1. Introduction
Epidemiologic and experimental animal studies have shown that stress can influence tumor growth [
1,
2]; however, the biological mechanisms underlying such effects are not well understood, and the clinical significance of stress on progression of human disease remains controversial. Data from animal models suggest that stress modulates the growth of certain tumors via neuroendocrine regulation of the immune response to tumor cells [
1]. Recent studies indicate that adrenergic fibers from the sympathetic nervous system, acting through stromal β2 and β3 adrenergic receptors, promote tumor cell survival during the initial phases of prostate cancer development [
3,
4]. Innervation patterns of human prostate cancer support this hypothesis: Sympathetic fibers accumulate in normal tissues and infiltrate the tumor edge [
5].
Recent epidemiological data indicate that β2 blocker intake is associated with improved survival of prostate cancer patients [
6] and that hypertension and high blood pressure can increase prostate cancer development [
7], suggesting that the use of beta blockers could benefit to these patients.
The β adrenergic receptors signal through the cAMP/PKA pathway, a pathway known to induce neuroendocrine differentiation (NED) in LNCaP prostate cancer cell lines [
8]. Neuroendocrine (NE) cells are a minor cell population in the epithelial compartment of normal prostate glands; the population of NE-like cells is higher in prostate tumor lesions and correlates with tumor progression, poor prognosis, and the androgen-independent state [
9]. NE-like cells have higher levels of tumoral marker AMACR, SYN, and Bcl-2, which is indicative of resistance to apoptosis, than NE cells [
9]. DLL-3 expression, another marker of NE cells in several tissues, including prostate, [
10,
11] is increased following PKA pathway activation [
12].
Recent studies have shown that an increase in NE cell density occurs before development of benign prostate hyperplasia (BPH) in spontaneously hypertensive rats [
13]. BPH is a non-lethal disease affecting the prostatic transition zone where adenomatous nodules mainly appear. NE cells are distributed around small adenomas and influence the initial growth of BPH in human patients [
14]. Interestingly, about 20% of all prostate cancers originate in transition zone [
15]. Furthermore, AMACR expression is detected within evolving carcinomas in some BPH nodules and in fully developed grade 1 carcinomas [
16]. In most cases, prostatic biopsies used to screen for prostate cancer target the peripheral zone, where most cancers occur, and transition zone cancer is often detected during BPH surgery (10% incidence among all BPH surgeries) [
17].
We speculated that β2 adrenergic receptors influence the development of NE-like cells in BPH and that β2 blockers may reduce NED in this zone. We therefore investigated the relationship between NED and BPH in vitro. BPH-1 prostate cells were challenged with a β adrenergic receptor agonist isoproterenol (ISO) or with the combination of isobutyl-methyl-xanthine (IBMX) and forskolin (FSK) to stimulate the cAMP/PKA pathway. NE-like cell markers were analyzed to detect differentiation. By RT-qPCR and western blot, we showed that these treatments resulted in the expression of NED markers in these cells. Furthermore, we showed that β2 blockers antagonized the effects of β2 adrenergic receptor agonists.
2. Materials and Methods
Cell Culture
BPH-1, RWPE-1, LNCaP, DU-145, and PC-3 cells were purchased from ATCC (Manassas, USA) and used before passage 20. They were maintained in DMEM with Glutamax-I supplemented with 200,000 U/ml penicillin and 100 μg/ml streptomycin (Gibco, Les Ulis, France), and with 10% fetal calf serum (Eurobio Scientific, Les Ulis, France).
Cell Proliferation, Cytotoxicity, and Viability
For proliferation, BPH-1 (400 cells/well), PC-3 (1200 cells/well), DU-145 (1200 cells/well), LNCaP (2000 cells/well), and RWPE-1 (1200 cells/well) cells were seeded in 96-well plates. After 3 days of culture, for all cells except the BPH-1 cells, the medium was replaced with the same culture medium containing the test compounds and Red NucLight (1/1000) for staining of nuclei. Plates were transferred to an Incucyte plate reader (Sartorius, Dourdan, France) for an additional 4 days. The Incucyte software allowed detection and numeration of red nuclei every hour over this 4-day period. Results given here are the values at the end of the 4-day period of acquisition. For BPH-1 cells, cell proliferation was assessed by measuring the cell confluence using the same software. BPH-1 confluence was always less than 80% at the end of the experiments. BPH-1 cell viability was assessed using Trypan Blue staining quantified on a Biorad TC 20 (Bio-Rad, Marnes-la-Coquette, France) after 4 days of treatment with appropriate drugs.
Western Blot and qRT-PCR
IHC and qRT-PCR experiments were performed as earlier described [
18]. Protein samples (30 µg) were electrophoresed then transferred onto nitrocellulose membranes (Bio-Rad), and membranes were incubated with primary antibodies (
Table S1).
RNA (250 ng) was reverse transcribed using PrimeScript™ RT Master Mix #RR036A (Takara Bio Europe SAS, Saint Germain en Laye, France), The cDNAs were then subjected to real-time PCR amplification using gene-specific primers (0.5 µM final concentration) purchased from Sigma-Aldrich (Saint-Quentin-Fallavier, France) (
Table S2).
PPIA, which encodes Cyclophilin A, was analyzed as a control in each reaction. Experiments were performed in duplicate, and the results are expressed as means ± S.D.
Statistical Analysis
A paired-samples t-test was used for statistical analyses of differences in cell proliferation. As the Incucyte technique allowed high-throughput screening, each set of data was compared to its own control on the same plate and overall differences were tested using the statistical test. As control values differed from one plate to another (Supp.
Figure S1A), we normalized the data to controls on each plate. Results are expressed as means ± S.E.M. A one-way ANOVA was used for statistical analysis of western blot data with *, ** and *** corresponding to p values <0.05, <0.01 and <0.001 respectively.
Compounds
IBMX, FSK, ISO, propranolol, norepinephrine, epinephrine, and phenylephrine were purchased from Sigma-Aldrich, carvedilol and atenolol were purchases from Cayman Chemicals (Interchim, Montluçon, France), mirabegron was purchased from Tocris (BioTechne SA, Noyal Châtillon sur Seiche, France), and NucLight was purchased from Sartorius (Dourdan, France).
3. Results
Stimulation of the cAMP/PKA Pathway Inhibits Proliferation of Prostate Cancer Cells
As the aim of this study was to investigate whether stimulation of the cAMP/PKA pathway could induce NED of BPH-1 cells, we first evaluated whether the combined effects of IBMX and FSK reduced proliferation of these cells; this combination was previously been shown to do so in other cell lines [
14]. DMSO, which was used as a vehicle, did not affect the rate of cell proliferation compared to distilled water. The mean proliferation rates corresponded to a 12.0 ± 1.0 and 12.0 ± 0.9 fold increase form initial values (n=30) without and with 0.02% DMSO, respectively (Supp.
Figure S1A, left panel), corresponding to 3 to 4 cell cycles over a 96-h period of time, with a range in proliferation rate of approximately 4 to 28 fold increase in number of cells treated or not with DMSO (Supp.
Figure S1A, right panel). Normalization to cells not cultured with DMSO showed a range from 0.8 to 1.2 units (Supp.
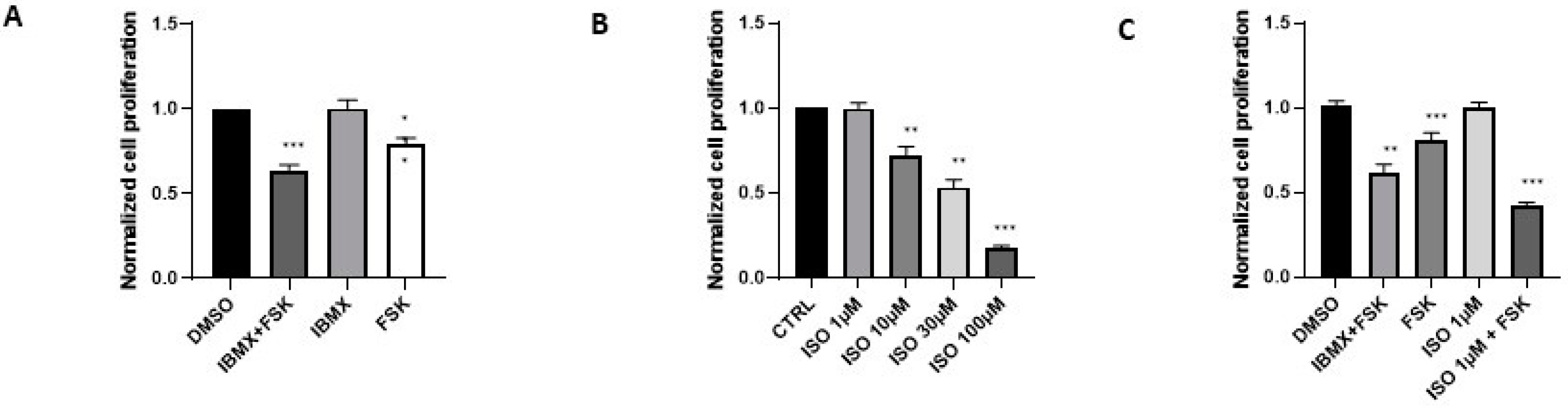
Figure S1B), and drug effects were therefore compared to proliferation rates in their respective control conditions throughout this study. The combined effects of 100 µM IBMX and 10 µM FSK reduced cell proliferation by 40%, whereas IBMX alone no effect and FSK reduced proliferation by about 20% (
Figure 1A). The combination of IBMX and FSK also reduced proliferation of RWPE-1, LNCaP, DU-145, and PC-3 cells, other prostate lines, with comparable efficiency (Supp.
Figure 1C). These results indicate that blocking cAMP degradation with IBMX was less effective than adenylate cyclase activation with FSK in halting cell proliferation but that the combination was more effective than either drug alone. Importantly, the combination had no effect on cell viability (Supp.
Figure S2)
Agonists of β Adrenergic Receptors Reduce Prostate Cancer Cell Proliferation
IBMX and FSK mimic the effects of β2 adrenergic receptor agonists such as ISO. Therefore, we tested the effect of ISO on BPH-1 cell proliferation. ISO treatment reduced BPH-1 cell proliferation in a dose-dependent manner (
Figure 1B). The large decrease in cell proliferation observed at 100 µM ISO was associated with a decrease in cell viability, but lower concentrations of ISO did not kill cells (Supp.
Figure S2). The ISO-induced decrease in BHP-1 cell proliferation was also observed in other prostate cell lines (Supp.
Figure S3). We next tested the combined effects of a low concentration of ISO and FSK. ISO at 1 µM, a concentration that did not inhibit cell proliferation, combined with 10 µM FSK induced a 60% reduction in cell proliferation, a larger decrease than that observed in the presence of the combination of 100 µM IBMX and 10 µM FSK (
Figure 1C).
Norepinephrine and epinephrine are natural neurotransmitters released from nerve terminals that do not discriminate between α and β adrenergic receptors. Both norepinephrine and epinephrine inhibited BPH-1 proliferation (Supp.
Figure S4). As the α1 adrenergic receptors agonist phenylephrine had no effect on cell proliferation (Supp.
Figure S4), norepinephrine and epinephrine appear to act exclusively through β2 adrenergic receptors in the prostate cancer cells. Mirabegron, a β3 adrenergic receptor agonist, caused a small reduction in BPH-1 cell proliferation at 30 µM (Supp.
Figure S4). Some studies have shown that
ADRB3 is expressed in prostate tissue [
26], but we cannot rule out an effect of mirabegron through β2 adrenergic receptors at this concentration, and this will need further investigation. In summary, these experiments showed here that reduction in BPH-1 cell proliferation is associated with β2 adrenergic receptor activation.
Beta Blockers Reduce Proliferation of BPH-1 Cells
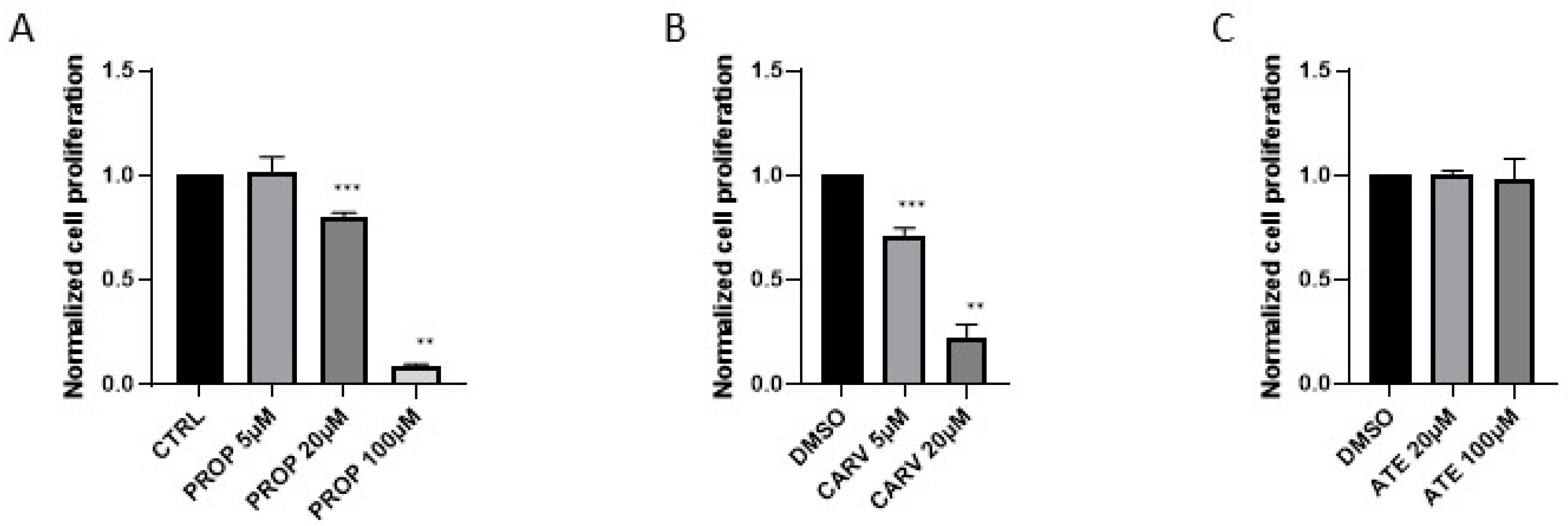
Next we tested whether beta blockers inhibit cell proliferation independently of β adrenergic receptor stimulation by ISO. To do so, we used two different β2 blockers and one β1 blocker. Atenolol, a β1 blocker, had no effect on BPH-1 cell proliferation at concentrations up to 100 µM, but both β2 blockers, propranolol and carvedilol, reduced cell proliferation at 20 and 5 µM, respectively (
Figure 2). Increasing the concentration of these two β2 blockers further inhibited cell proliferation via a mechanism that could involve mitochondrial bioenergetics as suggested based on studies of medulloblastoma [
27]. Tests in three other prostate cell lines confirmed our observations (Supp.
Figure S5). Thus, β2 blockers affect prostate cancer cell proliferation independently of β2 adrenergic receptor activation as shown previously in gastric adenocarcinoma, colon adenocarcinoma, and non-small cell lung cancer [
27]. However, the mechanisms of action of these blockers in BPH-1 cells and other prostate cell lines remain unknown.
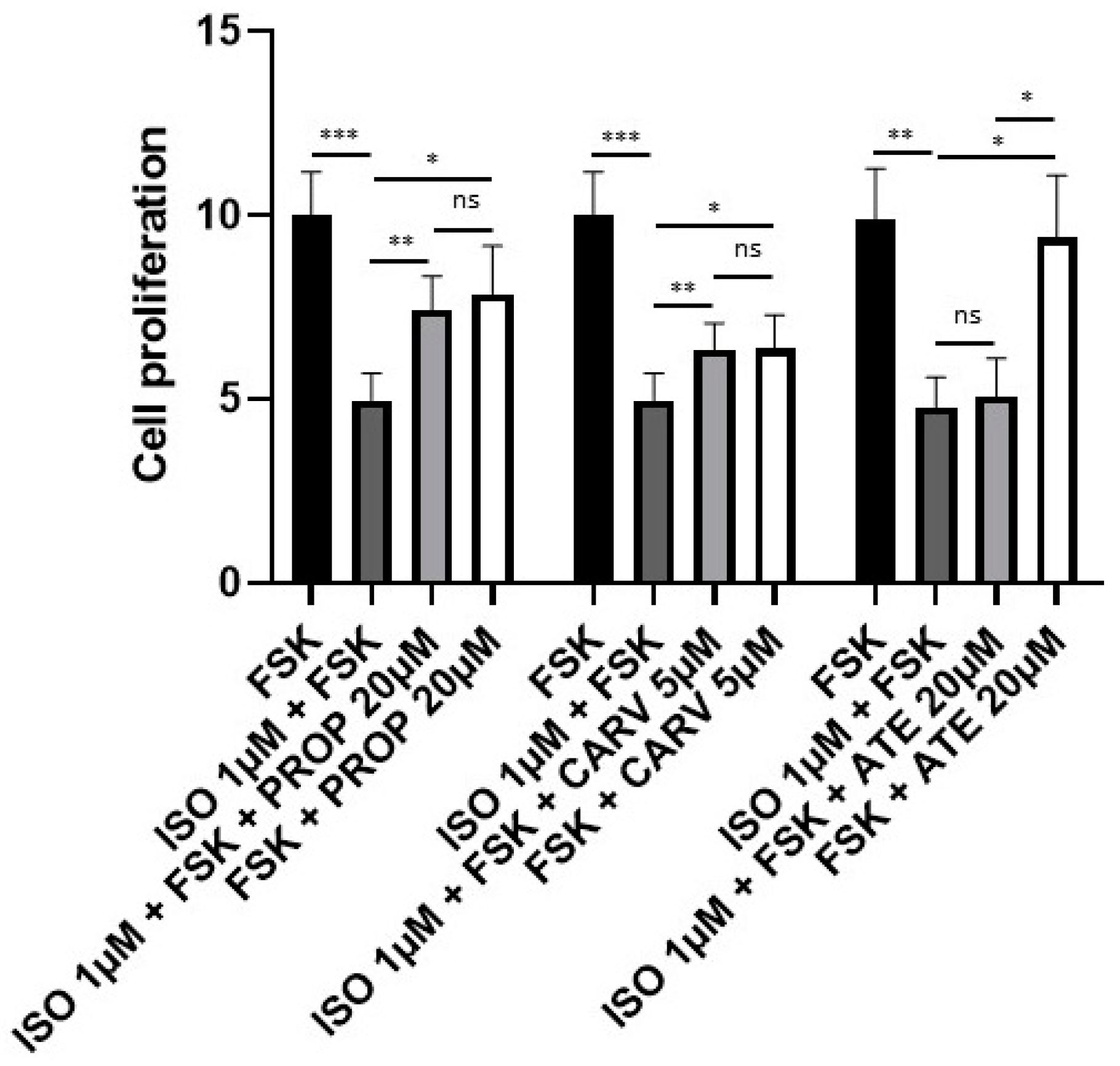
Next, we tested whether these beta blockers could reverse the inhibitory effect of the combination of ISO and FSK on proliferation of BPH-1 cells. As shown before, 1 µM ISO combined with 10 µM FSK induced a 60% decrease in cell proliferation. This was totally reversed in the presence of 5 µM carvedilol and almost completely reversed in the presence of 20 µM propranolol (
Figure 3). Thus, under conditions that result in activation of the cAMP/PKA pathway, the beta blockers maintain normal levels of proliferation. Atenolol had no effect on cell proliferation inducted by ISO and FSK (
Figure 3). This confirmed that β2 adrenergic receptors are responsible for inhibition of proliferation of BHP-1 cells.
Activation of β Adrenergic Receptors Induces Neuroendocrine Differentiation of Prostate Cancer Cells
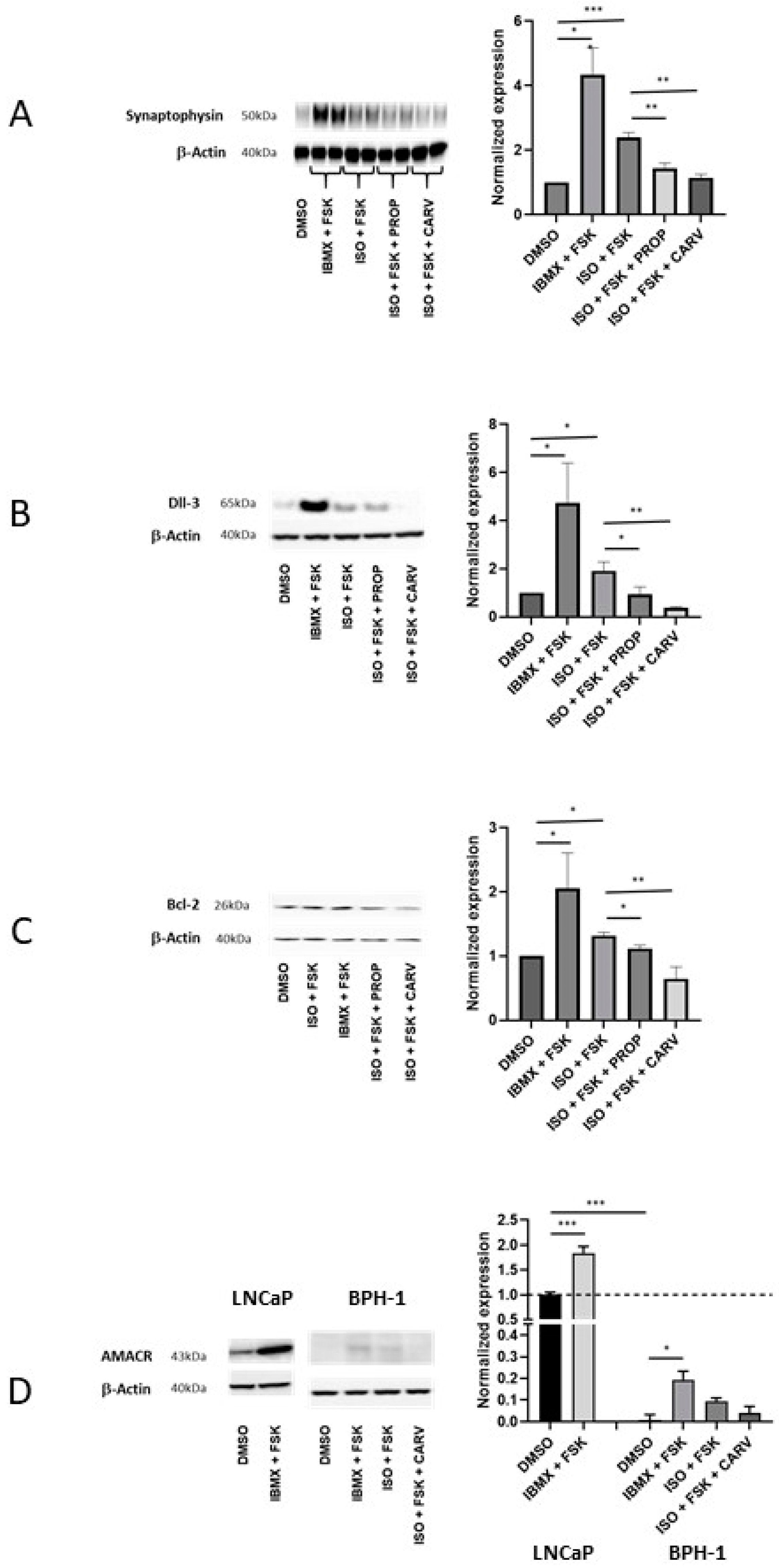
Markers of NED include SYN, Dll-3, Bcl-2, and AMACR. We therefore evaluated how activation of β adrenergic receptors and beta blockers affected the expression of these proteins. BPH-1 cells were treated for 10 days with the combination of IBMX and FSK or the combination of ISO and FSK in the presence or absence of β2 blockers. SYN, Dll-3, and Bcl-2 proteins were significantly increased in the presence of the combination of IBMX and FSK and in the presence of the combination of ISO and FSK (
Figure 4). The β2 blockers 20 µM propranolol and 5 µM carvedilol both decreased the effects of the ISO and FSK combination with a clearer effect for carvedilol, the more active blocker of cell proliferation, than propranolol. Another feature of NED differentiation is AMACR expression. AMACR was barely detectable in BHP-1 cells; it is expressed at much higher levels in LNCaP cells (
Figure 4D).
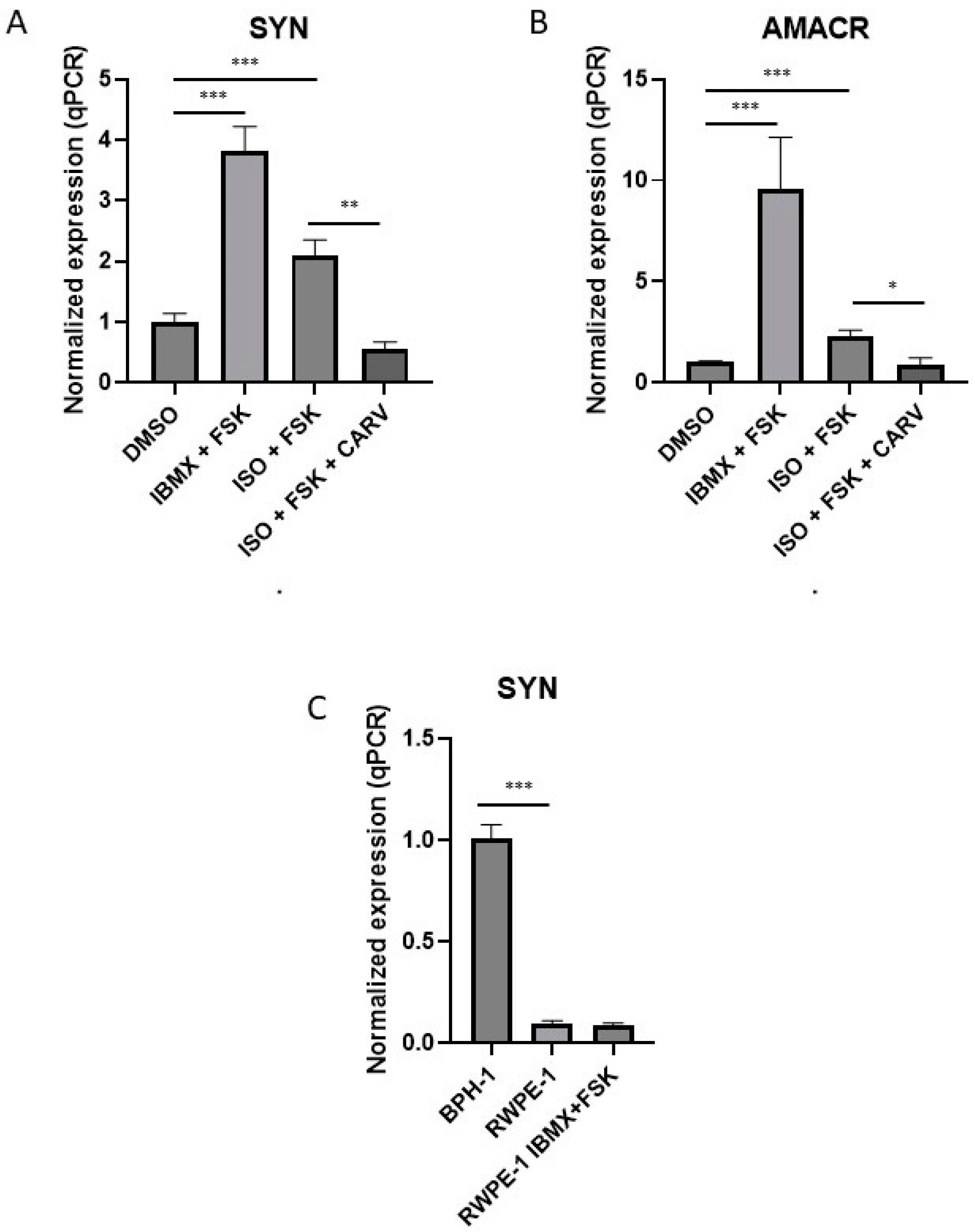
By western blot, AMACR levels in BHP-1 cells were increased by treatment with IBMX and FSK and with ISO and FSK, but differences were not significant in the presence and absence of carvedilol. qPCR experiments, however, showed that the mRNAs encoding AMACR and SYN were increased in the presence of the combination of IBMX and FSK and by the combination of ISO and FSK and that the latter effect was reversed by 5 µM carvedilol (
Figure 5A and B). Interestingly,
SYN mRNA expression was not increased in RWPE-1 cells under IBMX and FSK treatment, suggesting that NED does not occur in normal prostate cells under these conditions (
Figure 5C).
SYN mRNA expression in RWPE-1 was about 90% lower than in BPH-1 cells (
Figure 5C). Overall, these data showed that BPH-1 cells are more susceptible than normal non-tumoral cells such as RWPE-1 to differentiation into NE-like cells upon β2 adrenergic receptor stimulation.
4. Discussion
Here we provide the first evidence that NE-like cells can arise from a BPH model cell line. Although the presence of NE-like cells correlates with poor prognosis for prostate cancer patients, these cells are present not only during the later phase of this disease, as described before [
28], but may also precede the occurrence of BPH in hypertensive patients [
21]. Previous work demonstrated a link between β2 adrenergic receptor expression and activity and the presence of NE-like cells during the late phase of prostate cancer [
29], and our data suggest that β2 adrenergic receptor activation results in NED in the BPH transition zone. It is known that these β2 adrenergic receptors are present in prostate tissues, and their expression is correlated to the grade of prostate cancer [
29]. Indirect evidence of their activity comes from the well-known BPH treatment using α adrenergic receptor blockers [
30]. Considered together, these data suggest that there is a constitutive release of neurotransmitters in the prostate transition zone.
Blockers of β1 adrenergic receptors are preferentially used to treat hypertension, and their lack of effect in our study suggests that the use of β2 blockers to prevent NE-like cell appearance should be reconsidered. It is worth noting that the use of β2 blockers is beneficial in breast and lung cancers [
31,
32]. Βeta blocker concentration must be adjusted to prevent the cytotoxic effects observed with propranolol and carvedilol in BPH-1 cells, which were previously described in other tissues [
27]. Although β2 blocker side effects are well documented [
33], drug repurposing suggests that their use could benefit to BPH patients with adenocarcinoma as well as patients undergoing androgen deprivation therapy [
34].
It has recently become clear that stress responses, which occur during the immediate post-operative period and which result in the release of catecholamines such as epinephrine and norepinephrine, could mediate many of the adverse effects of surgery through their direct effects on the malignant tissue and through modulation of host physiology and immune competence [
1,
35]. Even if BPH and cancer are two separate prostate diseases, the effects of β2 adrenergic receptor agonists on BPH-1 cells shown in our study suggest that a systematic search for NE-like cells in the transition zone in these patients after BPH surgery or radical prostatectomy in a large cohort should be conducted. If these cells are present in a large subset of patients, β2 blocker use should be recommended.
The two neurotransmitters epinephrine and norepinephrine have similar affinities for both α and β adrenergic receptors, and α blockers such as tamsulosin and silodosin are used to treat BPH as they induce smooth muscle relaxation [
30]. BPH-1 cell proliferation was not affected by phenylephrine, a specific α1 adrenergic receptor agonist, suggesting that development of NE-like cells results specifically from activation of β2 adrenergic receptors.
Previous transcriptome profiling showed clear differences between normal and BPH tissues [
36]. As activating the cAMP/PKA pathway in RWPE-1 cells, a normal prostate tissue line, did not result in the activation of NE-like markers in our hands, we speculate that BPH tissues differ from healthy tissues in their response to β2 adrenergic receptor stimulation. The mechanism by which LNCaP and BPH-1 cells are transformed into NE-like cells is not fully understood, and it will be of interest to determine why RWPE-1 and BPH-1 cells respond differently to β2 adrenergic receptor agonists. AMACR expression was detected by qPCR and western blot in BPH-1 cells treated with β2 adrenergic receptor agonists and cAMP/PKA pathway activators for 10 days. The low level of AMACR expression in BPH-1 cells compared to LNaP cells suggests that additional triggers or a longer duration of stimulation may be needed to fully transform BPH-1 cells into NE-like cells. Very low levels of AMACR protein expression were observed in previous studies of RWPE-1 and BPH-1 cells [
37].
CREB is a relay for the cAMP/PKA pathway that triggers NED [
38]. Androgen deprivation therapy and radiotherapy induce NED via the CREB pathway [
39,
40,
41]. Enzalutamide increases NED in LNCaP cells [
42], and clinical studies have demonstrated that this drug increases the frequency of NE-like cells leading to poorer outcome for men with prostate cancer [
43]. In contrast, the use of antihypertensive medication significantly increased survival of patients with metastatic castration-resistant prostate cancer [
44]. NED is associated to a decrease in androgen receptor expression [
15], and as all these treatments as well as stress leads to an increase in NE-like cells, this may explain why late prostate cancer becomes insensitive to androgen deprivation therapy [
15,
45,
46]. For patients with metastatic castration-resistant prostate cancer treated with abiraterone or docetaxel, NED and the proportion of NE-like cells were critical predictive factors, so NED might serve as a biomarker to predict outcomes and to guide treatment decisions [
47].
5. Conclusions
Stress is positively correlated with cancer initiation, progression, and metastasis in several types of cancer [
48,
49,
50]. Our data indicate that stress neurotransmitters such as epinephrine and norepinephrine trigger NED even in cells that serve as a model for BHP. This mechanism, which involve cAMP/PKA and CREB pathways, may underly stress-mediated induction of other types of cancer. Combining use of enzalutamide (and other drugs used to treat prostate cancer) and β2 blockers may reduce the appearance of NE-like cells, which are associated with poor outcome in this disease [
51].
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org, Figure S1: Combination treatment with IBMX and FSK reduces BPH-1 cell proliferation. (A) Left: Mean increase in BPH-1 cells after 96h cultured without (CTRL) and with 0.02% DMSO (n=30). Right: Plot of BPH-1 cell number increase after 96h revealing variability (range 4 to 28). (B) Left: Plot of normalized DMSO values to mean distilled water values versus time. Right: Plot of normalized control values revealing variability (range 0.78 to 1.24). (C) Left: Proliferation of RWPE-1, BPH-1, LNCaP, DU-145, and PC-3 cells at 96 h in the presence of IBMX and FSK normalized to proliferation in DMSO (n=3, 23, 16, 8, and 8, respectively). Right: Numbers of RWPE-1 cells (log scale) treated with DMSO or the combination of IBMX and FSK versus time (n=4).; Figure S2: β-adrenergic receptor agonists have little effect on viability of BPH-1 cells. Cell viability was assessed after 4 days in the presence of the IBMX and FSK (I+F), ISO), propranolol (PROP), or carvedilol (CARV) (n=6 to 8 for treatments and n=32 for untreated control cells). Figure S3: The β-adrenergic receptor agonist isoproterenol reduces proliferation of prostate cancer lines. Normalized cell proliferation was assessed as a function of concentration of ISO for RWPE-1 (n=3-4), LNCaP (n=6-10), and PC-3 (n=4-5) prostate cell lines. Figure S4: Activation of the β2-adrenergic receptor blocks BPH-1 cell proliferation. Cell proliferation measured HOW in the presence of epinephrine (EPI, n=4-7), norepinephrine (NOR, n=7-8), phenylephrine (PHE, n=3-4), and mirabegron (MBG, n=7). Figure S5: Beta blockers inhibit prostate cancer cell proliferation. Normalized cell proliferation as measured HOW in RWPE-1 (n=3-4), LNCaP (n=4-8), and PC-3 (n=3-4) prostate cell lines in the presence of β2 blockers 20 µM propranolol (PROP) and 5 µM carvedilol (CARV) and 20 µM atenolol (ATE). Table S1: List of antibodies used with references, clone numbers, suppliers and dilutions. Table S2: List of primers for real-time PCR amplification using gene-specific primers (0.5 µM final concentration) purchased from Sigma-Aldrich (Saint-Quentin-Fallavier, France). PPIA, which encodes Cyclophilin A, was analyzed as a control in each reaction.
Author Contributions
Conceptualization, T.C.; methodology, T.C., N.P., L.N.G.C., C.L. and A.S.A.; validation, T.C., N.P. and A.S.A.; formal analysis, T.C.; investigation, T.C.; resources, E.P., E.P.L. and W.Z.; data curation, T.C., N.P. and A.S.A.; writing—original draft preparation, T.C.; writing—review and editing, T.C., A.S.A, M.B., V.G. and J.A.; supervision, T.C.; project administration, T.C.; funding acquisition, T.C.. All authors have read and agreed to the published version of the manuscript. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research was funded by Association pour la recherche sur les tumeurs de la prostate (ARTP).
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
Not applicable
Acknowledgments
The authors are grateful to Jacqueline R. Wyatt for editing the manuscript and Natascha Pigat (Institut Necker Enfant Malades) for help with the Figures.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Antoni, M.H.; Lutgendorf, S.K.; Cole, S.W.; Dhabhar, F.S.; Sephton, S.E.; McDonald, P.G.; Stefanek, M.; Sood, A.K. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 2006, 6, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eliyahu, S. The promotion of tumor metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain Behav Immun 2003, 17 Suppl 1, S27–36. [Google Scholar] [CrossRef]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 2006, 12, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Bautista, M.; Shrestha, S.; Elhasasna, H.; Chaphekar, T.; Vizeacoumar, F.S.; Krishnan, A. Sympathetic signaling facilitates progression of neuroendocrine prostate cancer. Cell Death Discov 2021, 7, 364. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- March, B.; Faulkner, S.; Jobling, P.; Steigler, A.; Blatt, A.; Denham, J.; Hondermarck, H. Tumour innervation and neurosignalling in prostate cancer. Nat Rev Urol 2020, 17, 119–130. [Google Scholar] [CrossRef] [PubMed]
- McVary, K.T.; McKenna, K.E.; Lee, C. Prostate innervation. Prostate Suppl 1998, 8, 2–13. [Google Scholar] [CrossRef]
- Cruickshank, J.M. The Role of Beta-Blockers in the Treatment of Hypertension. Adv Exp Med Biol 2017, 956, 149–166. [Google Scholar] [CrossRef]
- Grytli, H.H.; Fagerland, M.W.; Fossa, S.D.; Tasken, K.A. Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol 2014, 65, 635–641. [Google Scholar] [CrossRef]
- Grytli, H.H.; Fagerland, M.W.; Fossa, S.D.; Tasken, K.A.; Haheim, L.L. Use of beta-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate 2013, 73, 250–260. [Google Scholar] [CrossRef]
- Liang, Z.; Xie, B.; Li, J.; Wang, X.; Wang, S.; Meng, S.; Ji, A.; Zhu, Y.; Xu, X.; Zheng, X.; et al. Hypertension and risk of prostate cancer: a systematic review and meta-analysis. Sci Rep 2016, 6, 31358. [Google Scholar] [CrossRef] [PubMed]
- Loughlin, K.R. Calcium channel blockers and prostate cancer. Urol Oncol 2014, 32, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Braadland, P.R.; Urbanucci, A. Chromatin reprogramming as an adaptation mechanism in advanced prostate cancer. Endocr Relat Cancer 2019, 26, R211–R235. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.E.; Deeble, P.D.; Lakhani, S.; Parsons, S.J. Acquisition of neuroendocrine characteristics by prostate tumor cells is reversible: implications for prostate cancer progression. Cancer Res 1999, 59, 3821–3830. [Google Scholar] [PubMed]
- Yuan, T.C.; Veeramani, S.; Lin, M.F. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer 2007, 14, 531–547. [Google Scholar] [CrossRef]
- Kemble, J.; Kwon, E.D.; Karnes, R.J. Addressing the need for more therapeutic options in neuroendocrine prostate cancer. Expert Rev Anticancer Ther 2023, 23, 177–185. [Google Scholar] [CrossRef]
- Yao, J.; Bergsland, E.; Aggarwal, R.; Aparicio, A.; Beltran, H.; Crabtree, J.S.; Hann, C.L.; Ibrahim, T.; Byers, L.A.; Sasano, H.; et al. DLL3 as an Emerging Target for the Treatment of Neuroendocrine Neoplasms. Oncologist 2022, 27, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Molloy, M.E.; Aaron, W.H.; Barath, M.; Bush, M.C.; Callihan, E.C.; Carlin, K.; Cremin, M.; Evans, T.; Gamez Guerrero, M.; Hemmati, G.; et al. HPN328, a Trispecific T Cell-activating Protein Construct Targeting DLL3-Expressing Solid Tumors. Mol Cancer Ther 2024. [Google Scholar] [CrossRef]
- Puca, L.; Gavyert, K.; Sailer, V.; Conteduca, V.; Dardenne, E.; Sigouros, M.; Isse, K.; Kearney, M.; Vosoughi, A.; Fernandez, L.; et al. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci Transl Med 2019, 11. [Google Scholar] [CrossRef]
- Coles, G.L.; Cristea, S.; Webber, J.T.; Levin, R.S.; Moss, S.M.; He, A.; Sangodkar, J.; Hwang, Y.C.; Arand, J.; Drainas, A.P.; et al. Unbiased Proteomic Profiling Uncovers a Targetable GNAS/PKA/PP2A Axis in Small Cell Lung Cancer Stem Cells. Cancer Cell 2020, 38, 129–143. [Google Scholar] [CrossRef]
- Kyoda, Y.; Ichihara, K.; Hashimoto, K.; Kobayashi, K.; Fukuta, F.; Masumori, N. Sustained density of neuroendocrine cells with aging precedes development of prostatic hyperplasia in spontaneously hypertensive rats. BMC Urol 2019, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Kyoda, Y.; Ichihara, K.; Hashimoto, K.; Kobayashi, K.; Fukuta, F.; Masumori, N. Distribution of Neuroendocrine Cells in the Transition Zone of the Prostate. Adv Urol 2017, 2017, 8541697. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Thomas, I.C.; Nolley, R.; Ferrari, M.; Brooks, J.D.; Leppert, J.T. Biologic differences between peripheral and transition zone prostate cancer. Prostate 2015, 75, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Leav, I.; McNeal, J.E.; Ho, S.M.; Jiang, Z. Alpha-methylacyl-CoA racemase (P504S) expression in evolving carcinomas within benign prostatic hyperplasia and in cancers of the transition zone. Hum Pathol 2003, 34, 228–233. [Google Scholar] [CrossRef]
- Coman, R.; Anract, J.; Pinar, U.; Sibony, M.; Peyromaure, M.; Delongchamps, B. Is the systematic histological analysis of benign prostatic hyperplasia surgical specimen always necessary? Int Urol Nephrol 2022, 54, 1485–1489. [Google Scholar] [CrossRef]
- Berkowitz, D.E.; Nardone, N.A.; Smiley, R.M.; Price, D.T.; Kreutter, D.K.; Fremeau, R.T.; Schwinn, D.A. Distribution of beta 3-adrenoceptor mRNA in human tissues. Eur J Pharmacol 1995, 289, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Talbot, J.; Piris, P.; Grand, M.L.; Montero, M.P.; Matteudi, M.; Agavnian-Couquiaud, E.; Appay, R.; Keime, C.; Williamson, D.; et al. Beta-blockers disrupt mitochondrial bioenergetics and increase radiotherapy efficacy independently of beta-adrenergic receptors in medulloblastoma. EBioMedicine 2022, 82, 104149. [Google Scholar] [CrossRef]
- Puca, L.; Vlachostergios, P.J.; Beltran, H. Neuroendocrine Differentiation in Prostate Cancer: Emerging Biology, Models, and Therapies. Cold Spring Harb Perspect Med 2019, 9. [Google Scholar] [CrossRef]
- Braadland, P.R.; Ramberg, H.; Grytli, H.H.; Urbanucci, A.; Nielsen, H.K.; Guldvik, I.J.; Engedal, A.; Ketola, K.; Wang, W.; Svindland, A.; et al. The beta(2)-Adrenergic Receptor Is a Molecular Switch for Neuroendocrine Transdifferentiation of Prostate Cancer Cells. Mol Cancer Res 2019, 17, 2154–2168. [Google Scholar] [CrossRef]
- Lepor, H.; Kazzazi, A.; Djavan, B. alpha-Blockers for benign prostatic hyperplasia: the new era. Curr Opin Urol 2012, 22, 7–15. [Google Scholar] [CrossRef]
- Melhem-Bertrandt, A.; Chavez-Macgregor, M.; Lei, X.; Brown, E.N.; Lee, R.T.; Meric-Bernstam, F.; Sood, A.K.; Conzen, S.D.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 2011, 29, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M.; Cole, B. Regulation of cell proliferation by beta-adrenergic receptors in a human lung adenocarcinoma cell line. Carcinogenesis 1989, 10, 1753–1755. [Google Scholar] [CrossRef] [PubMed]
- Colunga, K.N.; Reinert, J.P. A Review of Beta-Blocker Toxicity and Management Strategies. Sr Care Pharm 2020, 35, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, F.; Sukhatme, V.P.; Bouche, G. Drug repurposing in oncology--patient and health systems opportunities. Nat Rev Clin Oncol 2015, 12, 732–742. [Google Scholar] [CrossRef]
- Glasner, A.; Avraham, R.; Rosenne, E.; Benish, M.; Zmora, O.; Shemer, S.; Meiboom, H.; Ben-Eliyahu, S. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol 2010, 184, 2449–2457. [Google Scholar] [CrossRef]
- Liu, D.; Shoag, J.E.; Poliak, D.; Goueli, R.S.; Ravikumar, V.; Redmond, D.; Vosoughi, A.; Fontugne, J.; Pan, H.; Lee, D.; et al. Integrative multiplatform molecular profiling of benign prostatic hyperplasia identifies distinct subtypes. Nat Commun 2020, 11, 1987. [Google Scholar] [CrossRef]
- Festuccia, C.; Mancini, A.; Gravina, G.L.; Scarsella, L.; Llorens, S.; Alonso, G.L.; Tatone, C.; Di Cesare, E.; Jannini, E.A.; Lenzi, A.; et al. Antitumor effects of saffron-derived carotenoids in prostate cancer cell models. Biomed Res Int 2014, 2014, 135048. [Google Scholar] [CrossRef]
- Delgado-Gonzalez, E.; Sanchez-Tusie, A.A.; Morales, G.; Aceves, C.; Anguiano, B. Triiodothyronine Attenuates Prostate Cancer Progression Mediated by beta-Adrenergic Stimulation. Mol Med 2016, 22, 1–11. [Google Scholar] [CrossRef]
- Hu, C.D.; Choo, R.; Huang, J. Neuroendocrine differentiation in prostate cancer: a mechanism of radioresistance and treatment failure. Front Oncol 2015, 5, 90. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, Z.; Kimball, H.; Qu, F.; Berlind, K.; Stopsack, K.H.; Lee, G.M.; Choueiri, T.K.; Kantoff, P.W. Abiraterone Acetate Induces CREB1 Phosphorylation and Enhances the Function of the CBP-p300 Complex, Leading to Resistance in Prostate Cancer Cells. Clin Cancer Res 2021, 27, 2087–2099. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, D.; Zhou, T.; Song, H.; Hulsurkar, M.; Su, N.; Liu, Y.; Wang, Z.; Shao, L.; Ittmann, M.; et al. Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB-EZH2-TSP1 pathway in prostate cancers. Nat Commun 2018, 9, 4080. [Google Scholar] [CrossRef] [PubMed]
- Bery, F.; Cancel, M.; Gueguinou, M.; Potier-Cartereau, M.; Vandier, C.; Chantome, A.; Guibon, R.; Bruyere, F.; Fromont, G.; Maheo, K. Zeb1 and SK3 Channel Are Up-Regulated in Castration-Resistant Prostate Cancer and Promote Neuroendocrine Differentiation. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Mori, J.O.; Shafran, J.S.; Stojanova, M.; Katz, M.H.; Gignac, G.A.; Wisco, J.J.; Heaphy, C.M.; Denis, G.V. Novel forms of prostate cancer chemoresistance to successful androgen deprivation therapy demand new approaches: Rationale for targeting BET proteins. Prostate 2022, 82, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Fiala, O.; Hosek, P.; Korunkova, H.; Hora, M.; Kolar, J.; Sorejs, O.; Topolcan, O.; Filipovsky, J.; Liska, V.; Santoni, M.; et al. Concomitant antihypertensive medication and outcome of patients with metastatic castration-resistant prostate cancer receiving enzalutamide or abiraterone acetate. Cancer Med 2024, 13. [Google Scholar] [CrossRef]
- Cacciatore, A.; Albino, D.; Catapano, C.V.; Carbone, G.M. Preclinical Models of Neuroendocrine Prostate Cancer. Curr Protoc 2023, 3, e742. [Google Scholar] [CrossRef]
- de Kouchkovsky, I.; Chan, E.; Schloss, C.; Poehlein, C.; Aggarwal, R. Diagnosis and management of neuroendocrine prostate cancer. Prostate 2024, 84, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhao, J.; Zhao, F.; Liu, H.; Yin, W.; Zhu, S.; Nie, L.; Sun, G.; Zheng, L.; Liu, Z.; et al. Neuroendocrine differentiation predicts the therapeutic efficacy of abiraterone and docetaxel as first-line therapy in metastatic castration-resistant prostate cancer. J Cancer Res Clin Oncol 2023, 149, 7247–7258. [Google Scholar] [CrossRef]
- Abate, M.; Citro, M.; Caputo, M.; Pisanti, S.; Martinelli, R. Psychological Stress and Cancer: New Evidence of An Increasingly Strong Link. Transl Med UniSa 2020, 23, 53–57. [Google Scholar] [CrossRef]
- Oh, H.M.; Son, C.G. The Risk of Psychological Stress on Cancer Recurrence: A Systematic Review. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Cole, S.W.; Sood, A.K. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res 2012, 18, 1201–1206. [Google Scholar] [CrossRef]
- Sivanesan, S.; Tasken, K.A.; Grytli, H.H. Association of beta-Blocker Use at Time of Radical Prostatectomy With Rate of Treatment for Prostate Cancer Recurrence. JAMA Netw Open 2022, 5, e2145230. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).