: Aquaculture Growth across Regions, Species, and Countries. Reviews in Fisheries Science & Aquaculture. 2020, 28, 107–116. [Google Scholar] [CrossRef]
- Dabi, M.; Dzorvakpor, S.E.A. The Impact of Aquaculture on the Environment: A Ghanaian Perspective. The International Journal of Science & Technology. 2015, 3. Retrieved on February 20 2024. https://www.internationaljournalcorner.com/index.php/theijst/article/view/124516.
- Amoah, A.; Dorm-Adzobu, C. Application of contingent valuation method (CVM) in determining demand for improved rainwater in coastal savanna region of Ghana, West Africa. Journal of Economics and sustainable development. 2013, 4, 1–24. [Google Scholar]
- Zornu, J.; Tavornpanich, S.; Shimaa, A.E.; Addo, S.; Nyaga, P.; Dverdal, M.J.; Norheim, K.; Brun, E.; Cudjoe, K.S. Bridging knowledge gaps in fish health management through education, research, and biosecurity. Frontiers in Sustainable Food Systems. 2023, 7. [Google Scholar] [CrossRef]
- Zornu, J.; Oyih, M.; Binde, M.; Viglo, J.; Agbekpornu, H.; Nkansa, M.; Tavornpanich, S.; Norheim, K.; Brun, E.; Cudjoe, K.S. Stakeholder perspectives on the 2023 Ghana national aquaculture development plan: an integration within the ecosystem approach framework. Aquaculture, Fish and Fisheries. 2023, 3, 459–471. [Google Scholar] [CrossRef]
- Hishamunda, N.; Ridler, N.; Bueno, P.; Yap, W. Commercial aquaculture in Southeast Asia: some policy lessons. Food Policy. 2009, 34, 102–107. [Google Scholar] [CrossRef]
- Chironjib, S.S.C.; Puja, R. Aquaculture practices in Bangladesh: A synopsis on prospects, productivity, and problems. Journal of the World Aquaculture Society. 2024, 55, 4–25. [Google Scholar] [CrossRef]
- Rothuis, A.J., van Duijn, A.P., Roem, A.J., Ouwehand, A., van der Pijl, W., Rurangwa, E. Aquaculture business opportunities in Egypt. Wageningen UR. 2013. Available on: https://edepot.wur.nl/258663. (Accessed February 8, 2024).
- Li, X.; Zhang, Y.; Chen, Y. Environmental benefits of pond aquaculture: A review of nutrient management approaches. Aquaculture Research. 2019, 50, 691–705. [Google Scholar]
- Bosma, R.H.; Verdegem, M.C.J. Sustainable aquaculture in ponds: Principles, practices and limits. Livestock Science. 2011, 139, 58–68. [Google Scholar] [CrossRef]
- Hasimuna, O.; Maulu, S.; Nawanzi, K.; Lundu, B.; Mphande, J.; Phiri, C.; Kikamba, E.; Siankwilimba, E.; Siavwapa, S.; Chibesa, M. Integrated agriculture-aquaculture as an alternative to improving small-scale fish production in Zambia. Frontiers in Sustainable Food Systems. 2023, 7. [Google Scholar] [CrossRef]
- Environmental Impact Assessment (EIA). Legislation Act 490, legal backing for established EIA system to be implemented in Ghana. 1994. Available online: https://www.eia.nl/documenten/00000062.docx. (Accessed April 29, 2024).
- Environmental Assessment Regulations. LI. 1625, Environmental Assessment Regulations. 1999. Available online: https://faolex.fao.org/docs/pdf/gha78169.pdf. (Accessed April 29, 2024).
- Fisheries Act. Act 625, License for aquaculture and recreational fishing. Available online: 2002. Available online: https://faolex.fao.org/docs/pdf/gha34737.pdf. (Accessed April 29, 2024).
- Fisheries Regulations. LI. 1968, Fish seed production certificate and fish transfer permit. 2010. Available online: https://faolex.fao.org/docs/pdf/gha151991.pdf. (Accessed April 29, 2024).
- Abhishek, G.; Suresh, K.; Gurjar, S.C. Circular system of resource recovery and reverse logistics approach: key to zero waste and zero landfill. Advanced Organic Waste Management. 2022, 365–381. [Google Scholar] [CrossRef]
- OECD. Recommendation of the Council on the Implementation of the Polluter-Pays Principle, OECD/LEGAL/0132. 2022. Available on: https://legalinstruments.oecd.org/public/doc/11/11.en.pdf. (Accessed April 30, 2024).
- Luppi, B.; Parisi, F.; Rajagopalan, S. The rise and fall of the polluter-pays principle in developing countries. International Review of Law & Economics. 2012, 32, 135–144. [Google Scholar] [CrossRef]
- Hishamunda, N.; Ridler, N.; Martone, E. Policy and governance in aquaculture: lessons learned and way forward. FAO Fisheries and Aquaculture Technical Paper No. 577. Rome, FAO. 2014.
- Abarike, E.A. Review of Ghana’s Aquaculture Industry. Journal of Aquaculture Research and Development. 2018, 9, 545. [Google Scholar] [CrossRef]
- Anane-Taabeah, G.; Frimpong, E.A.; Amisah, S.; Agbo, N. Constraints and opportunities in cage aquaculture in Ghana. In: L. Liping and K. Fitzsimmons (Eds.), Better science, better fish, better life: Proceedings of the ninth international symposium on tilapia in aquaculture. 2011. pp. 182–190. April 22–24, Shanghai. AquaFish Collaborative Support Program.
- Aguilar-Manjarrez, J.; Soto, D.; Brummett, R.
- Kashindye, B.B.; Nsinda, P.; Kayanda, R.; Ngupula, G.W.; Mashafi, C.A.; Ezekiel, C.N. Environmental impacts of cage culture in Lake Victoria: the case of Shirati Bay-Sota, Tanzania. Springerplus. 2015, 4, 475. [Google Scholar] [CrossRef]
- Masser, M. What is cage culture? SRAC publication No. 160. 2008. https://www.ncrac.org/files/inline-files/SRAC0160.pdf.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




