Submitted:
09 September 2024
Posted:
11 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Cell Viability Methods
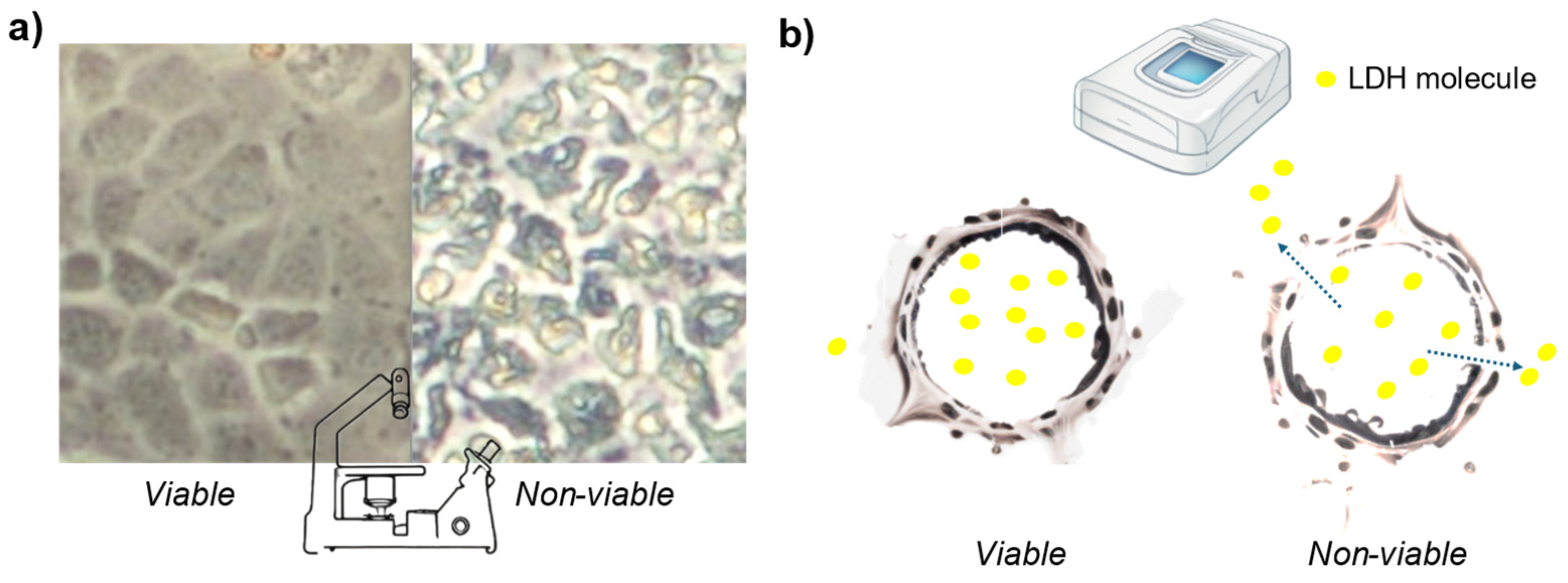
2.1. Structural Cell Damage (Non-Invasive)
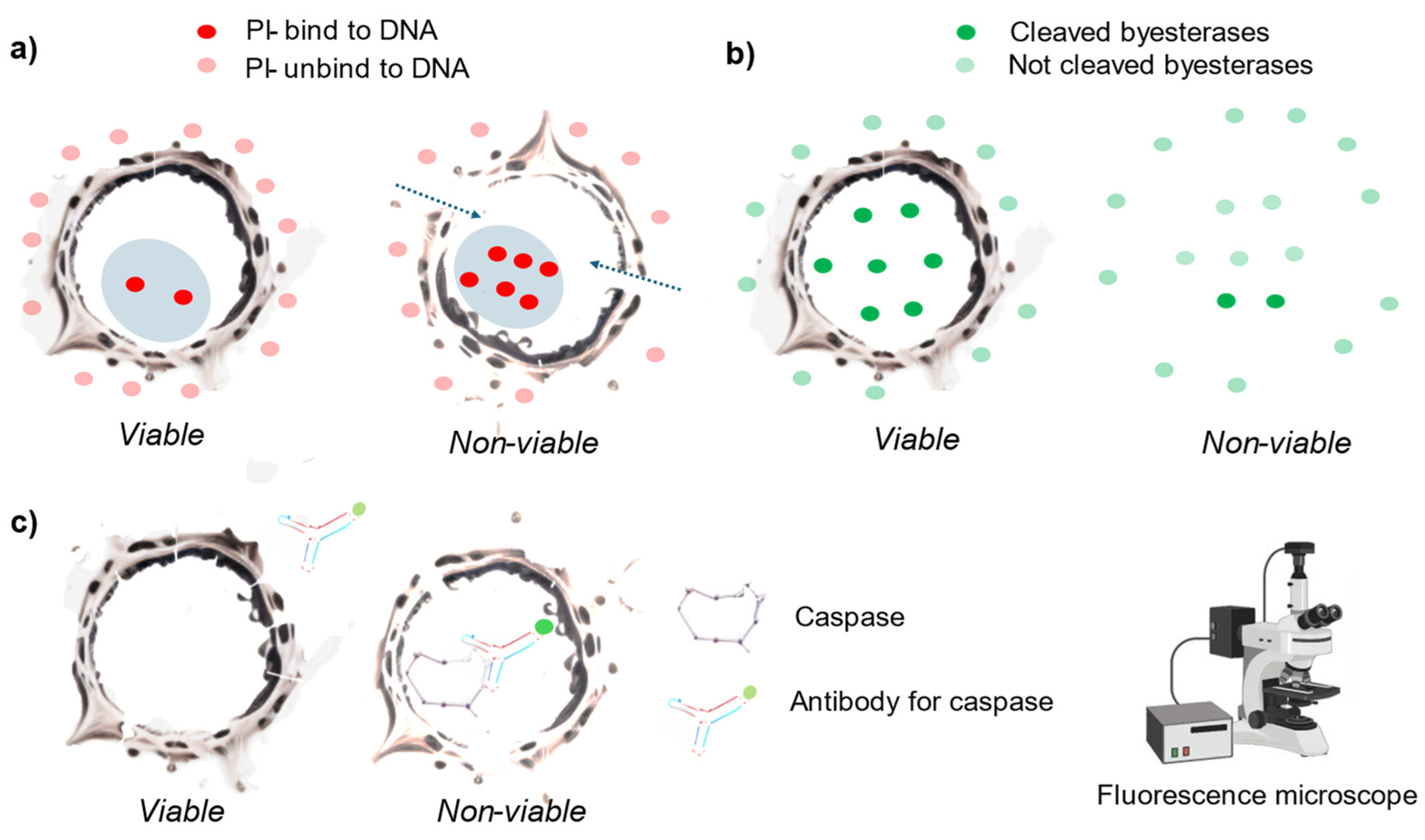
2.2. Structural Cell Damage (Invasive)
2.3. Cell Growth
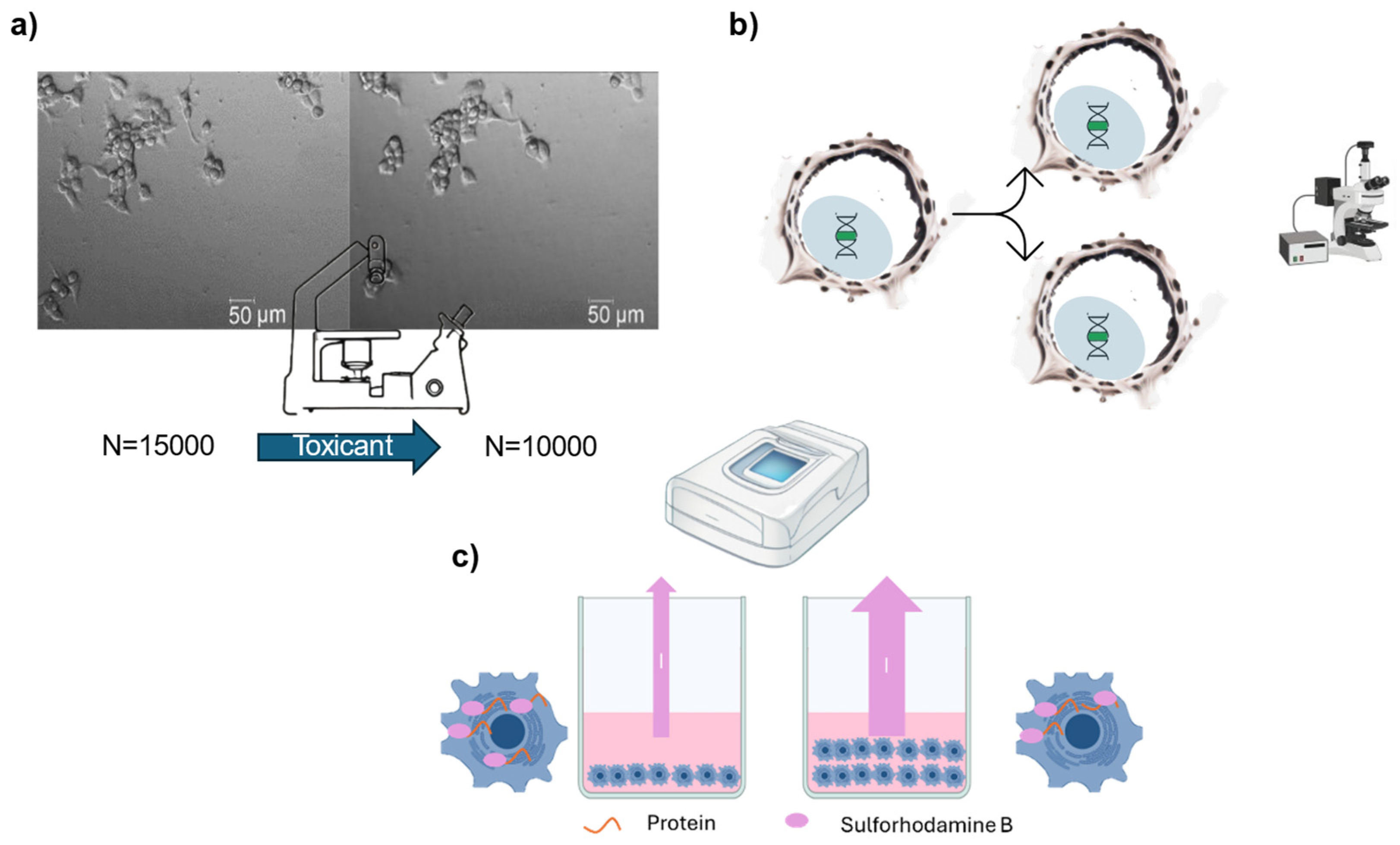
2.4. Cellular Metabolism
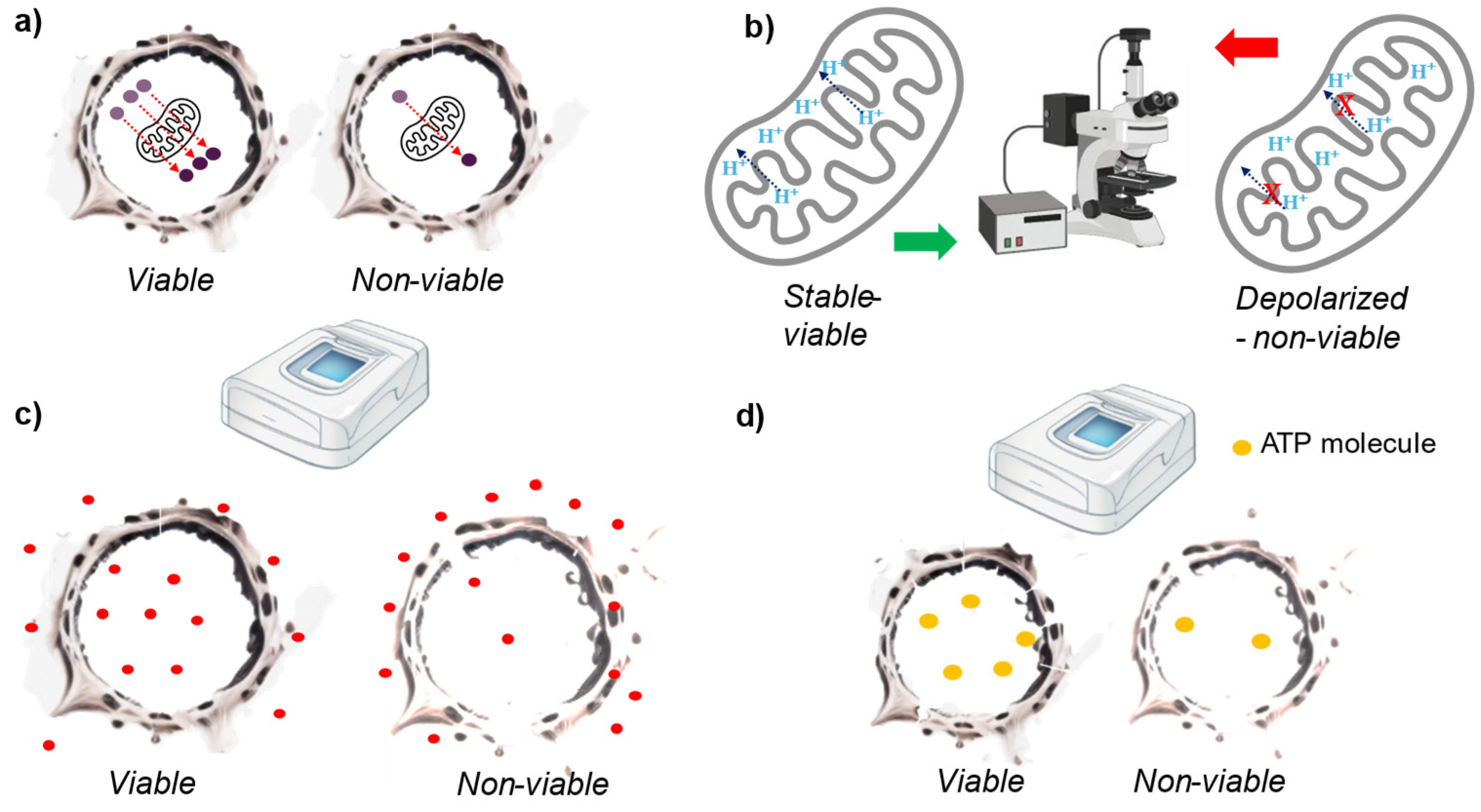
2.5. Membrane Potential
2.5.1. The Membrane Potential Cell Viability Assay (MPCVA)
2.5.2. The DD Cell-Tox Method
3. Discussion
4. Conclusions
5. Future Directions
Conflicts of Interest
References
- Adan, A.; Kiraz, Y.; Baran, Y. , Cell Proliferation and Cytotoxicity Assays. Curr Pharm Biotechnol 2016, 17, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Pognan, F.; Beilmann, M.; Boonen, H. C. M.; Czich, A.; Dear, G.; Hewitt, P.; Mow, T.; Oinonen, T.; Roth, A.; Steger-Hartmann, T.; Valentin, J. P.; Van Goethem, F.; Weaver, R. J.; Newham, P. , The evolving role of investigative toxicology in the pharmaceutical industry. Nat Rev Drug Discov 2023, 22, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Andrais, B.; Murray, D. , Viability Assessment Following Anticancer Treatment Requires Single-Cell Visualization. Cancers (Basel) 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, Stuart L. ; Kotz, Joanne D.; Li, M.; Aubé, J.; Austin, Christopher P.; Reed, John C.; Rosen, H.; White, E. L.; Sklar, Larry A.; Lindsley, Craig W.; Alexander, Benjamin R.; Bittker, Joshua A.; Clemons, Paul A.; de Souza, A.; Foley, Michael A.; Palmer, M.; Shamji, Alykhan F.; Wawer, Mathias J.; McManus, O.; Wu, M.; Zou, B.; Yu, H.; Golden, Jennifer E.; Schoenen, Frank J.; Simeonov, A.; Jadhav, A.; Jackson, Michael R.; Pinkerton, Anthony B.; Chung, Thomas D. Y.; Griffin, Patrick R.; Cravatt, Benjamin F.; Hodder, Peter S.; Roush, William R.; Roberts, E.; Chung, D.-H.; Jonsson, Colleen B.; Noah, James W.; Severson, William E.; Ananthan, S.; Edwards, B.; Oprea, Tudor I.; Conn, P. J.; Hopkins, Corey R.; Wood, Michael R.; Stauffer, Shaun R.; Emmitte, Kyle A.; Brady, Linda S.; Driscoll, J.; Li, Ingrid Y.; Loomis, Carson R.; Margolis, Ronald N.; Michelotti, E.; Perry, Mary E.; Pillai, A.; Yao, Y., Advancing Biological Understanding and Therapeutics Discovery with Small-Molecule Probes. Cell 2015, 161, 1252–1265. [Google Scholar] [CrossRef]
- Madorran, E.; Stozer, A.; Bevc, S.; Maver, U. , In vitro toxicity model: Upgrades to bridge the gap between preclinical and clinical research. Bosn J Basic Med Sci 2019. [Google Scholar] [CrossRef]
- Oltvai, Z. N.; Barabási, A.-L.; Jeong, H.; Tombor, B.; Albert, R. , The large-scale organization of metabolic networks. Nature 2000, 407, 651–654. [Google Scholar] [CrossRef]
- Galluzzi, L.; Pedro, J. M. B.-s.; Vitale, I.; Aaronson, S. A.; Abrams, J. M.; Adam, D.; Alnemri, E. S.; Altucci, L.; Andrews, D. , Essential versus accessory aspects of cell death : recommendations of the NCCD 2015. 2015, 58-73. [CrossRef]
- Riss, T.; Niles, A.; Moravec, R.; Karassina, N.; Vidugiriene, J. , Markossian, S.; Grossman, A.; Arkin, M.; Auld, D.; Austin, C.; Baell, J.; Brimacombe, K.; Chung, T. D. Y.; Coussens, N. P.; Dahlin, J. L.; Devanarayan, V.; Foley, T. L.; Glicksman, M.; Gorshkov, K.; Haas, J. V.; Hall, M. D.; Hoare, S.; Inglese, J.; Iversen, P. W.; Lal-Nag, M.; Li, Z.; Manro, J. R.; McGee, J.; McManus, O.; Pearson, M.; Riss, T.; Saradjian, P.; Sittampalam, G. S.; Tarselli, M.; Trask, O. J., Jr.; Weidner, J. R.; Wildey, M. J.; Wilson, K.; Xia, M.; Xu, X., Eds. Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda (MD), 2004.Cells. In Assay Guidance Manual, Markossian, S.; Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T. D. Y., Coussens, N. P., Dahlin, J. L., Devanarayan, V., Foley, T. L., Glicksman, M., Gorshkov, K., Haas, J. V., Hall, M. D., Hoare, S., Inglese, J., Iversen, P. W., Lal-Nag, M., Li, Z., Manro, J. R., McGee, J., McManus, O., Pearson, M., Riss, T., Saradjian, P., Sittampalam, G. S., Tarselli, M., Trask, O. J., Jr., *!!! REPLACE !!!*, Weidner, J. R., Wildey, M. J., Wilson, K., Xia, M., Eds.; Xu, X., Eds. Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda (MD), 2004; Xu, X., Eds. Eli Lilly &. [Google Scholar]
- OECD, In vitro toxicological tests. 2016.
- Uzman, A. , Molecular biology of the cell (4th ed.): Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., and Walter, P. Biochemistry and Molecular Biology Education 2003, 31, 212–214. [Google Scholar] [CrossRef]
- Chalmers, S.; Saunter, C.; Wilson, C.; Coats, P.; Girkin, J. M.; McCarron, J. G. , Mitochondrial motility and vascular smooth muscle proliferation. Arterioscler Thromb Vasc Biol 2012, 32, 3000–3011. [Google Scholar] [CrossRef]
- Seal, S.; Yang, H.; Vollmers, L.; Bender, A. , Comparison of Cellular Morphological Descriptors and Molecular Fingerprints for the Prediction of Cytotoxicity- and Proliferation-Related Assays. Chemical Research in Toxicology 2021, 34, 422–437. [Google Scholar] [CrossRef]
- Munoz, L. E.; Maueröder, C.; Chaurio, R.; Berens, C.; Herrmann, M.; Janko, C. , Colourful death: six-parameter classification of cell death by flow cytometry--dead cells tell tales. Autoimmunity 2013, 46, 336–341. [Google Scholar] [CrossRef]
- Kühn, J.; Shaffer, E.; Mena, J.; Breton, B.; Parent, J.; Rappaz, B.; Chambon, M.; Emery, Y.; Magistretti, P.; Depeursinge, C.; Marquet, P.; Turcatti, G. , Label-free cytotoxicity screening assay by digital holographic microscopy. Assay Drug Dev Technol 2013, 11, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kasprowicz, R.; Suman, R.; O’Toole, P. , Characterising live cell behaviour: Traditional label-free and quantitative phase imaging approaches. The International Journal of Biochemistry & Cell Biology 2017, 84, 89–95. [Google Scholar] [CrossRef]
- Louzao, M. C.; Ares, I. R.; Vieytes, M. R.; Valverde, I.; Vieites, J. M.; Yasumoto, T.; Botana, L. M. , The cytoskeleton, a structure that is susceptible to the toxic mechanism activated by palytoxins in human excitable cells. Febs j 2007, 274, 1991–2004. [Google Scholar] [CrossRef] [PubMed]
- Restrepo-Angulo, I.; De Vizcaya-Ruiz, A.; Camacho, J. , Ion channels in toxicology. Journal of Applied Toxicology 2010, 30, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A. B.; Dobson, E. T. A.; Rueden, C. T.; Tomancak, P.; Jug, F.; Eliceiri, K. W. , The ImageJ ecosystem: Open-source software for image visualization, processing, and analysis. Protein Sci 2021, 30, 234–249. [Google Scholar] [CrossRef]
- Zuba-Surma, E. K.; Kucia, M.; Abdel-Latif, A.; Lillard, J. W., Jr.; Ratajczak, M. Z. , The ImageStream System: a key step to a new era in imaging. Folia Histochem Cytobiol 2007, 45, 279–290. [Google Scholar]
- Urbanska, M.; Muñoz, H. E.; Shaw Bagnall, J.; Otto, O.; Manalis, S. R.; Di Carlo, D.; Guck, J. , A comparison of microfluidic methods for high-throughput cell deformability measurements. Nature Methods 2020, 17, 587–593. [Google Scholar] [CrossRef]
- Devendran, C.; Carthew, J.; Frith, J. E.; Neild, A. , Cell Adhesion, Morphology, and Metabolism Variation via Acoustic Exposure within Microfluidic Cell Handling Systems. Adv Sci (Weinh) 2019, 6, 1902326. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P. D. , Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb Protoc.
- Legrand, C.; Bour, J. M.; Jacob, C.; Capiaumont, J.; Martial, A.; Marc, A.; Wudtke, M.; Kretzmer, G.; Demangel, C.; Duval, D.; et al. , Lactate dehydrogenase (LDH) activity of the cultured eukaryotic cells as marker of the number of dead cells in the medium [corrected]. J Biotechnol 1992, 25, 231–243. [Google Scholar] [CrossRef]
- Mishra, D.; Banerjee, D. , Lactate Dehydrogenases as Metabolic Links between Tumor and Stroma in the Tumor Microenvironment. Cancers (Basel). [CrossRef]
- Fotakis, G.; Timbrell, J. A. , In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Galluzzi, L.; Aaronson, S. A.; Abrams, J.; Alnemri, E. S.; Andrews, D. W.; Baehrecke, E. H.; Bazan, N. G.; Blagosklonny, M. V.; Blomgren, K.; Borner, C.; Bredesen, D. E.; Brenner, C.; Castedo, M.; Cidlowski, J. A.; Ciechanover, A.; Cohen, G. M.; De Laurenzi, V.; De Maria, R.; Deshmukh, M.; Dynlacht, B. D.; El-Deiry, W. S.; Flavell, R. A.; Fulda, S.; Garrido, C.; Golstein, P.; Gougeon, M. L.; Green, D. R.; Gronemeyer, H.; Hajnoczky, G.; Hardwick, J. M.; Hengartner, M. O.; Ichijo, H.; Jaattela, M.; Kepp, O.; Kimchi, A.; Klionsky, D. J.; Knight, R. A.; Kornbluth, S.; Kumar, S.; Levine, B.; Lipton, S. A.; Lugli, E.; Madeo, F.; Malomi, W.; Marine, J. C.; Martin, S. J.; Medema, J. P.; Mehlen, P.; Melino, G.; Moll, U. M.; Morselli, E.; Nagata, S.; Nicholson, D. W.; Nicotera, P.; Nunez, G.; Oren, M.; Penninger, J.; Pervaiz, S.; Peter, M. E.; Piacentini, M.; Prehn, J. H.; Puthalakath, H.; Rabinovich, G. A.; Rizzuto, R.; Rodrigues, C. M.; Rubinsztein, D. C.; Rudel, T.; Scorrano, L.; Simon, H. U.; Steller, H.; Tschopp, J.; Tsujimoto, Y.; Vandenabeele, P.; Vitale, I.; Vousden, K. H.; Youle, R. J.; Yuan, J.; Zhivotovsky, B.; Kroemer, G. , Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell death and differentiation 2009, 16, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, S.; Vandeplassche, E.; Ostyn, L.; Coenye, T.; Crabbé, A. , Bacterial Interference With Lactate Dehydrogenase Assay Leads to an Underestimation of Cytotoxicity. Front Cell Infect Microbiol 2020, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Farhana, A.; Lappin, S. L. , Biochemistry, Lactate Dehydrogenase. In StatPearls, StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.: Treasure Island (FL) ineligible companies. Disclosure: Sarah Lappin declares no relevant financial relationships with ineligible companies., 2024.
- Azqueta, A.; Stopper, H.; Zegura, B.; Dusinska, M.; Møller, P. , Do cytotoxicity and cell death cause false positive results in the in vitro comet assay? Mutat Res Genet Toxicol Environ Mutagen 2022, 881, 503520. [Google Scholar] [CrossRef] [PubMed]
- Pappenheimer, A. M. , EXPERIMENTAL STUDIES UPON LYMPHOCYTES : I. THE REACTIONS OF LYMPHOCYTES UNDER VARIOUS EXPERIMENTAL CONDITIONS. J Exp Med 1917, 25, 633–650. [Google Scholar] [CrossRef]
- Owen, S. C.; Doak, A. K.; Ganesh, A. N.; Nedyalkova, L.; McLaughlin, C. K.; Shoichet, B. K.; Shoichet, M. S. , Colloidal drug formulations can explain “bell-shaped” concentration-response curves. ACS Chem Biol 2014, 9, 777–784. [Google Scholar] [CrossRef]
- Kuijpers, L.; van Veen, E.; van der Pol, L. A.; Dekker, N. H. , Automated cell counting for Trypan blue-stained cell cultures using machine learning. PLoS One 2023, 18, e0291625. [Google Scholar] [CrossRef]
- Chiaraviglio, L.; Kirby, J. E. , Evaluation of impermeant, DNA-binding dye fluorescence as a real-time readout of eukaryotic cell toxicity in a high throughput screening format. Assay Drug Dev Technol 2014, 12, 219–228. [Google Scholar] [CrossRef]
- Foglieni, C.; Meoni, C.; Davalli, A. M. , Fluorescent dyes for cell viability: an application on prefixed conditions. Histochem Cell Biol 2001, 115, 223–229. [Google Scholar] [CrossRef]
- Boyd, V.; Cholewa, O. M.; Papas, K. K. , Limitations in the Use of Fluorescein Diacetate/Propidium Iodide (FDA/PI) and Cell Permeable Nucleic Acid Stains for Viability Measurements of Isolated Islets of Langerhans. Curr Trends Biotechnol Pharm 2008, 2, 66–84. [Google Scholar]
- Inde, Z.; Rodencal, J.; Dixon, S. J. , Quantification of drug-induced fractional killing using high-throughput microscopy. STAR Protoc 2021, 2, 100300. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Bedner, E.; Traganos, F. , Difficulties and pitfalls in analysis of apoptosis. Methods Cell Biol 2001, 63, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Zamai, L.; Mazzotti, G.; Cataldi, A.; Falcieri, E. , Differential kinetics of propidium iodide uptake in apoptotic and necrotic thymocytes. Histochemistry 1993, 100, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Zamai, L.; Falcieri, E.; Marhefka, G.; Vitale, M. , Supravital exposure to propidium iodide identifies apoptotic cells in the absence of nucleosomal DNA fragmentation. Cytometry 1996, 23, 303–311. [Google Scholar] [CrossRef]
- Madorran, E.; Stožer, A.; Arsov, Z.; Maver, U.; Rožanc, J. , A Promising Method for the Determination of Cell Viability: The Membrane Potential Cell Viability Assay. 2022, 11, 2314. [CrossRef]
- Castro-Concha, L. A.; Escobedo, R. M.; de Miranda-Ham, M. L. , Loyola-Vargas, V. M.; Vázquez-Flota, F., Eds. Humana Press: Totowa, NJ, 2006; pp 71-76.Cultures. In Plant Cell Culture Protocols, Loyola-Vargas, V. M.; Vázquez-Flota, F., Eds. Humana Press: Totowa, NJ, 2006; Vázquez-Flota, F., Eds. Humana Press: Totowa, NJ, 2006. [Google Scholar]
- Kumar, P.; Srivastava, N.; Pande, M.; Prasad, J. K.; Sirohi, A. S. , Srivastava, N.; Pande, M., Eds. Springer Singapore: Singapore, 2017; pp 57-71.Integrity. In Protocols in Semen Biology (Comparing Assays), Srivastava, N.; Pande, M., Eds. Springer Singapore: Singapore, 2017; Pande, M., Eds. Springer Singapore: Singapore, 2017. [Google Scholar]
- Michel, C. C. , Chapter 9 - Microvascular Permeability and the Exchange of Water and Solutes Across Microvascular Walls. In Seldin and Giebisch’s The Kidney (Fifth Edition), Alpern, R. J.; Moe, O. W.; Caplan, M., Eds. Academic Press: 2013; pp 263-290.
- Heymann, E.; Mentlein, R.; Schmalz, R.; Schwabe, C.; Wagenmann, F. , A method for the estimation of esterase synthesis and degradation and its application to evaluate the influence of insulin and glucagon. Eur J Biochem 1979, 102, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Kari, S.; Subramanian, K.; Altomonte, I. A.; Murugesan, A.; Yli-Harja, O.; Kandhavelu, M. , Programmed cell death detection methods: a systematic review and a categorical comparison. Apoptosis 2022, 27, 482–508. [Google Scholar] [CrossRef]
- Ai, X.; Butts, B.; Vora, K.; Li, W.; Tache-Talmadge, C.; Fridman, A.; Mehmet, H. , Generation and characterization of antibodies specific for caspase-cleaved neo-epitopes: a novel approach. Cell death & disease 2011, 2, e205. [Google Scholar] [CrossRef]
- Bajt, M. L.; Cover, C.; Lemasters, J. J.; Jaeschke, H. , Nuclear Translocation of Endonuclease G and Apoptosis-Inducing Factor during Acetaminophen-Induced Liver Cell Injury. Toxicological Sciences 2006, 94, 217–225. [Google Scholar] [CrossRef]
- Rieger, A. M.; Nelson, K. L.; Konowalchuk, J. D.; Barreda, D. R. , Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. Journal of visualized experiments : JoVE.
- Gao, J.; Xiong, A.; Liu, J.; Li, X.; Wang, J.; Zhang, L.; Liu, Y.; Xiong, Y.; Li, G.; He, X. , PANoptosis: bridging apoptosis, pyroptosis, and necroptosis in cancer progression and treatment. Cancer Gene Therapy 2024, 31, 970–983. [Google Scholar] [CrossRef]
- Kist, M.; Vucic, D. , Cell death pathways: intricate connections and disease implications. The EMBO journal 2021, 40, e106700. [Google Scholar] [CrossRef]
- Vembadi, A.; Menachery, A.; Qasaimeh, M. A. , Cell Cytometry: Review and Perspective on Biotechnological Advances. Front Bioeng Biotechnol 2019, 7, 147. [Google Scholar] [CrossRef]
- Luk, H.-Y.; McFarlin, B. K.; Vingren, J. L. , Using image-based flow cytometry to monitor satellite cells proliferation and differentiation in vitro. Methods (San Diego, Calif.) 2017, 112, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Flomerfelt, F. A.; Gress, R. E. , Analysis of Cell Proliferation and Homeostasis Using EdU Labeling. Methods in molecular biology (Clifton, N.J.) 2016, 1323, 211–220. [Google Scholar] [PubMed]
- Levkoff, L. H.; Marshall, G. P., 2nd; Ross, H. H.; Caldeira, M.; Reynolds, B. A.; Cakiroglu, M.; Mariani, C. L.; Streit, W. J.; Laywell, E. D. , Bromodeoxyuridine inhibits cancer cell proliferation in vitro and in vivo. Neoplasia 2008, 10, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Haskins, J. S.; Su, C.; Maeda, J.; Walsh, K. D.; Haskins, A. H.; Allum, A. J.; Froning, C. E.; Kato, T. A. , Evaluating the Genotoxic and Cytotoxic Effects of Thymidine Analogs, 5-Ethynyl-2’-Deoxyuridine and 5-Bromo-2’-Deoxyurdine to Mammalian Cells. International journal of molecular sciences.
- Vichai, V.; Kirtikara, K. , Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. , Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harbor Protocols, 0873. [Google Scholar] [CrossRef]
- Mani, S.; Bharagava, R. N. , Exposure to Crystal Violet, Its Toxic, Genotoxic and Carcinogenic Effects on Environment and Its Degradation and Detoxification for Environmental Safety. Rev Environ Contam Toxicol 2016, 237, 71–104. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G. J.; Cloos, J. , Cell sensitivity assays: the MTT assay. Methods in molecular biology (Clifton, N.J.) 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Alépée, N.; Grandidier, M. H.; Cotovio, J. , Sub-categorisation of skin corrosive chemicals by the EpiSkin™ reconstructed human epidermis skin corrosion test method according to UN GHS: revision of OECD Test Guideline 431. Toxicol In Vitro 2014, 28, 131–145. [Google Scholar] [CrossRef]
- Hamid, R.; Rotshteyn, Y.; Rabadi, L.; Parikh, R.; Bullock, P. , Comparison of alamar blue and MTT assays for high through-put screening. 2004, 18, 703–710. [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. , The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. International journal of molecular sciences. [CrossRef]
- Karakaş, D.; Ari, F.; Ulukaya, E. , The MTT viability assay yields strikingly false-positive viabilities although the cells are killed by some plant extracts. Turk J Biol 2017, 41, 919–925. [Google Scholar] [CrossRef]
- Wang, P.; Henning, S. M.; Heber, D. , Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS One 2010, 5, e10202. [Google Scholar] [CrossRef]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D. K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B. S.; Bhatt, A. N. , Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Scientific reports 2018, 8, 1531. [Google Scholar] [CrossRef]
- Damiani, E.; Solorio, J. A.; Doyle, A. P.; Wallace, H. M. , How reliable are in vitro IC(50) values? Values vary with cytotoxicity assays in human glioblastoma cells. Toxicol Lett 2019, 302, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Perelman, A.; Wachtel, C.; Cohen, M.; Haupt, S.; Shapiro, H.; Tzur, A. , JC-1 : alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. 2012, 3, e430–e437. [CrossRef]
- Keil, V. C.; Funke, F.; Zeug, A.; Schild, D.; Müller, M. , Ratiometric high-resolution imaging of JC-1 fluorescence reveals the subcellular heterogeneity of astrocytic mitochondria. Pflugers Arch 2011, 462, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Perry, S. W.; Norman, J. P.; Barbieri, J.; Brown, E. B.; Gelbard, H. A. , Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Zuang, V. , The neutral red release assay: a review. Altern Lab Anim 2001, 29, 575–599. [Google Scholar] [CrossRef]
- Ates, G.; Vanhaecke, T.; Rogiers, V.; Rodrigues, R. M. , Assaying Cellular Viability Using the Neutral Red Uptake Assay. Methods in molecular biology (Clifton, N.J.) 2017, 1601, 19–26. [Google Scholar] [CrossRef]
- Seglen, P. O. , Inhibitors of lysosomal function. Methods in enzymology 1983, 96, 737–764. [Google Scholar] [CrossRef]
- Hu, W.; Culloty, S.; Darmody, G.; Lynch, S.; Davenport, J.; Ramirez-Garcia, S.; Dawson, K.; Lynch, I.; Doyle, H.; Sheehan, D. , Neutral red retention time assay in determination of toxicity of nanoparticles. Marine environmental research 2015, 111, 158–161. [Google Scholar] [CrossRef]
- Fan, F.; Wood, K. V. , Bioluminescent Assays for High-Throughput Screening. ASSAY and Drug Development Technologies 2007, 5, 127–136. [Google Scholar] [CrossRef]
- Madorran, E.; Kocbek Šaherl, L.; Rakuša, M.; Takač, I.; Munda, M. , Finding a Direct Method for a Dynamic Process: The DD (Direct and Dynamic) Cell-Tox Method. International journal of molecular sciences 2024, 25, 5133. [Google Scholar] [CrossRef]
- Cho, M. H.; Niles, A.; Huang, R.; Inglese, J.; Austin, C. P.; Riss, T.; Xia, M. , A bioluminescent cytotoxicity assay for assessment of membrane integrity using a proteolytic biomarker. Toxicol In Vitro 2008, 22, 1099–1106. [Google Scholar] [CrossRef]
- Zhu, S.; Barbe, M. F.; Liu, C.; Hadjiargyrou, M.; Popoff, S. N.; Rani, S.; Safadi, F. F.; Litvin, J. , Periostin-like-factor in osteogenesis. Journal of cellular physiology 2009, 218, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. , Trypan Blue Exclusion Test of Cell Viability. Curr Protoc Immunol 2015, 111, A3. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yang, J.; Zhang, Y.; Xi, Y.; Wen, X.; Yuan, D.; Wang, Y.; Wei, C.; Wang, R.; Wu, L.; Li, H.; Xu, C. , Exogenous H(2)S restores ischemic post-conditioning-induced cardioprotection through inhibiting endoplasmic reticulum stress in the aged cardiomyocytes. Cell & bioscience 2017, 7, 67. [Google Scholar] [CrossRef]
- Chazotte, B. , Labeling nuclear DNA with hoechst 33342. Cold Spring Harb Protoc, 5557. [Google Scholar] [CrossRef]
- Wlodkowic, D.; Akagi, J.; Dobrucki, J.; Errington, R.; Smith, P. J.; Takeda, K.; Darzynkiewicz, Z. , Kinetic viability assays using DRAQ7 probe. Current protocols in cytometry, 9. [CrossRef]
- Da Costa, R.; Redmann, K.; Schlatt, S. , Simultaneous detection of sperm membrane integrity and DNA fragmentation by flow cytometry: A novel and rapid tool for sperm analysis. Andrology 2021, 9, 1254–1263. [Google Scholar] [CrossRef]
- Jannoo, R.; Xia, Z.; Row, P. E.; Kanamarlapudi, V. , Targeting of the Interleukin-13 Receptor (IL-13R)α2 Expressing Prostate Cancer by a Novel Hybrid Lytic Peptide. Biomolecules. [CrossRef]
- Wlodkowic, D.; Faley, S.; Darzynkiewicz, Z.; Cooper, J. M. , Real-time cytotoxicity assays. Methods in molecular biology (Clifton, N.J.) 2011, 731, 285–291. [Google Scholar]
- Gustafson, D. L.; Viola, L. O.; Towers, C. G.; Das, S.; Duval, D. L.; Van Eaton, K. M. , Sensitivity of osteosarcoma cell lines to autophagy inhibition as determined by pharmacologic and genetic manipulation. Veterinary and comparative oncology 2023, 21, 726–738. [Google Scholar] [CrossRef]
- Lee-MacAry, A. E.; Ross, E. L.; Davies, D.; Laylor, R.; Honeychurch, J.; Glennie, M. J.; Snary, D.; Wilkinson, R. W. , Development of a novel flow cytometric cell-mediated cytotoxicity assay using the fluorophores PKH-26 and TO-PRO-3 iodide. J Immunol Methods. [CrossRef]
- Neri, S.; Mariani, E.; Meneghetti, A.; Cattini, L.; Facchini, A. , Calcein-acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clinical and diagnostic laboratory immunology 2001, 8, 1131–1135. [Google Scholar] [CrossRef]
- Yang, W.; Mu, B.; You, J.; Tian, C.; Bin, H.; Xu, Z.; Zhang, L.; Ma, R.; Wu, M.; Zhang, G.; Huang, C.; Li, L.; Shao, Z.; Dai, L.; Désaubry, L.; Yang, S. , Non-classical ferroptosis inhibition by a small molecule targeting PHB2. Nature Communications 2022, 13, 7473. [Google Scholar] [CrossRef]
- Virág, L.; Kerékgyártó, C.; Fachet, J. , A simple, rapid and sensitive fluorimetric assay for the measurement of cell-mediated cytotoxicity. Journal of Immunological Methods 1995, 185, 199–208. [Google Scholar] [CrossRef]
- Masson-Meyers, D. S.; Bumah, V. V.; Enwemeka, C. S. , A comparison of four methods for determining viability in human dermal fibroblasts irradiated with blue light. J Pharmacol Toxicol Methods 2016, 79, 15–22. [Google Scholar] [CrossRef]
- Im, K.; Mareninov, S.; Diaz, M. F. P.; Yong, W. H. , An Introduction to Performing Immunofluorescence Staining. Methods in molecular biology (Clifton, N.J.) 2019, 1897, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Mfouo-Tynga, I.; Houreld, N. N.; Abrahamse, H. , Evaluation of cell damage induced by irradiated Zinc-Phthalocyanine-gold dendrimeric nanoparticles in a breast cancer cell line. Biomedical journal 2018, 41, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, I.; Batra, S. K. , Protocol for Apoptosis Assay by Flow Cytometry Using Annexin V Staining Method. Bio-protocol. [CrossRef]
- Huang, Q.; Wang, L.; Ran, Q.; Wang, J.; Wang, C.; He, H.; Li, L.; Qi, H. , Notopterol-induced apoptosis and differentiation in human acute myeloid leukemia HL-60 cells. Drug design, development and therapy 2019, 13, 1927–1940. [Google Scholar] [CrossRef] [PubMed]
- Peters, T.; Lindenmaier, H.; Haefeli, W. E.; Weiss, J. , Interaction of the mitotic kinesin Eg5 inhibitor monastrol with P-glycoprotein. Naunyn-Schmiedeberg’s Archives of Pharmacology 2006, 372, 291–299. [Google Scholar] [CrossRef]
- Ngamwongsatit, P.; Banada, P. P.; Panbangred, W.; Bhunia, A. K. , WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. Journal of Microbiological Methods 2008, 73, 211–215. [Google Scholar] [CrossRef]
- Huyck, L.; Ampe, C.; Van Troys, M. , The XTT cell proliferation assay applied to cell layers embedded in three-dimensional matrix. Assay Drug Dev Technol 2012, 10, 382–392. [Google Scholar] [CrossRef]
- Bonnier, F.; Keating, M. E.; Wróbel, T. P.; Majzner, K.; Baranska, M.; Garcia-Munoz, A.; Blanco, A.; Byrne, H. J. , Cell viability assessment using the Alamar blue assay: A comparison of 2D and 3D cell culture models. Toxicology in Vitro 2015, 29, 124–131. [Google Scholar] [CrossRef]
- Desai, S.; Grefte, S.; van de Westerlo, E.; Lauwen, S.; Paters, A.; Prehn, J. H. M.; Gan, Z.; Keijer, J.; Adjobo-Hermans, M. J. W.; Koopman, W. J. H. , Performance of TMRM and Mitotrackers in mitochondrial morphofunctional analysis of primary human skin fibroblasts. Biochimica et Biophysica Acta (BBA) - Bioenergetics 2024, 1865, 149027. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J. L. , Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Caldwell, J. C. , cell death, or nutrient supply. In Tumour Site Concordance and Mechanisms of Carcinogenesis, Baan, R. A.; Stewart, B. W., Ed.; Straif, K., Eds. International Agency for Research on Cancer © International Agency for Research on Cancer, 2019. For more information contact publications@iarc.fr.: Lyon (FR), 2019. [Google Scholar]
- Zhao, J.; Yu, H. Q.; Ge, F. Q.; Zhang, M. R.; Song, Y. C.; Guo, D. D.; Li, Q. H.; Zhu, H.; Hang, P. Z. , 7,8,3’-Trihydroxyflavone prevents doxorubicin-induced cardiotoxicity and mitochondrial dysfunction via activating Akt signaling pathway in H9c2 cells. Cell Signal 2023, 112, 110924. [Google Scholar] [CrossRef]
- Elmore, S. , Apoptosis: a review of programmed cell death. Toxicol Pathol 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Rainieri, S.; Conlledo, N.; Langerholc, T.; Madorran, E.; Sala, M.; Barranco, A. , Toxic effects of perfluorinated compounds at human cellular level and on a model vertebrate. Food and Chemical Toxicology 2017. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, A.; Wittmann, T. , Fluorescence live cell imaging. Methods Cell Biol 2014, 123, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Prochazkova, M.; Kim, Y. S.; Jiang, C.; Ma, J.; Moses, L.; Martin, K.; Pham, V.; Zhang, N.; Highfill, S. L.; Somerville, R. P.; Stroncek, D. F.; Jin, P. , Assessment and comparison of viability assays for cellular products. Cytotherapy 2024, 26, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Afjei, R.; Massoud, T. F.; Paulmurugan, R. , Comparison of cell-based assays to quantify treatment effects of anticancer drugs identifies a new application for Bodipy-L-cystine to measure apoptosis. Scientific reports 2018, 8, 16363. [Google Scholar] [CrossRef]
| Method (Group) | Principle | Endpoint (Instrument) |
Advantages | Disadvantages |
|---|---|---|---|---|
| Structural cell damage (non-invasive) | ||||
| Optical microscope (1) | Changes in the morphology of the cell. | Observe changes in the morphology of the cell (optical microscope). | Quick, cheap and it could be automatized. | Many artifacts affect the accuracy of the assay. |
| Release of intracellular compounds (2) | Measure the release of compounds into the cell culture medium due to damaged cell membrane. | Absorbance or luminescence of the compound (spectrophotometer or luminometer). | Quick, non-invasive. | High background level and potential false positive events. |
| Structural cell damage | ||||
| Trypan blue (3) | Viable cells exclude the trypan blue dye. | Observation of stained cells (optical microscope). | Cheap, it could be automatized. | High rates of false positives and false negatives. |
| Lipid-soluble dyes (4) | Hydrolysis of the dye by intracellular esterase. | Fluorescence intensity of reduced products (Fluorescence microscope, Flow cytometer). | Quick, versatile (analysis of single cell or cell population in different instruments) | High rate of false-positives. |
| Propidium Iodide (5) | The dyes are impermeant only to live cell. | Fluorescence intensity of the internalized dye (Fluorescence microscope, Flow cytometer). | Quick, many references. | High rate of false-positives. |
| Live/Dead assay (6) | It has two dyes: one impermeant only to live cells and the second is cleaved by esterases within the cell. | Fluorescence intensity of internalized dye and reduced dye (Fluorescence microscope, Flow cytometer). | Quick, versatile, better accuracy than single dyes alone. | High rate of false-positives |
| Antibodies to cell-death-associated pathways (7) | Determine the presence of molecules associated to cell death pathways. | Fluorescence intensity of the dye attached to the antibody (Fluorescence microscope, Flow cytometer). | Specific, determines the type of the program cell death. | Expensive, unable to determine the viability of the cells with a different cell death pathway. |
| Cell growth | ||||
| Cell division (8) | Difference in cell number before and after the exposure | Counting cell number in a population (microscope, flow cytometer, cell counter) | Cheap, accurate and straightforward. | Time-consuming. |
| BrdU/EdU (9) | Daughter cells contained BrdU intercalated in their DNA. | Fluorescence intensity of BrdU (Fluorescence microscope, Flow cytometer). | High sensitivity no. | Toxic, impairs cell division. |
| Sulforhodamine B (SRB) and crystal violet (10) | The amount of dye is proportional to the cells (cell proliferation). | Absorbance of the dye (spectrophotometer) | Quick, cheap. | Toxic. Measures cell mass, not cell viability. |
| Cellular metabolism | ||||
| MTT (11) | Reduction of the tetrazolium dye to formazan. | Absorbance of the formazan product (spectrophotometer). | Quick, cheap, high throughput and many references. | Many artifacts affect the accuracy of the assay. |
| Alamar blue (12) | Resazurin reduction to resorufin. | Luminescence measurement of resorufin (Fluorescence microscope and Flow cytometer). | Quick, cheap and high throughput | Naturally occurring molecules disrupt the assay. |
| JC-1, TMRE, MitoTracker (13) | Mitochondrial membrane potential (lost in non-viable cells). | Fluorescence emission of the dye proportional to the mitochondrial membrane potential (Fluorescence microscope and Flow cytometer). | Fast, cheap and high throughput. | Prone to bleaching, quenching and unquenching. Accuracy issues in certain situations. |
| Neutral Red (14) | Viable cells incorporate and bind the neutral red dye. | Absorbance of the incorporated dye (spectrophotometer). | Quick, cheap and standardize. | Not a good correlation. Lysosomal activity affects its accuracy. |
| ATP production (15) | ATP production is correlated to cell viability. | Luminescence measurement of released ATP (Luminometer). | Live imaging, non-invasive and high throughput | Many artifacts affect the accuracy of the assay. |
| Cell membrane potential | ||||
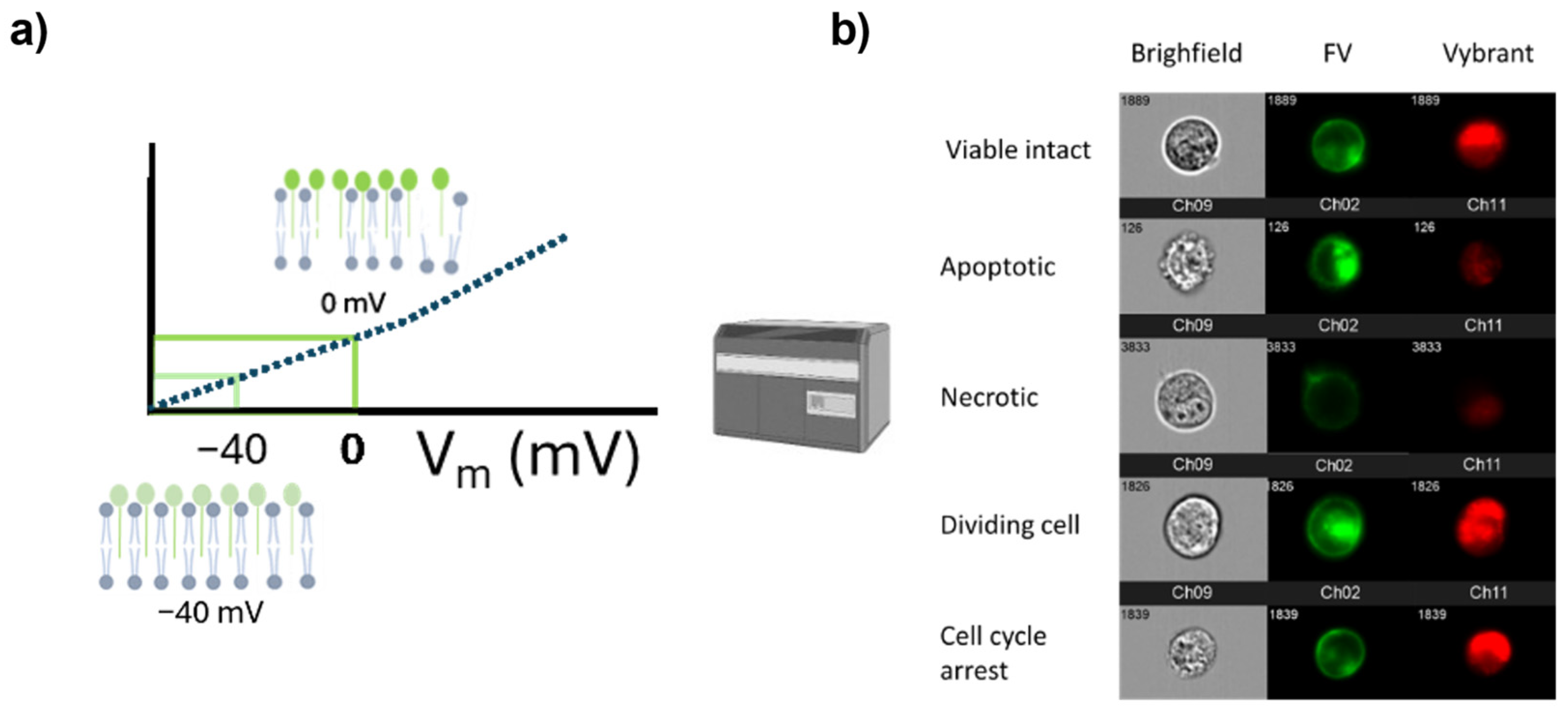
| MPCVA (16) | The cell membrane potential determines cell membrane integrity. | Fluorescence intensity of the dyes (Fluorescence microscope, Flow cytometer). | Direct determination. Live imaging | Not tested in various alternative situations. |
| DD Cell-Tox (17) | The cell membrane potential determines cell membrane integrity and DNA content the doubling cells. | Fluorescence intensity of the dyes (Fluorescence microscope, Flow cytometer). | Direct determination. Considers the cell population dynamics, and the various outcomes triggered by toxic compounds. | Not tested in various alternative situations. Not suitable for long periods of cultivation. |
| Group | Method | Reference |
|---|---|---|
| 1 | Label-free imaging | [15] |
| 2 | LDH | [23] |
| 2 | CytoToxTM | [76] |
| 2 | Toxi-Light®® | [76] |
| 2 | aCella™ - TOX | [76] |
| 2 | CyQUANT™ | [77] |
| 3 | Trypan blue | [78] |
| 4 | Propidium iodide | [48] |
| 4 | Hoechst 33342 | [79,80] |
| 4 | DRAQ7 | [81] |
| 4 | Acridine orange | [82] |
| 4 | CellTox | [83] |
| 4 | SYTOX | [84] |
| 4 | YO-YO | [85] |
| 4 | TO-PRO-3 Iodide | [86] |
| 5 | Calcein AM | [87] |
| 5 | CytoCalcein™ | [88] |
| 5 | 4-methylumbelliferyl heptanoate (MUH) assay | [89] |
| 6 | Live/Dead assay | [90] |
| 7 | Antibodies | [91] |
| 7 | ApoTox-Glo | [92] |
| 7 | Annexin V | [93] |
| 8 | Cell division counting | [52] |
| 9 | BrdU assay | [53] |
| 9 | Edu assay | [53] |
| 10 | Sulforhodamine B | [94] |
| 10 | Crystal Violet | [95] |
| 11 | MTT assay | [59] |
| 11 | WST-1 assay | [96] |
| 11 | MTS assay | [8] |
| 11 | XTT assay | [97] |
| 12 | Alamar Blue | [98] |
| 13 | JC-1 | [67] |
| 13 | TMRE | [99] |
| 13 | Mitotracker | [99] |
| 14 | Neutral red | [100] |
| 15 | ATP assay | [8] |
| 16 | MPCVA | [40] |
| 17 | DD Cell-Tox | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





