1. Introduction
Systemic-to-pulmonary collaterals (SPC) are frequently encountered in congenital heart disease (CHD). Also referred to as “major aorto-pulmonary collateral arteries (MAPCA)”, arterial SPC usually originate as irregular vessels from the thoracic aorta or its branches, namely, from the subclavian, internal mammary, thyroid, or carotid arteries [
1]. Venous SPC often derive from the caval or other main thoracic veins. Typically, SPC occur in cyanotic CHD with impaired pulmonary perfusion, especially in univentricular physiology. Chronic hypoxaemia as well as reduced or non-pulsatile pulmonary blood flow and inflammatory effects are assumed to promote the development of SPC [
2,
3]. Moreover, premature neonates and infants can be affected by arterial collaterals. In noncyanotic CHD, SPC are rare [
4]. Nevertheless, the pathogenetic factors are still not completely understood.
Irregular collaterals emerge in a great variety of shapes, sizes, and number. They may present as unifocal or multifocal singular vessels as well as highly branched collateral networks. Due to the markedly different individual characteristics, a general classification of SPC is difficult [
5,
6].
In case of collateral-dependent pulmonary perfusion such as in selected types of pulmonary atresia, arterial SPC develop already during the embryonic phase and are considered as essential SPC. Non-essential SPC may evolve at any stage in complex CHD from infancy to adulthood. The latter collaterals are responsible for excessive pulmonary blood flow resulting in hyperaemia and increased pulmonary pressure with the risk for haemoptysis or severe haemorrhages [
7]. As well, cardiac volume and pressure overload may occur [
8].
Treatment of non-essential arterial and venous SPC is handled very differently [
2]. In many centres, SPC are the target of consistent percutaneous embolization. For transcatheter occlusion, different devices are used such as coils, vascular plugs, duct and septal occluders, or particles [
2,
8,
9,
10]. Selecting the appropriate method of SPC embolization is a challenge that depends on the location, size and course of the collaterals as well as the availability of devices suitable in size. Individual solutions have to be chosen. SPC with small diameters that can nevertheless supply extensive collateral networks as well as tortuous SPC may particularly be difficult to embolize. In these cases, Concerto™ Helix nylon-fibred microcoils (CHM) are used in our institution, which to our knowledge has not yet been reported.
The aim of our study was to describe the technique and to evaluate the applicability, efficacy, safety and potential complications of using CHM for embolization of SPC. The patient population as well as the sizes and numbers of the implanted coils, primary outcome, and any complications of the embolization were analyzed.
2. Materials and Methods
2.1. Study Design
This retrospective single-centre study was performed at a tertiary care medical centre (Saarland University Medical Center), after approval from the local ethics committee of the Saarland, Saarbruecken, Germany (file number 154/23).
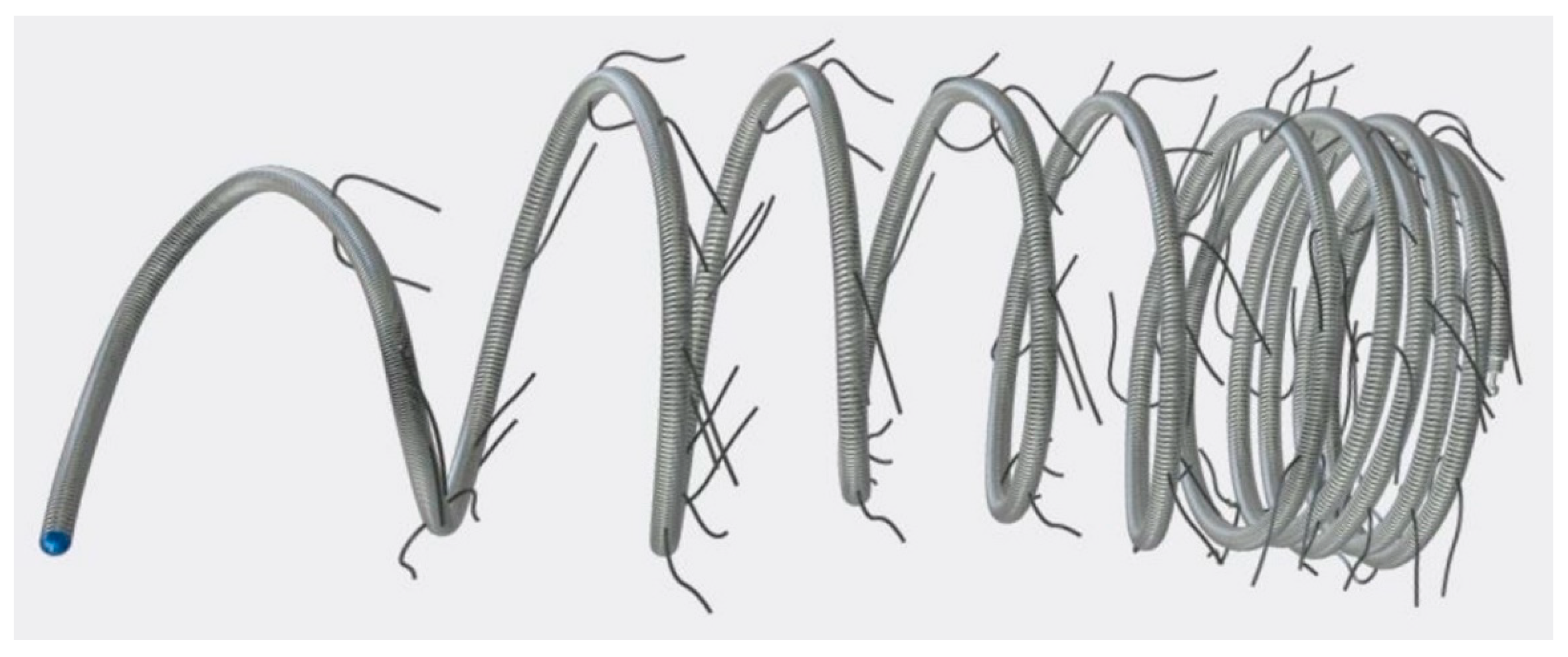
We included all patients who underwent transcatheter embolization of SPC using Concerto™ Helix nylon-fibered microcoils (CHM; Micro Therapeutics, Inc., ev3 Neurovascular, Irvine, CA, USA) in our institution from January 2016 to December 2023. The mechanically detachable CHM consist of a platinum alloy and are provided with nylon fibres. They are available in various diameters (from 2 to 10 mm) and lengths (from 3 to 30 cm).
Figure 1 shows a CHM.
Parents or patients of legal age had given written informed consent for the intervention. Based on the retrospective nature of this data analysis, written informed consent for the study was waived.
We reviewed the relevant patients’ files as well as the angiographic records and post-interventional x-rays in order to obtain the following data:
- -
patients’ characteristics (sex, body weight, age at intervention),
- -
ventricular morphology (biventricular or univentricular anatomy),
- -
preceding surgery,
- -
origin and diameter of the embolized SPC,
- -
number and size of the implanted CHM,
- -
oversizing of the CHM,
- -
primary success of the embolization,
- -
follow-up,
- -
complications (vascular injuries, haemorrhage, coil migration, non-target embolization, thrombosis, embolism, mortality).
2.2. Catheterization and Transcatheter Embolization
Under sedation, percutaneous catheterization was performed via femoral vessel puncture and insertion of 4 French sheaths. Four French angiographic catheters (Multipurpose A Special or MPA 2 or Pigtail; Cordis Corporation, Miami Lakes, FL, USA) were used for angiography of the thoracic aorta and its branches respectively the caval and the brachiocephalical veins as well as the SPC in order to locate the collaterals and to measure their diameters. SPC were assessed as relevant if they supplied pulmonary parenchyma, if pulmonary vascular contrast was visible, or if there was an antegrade pulmonary perfusion washout [
9,
11]. To achieve probing of the SPC as deep as possible, guide wires (0.014’’, Whisper™ ES or LS; Abbott Medical, Santa Clara, CA, USA) and microcatheters (Rebar™-18; Micro Therapeutics, Inc., ev3 Neurovascular, Irvine, CA, USA) were used. Through the latter, the CHM were pushed and deployed into the target vessel
aiming at a densely packed coil formation. They were then mechanically released by retracting the restraining wire within the pusher.
The correct position and the required configuration of the CHM were fluoroscopically assessed during the intervention. Following, the residual contrast medium flow and potential extravasation were controlled by selective SPC angiography to review the effectiveness as well as potential vessel injury.
Successful closure was defined as total or subtotal occlusion (i.e., minimal residual contrast shunt). Heparin (100 IU per kilogram body weight) and antibiotic prophylaxis were administered during the procedure. Post-interventionally,
the patients were monitored in hospital for 48 hours before discharge. A sports ban was imposed for 4 weeks depending on age. Clinical follow-up and echocardiography were carried out after 4 weeks and 3 months, thereafter in individual intervals.
Possible coil migration was evaluated by follow-up chest x-ray or in subsequent angiographies.
CHM have been preferred over other devices (e.g., different coils or vascular plugs) in SPC with small diameters (≤ 4 mm) as well as with tortuous morphology or if probing was only possible using microcatheters. The lengths of the landing zones are usually difficult to measure in tortuous and curved SPC. Therefore, they were mostly estimated in relation to the diameter. Multiple CHM were inserted if the landing zones were not completely filled by the first one or to occlude additional collateral branches. The CHM has to be oversized in order to ensure a stable coil position within the target. Notably, there are no strict recommendations regarding the sizing.
Microsoft© Excel 2016 was used for the statistical analyses. Data analysis is purely descriptive. The data is given as an absolute number and percentage or median and range.
3. Results
3.1. Patients’ Characteristics
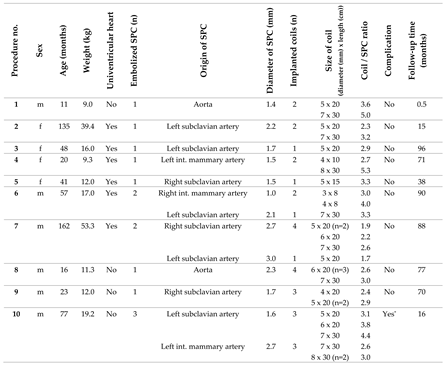
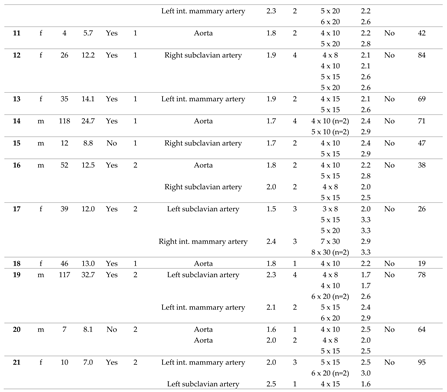
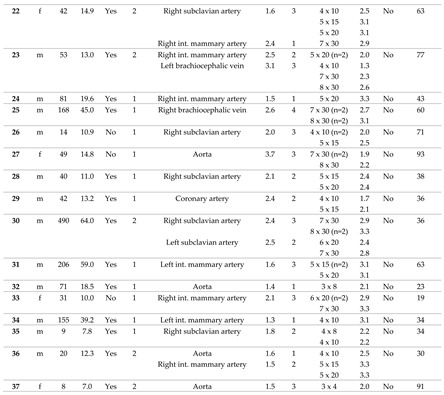
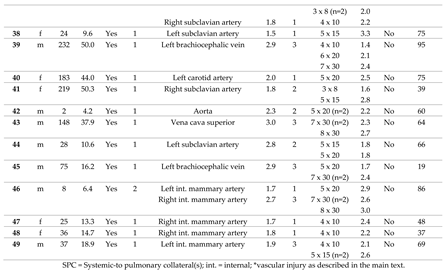
In 38 consecutive patients (thereof 65.8 % male), CHM had been implanted into 64 SPC in a total of 49 procedures. In 29 patients only one procedure was performed. Two separate sessions were performed in 7 patients, and 3 sessions in 2 patients, each at different ages. The median age at the time of intervention was 41 months (range 2 - 490), and the median body weight was 13.2 kg (range 4.2 – 64.0). The cases are described in detail in
Table 1.
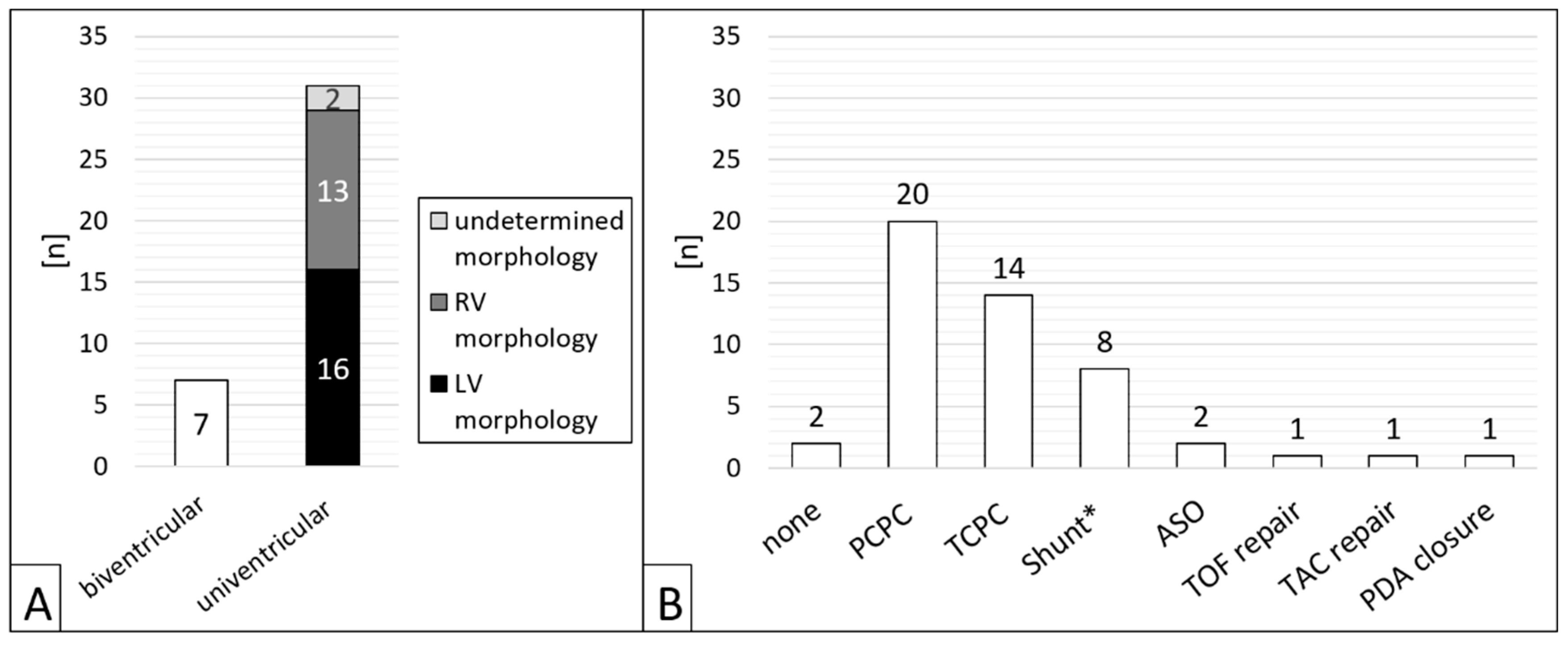
Of 38 patients, n = 31 (81.6 %) had CHD with single ventricle anatomy (
Figure 2A). Regarding the procedures, a single ventricle heart was present in 81.6 % (n = 40/49). Cardiac surgery preceded in n = 47/49 (95.1 %) interventions (figure 2B), most of which cavo-pulmonary connections (PCPC) or total cavo-pulmonary connections (TCPC, Fontan circulation) (n = 34/49, 69.4 %).
3.2. Characteristics of the Target Vessels, Characteristics of the Used Coils, and Coil Oversizing
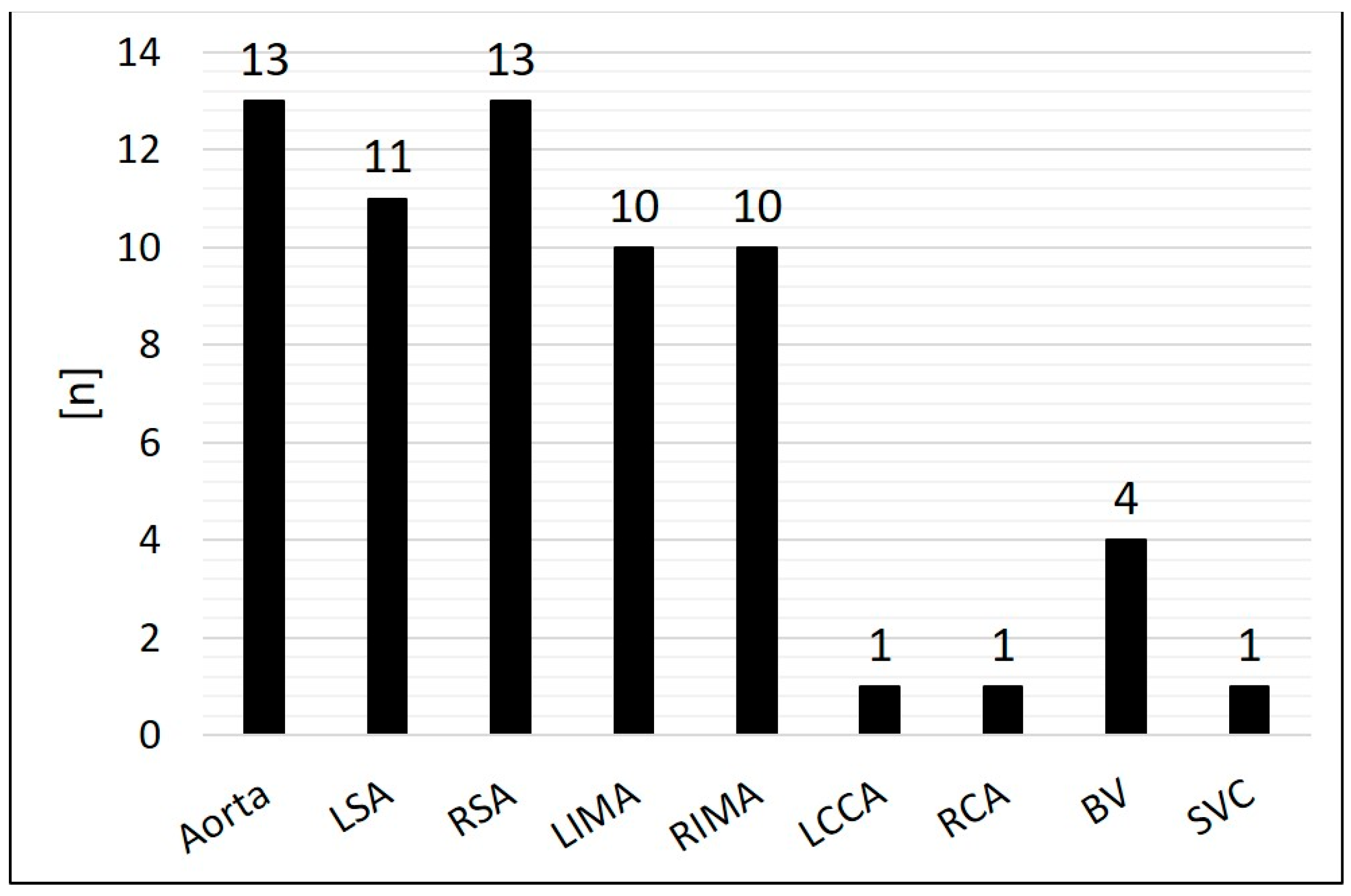
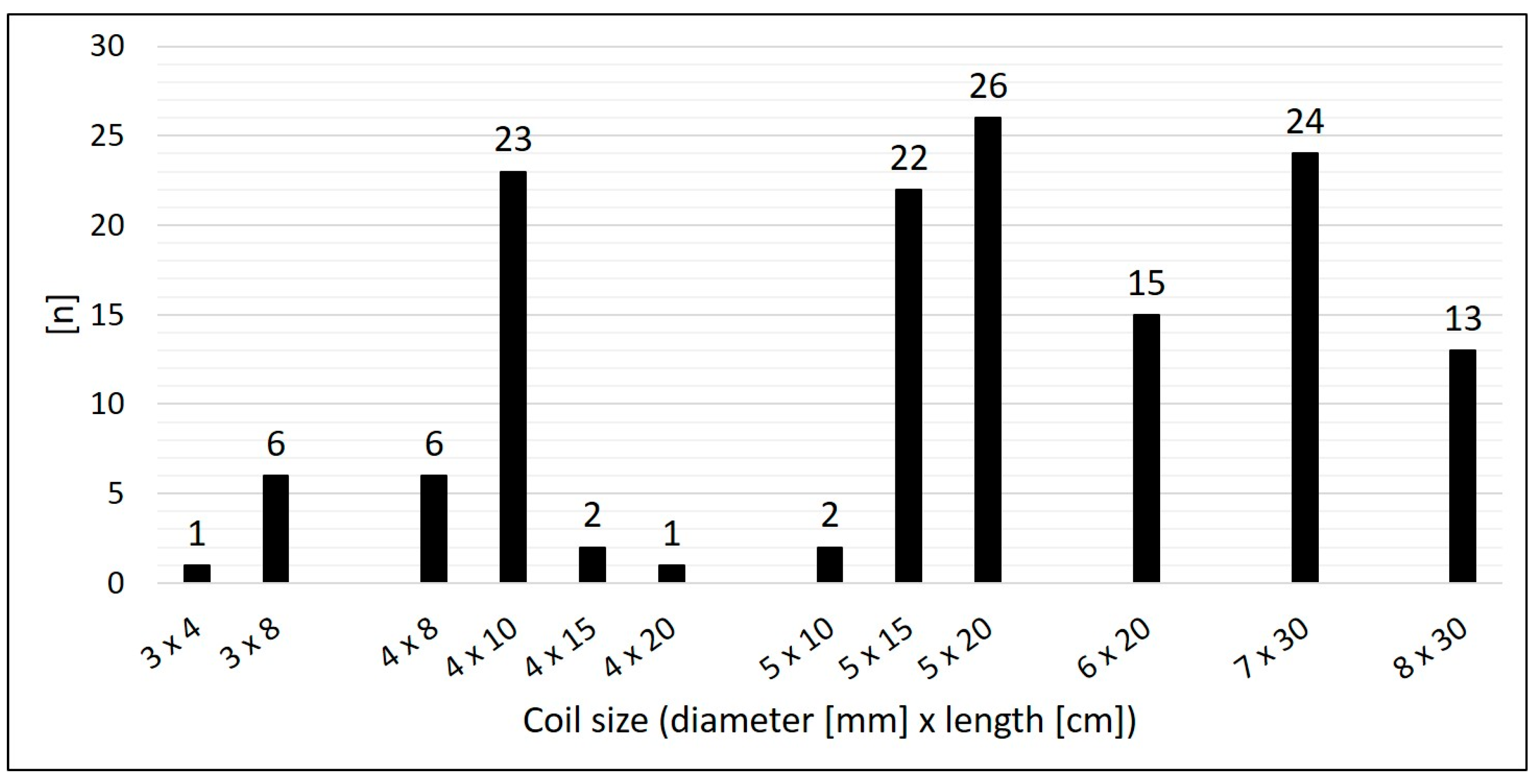
Arterial SPC originating from the thoracic aorta or its branches were present in 92.2 % (n = 59/64); 7.8 % (n = 5/64) were venous SPC from the superior vena cava or from the brachiocephalic vein (
Figure 3). The median diameter of the target SPC was 1.95 mm (range 1.0 – 3.7). A total number of 141 CHM were inserted. The coil diameters were 3 to 8 mm, with 5 mm (n = 50; 35.5 %) being the most commonly used; 3 mm was used in n = 7 (5.0 %); 4 mm in n = 32 (22.7%); 6 mm in n = 15 (10.6 %); 7 mm in n = 24 (17.0 %); and 8 mm in n = 13 (9.2 %) (
Figure 4).
Median oversize of the coil diameter compared to the collateral diameter (CHM / SPC ratio) was 2.6-fold (range 1.3 – 5.3).
3.3. Outcome, Complications, and Follow-Up
The immediate technical success rate was 100 % with total or subtotal closure of the SPC in all patients.
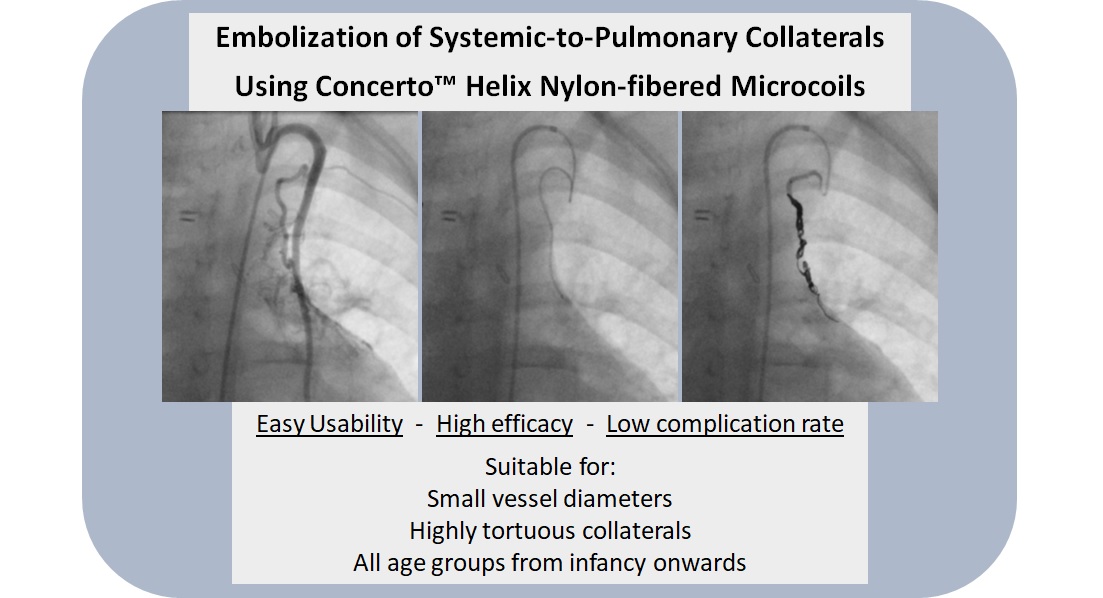
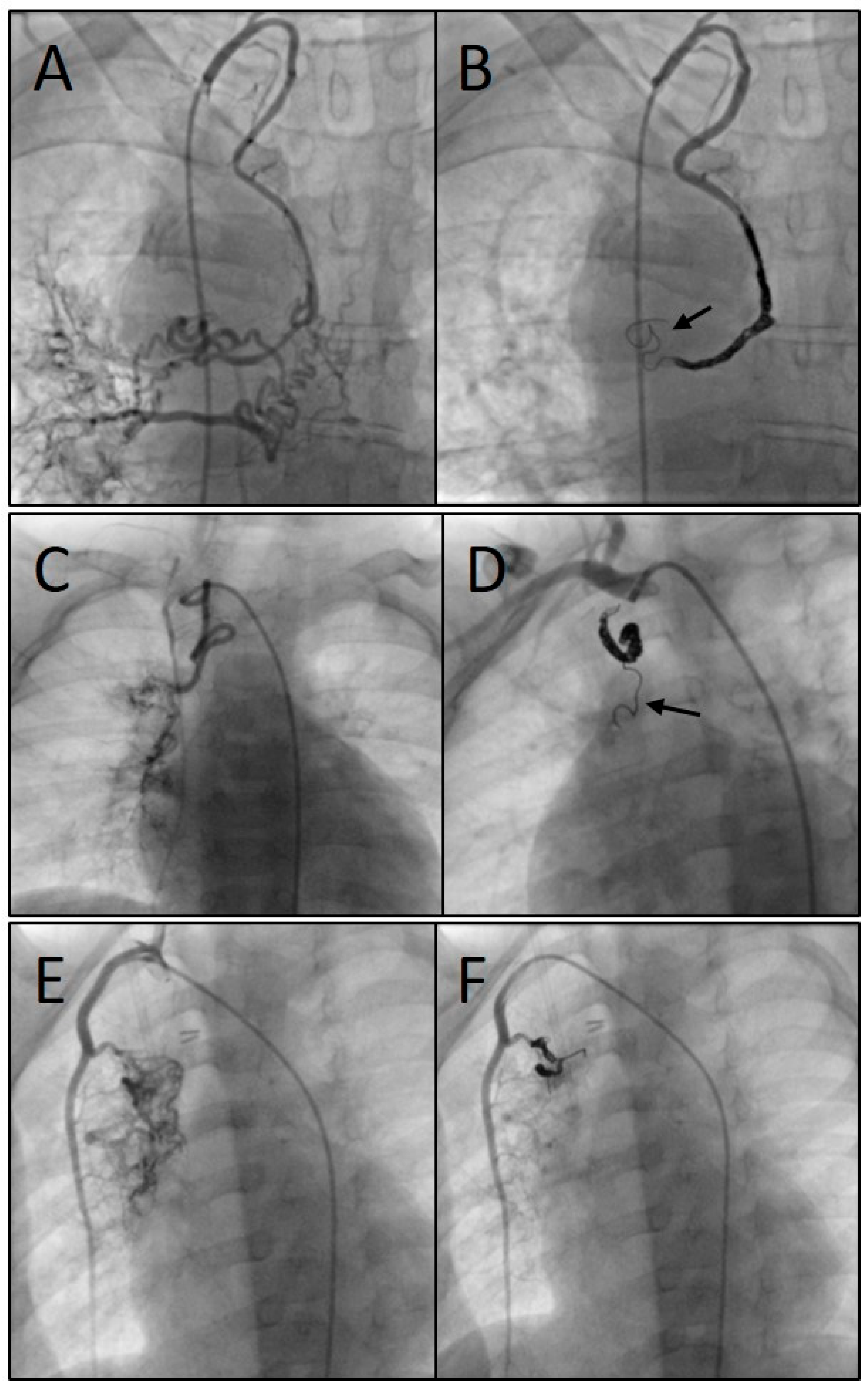
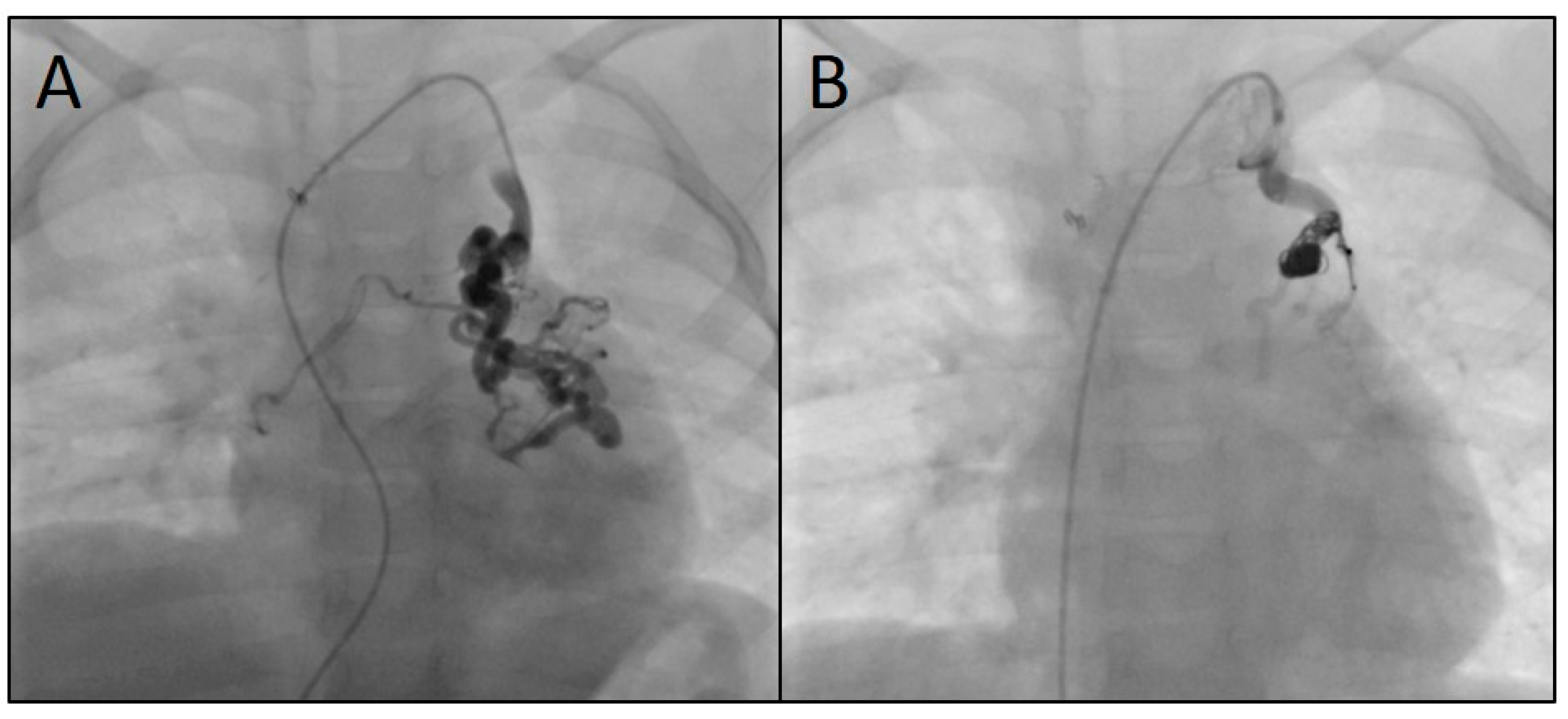
Figure 5 shows three angiograms illustrating embolizations of arterial SPC;
Figure 6 shows a venous SPC embolization. In 24/49 (49.0 %) procedures, a small portion of the distal CHM formed a stretched instead of a coiled structure (see
Figure 5B and 5D). However, the remaining proximal part of the CHM could be stably deployed even in these cases and formed the desired densely packed configuration. Neither non-target embolization nor coil migration occurred during the intervention or in the follow-up. In 47 of 49 cases, follow-up chest x-ray or fluoroscopy were performed where a persistently stable CHM position could be detected in all cases. Thrombosis or embolism did not occur. There were no serious vascular injuries, no need for additional hospitalization and no mortality. The duration of hospital stay (discharge after 48 hours of monitoring) was not longer than usual in our institution for patients undergoing interventional catheterization.
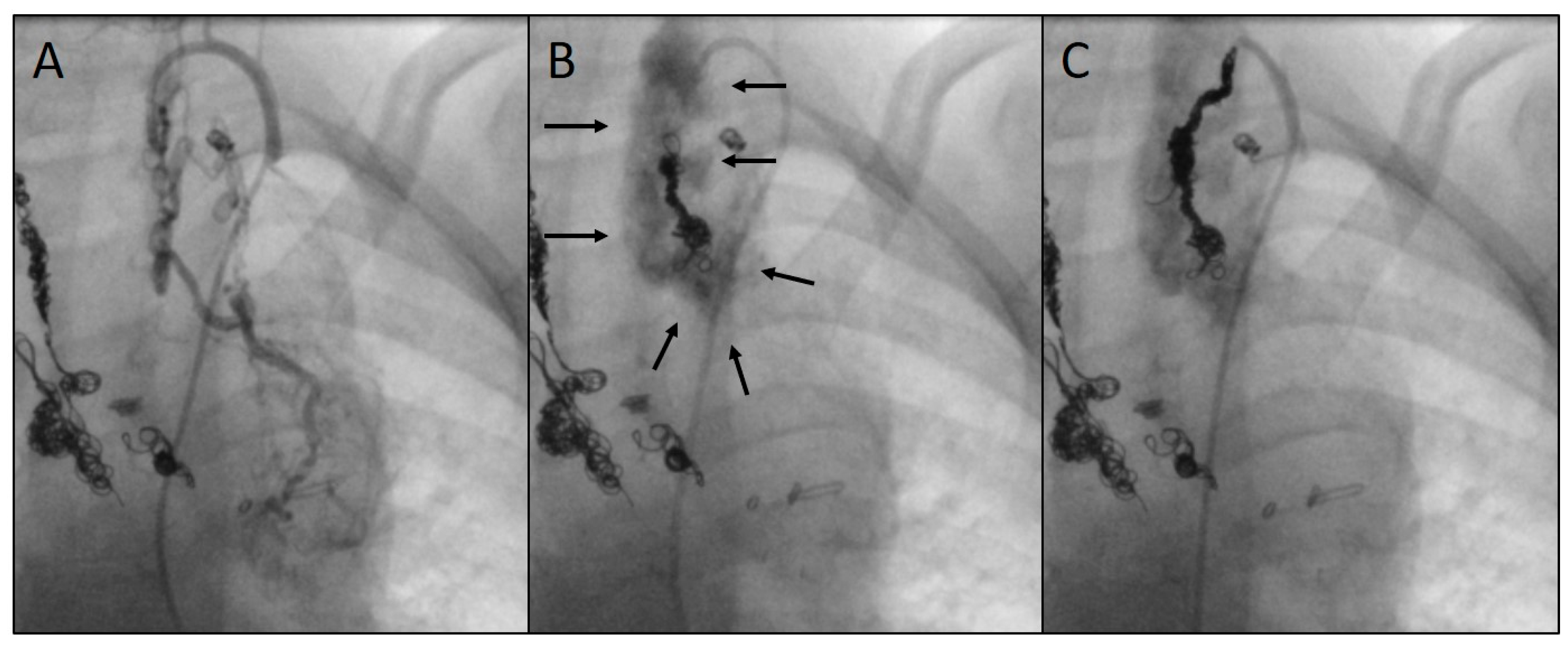
As a complication, there was one vascular injury in a patient who underwent CHM embolization of a SPC originating from the left subclavian artery (
Table 1, procedure no. 10). After deploying of the first CHM (diameter 5 mm, oversize 3.1-fold), there was a contrast extravasation surrounding the target vessel. Two more CHM were promptly inserted into the proximal landing zone, whereupon no more extravasation was detectable (
Figure 7). The patient was stable during the entire procedure as well as in the post-interventional monitoring. Clinical symptoms did not occur. The coils remained stable in the desired position.
For all patients, median follow-up time was 63.0 months (range 0.5 – 96.0). During this time, no complications related to the SPC embolization occurred. Independently of the procedure, one child died three weeks later in connection with cardiac surgery.
4. Discussion
Abnormal blood supply to the lungs in patients with severe congenital heart or vascular malformations leads to pulmonary hyperaemia and increased pulmonary resistance particularly when undergoing several stages of surgical palliation. Moreover, systemic-to-pulmonary collaterals (SPC) lead to increased pulmonary venous returns during cardiac surgery and cardiopulmonary bypass. Several approaches are used to close abnormal vessels during interventional catheterizations.
We report on our institutional experiences with a novel approach to transcatheter embolization of SPC using Concerto™ Helix nylon-fibered microcoils (CHM). With a total number of 141 CHM, 64 SPC had been occluded in 38 consecutive patients. Embolization was successful in all cases. The duration of hospital stay was not prolonged compared to other interventional SPC embolization procedures in our institution. The vast majority of the SPC (n = 57/64) derived either from the aorta, the subclavian or internal mammary arteries which are also the most common origins according to the published literature [
1,
11]. There was one complication in the form of a vascular injury without clinical relevance. When probing SPC, it has to be taken into account that these are vulnerable vessels with abnormally thin vascular walls, and that vasoconstriction can occur during the intervention. Careful vessel probing should be performed using microcatheters aiming at distal coil implantation. Especially small SPC, which nevertheless may feed extensive collateral networks, as well as highly tortuous SPC can be embolized selectively using this technique. In spite of the above-mentioned complication, CHM appear to be particularly suitable due to their soft, flexible and adaptable structure. Severe complications did not occur in the described population. It is assumed that embolizations as distal as possible prevent recurrence of SPC which is achieved well using particle embolization according to previous studies [
9]. In long landing zones with additional branches, several CHM should be inserted if necessary. Embolization of the entire internal mammary arteries as feeding vessels can be avoided by selective SPC closure. Brown et al. have previously reported on the favourable use of micro-catheters in difficult target vessels [
12].
The delivery mechanism of the CHM was easy to operate, there were no complications associated with the coil release in the described population. The absence of screw threads in CHM facilitates the deployment. Notably, neither non-target embolization nor coil migration occurred. It appeared advantageous that the deployed CHM are densely packed within the SPC and thus adapt to the course of the SPC forming an almost “cast-like” structure. As a result, extensive vessel obstruction occurs without delay. The initial portion of the CHM may be inserted in a stretched shape without forming a coiled configuration. A possible cause for this may be that the SPC usually taper distally so that the coils cannot unfold. This does neither affect the stability nor the efficacy of the CHM as long as the main part takes the desired shape. Advantageously, the nylon fibres have been shown to promote faster thrombin generation, which should lead to definitive SPC closure [
13,
14].
Both the tapering diameters of the collaterals and the potential vasoconstriction during the angiography make it difficult to select the appropriate CHM size. Therefore, the selection depended to a certain extent on the interventionalist’s experience. Coil oversizing results in stable positioning similar to other types of embolizing devices [
10]. Given the 1.3 to 5.3-fold diameter of the CHM versus the SPC in our patients, a wide range of CHM / SPC ratios appears to be acceptable. The large selection of available CHM sizes has to be seen as an advantage especially because both the sizes and morphology of SPC are widespread. In long landing zones, either long or multiple CHM have to be used. Implantation close to the branching or even protruding into the feeding vessel must be strongly avoided in order to prevent dislocation and non-target embolization. As the CHM can be inserted via small catheters and thus via small vascular accesses, they are not only appropriate for children and adults of all age groups and body sizes, but also particularly for neonates.
According to the published literature, the management of SPC is handled very differently by interventional cardiologists [
2,
15]. On the one hand, earlier studies suggested that SPC have no significant pathophysiological relevance or that new ones developed quickly after SPC closure anyway [
16,
17]. Furthermore, the haemodynamic significance of individual SPC is undoubtedly difficult to measure [
18]. Some studies suggest that SPC can be better assessed by magnetic resonance imaging than by cardiac catheterization [
19,
20].
On the other hand, recent studies have shown that SPC are associated with a higher morbidity in children with CHD. Especially for patients with PCPC and TCPC, SPC are deleterious and are therefore consistently embolized in many centres [
9,
11]. Dori et al. demonstrated a favourable decrease of pulmonary blood flow after closure of SPC in PCPC patients [
21]. With underlying passive pulmonary perfusion in these patients, counter flow via arterial collaterals can lead to impaired pulmonary blood flow. Prolonged postoperative hospital stays and higher incidence for pleural effusion are described [
22]. In the long term, failing Fontan circulation can be promoted [
23,
24,
25]. As veno-venous collaterals are associated with cyanosis and an increased risk for plastic bronchitis, they should also be embolized in these patients [
26,
27]. Moreover, SPC are responsible for morbidity as well in other CHD, e.g. in children undergoing arterial switch operation [
28,
29]. The majority of patients in the population described above had univentricular anatomy (n = 31/38), and most SPC embolizations were preceded by PCPC or TCPC surgery.
Of note, essential SPC have to be strictly differentiated as they have to be conserved in collateral-depending pulmonary circulation respectively have to be surgically unifocalized [
30,
31].
There are several limitations in the present study. The numbers of both the patients and the procedures are still low. Although the SPC diameters were measured, the selection of the implanted coils depended on the experience of the interventionalist. A specific recommendation regarding the CHM sizes is difficult to provide; further studies with a larger population are necessary. Other limitations are the retrospective approach and the single-centre character of the study.
We conclude that Concerto™ Helix nylon-fibered microcoils are simple and safe to use with a high efficacy and a low complication rate. They are suitable in embolization of systemic-to-pulmonary collaterals, particularly with small diameters, highly tortuous morphologies, and variable landing zones. As they are inserted via small vessel accesses, they can be used in patients of a wide range of age groups and body weights.
Author Contributions
Conceptualization, J.P., A.G. and H.A.-K.; methodology, J.P. and H.A.-K.; software, J.P. and H.A.-K.; validation, J.P., M.P., A.R. and H.A.-K.; formal analysis, J.P., M.P. and H.A.-K.; investigation, J.P. and A.G.; resources, J.P., M.P., A.R. and H.A.-K.; data curation, J.P. and A.G.; writing—original draft preparation, J.P. and A.G.; writing—review and editing, M.P., A.R. and H.A.-K.; visualization, J.P.; supervision, H. A.-K.; project administration, H. A.-K.; funding acquisition, H. A.-K. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge support by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) and Saarland University within the funding programme Open Access Publishing.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved on 8 August 2023 by the local ethics committee of the Saarland, Saarbruecken, Germany (file number 154/23).
Informed Consent Statement
Parents or patients of legal age had given written informed consent for the intervention. Based on the retrospective nature of this data analysis, written informed consent for the study was waived.
Data Availability Statement
The data underlying the present study are available on request (corresponding author).
Conflicts of Interest
The authors declare no conflicts of interest. There are neither contracts with companies nor are funds provided by the industry.
Abbreviations
| CHD |
congenital heart disease |
| CHM |
Concerto™ Helix microcoil(s) (nylon-fibered) |
| MAPCA |
major aorto-pulmonary collateral arteries |
| PCPC |
partial cavo-pulmonary connection |
| SPC |
systemic-to-pulmonary collateral(s) |
| TCPC |
total cavo-pulmonary connection |
References
- Alex, A., et al., Major Aortopulmonary Collateral Arteries. Radiol Cardiothorac Imaging, 2022. 4(1): p. e210157. [CrossRef]
- Mohammad Nijres, B., et al., Aortopulmonary Collaterals in Single Ventricle Physiology: Variation in Understanding Occlusion Practice Among Interventional Cardiologists. Pediatr Cardiol, 2020. 41(8): p. 1608-1616. [CrossRef]
- Schmiel, M., et al., Impact of Anatomical Sub-types and Shunt Types on Aortopulmonary Collaterals in Hypoplastic Left Heart Syndrome. Semin Thorac Cardiovasc Surg, 2023. 35(4): p. 746-756. [CrossRef]
- Yang, F., et al., Aortopulmonary Collateral Arteries in Noncyanotic Congenital Heart Disease. Ann Thorac Surg, 2022. 113(2): p. e125-e127. [CrossRef]
- Adamson, G.T., et al., Angiographic Anatomy of Major Aortopulmonary Collateral Arteries and Association With Early Surgical Outcomes in Tetralogy of Fallot. J Am Heart Assoc, 2020. 9(24): p. e017981. [CrossRef]
- Adamson, G.T., et al., Comprehensive diagnostic catheterization in children with major aortopulmonary collateral arteries: A review of catheterization technique and anatomic nomenclature. Catheter Cardiovasc Interv, 2022. 99(4): p. 1129-1137. [CrossRef]
- Averin, K., et al., Life-threatening airway bleeding after palliation of single ventricle congenital heart disease. Heart, 2018. 104(3): p. 254-260. [CrossRef]
- Van De Bruaene, A. and W. Budts, Collaterals in congenital heart disease: when and how to treat? Cardiovasc Diagn Ther, 2023. 13(2): p. 418-426. [CrossRef]
- Batlivala, S.P., W.E. Briscoe, and M.R. Ebeid, Particle embolization of systemic-to-pulmonary collateral artery networks in congenital heart disease: Technique and special considerations. Ann Pediatr Cardiol, 2018. 11(2): p. 181-186. [CrossRef]
- Pfeifer, J., et al., Transcatheter Embolization in Congenital Cardiovascular Malformations—Variable Use of Vascular Plugs. Cardiovascular Therapeutics, 2024. 2024(1): p. 4778469. [CrossRef]
- Schmiel, M., et al., Aortopulmonary collaterals in single ventricle: incidence, associated factors and clinical significance. Interact Cardiovasc Thorac Surg, 2022. 35(2). [CrossRef]
- Brown, S.C., et al., Use of a microcatheter in a telescopic system to reach difficult targets in complex congenital heart disease. Catheter Cardiovasc Interv, 2009. 73(5): p. 676-81. [CrossRef]
- Girdhar, G., et al., In-vitro thrombogenicity assessment of polymer filament modified and native platinum embolic coils. J Neurol Sci, 2014. 339(1-2): p. 97-101. [CrossRef]
- Trerotola, S.O., G.A. Pressler, and C. Premanandan, Nylon Fibered Versus Non-Fibered Embolization Coils: Comparison in a Swine Model. J Vasc Interv Radiol, 2019. 30(6): p. 949-955. [CrossRef]
- Goldstein, B.H., et al., Practice Variation in Single-Ventricle Patients Undergoing Elective Cardiac Catheterization: A Report from the Congenital Cardiac Catheterization Project on Outcomes (C3PO). Congenit Heart Dis, 2016. 11(2): p. 122-35. [CrossRef]
- Bradley, S.M., Management of aortopulmonary collateral arteries in Fontan patients: routine occlusion is not warranted. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu, 2002. 5: p. 55-67. [CrossRef]
- Bradley, S.M., et al., Aortopulmonary collateral flow in the Fontan patient: does it matter? Ann Thorac Surg, 2001. 72(2): p. 408-15. [CrossRef]
- Powell, A.J., Aortopulmonary collaterals in single-ventricle congenital heart disease: how much do they count? Circ Cardiovasc Imaging, 2009. 2(3): p. 171-3. [CrossRef]
- Prakash, A., Significance of systemic to pulmonary artery collaterals in single ventricle physiology: new insights from CMR imaging. Heart, 2012. 98(12): p. 897-9. [CrossRef]
- Ridderbos, F.S., et al., Quantification of systemic-to-pulmonary collateral flow in univentricular physiology with 4D flow MRI. Cardiol Young, 2023. 33(9): p. 1634-1642. [CrossRef]
- Dori, Y., et al., Acute effects of embolizing systemic-to-pulmonary arterial collaterals on blood flow in patients with superior cavopulmonary connections: a pilot study. Circ Cardiovasc Interv, 2013. 6(1): p. 101-6. [CrossRef]
- Grosse-Wortmann, L., et al., Aortopulmonary collateral flow volume affects early postoperative outcome after Fontan completion: a multimodality study. J Thorac Cardiovasc Surg, 2012. 144(6): p. 1329-36. [CrossRef]
- Ascuitto, R.J. and N.T. Ross-Ascuitto, Systematic-to-pulmonary collaterals: a source of flow energy loss in Fontan physiology. Pediatr Cardiol, 2004. 25(5): p. 472-81. [CrossRef]
- Glatz, A.C., et al., Systemic-to-pulmonary collateral flow, as measured by cardiac magnetic resonance imaging, is associated with acute post-Fontan clinical outcomes. Circ Cardiovasc Imaging, 2012. 5(2): p. 218-25. [CrossRef]
- Osawa, T., et al., Impact of aortopulmonary collaterals on adverse events after total cavopulmonary connection. Eur J Cardiothorac Surg, 2023. 64(6). [CrossRef]
- Abdel-Aziz, D., et al., Catheter interventional closure of veno-venous collaterals in cyanotic patients after partial cavopulmonary shunts in pediatric patients: clinical practice review. Cardiovasc Diagn Ther, 2023. 13(3): p. 599-608. [CrossRef]
- Nguyen Cong, M.B.H., et al., Impact of veno-venous collaterals on outcome after the total cavopulmonary connection. Int J Cardiol, 2024. 410: p. 132229. [CrossRef]
- Doulamis, I.P., et al., Major Aortopulmonary Collateral Arteries Requiring Percutaneous Intervention Following the Arterial Switch Operation: A Case Series and Systematic Review. World J Pediatr Congenit Heart Surg, 2022. 13(2): p. 146-154. [CrossRef]
- Wipf, A., et al., Aortopulmonary collaterals in neonates with d-transposition of the great arteries - Clinical significance early after arterial switch operation. Int J Cardiol, 2018. 258: p. 237-242. [CrossRef]
- Mainwaring, R.D., Midline unifocalization for pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. J Thorac Dis, 2020. 12(3): p. 1263-1273. [CrossRef]
- Patrick, W.L., et al., Major Aortopulmonary Collateral Arteries With Anatomy Other Than Pulmonary Atresia/Ventricular Septal Defect. Ann Thorac Surg, 2017. 104(3): p. 907-916. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).