Submitted:
09 September 2024
Posted:
10 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Overview of Human Papillomavirus (HPV) and Its Implications in Cancer Development
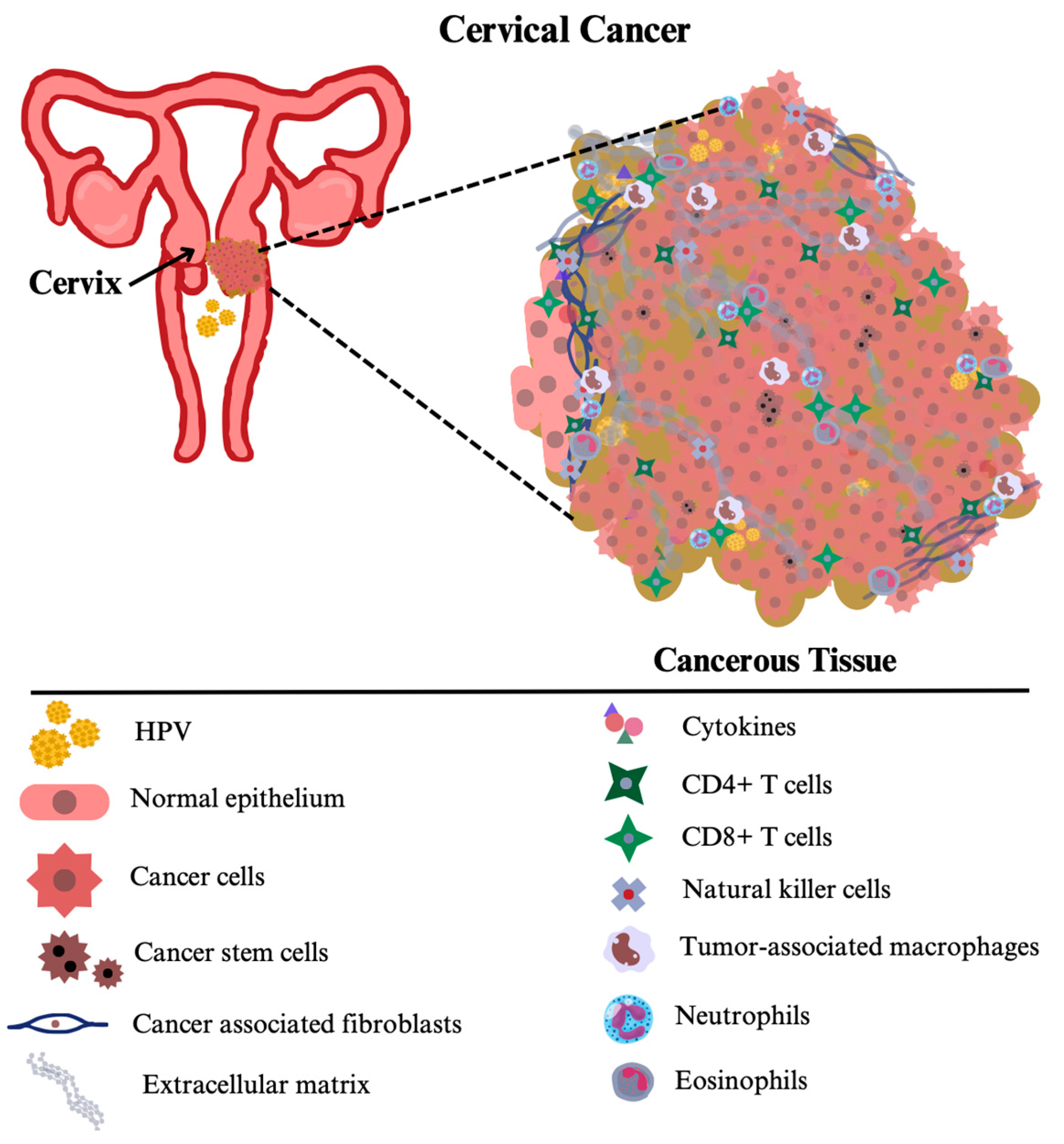
3. The role of HPV on the Tumor Microenvironment
4. Characterization of the Tumor Microenvironment in HPV-Associated Cancers
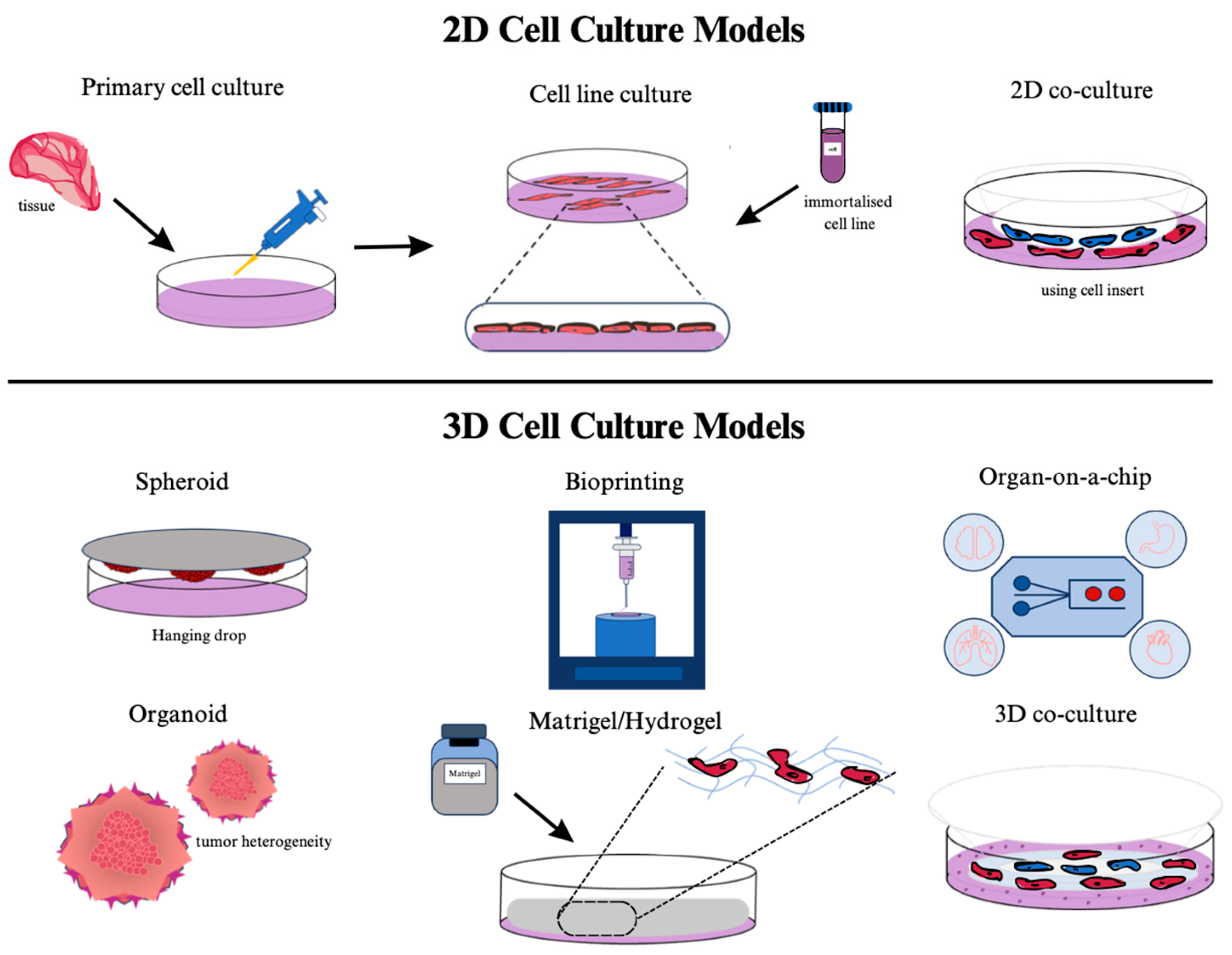
5. Utilization of 3D In Vitro Models in HPV-Related Cancer Research
6. 3D Modelling of HPV-Induced Cancers
Cervical Cancers
Oropharyngeal Cancers
| HPV-related cancer types | Culturing Model | Used Cells | ECM Matrix | References |
|---|---|---|---|---|
| CVC | Hydrogel | Hela cells | Gelatin/alginate/fibrinogen | [138] |
| CVC | Spheroid System | HeLa (HPV18), CaSki (HPV16), SiHa (HPV16), C33A (non-HPV), HT3 (non-HPV) |
Bovine collagen I matrix | [139] |
| CVC | Scaffold | human keratinocyte cell line (NTERT), cervical cancer cell line (C33A) |
Human skin (Euro Skin Bank, Netherlands) | [140] |
| CVC | Scaffold | SiHa (ATCC® HTB-35), Cervical CAF isolated from squamous intraepithelial lesion, and invasive cervical carcinoma biopsies |
Fibronectin solution human fibroblasts | [141] |
| CVC | Hydrogel | Hela cells (ATCC® CCL-2™) | Peptide-based | [64] |
| CVC | Hydrogel | SiHa and Hela cells | Matrigel matrix (BD Biosciences, USA) | [142] |
| CVC | Scaffold | primary human cervical carcinoma | Collagen, glycosaminoglycan, and elastin | [144] |
| CVC and OSCC | Organotypic Raft | primary epithelial cells (human tonsillar keratinocytes or human cervical keratinocytes), J2 fibroblasts, Human fibroblasts hTERT |
Collagen-fibroblast mixture | [143] |
| OSCC | Scaffold | UPCI:SCC090 (CRL-3239), UM-SCC-6 Squamous Carcinoma Cell Line |
Type I collagen | [145] |
| OSCC | Scaffold | Normal human oral keratinocytes and fibroblasts were isolated from oral mucosa biopsies, Primary alveolar human osteoblasts were isolated from aspirated waste bone chips |
Collagen mixture and fibrin adhesive sealant | [147] |
| OSCC | Spheroid System | D20, FaDu, DOK, Cal27 (CRL-2095) and, Normal oral keratinocytes and fibroblasts were isolated from biopsies |
Human cadaver skin(Euroskin, Beverwijk, Holland) | [146] |
| HNSCC | Organoid | Individual patient’s tumors | - | [152] |
| Human tongue squamous cell carcinoma | Organotypic Culture | Individual patient’s tumors (keratinocytes and fibroblasts) | Collagen Matrix | [153] |
| Human tongue cancer tissue | Silica fibre sheet | HSC-3 and HSC-4 | - | [148] |
| Human oral cancer | Microfluidic chip | OEC-M1 | - | [150] |
| Human oral cancer | Hydrogel | Human primary normal oral fibroblasts human oral squamous carcinoma cell lines |
Collagen Matrix | [151] |
Colorectal Cancer
Skin Cancer
7. Limitations and Future Directions in 3D Model-Based Cancer Research
8. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riou, G.; Bourhis, J.; Favre, M.; Orth, G.; Jeannel, D.; Le Doussal, V. Association between Poor Prognosis in Early-Stage Invasive Cervical Carcinomas and Non-Detection of HPV DNA. The Lancet 1990, 335, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Canfell, K.; Kim, J.J.; Brisson, M.; Keane, A.; Simms, K.T.; Caruana, M.; Burger, E.A.; Martin, D.; Nguyen, D.T.N.; Bénard, É.; et al. Mortality Impact of Achieving WHO Cervical Cancer Elimination Targets: A Comparative Modelling Analysis in 78 Low-Income and Lower-Middle-Income Countries. The Lancet 2020, 395, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Pina Brianti 1, E.D.F. 1 Review of HPV-Related Diseases and Cancers - PubMed Available online:. Available online: https://pubmed.ncbi.nlm.nih.gov/28368072/ (accessed on 19 April 2024).
- Muñoz, N.; Castellsagué, X.; de González, A.B.; Gissmann, L. Chapter 1: HPV in the Etiology of Human Cancer. Vaccine 2006, 24 (Suppl 3). [Google Scholar] [CrossRef]
- Stanley, M.A.; Pett, M.R.; Coleman, N. HPV: From Infection to Cancer. Biochem Soc Trans 2007, 35, 1456–1460. [Google Scholar] [CrossRef]
- de Sanjose, S.; Serrano, B.; Tous, S.; Alejo, M.; Loveras, B.L.; Quiros, B.; Clavero, O.; Vidal, A.; Ferrandiz-Pulido, C.; Pavon, M.A.; et al. Burden of Human Papillomavirus (HPV)-Related Cancers Attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI Cancer Spectr 2018, 2. [Google Scholar] [CrossRef]
- Potale, Y.; Kasat, Y.K.; Kumar, A.; Ahmad, F. Unravelling the Impact of Human Papillomavirus (HPV): A Comprehensive Exploration of Its Role in Cancer Progression and Global Health Challenges. [CrossRef]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Human Papillomavirus (HPV) Available online:. Available online: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/human-papillomavirus (accessed on 29 February 2024).
- Krasniqi, E.; Barba, M.; Venuti, A.; Pizzuti, L.; Cappuzzo, F.; Landi, L.; Carpano, S.; Marchetti, P.; Villa, A.; Vizza, E.; et al. Circulating HPV DNA in the Management of Oropharyngeal and Cervical Cancers: Current Knowledge and Future Perspectives. J Clin Med 2021, 10, 1525. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D Tumor Spheroids: An Overview on the Tools and Techniques Used for Their Analysis. Biotechnol Adv 2016, 34, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N. Human Papillomavirus and Cancer: The Epidemiological Evidence. J Clin Virol 2000, 19, 1–5. [Google Scholar] [CrossRef]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K. V.; Snijders, P.J.F.; Meijer, C.J.L.M. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N Engl J Med 2003, 348, 518–527. [Google Scholar] [CrossRef]
- Zibako, P.; Tsikai, N.; Manyame, S.; Ginindza, T.G. Cervical Cancer Management in Zimbabwe (2019–2020). PLoS One 2022, 17, e0274884. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. World Health Organization 2020, 1–56.
- Hardefeldt, H.A.; Cox, M.R.; Eslick, G.D. Association between Human Papillomavirus (HPV) and Oesophageal Squamous Cell Carcinoma: A Meta-Analysis. Epidemiol Infect 2014, 142, 1119–1137. [Google Scholar] [CrossRef] [PubMed]
- Melo, B.A. de C.; Vilar, L.G.; Oliveira, N.R. de; Lima, P.O. de; Pinheiro, M. de B.; Domingueti, C.P.; Pereira, M.C. Human Papillomavirus Infection and Oral Squamous Cell Carcinoma - a Systematic Review. Braz J Otorhinolaryngol 2021, 87, 346–352. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Tornesello, M.L.; Perri, F.; Buonaguro, L.; Ionna, F.; Buonaguro, F.M.; Caponigro, F. HPV-Related Oropharyngeal Cancers: From Pathogenesis to New Therapeutic Approaches. Cancer Lett 2014, 351, 198–205. [Google Scholar] [CrossRef]
- Daling, J.R.; Madeleine, M.M.; Johnson, L.G.; Schwartz, S.M.; Shera, K.A.; Wurscher, M.A.; Carter, J.J.; Porter, P.L.; Galloway, D.A.; McDougall, J.K. Human Papillomavirus, Smoking, and Sexual Practices in the Etiology of Anal Cancer. Cancer 2004, 101, 270–280. [Google Scholar] [CrossRef]
- Kirgan, D.; Manalo, P.; Hall, M.; Mcgregor, B. Association of Human Papillomavirus and Colon Neoplasms. Archives of Surgery 1990, 125, 862–865. [Google Scholar] [CrossRef]
- Burgers, J.K.; Badalament, R.A.; Drago, J.R. PENILE CANCER: Clinical Presentation, Diagnosis, and Staging. Urologic Clinics of North America 1992, 19, 247–256. [Google Scholar] [CrossRef]
- Gregoire, L.; Cubilla, A.L.; Reuter, V.E.; Haas, G.P.; Lancaster, W.D. Preferential Association of Human Papillomavirus With High-Grade Histologic Variants of Penile-Invasive Squamous Cell Carcinoma. JNCI: Journal of the National Cancer Institute 1995, 87, 1705–1709. [Google Scholar] [CrossRef]
- Rubin, M.A.; Kleter, B.; Zhou, M.; Ayala, G.; Cubilla, A.L.; Quint, W.G.V.; Pirog, E.C. Detection and Typing of Human Papillomavirus DNA in Penile Carcinoma: Evidence for Multiple Independent Pathways of Penile Carcinogenesis. Am J Pathol 2001, 159, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.A.; McGregor, J.M.; Proby, C.M.; Breuer, J. Human Papillomavirus and the Development of Non-Melanoma Skin Cancer. J Clin Pathol 1999, 52, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsagué, X. Skin Cancer: Epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol Ther (Heidelb) 2017, 7, 5–19. [Google Scholar] [CrossRef]
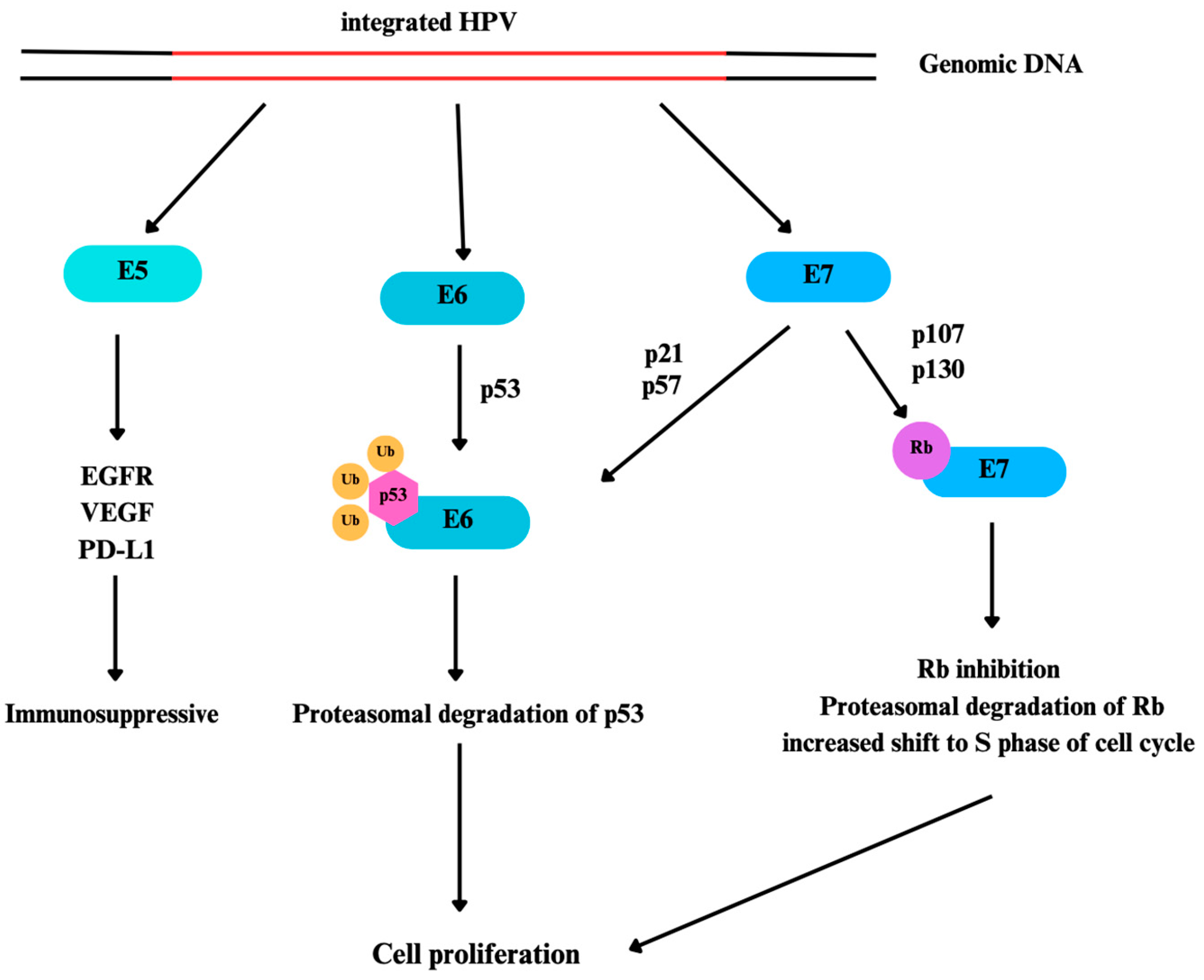
- Tomaić, V. Functional Roles of E6 and E7 Oncoproteins in HPV-Induced Malignancies at Diverse Anatomical Sites. Cancers (Basel) 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Cosper, P.F.; Bradley, S.; Luo, L.; Kimple, R.J. Biology of HPV Mediated Carcinogenesis and Tumor Progression. Semin Radiat Oncol 2021, 31, 265. [Google Scholar] [CrossRef]
- Lechner, M.S.; Laimins, L.A. Inhibition of P53 DNA Binding by Human Papillomavirus E6 Proteins. J Virol 1994, 68, 4262–4273. [Google Scholar] [CrossRef]
- Thomas, M.; David, P.; Banks, L. The Role of the E6-P53 Interaction in the Molecular Pathogenesis of HPV. Oncogene 1999, 18, 7690–7700. [Google Scholar] [CrossRef]
- Mantovani, F.; Banks, L. The Human Papillomavirus E6 Protein and Its Contribution to Malignant Progression. Oncogene 2001, 20, 7874–7887. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kim, S.S.; Jones, R.N.; Zhang, L.; Guram, K.; Sharma, S.; Schoenberger, S.P.; Cohen, E.E.W.; Califano, J.A.; Sharabi, A.B. Human Papillomavirus E5 Suppresses Immunity via Inhibition of the Immunoproteasome and STING Pathway. Cell Rep 2023, 42. [Google Scholar] [CrossRef]
- Ashrafi, G.H.; Haghshenas, M.; Marchetti, B.; Campo, M.S. E5 Protein of Human Papillomavirus 16 Downregulates HLA Class I and Interacts with the Heavy Chain via Its First Hydrophobic Domain. Int J Cancer 2006, 119, 2105–2112. [Google Scholar] [CrossRef]
- Campo, M.S.; Graham, S. V.; Cortese, M.S.; Ashrafi, G.H.; Araibi, E.H.; Dornan, E.S.; Miners, K.; Nunes, C.; Man, S. HPV-16 E5 down-Regulates Expression of Surface HLA Class I and Reduces Recognition by CD8 T Cells. Virology 2010, 407, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, D.J.; Tremellen, K.P.; Jasper, M.J.; Gemzell-Danielsson, K.; Robertson, S.A. Seminal Fluid Induces Leukocyte Recruitment and Cytokine and Chemokine MRNA Expression in the Human Cervix after Coitus. The Journal of Immunology 2012, 188, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, M.B.; Katki, H.A.; Kreimer, A.R.; Sherman, M.E. Epidemiologic Analysis of Histologic Cervical Inflammation: Relationship to Human Papillomavirus Infections. Hum Pathol 2008, 39, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Potale, Y.; Kasat, Y.K.; Kumar, A.; Ahmad, F. Unravelling the Impact of Human Papillomavirus (HPV): A Comprehensive Exploration of Its Role in Cancer Progression and Global Health Challenges. [CrossRef]
- Faja, F.; Pallotti, F.; Bianchini, S.; Buonacquisto, A.; Cicolani, G.; Conflitti, A.C.; Fracella, M.; Cavallari, E.N.; Sciarra, F.; Pierangeli, A.; et al. Molecular Study of the Presence and Transcriptional Activity of HPV in Semen. J Endocrinol Invest 2024, 47, 557–570. [Google Scholar] [CrossRef]
- Capra, G.; Schillaci, R.; Bosco, L.; Roccheri, M.C.; Perino, A.; Ragusa, M.A. HPV Infection in Semen: Results from a New Molecular Approach. Epidemiol Infect 2019, 147, e177. [Google Scholar] [CrossRef]
- Pérez-Soto, E.; Fernández-Martínez, E.; Oros-Pantoja, R.; Medel-Flores, O.; Miranda-Covarrubias, J.C.; Sánchez-Monroy, V. Proinflammatory and Oxidative Stress States Induced by Human Papillomavirus and Chlamydia Trachomatis Coinfection Affect Sperm Quality in Asymptomatic Infertile Men. Medicina 2021, Vol. 57, Page 862 2021, 57, 862. [Google Scholar] [CrossRef]
- Giannini, S.L.; Al-Saleh, W.; Piron, H.; Jacobs, N.; Doyen, J.; Boniver, J.; Delvenne, P. Cytokine Expression in Squamous Intraepithelial Lesions of the Uterine Cervix: Implications for the Generation of Local Immunosuppression. Clin Exp Immunol 1998, 113, 183–189. [Google Scholar] [CrossRef]
- Torres-Poveda, K.; Bahena-Román, M.; Madrid-González, C.; Burguete-García, A.I.; Bermúdez-Morales, V.H.; Peralta-Zaragoza, O.; Madrid-Marina, V. Role of IL-10 and TGF-Β1 in Local Immunosuppression in HPV-Associated Cervical Neoplasia. World J Clin Oncol 2014, 5, 753. [Google Scholar] [CrossRef]
- Pahne-Zeppenfeld, J.; Schröer, N.; Walch-Rückheim, B.; Oldak, M.; Gorter, A.; Hegde, S.; Smola, S. Cervical Cancer Cell-Derived Interleukin-6 Impairs CCR7-Dependent Migration of MMP-9-Expressing Dendritic Cells. Int J Cancer 2014, 134, 2061–2073. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Current Biology 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- LaRosa, D.F.; Orange, J.S. 1. Lymphocytes. J Allergy Clin Immunol 2008, 121. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, B.; DeNardo, D.G.; Affara, N.I.; Coussens, L.M. Lymphocytes in Cancer Development: Polarization towards pro-Tumor Immunity. Cytokine Growth Factor Rev 2010, 21, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kravtsov, D.S.; Erbe, A.K.; Sondel, P.M.; Rakhmilevich, A.L. Roles of CD4+ T Cells as Mediators of Antitumor Immunity. Front Immunol 2022, 13, 972021. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T Cells in Cancer Immunosuppression - Implications for Anticancer Therapy. Nat Rev Clin Oncol 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Philip, M.; Schietinger, A. CD8+ T Cell Differentiation and Dysfunction in Cancer. Nat Rev Immunol 2022, 22, 209–223. [Google Scholar] [CrossRef]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front Immunol 2018, 9. [Google Scholar] [CrossRef]
- Fantini, M.; Arlen, P.M.; Tsang, K.Y. Potentiation of Natural Killer Cells to Overcome Cancer Resistance to NK Cell-Based Therapy and to Enhance Antibody-Based Immunotherapy. Front Immunol 2023, 14. [Google Scholar] [CrossRef]
- Portale, F.; Di Mitri, D. NK Cells in Cancer: Mechanisms of Dysfunction and Therapeutic Potential. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The Role of Macrophages in Cancer Development and Therapy. Cancers 2021, Vol. 13, Page 1946 2021, 13, 1946. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New Insights into M1/M2 Macrophages: Key Modulators in Cancer Progression. Cancer Cell Int 2021, 21, 1–7. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Descamps, G.; Seminerio, I.; Furgiuele, S.; Dequanter, D.; Mouawad, F.; Badoual, C.; Journe, F.; Saussez, S. HPV Involvement in the Tumor Microenvironment and Immune Treatment in Head and Neck Squamous Cell Carcinomas. Cancers 2020, Vol. 12, Page 1060 2020, 12, 1060. [Google Scholar] [CrossRef]
- Gorvel, L.; Olive, D. Tumor Associated Macrophage in HPV+ Tumors: Between Immunosuppression and Inflammation. Semin Immunol 2023, 65, 101671. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front Physiol 2018, 9, 324475. [Google Scholar] [CrossRef]
- Wu, L.; Saxena, S.; Singh, R.K. Neutrophils in the Tumor Microenvironment. Adv Exp Med Biol 2020, 1224, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Que, H.; Fu, Q.; Lan, T.; Tian, X.; Wei, X. Tumor-Associated Neutrophils and Neutrophil-Targeted Cancer Therapies. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 2022, 1877, 188762. [Google Scholar] [CrossRef]
- Dutta, A.; Bhagat, S.; Paul, S.; Katz, J.P.; Sengupta, D.; Bhargava, D. Neutrophils in Cancer and Potential Therapeutic Strategies Using Neutrophil-Derived Exosomes. Vaccines 2023, Vol. 11, Page 1028 2023, 11, 1028. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Munitz, A.; Ackerman, S.J.; Drake, M.G.; Jackson, D.J.; Wardlaw, A.J.; Dougan, S.K.; Berdnikovs, S.; Schleich, F.; Matucci, A.; et al. Eosinophils in Health and Disease: A State-of-the-Art Review. Mayo Clin Proc 2021, 96, 2694–2707. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Rothenberg, M.E. The Regulatory Function of Eosinophils. Microbiol Spectr 2016, 4. [Google Scholar] [CrossRef]
- Thippabhotla, S.; Zhong, C.; He, M. 3D Cell Culture Stimulates the Secretion of in Vivo like Extracellular Vesicles. Sci Rep 2019, 9. [Google Scholar] [CrossRef]
- Ghaffari, S.; Rezaei, N. Eosinophils in the Tumor Microenvironment: Implications for Cancer Immunotherapy. J Transl Med 2023, 21. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Castle, P.E. Human Papillomavirus: Epidemiology and Public Health. Arch Pathol Lab Med 2003, 127, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Brianti, P.; De Flammineis, E.; Mercuri, S.R. Review of HPV-Related Diseases and Cancers.
- Reusser, N.M.; Downing, C.; Guidry, J.; Tyring, S.K. HPV Carcinomas in Immunocompromised Patients. J Clin Med 2015, 4, 260–281. [Google Scholar] [CrossRef] [PubMed]
- Siegel Mph, R.L.; Giaquinto, A.N.; Ahmedin, |; Dvm, J.; Siegel, R.L. Cancer Statistics, 2024. CA Cancer J Clin 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Lin, L.W.; Chen, Y.W.; Ye, Y.L. The Expression and Prognostic Impact of Proinflammatory Cytokines and Their Associations with Carcinogens in Oropharyngeal Squamous Cell Carcinoma. Cancer Immunology, Immunotherapy 2020, 69, 549–558. [Google Scholar] [CrossRef]
- Turksma, A.; Bontkes, H.; van den Heuvel, H.; De Gruijl, T.; Von Blomberg, B.; Braakhuis, B.; Leemans, C.; Bloemena, E.; Meijer, C.; Hooijberg, E. Effector Memory T-Cell Frequencies in Relation to Tumour Stage, Location and HPV Status in HNSCC Patients. Oral Dis 2013, 19, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Matlung, S.E.; Wilhelmina Van Kempen, P.M.; Bovenschen, N.; Baarle, D. Van; Willems, S.M. Differences in T-Cell Infiltrates and Survival between HPV+ and HPV- Oropharyngeal Squamous Cell Carcinoma. Future Sci OA 2016, 2. [Google Scholar] [CrossRef]
- O’Higgins, C.; Ward, F.J.; Eid, R.A. Deciphering the Role of Regulatory CD4 T Cells in Oral and Oropharyngeal Cancer: A Systematic Review. Front Oncol 2018, 8, 402601. [Google Scholar] [CrossRef]
- Snietura, M.; Brewczynski, A.; Kopec, A.; Rutkowski, T. Infiltrates of M2-Like Tumour-Associated Macrophages Are Adverse Prognostic Factor in Patients with Human Papillomavirus-Negative but Not in Human Papillomavirus-Positive Oropharyngeal Squamous Cell Carcinoma. Pathobiology 2020, 87, 75–86. [Google Scholar] [CrossRef]
- Ljokjel, B.; Lybak, H.; Moe, S.E.; Berge, J.E.; Vintermyr, O.K.; Helgeland, L.; Haave, H.; Ljokjel, B.; Lybak, H.; Moe, S.E.; et al. Tumor HPV Status, Level of Regulatory T Cells and Macrophage Infiltration Predict up to 20-Year Non-Disease-Specific Survival in Oropharynx Squamous Cell Carcinoma Patients. Biomedicines 2022, Vol. 10, Page 2484 2022, 10, 2484. [Google Scholar] [CrossRef]
- Al-Sahaf, S.; Hendawi, N.B.; Ollington, B.; Bolt, R.; Ottewell, P.D.; Hunter, K.D.; Murdoch, C. Increased Abundance of Tumour-Associated Neutrophils in HPV-Negative Compared to HPV-Positive Oropharyngeal Squamous Cell Carcinoma Is Mediated by IL-1R Signalling. Frontiers in Oral Health 2021, 2, 604565. [Google Scholar] [CrossRef] [PubMed]
- Rimini, M.; Franco, P.; Bertolini, F.; Berardino, D.B.; giulia, Z.M.; Stefano, V.; Andrikou, K.; Arcadipane, F.; Napolitano, M.; Buno, L.V.; et al. The Prognostic Role of Baseline Eosinophils in HPV-Related Cancers: A Multi-Institutional Analysis of Anal SCC and OPC Patients Treated with Radical CT-RT. J Gastrointest Cancer 2023, 54, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Cervical Cancer Available online:. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer#:~:text=Key%20facts,%2D%20and%20middle%2Dincome%20countries. (accessed on 28 April 2024).
- van Luijk, I.F.; Smith, S.M.; Marte Ojeda, M.C.; Oei, A.L.; Kenter, G.G.; Jordanova, E.S. A Review of the Effects of Cervical Cancer Standard Treatment on Immune Parameters in Peripheral Blood, Tumor Draining Lymph Nodes, and Local Tumor Microenvironment. J Clin Med 2022, 11. [Google Scholar] [CrossRef]
- De Jong, A.; Van Poelgeest, M.I.E.; Van Der Hulst, J.M.; Drijfhout, J.W.; Fleuren, G.J.; Melief, C.J.M.; Renter, G.; Offringa, R.; Van Der Burg, S.H. Human Papillomavirus Type 16-Positive Cervical Cancer Is Associated with Impaired CD4+ T-Cell Immunity against Early Antigens E2 and E6. Cancer Res 2004, 64, 5449–5455. [Google Scholar] [CrossRef] [PubMed]
- I, A.; RO, A.; A, W.A.; S, A.A. Role of T Cells in Cervical Cancer. Bioinformation 2023, 19, 556–561. [Google Scholar] [CrossRef]
- Das, D.; Sarkar, B.; Mukhopadhyay, S.; Banerjee, C.; Mondal, S.B. An Altered Ratio of CD4+ And CD8+ T Lymphocytes in Cervical Cancer Tissues and Peripheral Blood – A Prognostic Clue? Asian Pac J Cancer Prev 2018, 19, 471. [Google Scholar] [CrossRef]
- B C Sheu 1, S.M.H. Reversed CD4/CD8 Ratios of Tumor-Infiltrating Lymphocytes Are Correlated with the Progression of Human Cervical Carcinoma - PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/10526283/ (accessed on 28 April 2024).
- Litwin, T.R.; Irvin, S.R.; Chornock, R.L.; Sahasrabuddhe, V. V.; Stanley, M.; Wentzensen, N. Infiltrating T-Cell Markers in Cervical Carcinogenesis: A Systematic Review and Meta-Analysis. Br J Cancer 2021, 124, 831–841. [Google Scholar] [CrossRef]
- Jang, S.; Jang, S.; Nam, E.; Lee, D.; Hong, J.; Kim, S.; Kim, S.; Kim, J.; Kim, Y.; Kim, J. Relationship between the Proportion of Natural Killer Cells in Peripheral Blood Lymphocytes and Risk Factors in Cervical Cancer. 2008, 26, 16546–16546. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, S.; Goswami, S.; Pei, X.; Xiang, L.; Zhang, X.; Yang, H. Clinical Significance of Peripheral Blood and Tumor Tissue Lymphocyte Subsets in Cervical Cancer Patients. BMC Cancer 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Petrillo, M.; Zannoni, G.F.; Martinelli, E.; Anchora, L.P.; Ferrandina, G.; Tropeano, G.; Fagotti, A.; Scambia, G. Polarisation of Tumor-Associated Macrophages toward M2 Phenotype Correlates with Poor Response to Chemoradiation and Reduced Survival in Patients with Locally Advanced Cervical Cancer. PLoS One 2015, 10. [Google Scholar] [CrossRef]
- Lee, H.W.; Choi, H.J.; Ha, S.J.; Lee, K.T.; Kwon, Y.G. Recruitment of Monocytes/Macrophages in Different Tumor Microenvironments. Biochim Biophys Acta 2013, 1835, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.C.; Rossetti, R.A.M.; Alvarez, K.L.F.; Carvalho, J.P.; Margarido, P.F.R.; Baracat, E.C.; Tacla, M.; Boccardo, E.; Yokochi, K.; Lorenzi, N.P.; et al. Lactate Secreted by Cervical Cancer Cells Modulates Macrophage Phenotype. J Leukoc Biol 2019, 105, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Tas, M.; Yavuz, A.; Ak, M.; Ozcelik, B. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Discriminating Precancerous Pathologies from Cervical Cancer. J Oncol 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Du, J.Q.; Zhang, F.; Wang, C.Q.; Zhu, J.F.; Xu, L.X.; Yang, Y.H.; Han, M.F.; Hu, Y. Effects of Peripheral Blood Neutrophil/Lymphocyte Ratio Levels and Their Changes on the Prognosis of Patients with Early Cervical Cancer. Front Oncol 2023, 13, 1139809. [Google Scholar] [CrossRef]
- Holub, K.; Biete, A. Impact of Systemic Inflammation Biomarkers on the Survival Outcomes of Cervical Cancer Patients. Clin Transl Oncol 2019, 21, 836–844. [Google Scholar] [CrossRef]
- Chadha, J.; Chahoud, J.; Spiess, P.E. An Update on Treatment of Penile Cancer. Ther Adv Med Oncol 2022, 14. [Google Scholar] [CrossRef]
- L, I.; R, D.M.; C, C.D.; A, M.A.S.; A, P.S.; D, L.D.M.; M, S.; S, B.; T, C.; D, B.; et al. Penile Carcinoma and HPV Infection (Review). Exp Ther Med 2020, 20. [Google Scholar] [CrossRef]
- Chahoud, J.; Netto, F.; Lazcano Segura, R.; Parra Cuentas, E.R.; Lu, X.; Rao, P.; Wistuba, I.I.; Pickering, C.R.; Pettaway, C.A. Tumor Immune Microenvironment Alterations in Penile Squamous Cell Carcinoma Using Multiplex Immunofluorescence and Image Analysis Approaches. Journal of Clinical Oncology 2020, 38, 4–4. [Google Scholar] [CrossRef]
- Ottenhof, S.R.; Djajadiningrat, R.S.; Thygesen, H.H.; Jakobs, P.J.; Józwiak, K.; Heeren, A.M.; de Jong, J.; Sanders, J.; Horenblas, S.; Jordanova, E.S. The Prognostic Value of Immune Factors in the Tumor Microenvironment of Penile Squamous Cell Carcinoma. Front Immunol 2018, 9. [Google Scholar] [CrossRef]
- Matthias Walter Tumor Microenvironment in Penile Cancer. 2020.
- Campos, M.M.; Ornellasde Souza, M.H.; Pires, V.; Scheiner, M.A.M.; Esteves, E.B.; Ornellas, A.A. Clinical Implications of Natural Killer Cytotoxicity in Patients with Squamous Cell Carcinoma of the Penis. Nat Immun 1998, 16, 256–262. [Google Scholar] [CrossRef]
- Chu, C.; Yao, K.; Lu, J.; Zhang, Y.; Chen, K.; Lu, J.; Zhang, C.Z.; Cao, Y. Immunophenotypes Based on the Tumor Immune Microenvironment Allow for Unsupervised Penile Cancer Patient Stratification. Cancers (Basel) 2020, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.M.; Ottenhof, S.R.; Horenblas, S.; van der Heijden, M.S.; Jordanova, E.S. Defining the Tumor Microenvironment of Penile Cancer by Means of the Cancer Immunogram. Eur Urol Focus 2019, 5, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Ozawa, M.; Tamura, Y.; Suzuki, T.; Suzuki, K.; Kurokawa, K.; Fukabori, Y.; Yamanaka, H. Tumor-Associated Tissue Eosinophilia of Penile Cancer. International Journal of Urology 2002, 9, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Lin, Y.J.; Chen, Y.C.; Liu, I.L.; You, S.L.; Hu, J.M.; Lin, T.C.; Chang, P.K.; Chen, C.Y.; Chou, Y.C.; et al. Human Papillomavirus and Risk of Colorectal Cancer: An Analysis of Nationwide Claims Data. Medicina (Kaunas) 2022, 58. [Google Scholar] [CrossRef]
- Damin, D.C.; Ziegelmann, P.K.; Damin, A.P. Human Papillomavirus Infection and Colorectal Cancer Risk: A Meta-Analysis. Colorectal Dis 2013, 15. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y.; Zhang, Y.; Ling, F.; Zheng, J.; Yao, X.; Lyu, Z.; Feng, H.; Li, Y. Comprehensive Analysis of a Novel Subtype of Immune Microenvironment-Derived HPV-Infected Colorectal Cancer. Microbes Infect 2024, 105315. [Google Scholar] [CrossRef]
- Ambrosio, M.R.; Niccolai, E.; Petrelli, F.; Di Gloria, L.; Bertacca, G.; Giusti, A.; Baldi, S.; Cavazzana, A.; Palmeri, M.; Perotti, B.; et al. Immune Landscape and Oncobiota in HPV-Associated Colorectal Cancer: An Explorative Study. Clin Exp Med 2023, 23, 5101–5112. [Google Scholar] [CrossRef]
- Gondal, T.A.; Chaudhary, N.; Bajwa, H.; Rauf, A.; Le, D.; Ahmed, S. Anal Cancer: The Past, Present and Future. Curr Oncol 2023, 30, 3232–3250. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, J.H.; Huynh, V.O.; Lin, D.; Hillman, R.T.; Abana, C.O.; El Alam, M.B.; Tomasic, K.C.; Karpinets, T. V.; Kouzy, R.; Phan, J.L.; et al. HPV-Related Anal Cancer Is Associated with Changes in the Anorectal Microbiome during Cancer Development. Front Immunol 2023, 14, 1051431. [Google Scholar] [CrossRef]
- Balermpas, P.; Martin, D.; Wieland, U.; Rave-Fränk, M.; Strebhardt, K.; Rödel, C.; Fokas, E.; Rödel, F. Human Papilloma Virus Load and PD-1/PD-L1, CD8+ and FOXP3 in Anal Cancer Patients Treated with Chemoradiotherapy: Rationale for Immunotherapy. Oncoimmunology 2017, 6. [Google Scholar] [CrossRef]
- Dhawan, N.; Afzal, M.Z.; Amin, M. Immunotherapy in Anal Cancer. Curr Oncol 2023, 30, 4538–4550. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Rödel, F.; Balermpas, P.; Rödel, C.; Fokas, E. The Immune Microenvironment and HPV in Anal Cancer: Rationale to Complement Chemoradiation with Immunotherapy. Biochim Biophys Acta Rev Cancer 2017, 1868, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Schernberg, A.; Escande, A.; Rivin Del Campo, E.; Ducreux, M.; Nguyen, F.; Goere, D.; Chargari, C.; Deutsch, E. Leukocytosis and Neutrophilia Predicts Outcome in Anal Cancer. Radiother Oncol 2017, 122, 137–145. [Google Scholar] [CrossRef]
- Toh, E.; Wilson, J.; Sebag-Montefiore, D.; Botterill, I. Neutrophil:Lymphocyte Ratio as a Simple and Novel Biomarker for Prediction of Locoregional Recurrence after Chemoradiotherapy for Squamous Cell Carcinoma of the Anus. Colorectal Dis 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- Erez, N.; Truitt, M.; Olson, P.; Hanahan, D. Article Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-KB-Dependent Manner. Cancer Cell 17 135–147. [CrossRef]
- Barros, M.R.; De Melo, C.M.L.; Barros, M.L.C.M.G.R.; De Cássia Pereira De Lima, R.; De Freitas, A.C.; Venuti, A. Activities of Stromal and Immune Cells in HPV-Related Cancers. Journal of Experimental & Clinical Cancer Research 2018 37:1 2018, 37, 1–18. [Google Scholar] [CrossRef]
- Cirri, P.; Chiarugi, P. Cancer-Associated-Fibroblasts and Tumour Cells: A Diabolic Liaison Driving Cancer Progression. Cancer and Metastasis Reviews 2011 31:1 2011, 31, 195–208. [Google Scholar] [CrossRef]
- Id, K.S.; Mcbride, A.A.; Munger, K. Changing Stem Cell Dynamics during Papillomavirus Infection: Potential Roles for Cellular Plasticity in the Viral Lifecycle and Disease. Viruses 2017, Vol. 9, Page 221 2017, 9, 221. [Google Scholar] [CrossRef]
- Greshock, J.; Nathanson, K.; Martin, A.M.; Zhang, L.; Coukos, G.; Weber, B.L.; Zaks, T.Z. Cancer Cell Lines as Genetic Models of Their Parent Histology: Analyses Based on Array Comparative Genomic Hybridization. Cancer Res 2007, 67, 3594–3600. [Google Scholar] [CrossRef]
- Perkel, J.M. Curing Cell Lines. Biotechniques 2011, 51, 85–90. [Google Scholar] [CrossRef]
- Kasai, F.; Hirayama, N.; Ozawa, M.; Iemura, M.; Kohara, A. Changes of Heterogeneous Cell Populations in the Ishikawa Cell Line during Long-Term Culture: Proposal for an in Vitro Clonal Evolution Model of Tumor Cells. Genomics 2016, 107, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.R.; Abdullatif, M.B.; Burnett, E.C.; Kempsell, K.E.; Conforti, F.; Tolley, H.; Collins, J.E.; Davies, D.E. Long Term Culture of the A549 Cancer Cell Line Promotes Multilamellar Body Formation and Differentiation towards an Alveolar Type II Pneumocyte Phenotype. PLoS One 2016, 11, e0164438. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.A.; Wu, A.; Zhang, H.; Levy, M.S.; Lye, G.J. Microwell Engineering Characterization for Mammalian Cell Culture Process Development. Biotechnol Bioeng 2010, 105, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, A.; Yang, X.; Gong, J.; Yu, M.; Yao, X.; Wang, H.; He, Y. 3D Cell Culture—Can It Be As Popular as 2D Cell Culture? Adv Nanobiomed Res 2021, 1, 2000066. [Google Scholar] [CrossRef]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D Cell Culture to Organs-on-Chips. Trends Cell Biol 2011, 21, 745–754. [Google Scholar] [CrossRef]
- Bielecka, Z.F.; Maliszewska-Olejniczak, K.; Safir, I.J.; Szczylik, C.; Czarnecka, A.M. Three-Dimensional Cell Culture Model Utilization in Cancer Stem Cell Research. Biological Reviews 2017, 92, 1505–1520. [Google Scholar] [CrossRef] [PubMed]
- Goodman, T.T.; Chee, P.N.; Suzie, H.P. 3-D Tissue Culture Systems for the Evaluation and Optimization of Nanoparticle-Based Drug Carriers. Bioconjug Chem 2008, 19, 1951–1959. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Stylianopoulos, T.; Boucher, Y.; Jain, R.K. Delivery of Molecular and Nanoscale Medicine to Tumors: Transport Barriers and Strategies. Annu Rev Chem Biomol Eng 2011, 2, 281–298. [Google Scholar] [CrossRef]
- Larue, L.; Beermann, F. Cutaneous Melanoma in Genetically Modified Animals. Pigment Cell Res 2007, 20, 485–497. [Google Scholar] [CrossRef]
- Cheon, D.J.; Orsulic, S. Mouse Models of Cancer. Annual Review of Pathology: Mechanisms of Disease 2011, 6, 95–119. [Google Scholar] [CrossRef]
- Herreros-Pomares, A.; Zhou, X.; Calabuig-Fariñas, S.; Lee, S.J.; Torres, S.; Esworthy, T.; Hann, S.Y.; Jantus-Lewintre, E.; Camps, C.; Zhang, L.G. 3D Printing Novel in Vitro Cancer Cell Culture Model Systems for Lung Cancer Stem Cell Study. Materials Science and Engineering: C 2021, 122, 111914. [Google Scholar] [CrossRef]
- Geiger, P.; Mayer, B.; Wiest, I.; Schulze, S.; Jeschke, U.; Weissenbacher, T. Binding of Galectin-1 to Breast Cancer Cells MCF7 Induces Apoptosis and Inhibition of Proliferation in Vitro in a 2D- and 3D- Cell Culture Model. BMC Cancer 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mosaad, E.; Chambers, K.; Futrega, K.; Clements, J.; Doran, M.R. Using High Throughput Microtissue Culture to Study the Difference in Prostate Cancer Cell Behavior and Drug Response in 2D and 3D Co-Cultures. BMC Cancer 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, D.; Wu, G.; Wu, J.; Lu, S.; Lo, J.; He, Y.; Zhao, C.; Zhao, X.; Zhang, H.; et al. Metastasis-on-a-Chip Mimicking the Progression of Kidney Cancer in the Liver for Predicting Treatment Efficacy. Theranostics 2020, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Y.; Guo, J.; Lu, W.; Wei, Q.; Zhao, Y. The Construction and Application of Three-Dimensional Biomaterials. Adv Biosyst 2020, 4, 1900238. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and Challenges for Use of Tumor Spheroids as Models to Test Drug Delivery and Efficacy. Journal of Controlled Release 2012, 164, 192–204. [Google Scholar] [CrossRef]
- Jang, S.; Jang, S.; Nam, E.; Lee, D.; Hong, J.; Kim, S.; Kim, S.; Kim, J.; Kim, Y.; Kim, J. Relationship between the Proportion of Natural Killer Cells in Peripheral Blood Lymphocytes and Risk Factors in Cervical Cancer. 2008, 26, 16546–16546. [Google Scholar] [CrossRef]
- Baek, M.H.; Kim, D.Y.; Kim, N.; Rhim, C.C.; Kim, J.H.; Nam, J.H. Incorporating a 3-Dimensional Printer into the Management of Early-Stage Cervical Cancer. J Surg Oncol 2016, 114, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Ding, Y.; Sheng, C.; Pi, Y.; Guo, Y.; Wu, A.; Zhang, Z. Application of 3D Printing in Cervical Cancer Brachytherapy. J Radiat Res Appl Sci 2022, 15, 18–24. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-Dimensional Printing of Hela Cells for Cervical Tumor Model in Vitro. Biofabrication 2014, 6, 035001. [Google Scholar] [CrossRef]
- Kalaivani, Muniandy; Annabel Dass, S.; Shamsuddin, S.; Mohana-Kumaran, N.; Balakrishnan, V.; Muniandy, K.; Asra Ahmad, Z.; Mohana Kumaran, N.; Author, C. Growth and Invasion of 3D Spheroid Tumor of HeLa and CasKi Cervical Cancer Cells. 2021. [Google Scholar] [CrossRef]
- Zuk, A.K.; Wen, X.; Dilworth, S.; Li, D.; Ghali, L. Modeling and Validating Three Dimensional Human Normal Cervix and Cervical Cancer Tissues in Vitro. J Biomed Res 2017, 31, 240. [Google Scholar] [CrossRef]
- De Gregorio, V.; La Rocca, A.; Urciuolo, F.; Annunziata, C.; Tornesello, M.L.; Buonaguro, F.M.; Netti, P.A.; Imparato, G. Modeling the Epithelial-Mesenchymal Transition Process in a 3D Organotypic Cervical Neoplasia. Acta Biomater 2020, 116, 209–222. [Google Scholar] [CrossRef]
- Lin, Z.; Li, X.; Shi, H.; Cao, R.; Zhu, L.; Dang, C.; Sheng, Y.; Fan, W.; Yang, Z.; Wu, S. Decoding the Tumor Microenvironment and Molecular Mechanism: Unraveling Cervical Cancer Subpopulations and Prognostic Signatures through ScRNA-Seq and Bulk RNA-Seq Analyses. Front Immunol 2024, 15, 1351287. [Google Scholar] [CrossRef]
- Jackson, R.; Maarsingh, J.D.; Herbst-Kralovetz, M.M.; Van Doorslaer, K. 3D Oral and Cervical Tissue Models for Studying Papillomavirus Host-Pathogen Interactions. Curr Protoc Microbiol 2020, 59, e129. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Yang, W.; Guo, H.; Dong, R.; Ren, L.; Li, S. A Cervical Cancer Tissue-Derived Decellularized Extracellular Matrix Scaffold for Cervical Cancer Tissue Reconstruction in Vitro. Journal of Southern Medical University 2023, 43, 157. [Google Scholar] [CrossRef]
- Miserocchi, G.; Cocchi, C.; De Vita, A.; Liverani, C.; Spadazzi, C.; Calpona, S.; Di Menna, G.; Bassi, M.; Meccariello, G.; De Luca, G.; et al. Three-Dimensional Collagen-Based Scaffold Model to Study the Microenvironment and Drug-Resistance Mechanisms of Oropharyngeal Squamous Cell Carcinomas. Cancer Biol Med 2021, 18, 502. [Google Scholar] [CrossRef] [PubMed]
- Colley, H.E.; Hearnden, V.; Jones, A. V.; Weinreb, P.H.; Violette, S.M.; MacNeil, S.; Thornhill, M.H.; Murdoch, C. Development of Tissue-Engineered Models of Oral Dysplasia and Early Invasive Oral Squamous Cell Carcinoma. British Journal of Cancer 2011 105:10 2011, 105, 1582–1592. [Google Scholar] [CrossRef]
- Almela, T.; Al-Sahaf, S.; Brook, I.M.; Khoshroo, K.; Rasoulianboroujeni, M.; Fahimipour, F.; Tahriri, M.; Dashtimoghadam, E.; Bolt, R.; Tayebi, L.; et al. 3D Printed Tissue Engineered Model for Bone Invasion of Oral Cancer. Tissue Cell 2018, 52, 71–77. [Google Scholar] [CrossRef]
- Noi, M.; Mukaisho, K.I.; Yoshida, S.; Murakami, S.; Koshinuma, S.; Adachi, T.; Machida, Y.; Yamori, M.; Nakayama, T.; Yamamoto, G.; et al. ERK Phosphorylation Functions in Invadopodia Formation in Tongue Cancer Cells in a Novel Silicate Fibre-Based 3D Cell Culture System. International Journal of Oral Science 2018 10:4 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Vincent-Chong, V.K.; Seshadri, M. Development and Radiation Response Assessment in A Novel Syngeneic Mouse Model of Tongue Cancer: 2D Culture, 3D Organoids and Orthotopic Allografts. Cancers 2020, Vol. 12, Page 579 2020, 12, 579. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.F.; Wu, M.H.; Hsu, C.W.; Chen, Y.D. Real-Time and Non-Invasive Impedimetric Monitoring of Cell Proliferation and Chemosensitivity in a Perfusion 3D Cell Culture Microfluidic Chip. Biosens Bioelectron 2014, 51, 16–21. [Google Scholar] [CrossRef]
- Igawa, K.; Izumi, K.; Sakurai, Y. Development of the Follow-Up Human 3D Oral Cancer Model in Cancer Treatment. BioTech 2023, Vol. 12, Page 35 2023, 12, 35. [Google Scholar] [CrossRef]
- Tanaka, N.; Osman, A.A.; Takahashi, Y.; Lindemann, A.; Patel, A.A.; Zhao, M.; Takahashi, H.; Myers, J.N. Head and Neck Cancer Organoids Established by Modification of the CTOS Method Can Be Used to Predict in Vivo Drug Sensitivity. Oral Oncol 2018, 87, 49–57. [Google Scholar] [CrossRef]
- Sawant, S.; Dongre, H.; Singh, A.K.; Joshi, S.; Costea, D.E.; Mahadik, S.; Ahire, C.; Makani, V.; Dange, P.; Sharma, S.; et al. Establishment of 3D Co-Culture Models from Different Stages of Human Tongue Tumorigenesis: Utility in Understanding Neoplastic Progression. PLoS One 2016, 11, e0160615. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van Den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-Term Expansion of Epithelial Organoids from Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.N.; Al-Saffar, A.Z.; Jasim, S.M.; Sulaiman, G.M. Comparative Analysis between 2D and 3D Colorectal Cancer Culture Models for Insights into Cellular Morphological and Transcriptomic Variations. Scientific Reports 2023 13:1 2023, 13, 1–16. [Google Scholar] [CrossRef]
- Koch, J.; Mönch, D.; Maaß, A.; Gromoll, C.; Hehr, T.; Leibold, T.; Schlitt, H.J.; Dahlke, M.H.; Renner, P. Three Dimensional Cultivation Increases Chemo- and Radioresistance of Colorectal Cancer Cell Lines. PLoS One 2021, 16, e0244513. [Google Scholar] [CrossRef] [PubMed]
- Magdeldin, T.; López-Dávila, V.; Villemant, C.; Cameron, G.; Drake, R.; Cheema, U.; Loizidou, M. The Efficacy of Cetuximab in a Tissue-Engineered Three-Dimensional in Vitro Model of Colorectal Cancer. J Tissue Eng 2014, 5. [Google Scholar] [CrossRef]
- Cives, M.; Mannavola, F.; Lospalluti, L.; Sergi, M.C.; Cazzato, G.; Filoni, E.; Cavallo, F.; Giudice, G.; Stucci, L.S.; Porta, C.; et al. Non-Melanoma Skin Cancers: Biological and Clinical Features. Int J Mol Sci 2020, 21, 1–24. [Google Scholar] [CrossRef]
- Berning, M.; Prätzel-Wunder, S.; Bickenbach, J.R.; Boukamp, P. Three-Dimensional In Vitro Skin and Skin Cancer Models Based on Human Fibroblast-Derived Matrix. https://home.liebertpub.com/tec 2015, 21, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Vörsmann, H.; Groeber, F.; Walles, H.; Busch, S.; Beissert, S.; Walczak, H.; Kulms, D. Development of a Human Three-Dimensional Organotypic Skin-Melanoma Spheroid Model for in Vitro Drug Testing. Cell Death & Disease 2013 4:7 2013, 4, e719–e719. [Google Scholar] [CrossRef]
- Hill, D.S.; Robinson, N.D.P.; Caley, M.P.; Chen, M.; O’Toole, E.A.; Armstrong, J.L.; Przyborski, S.; Lovat, P.E. A Novel Fully Humanized 3D Skin Equivalent to Model Early Melanoma Invasion. Mol Cancer Ther 2015, 14, 2665–2673. [Google Scholar] [CrossRef]
- Visalakshan, R.M.; Lowrey, M.K.; Sousa, M.G.C.; Helms, H.R.; Samiea, A.; Schutt, C.E.; Moreau, J.M.; Bertassoni, L.E. Opportunities and Challenges to Engineer 3D Models of Tumor-Adaptive Immune Interactions. Front Immunol 2023, 14, 1162905. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chaudhuri, O. Modeling the Tumor Immune Microenvironment for Drug Discovery Using 3D Culture. APL Bioeng 2021, 5, 10903. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, Vol. 12, Page 1186 2020, 12, 1186. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, S.F.; Wang, J.; Li, X.; Wang, H.; Pu, W.Y.; Zhang, H.; Zhuang, Z.X. Effect of Environmental Factors on Chemoresistance of HepG2 Cells by Regulating Hypoxia-Inducible Factor-1α. Chin Med J (Engl) 2012, 125, 1095–1103. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, X.P.; Luo, S.F.; Guan, J.; Zhang, W.G.; Zhang, B.X. Involvement of Hypoxia-Inducible Factor-1-Alpha in Multidrug Resistance Induced by Hypoxia in HepG2 Cells. J Exp Clin Cancer Res 2005, 24, 565–574. [Google Scholar]
- Wartenberg, M.; Frey, C.; Diedershagen, H.; Ritgen, J.; Hescheler, J.; Sauer, H. DEVELOPMENT OF AN INTRINSIC P-GLYCOPROTEIN-MEDIATED DOXORUBICIN RESISTANCE IN QUIESCENT CELL LAYERS OF LARGE, MULTICELLULAR PROSTATE TUMOR SPHEROIDS. J. Cancer 1998, 75, 855–863. [Google Scholar] [CrossRef]
- Milane, L.; Duan, Z.; Amiji, M. Role of Hypoxia and Glycolysis in the Development of Multi-Drug Resistance in Human Tumor Cells and the Establishment of an Orthotopic Multi-Drug Resistant Tumor Model in Nude Mice Using Hypoxic Pre-Conditioning. Cancer Cell Int 2011, 11, 1–16. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated PH: A Perfect Storm for Cancer Progression. Nature Reviews Cancer 2011 11:9 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.L.; Bissell, M.J. The Tumor Microenvironment Is a Dominant Force in Multidrug Resistance. Drug Resistance Updates 2012, 15, 39–49. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





