1. Introduction
Canada is the fourth-largest producer of barley (Hordeum vulgaris L.) worldwide. It ranks third in harvested area following wheat and canola, producing over 8.9 million tons of barley grain in 2023. This extensive cultivation is driven by barley’s important roles in animal feed, alcoholic beverage production, and human table consumption [
1]. In addition, barley is often cultivated in crop rotation and intercropping systems attributed to its strong adaptability to diverse climates and soil conditions. This makes it a vital component in efforts to develop sustainable agroecosystems in the face of unpredictable climate changes.
Drought stress is expected to become more frequent and intensive in the coming future. Therefore, it is critical to fill our knowledge gap on how drought stress impacts on different stages of barley growth and development and identify genotypes with better drought tolerance for future cultivar development. Drought stress can be induced artificially using chemicals such as polyethylene glycol 6000 (PEG) due to its ability to restrict the water uptake and decrease the overall osmotic potential [
2]. Thus, PEG can be used in vitro screening against drought conditions because it reduces water potential and simulate drought stress under in vitro condition [
3,
4,
5,
6]. Specifically, PEG hinders water transport through the xylem by effectively increasing the size of water molecules when added to water [
7]. The presence of growth in the PEG-induced drought stress condition is important because it indicates that even though drought conditions were present from the beginning of the treatment, the seedlings were still able to germinate and grow. The current study aimed to investigate the responses of ten barley genotypes to drought stress induced by PEG and identify drought-tolerant barley genotypes for potential use in breeding programs to develop drought-resistant barley varieties. We hypothesized that PEG-induced osmotic stress would reduce seed germination and alter root number and length of both root and shoot in barley genotypes.
2. Materials and Methods
2.1. Plant Materials
Ten barley genotypes of CH1808-ON-28, CH1808-ON-69, CH1818-PE-34, CH1819-PE-05, OB1864-ON-16, OB1870-ON-27, OB1877-ON-41, OB1878-ON-50, OB1879-ON-13, and OB1882-ON-75 were used in this study. These genotypes were advanced barley breeding lines collected from the Ottawa barley breeding program, Ottawa, ON.
2.2. Experimental Design
Before the experiment, 10 mL of distilled water was added to each Petri dish, followed by the addition of either 5 mL of distilled water (Control) or 5 mL of 25% PEG solution (Drought) after 24 hours. Uniformly sized seeds were selected, surface-sterilized in 1% (v/v) sodium hypochlorite solution for 5 minutes, rinsed three times with distilled water, and air-dried. Fifteen seeds of each genotype were placed ventral side down on double layers of filter papers in Petri dishes. The experiment was carried out in a randomized complete block design with three replications.
2.3. Trait Measurements
The number of germinated seeds, defined as those with both the plumule and radicle emerged to at least 2 mm, was recorded daily for seven days. On the 7th day, seed germination rate (GR), root number (RN), root length (RL), shoot length (SL) were measured for each Petri dish. In addition, germination drought tolerance index (GDTI) of each genotype was calculated as follows [
8]:
2.4. Statistical Analysis
The analysis of variance (ANOVA) was performed with SAS (SAS Institute, Cary Inc.) using the Mixed procedure. Drought treatment and genotype were treated as fixed effects while the replication was as the random effect. The differences between treatments and genotypes were investigated using Duncan’s multiple comparison at the level of p < 0.05. Shapiro-Wilk’s statistic was used to test the normality assumption on the residuals of each model, while residual plots were used to verify the homogeneity of variances.
3. Results
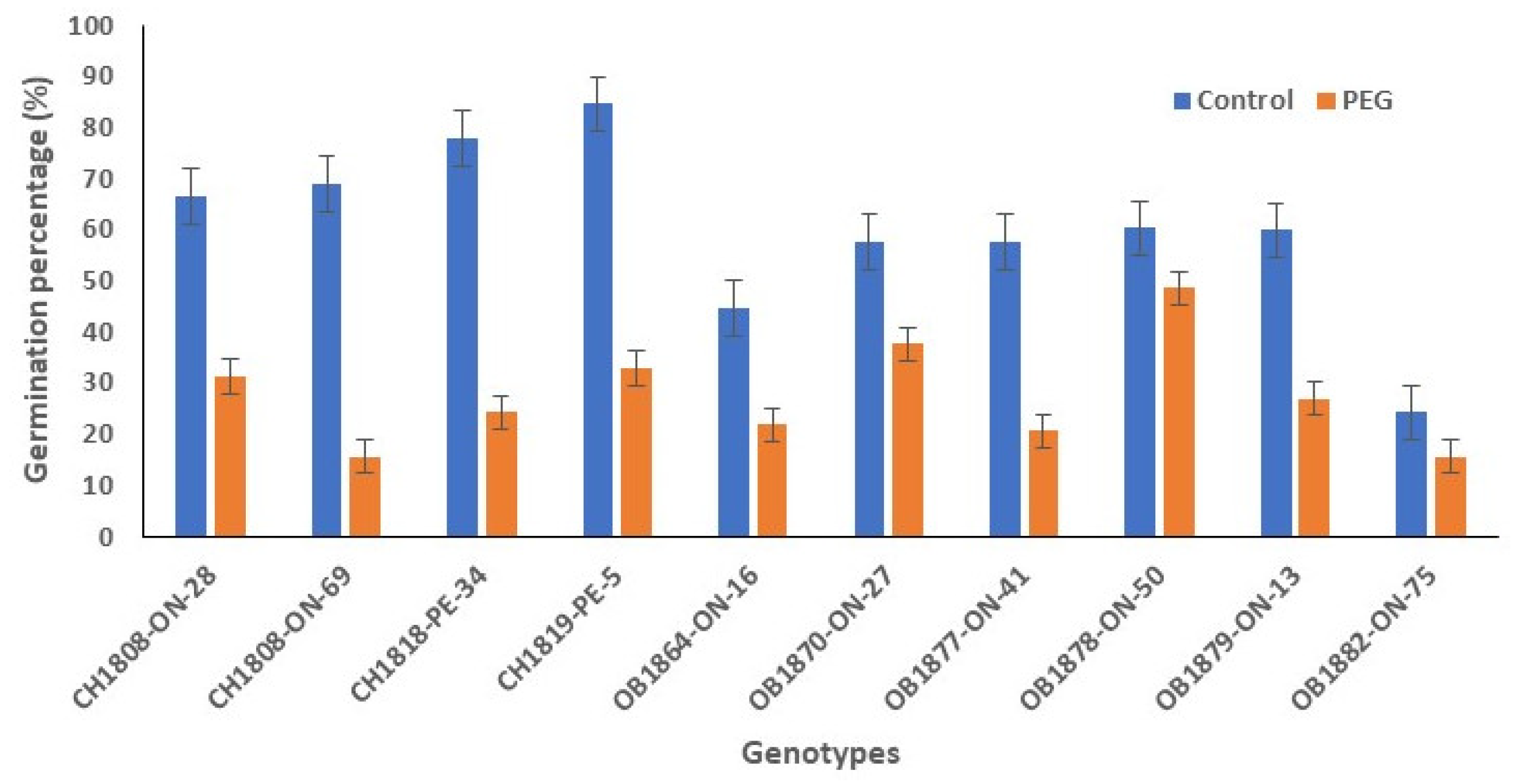
This rate gradually declined to 24%, with an average of 60% under the same growing environments. However, germination rate reduced to 27% on average under drought stress, ranging from 16% to 49%. All genotypes, except for OB1882-ON-75, exhibited a negative response to drought stress in terms of germination rate (
Figure 1). The genotype OB1878-ON-50 had the highest germination percent in PEG-induced drought stress condition.
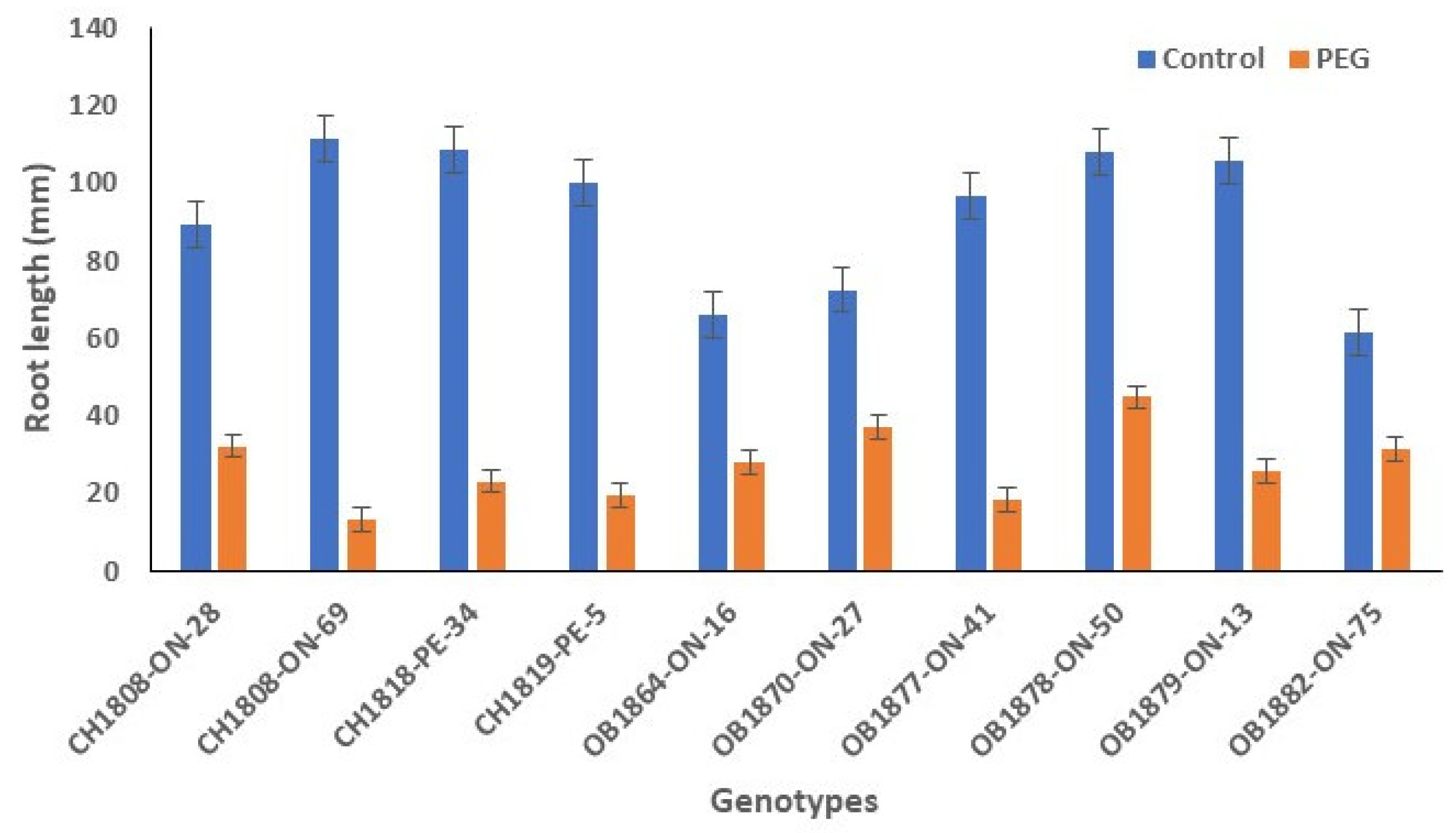
3.2. Root Length
Root length was significantly affected by drought treatment. The longest root, 111 mm, was observed for CH1808-ON-69 and gradually reduced to 62 mm for OB1882-ON-75 under control conditions. However, root length of drought-stressed seedlings decreased to 13-45 mm, with an average of 28 mm (
Figure 2). Of the 10 genotypes, OB1878-ON-50 had the longest root of 45 mm under PEG-induced drought stress.
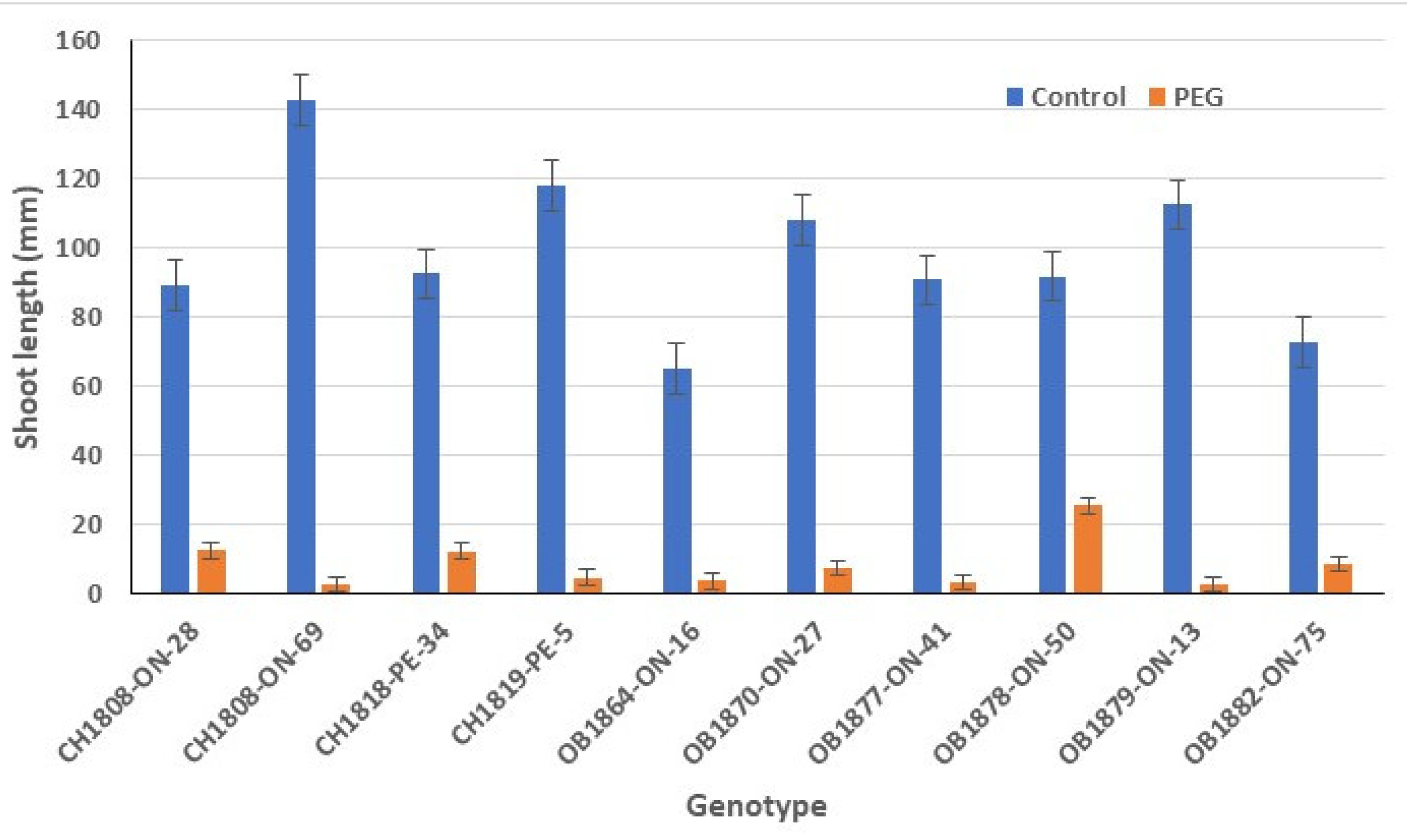
3.3. Shoot Length
There was a significant interaction effect between genotype and drought treatment on shoot length. PEG-induced conditions significantly shortened the shoot length in all genotypes (
Figure 3). The shoot length of genotypes under no-stress conditions varied between 65 mm and 143 mm, averaging 98 mm, which was over 12-fold greater than that under PEG-induced drought stress conditions. Genotype OB1878-ON-50 had the longest shoot of 25 mm under PEG-induced drought stress.
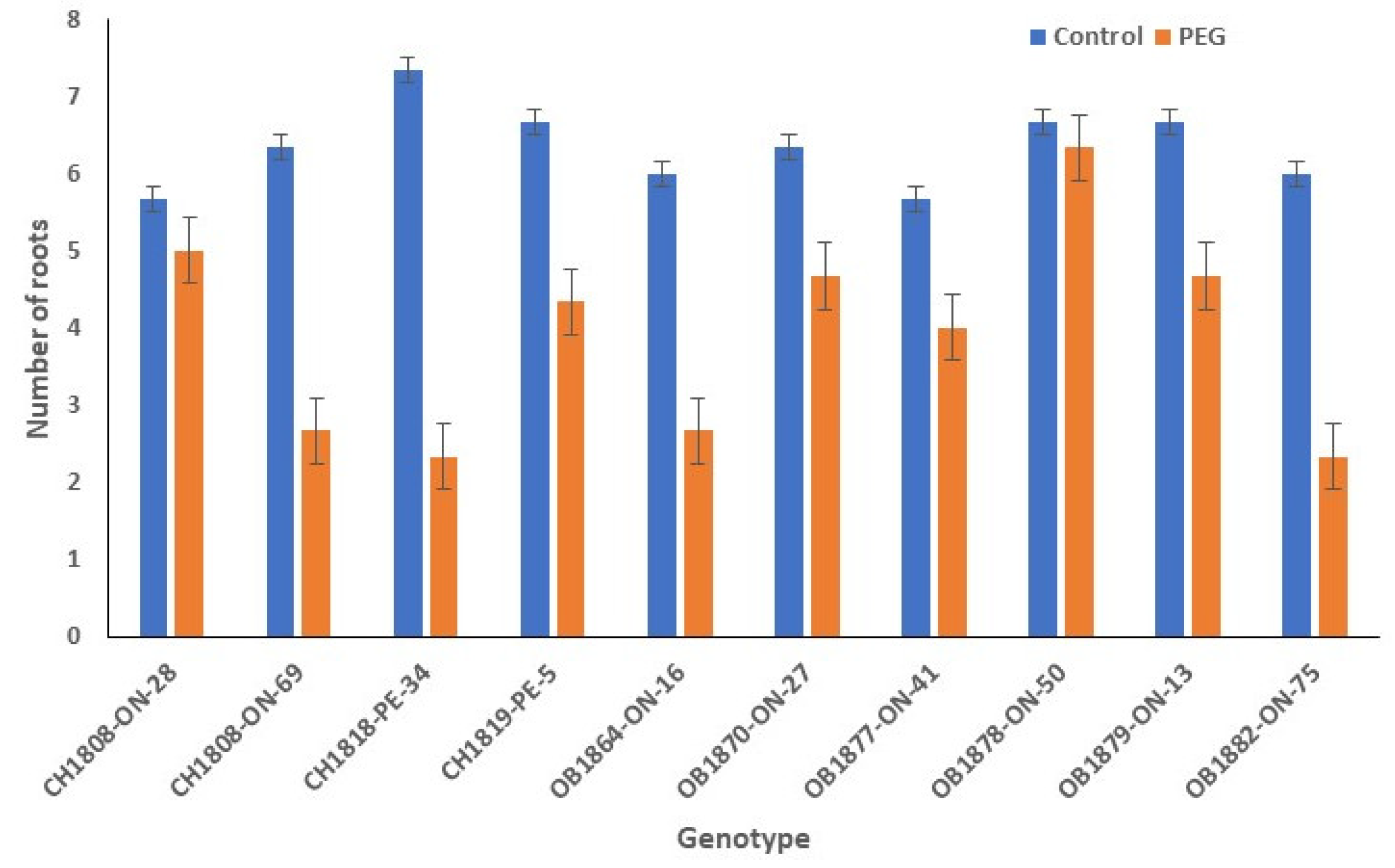
3.4. Number of Roots
The difference in root number between PEG-induced drought stress and no-stress conditions was more evenly distributed compared to other traits (
Figure 4). The number of roots under no-stress condition varied between 6 and 7, with an average of 6 roots per genotype. Under PEG-induced drought stress, root numbers ranged from 2 to 6 with an average of 4. Genotype CH1818-PE-34 had the highest number of roots, averaging roots of 7 under the no-stress condition. In contrast, OB1878-ON-50 had the highest number of roots under drought condition with an average number of 6. Notably, OB1878-ON-50 had the most consistent root number across both stress and no-stress conditions.
3.5. Germination drought Tolerance Index
To evaluate the drought response of the tested genotypes, GDTI was calculated by dividing its germination rate under stress condition by that under control condition. The index categorized the 10 genotypes into 4 groups of tolerant (GDTI > 0.80), moderately tolerant (0.60-0.79), moderately susceptible (0.40-0.59), and susceptible (GDTI <0.40). Among the tested genotypes, OB1878-ON-50 showed the highest GDTI while CH1808-ON-69 had the lowest value (
Table 1).
4. Discussion
Drought significantly impacts crop growth and development, leading to yield losses up to 80% [
9] due to its negative effects on plant morphological, physiological, and biochemical responses [
10]. With global warming, climate change-induced drought intensity and frequency are expected to increase in the coming years. This study aimed to examine the effects of PEG-induced drought stress and assess if different barley genotypes exhibited specific resistance towards drought stress.
When compared to all other studies, the common theme throughout all studies including this one is that the germination being affected by drought conditions, however results do vary. Previously, many researchers concluded that PEG-induced drought stress reduced the germination and other seedling traits in different crops [
3,
5,
6,
11,
12,
13]. Hellal, F. A. et al. [
12] reported that the increase in PEG concentrations decreased germination percentage of barley cultivars relative to the untreated barley cultivars. Similarly, Lateef et al. [
5] reported PEG-induced drought stress significantly minimized germination of 59 barley genotypes. Our results aligned with previous studies and provided further evidence that germination rate reduced with PEG-induced drought stress.
The findings indicated that genotypes showed reduced germination rate, root length, shoot length, and root number under PEG-simulated drought compared to those under favourable growing environments. Drought stress decreases the biosynthesis of key plant hormones, such as abscisic acid and gibberellins, which play crucial roles in promoting seed germination [
14,
15]. Similarly, root length was significantly shorter under PEG-induced drought condition than in the control; however, genotypes CH1818-PE-34 and OB1878-ON-50 maintained root lengths exceeding 15 mm. Sayed [
16] highlighted the roles of exotic alleles in enhancing root length under drought conditions, suggesting that these two genotypes may carry favourable alleles that contribute to increased root length under stress. In this study, barley genotypes showed slow shoot growth under PEG-induced drought, whereas fast shoot growth occurred under control conditions. This trend is likely due to the PEG limiting the seeds’ ability to absorb sufficient water for shoot growth and development. The number of roots did not change significantly between the control and PEG-induced drought stress condition. This could be attributed to the fact that both treatments received 10 ml of water 24 hours prior to the first PEG application, providing equal opportunities for germination to start.
This study identified OB1878-ON-50 as a promising drought-tolerant barley genotype as it excelled in 3 out 4 traits studied under drought conditions. This suggests that it could be used as a breeding material in future strategies to develop superior genotypes for regions with low precipitation. Future research can focus on in-depth genetic analysis to pinpoint specific loci or genetic regions responsible for drought resistance using methods such as QTL mapping and genome wide association studies. Additionally, developing molecular markers can support marker-assisted breeding for enhanced drought tolerance in barley.
Author Contributions
R.K. conceptualized and M.D.W. performed the experiment, collected data, and interpreted the results. B.S. and R.K. supervised the experiment. RK. wrote the manuscript with the assistance of G.W. R.K., G.W. and B.S. reviewed the manuscript.
Funding
This research was funded by the Agri-Food Innovation Support Program, a program developed under the Canadian Agricultural Partnership between the Barley Council of Canada and Agriculture and Agri-Food Canada.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Acknowledgments
Sincere appreciation is expressed to H. Morrison for technical assistance to conduct the experiment..
References
- Sullivan, P., E. Arendt and E. Gallagher. “The increasing use of barley and barley by-products in the production of healthier baked goods.” Trends in Food Science & Technology 29 (2013): 124-34. Available online: https://www.sciencedirect.com/science/article/pii/S0924224412002130. [CrossRef]
- Rai, M. K., R. K. Kalia, R. Singh, M. P. Gangola and A. K. Dhawan. “Developing stress tolerant plants through in vitro selection—an overview of the recent progress.” Environmental and Experimental Botany 71 (2011): 89-98. Available online: https://www.sciencedirect.com/science/article/pii/S0098847210002194. [CrossRef]
- Basal, O., A. Szabó and S. Veres. “Peg-induced drought stress effects on soybean germination parameters.” Journal of Plant Nutrition 43 (2020): 1768-79.
- Hellal, F., H. El-Shabrawi, M. Abd El-Hady, I. Khatab, S. El-Sayed and C. Abdelly. “Influence of peg induced drought stress on molecular and biochemical constituents and seedling growth of egyptian barley cultivars.” Journal of Genetic Engineering and Biotechnology 16 (2018): 203-12.
- Lateef, D., K. Mustafa and N. Tahir. “Screening of iraqi barley accessions under peg-induced drought conditions.” All Life 14 (2021): 308-32.
- Muscolo, A., M. Sidari, U. Anastasi, C. Santonoceto and A. Maggio. “Effect of peg-induced drought stress on seed germination of four lentil genotypes.” Journal of Plant Interactions 9 (2014): 354-63.
- Shivakrishna, P., K. A. Reddy and D. M. Rao. “Effect of peg-6000 imposed drought stress on rna content, relative water content (rwc), and chlorophyll content in peanut leaves and roots.” Saudi journal of biological sciences 25 (2018): 285-89.
- Badr, A., H. H. El-Shazly, R. A. Tarawneh and A. Börner. “Screening for drought tolerance in maize (Zea mays l.) germplasm using germination and seedling traits under simulated drought conditions.” Plants 9 (2020): 565. Available online: https://www.mdpi.com/2223-7747/9/5/565.
- Wen, G., B.-L. Ma, A. Vanasse, C. D. Caldwell, H. J. Earl and D. L. Smith. “Machine learning-based canola yield prediction for site-specific nitrogen recommendations.” Nutrient Cycling in Agroecosystems 121 (2021): 241-56.
- Iqbal, M. S., A. K. Singh and M. I. Ansari. “Effect of drought stress on crop production.” In New frontiers in stress management for durable agriculture. A. Rakshit, H. B. Singh, A. K. Singh, U. S. Singh and L. Fraceto. Singapore: Springer Singapore, 2020, 35-47.
- Ferioun, M., N. Srhiouar, S. Bouhraoua, N. El Ghachtouli and S. Louahlia. “Physiological and biochemical changes in moroccan barley (Hordeum vulgare l.) cultivars submitted to drought stress.” Heliyon 9 (2023): e13643. Available online: https://www.sciencedirect.com/science/article/pii/S2405844023008502. [CrossRef]
- Hellal, F. A., H. M. El-Shabrawi, M. Abd El-Hady, I. A. Khatab, S. A. A. El-Sayed and C. Abdelly. “Influence of peg induced drought stress on molecular and biochemical constituents and seedling growth of egyptian barley cultivars.” Journal of Genetic Engineering and Biotechnology 16 (2018): 203-12. Available online: https://www.sciencedirect.com/science/article/pii/S1687157X17300835. [CrossRef]
- Partheeban, C., C. Chandrasekhar, P. Jeyakumar, R. Ravikesavan and R. Gnanam. “Effect of peg induced drought stress on seed germination and seedling characters of maize (Zea mays l.) genotypes.” International Journal of Current Microbiology and Applied Sciences 6 (2017): 1095-104.
- Liao, Z., Y. Zhang, Q. Yu, W. Fang, M. Chen, T. Li, Y. Liu, Z. Liu, L. Chen and S. Yu. “Coordination of growth and drought responses by ga-aba signaling in rice.” New Phytologist 240 (2023): 1149-61.
- Zhou, Q., Y. Li, X. Wang, C. Yan, C. Ma, J. Liu and S. Dong. “Effects of different drought degrees on physiological characteristics and endogenous hormones of soybean.” Plants 11 (2022): 2282.
- Sayed, M. A. E.A. A. Qtl analysis for drought tolerance related to root and shoot traits in barley (Hordeum vulgare l.). Bonn: Universitäts-und Landesbibliothek Bonn, 2011.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).