Submitted:
09 September 2024
Posted:
11 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Description of starting materials
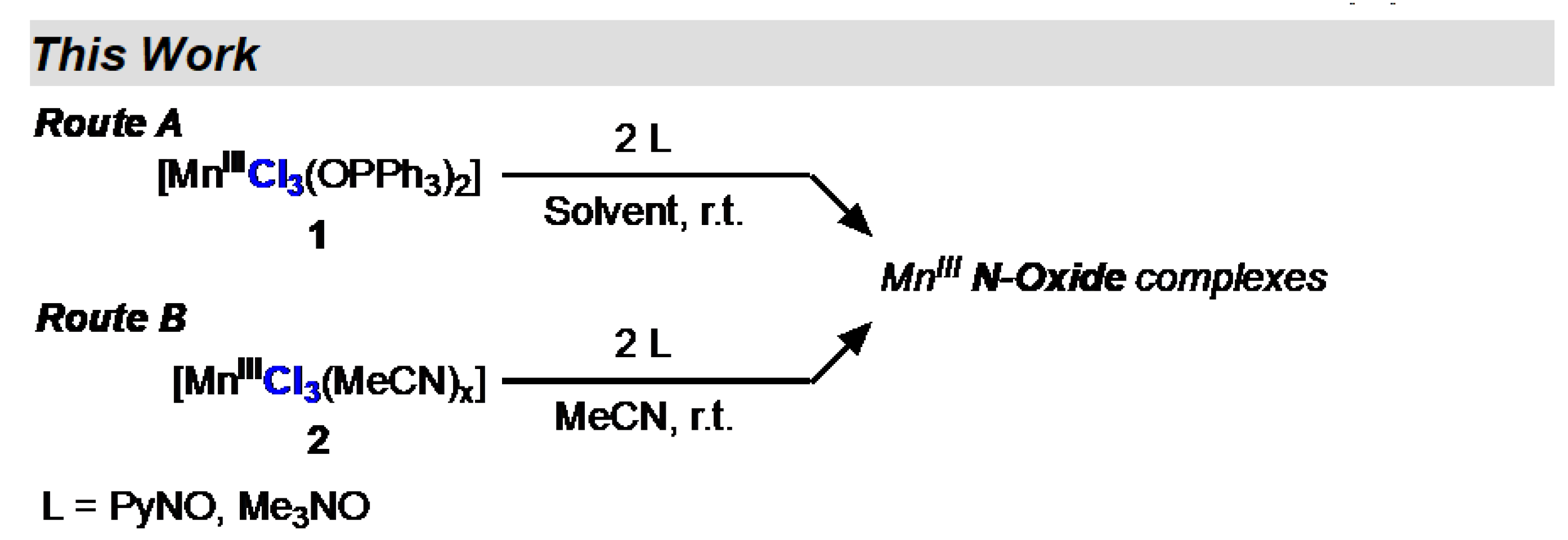
2.2. Synthesis of Mn(III) trimethylamine-N-oxide compounds
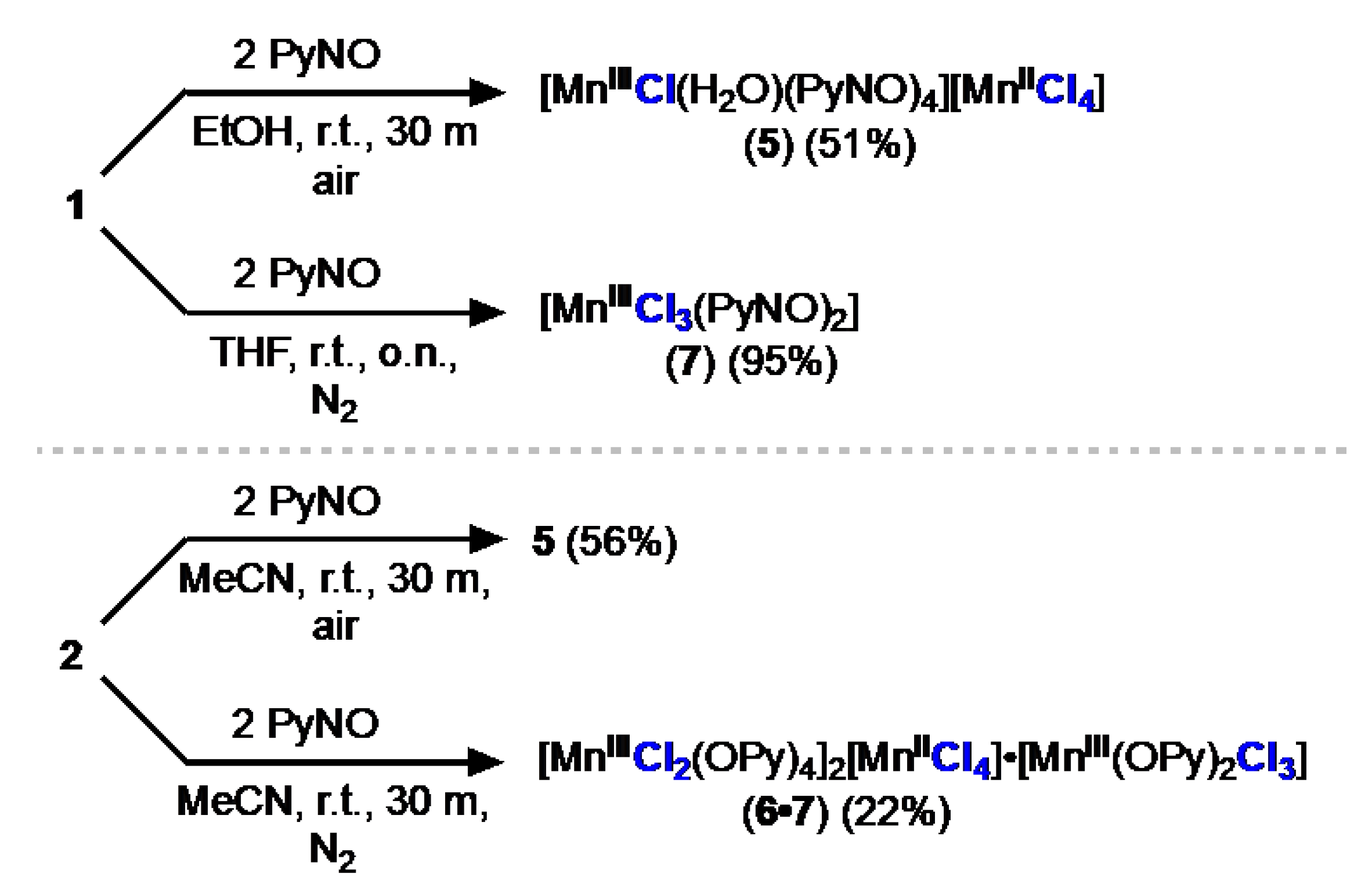
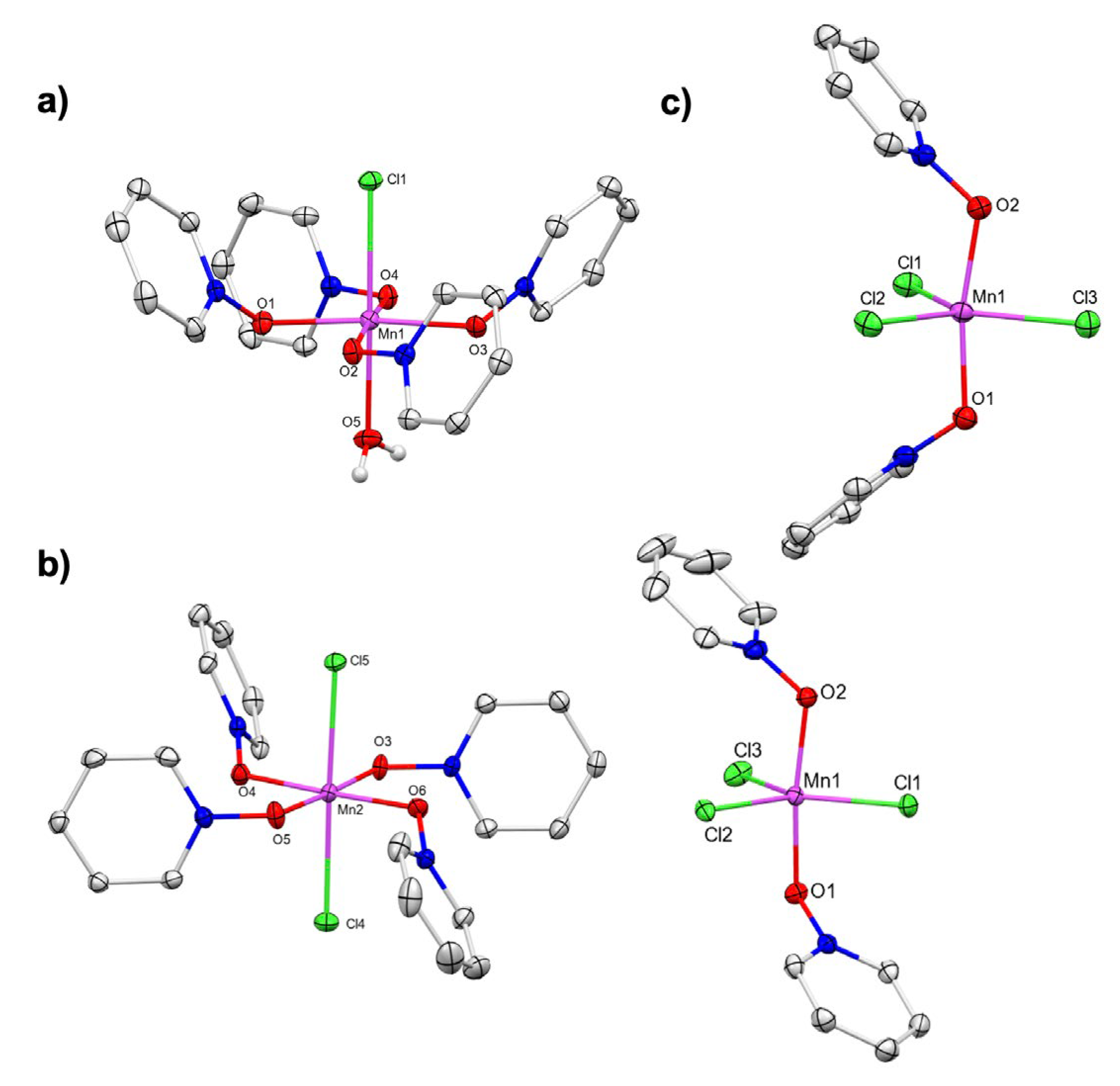
2.3. Synthesis of Mn(III) PyNO compounds
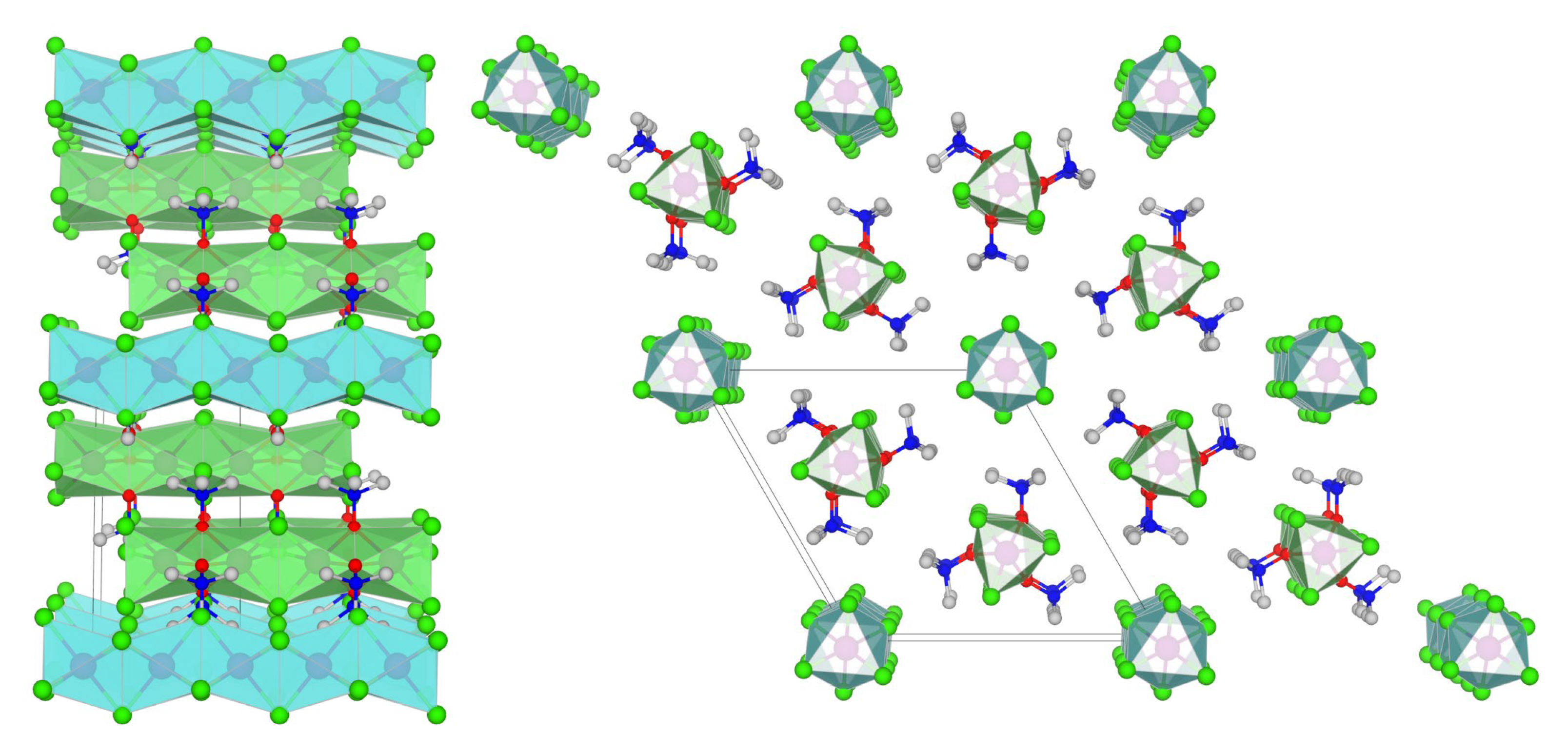
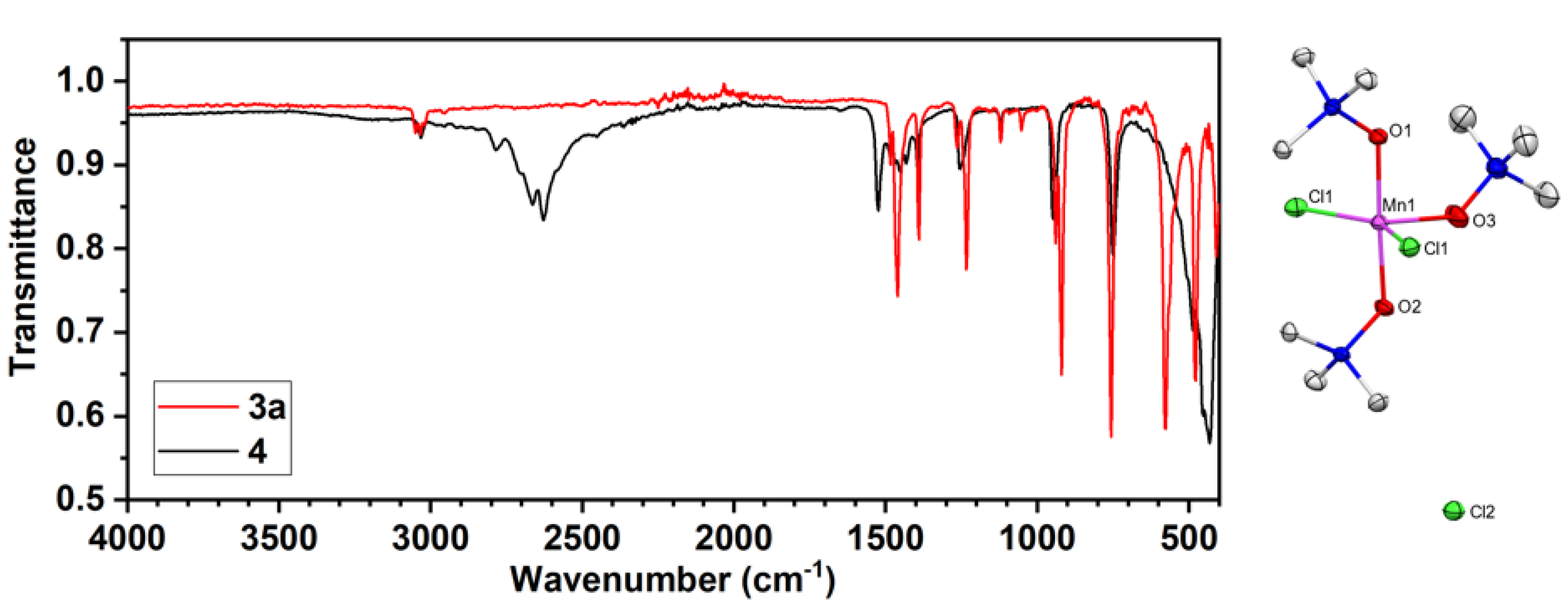
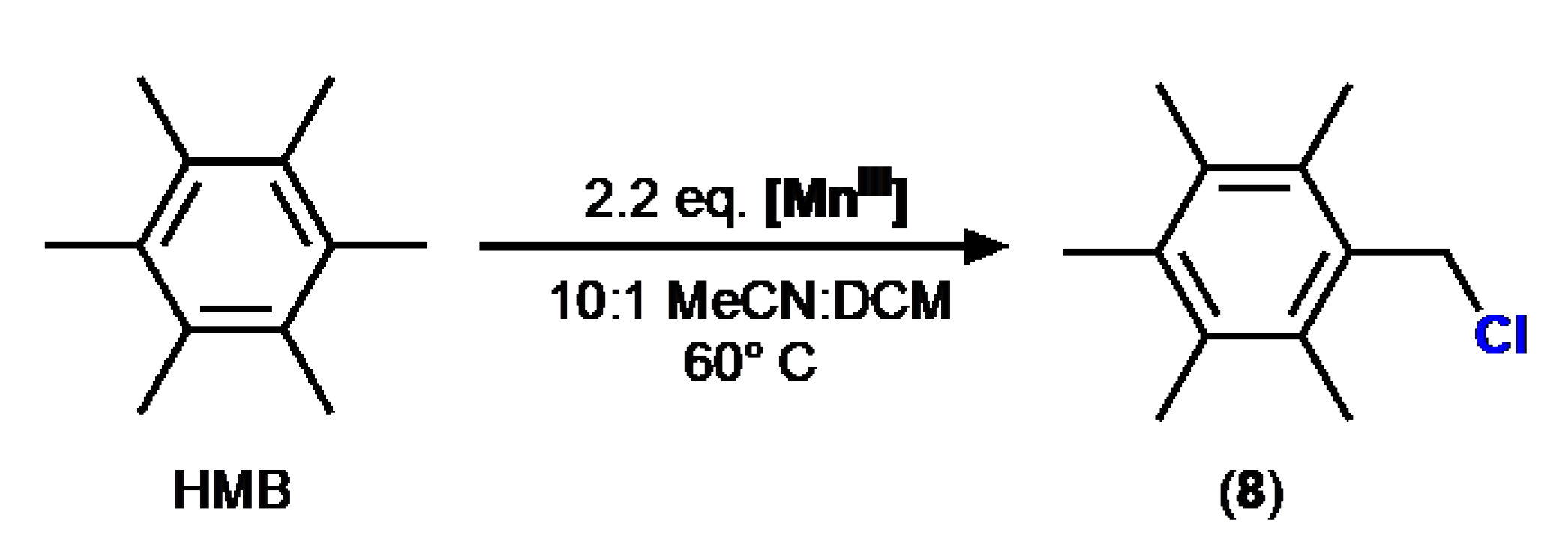
2.4. Reactivity of Mn(III) chloride compounds
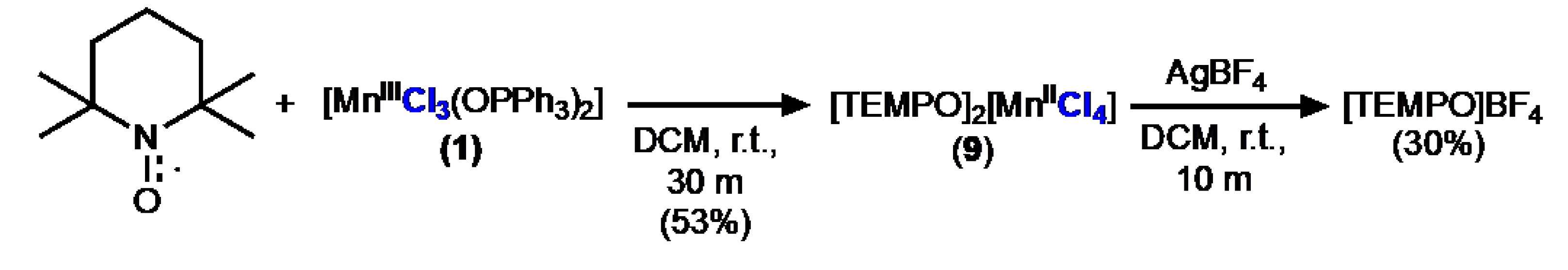
2.5. Reaction of 1 with 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO):
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
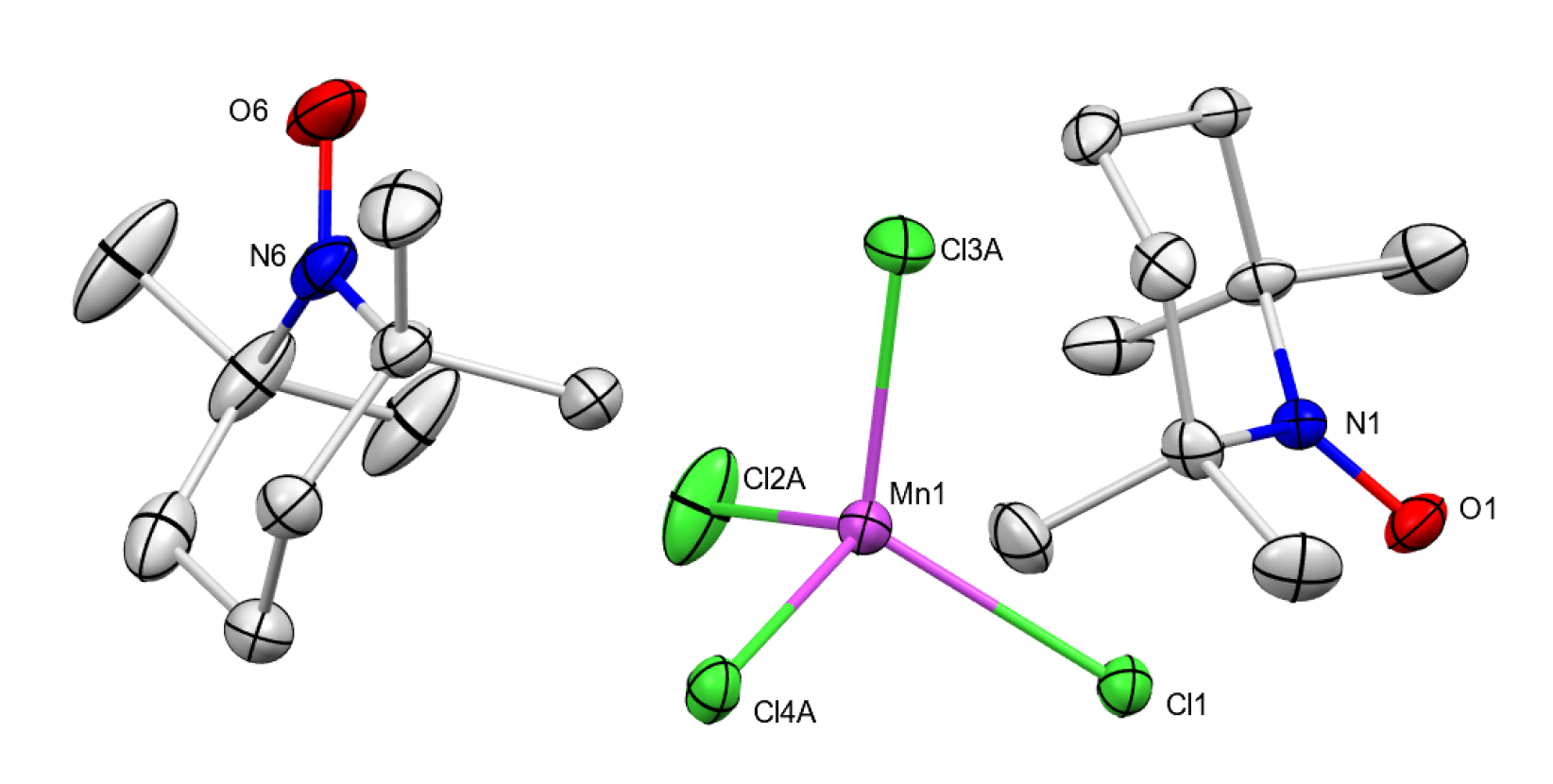
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
| 1 | (a) Lingappa, U.F.; Monteverde, D.R.; Magyar, J.S.; Valentine, J.S.; Fischer, W.W. How manganese empowered life with dioxygen (and vice versa). Free Radic. Biol. Med. 2019, 140, 113–125. (b) Zhu, W.; Richards, N.G.J. Biological functions controlled by manganese redox changes in mononuclear Mn-dependent enzymes. Essays Biochem. 2017, 61, 259-270. (c) Li, H.; Santos, F.; Butler, K.; Herndon, E.; A critical review on the multiple roles of manganese in stabilizing and destabilizing soil organic matter. Environ. Sci. Technol. 2021, 55, 12136–12152. |
| 2 | Alkene difunctionalization: (a) Fu, N.; Sauer, G. S.; Saha, A.; Loo, A.; Lin, S. Metal-catalyzed electrochemical diazidation of alkenes. Science 2017, 357, 575–579. (b) Sauer, G. S.; Lin, S. An Electrocatalytic approach to the radical difunctionalization of alkenes. ACS Catal. 2018, 8, 5175–5187. (c) Dong, X.; Roeckl, J. L.; Waldvogel, S. R.; Morandi, B. Merging shuttle reactions and paired electrolysis for reversible vicinal dihalogenations. Science 2021, 371, 507–514. |
| 3 | Alkene hydroxylation and epoxidation: (a) Philip, R. M.; Radhika, S.; Abdulla, C. M. A.; Anilkumar, G. Recent trends and prospects in homogeneous manganese-catalyzed epoxidation. Adv. Synth. Catal. 2021, 363, 1272–1289. (b) Eisink, N. N. H. M.; Browne, W. R. Chapter 10. Manganese-catalyzed dihydroxylation and epoxidation of olefins. In Manganese Catalysis in Organic Synthesis. Wiley-VCH, 2022, 323-343. |
| 4 | Radical functionalizations with Mn(OAc)3. (a) Demir, A.S.; Emrullahoglu, M. Manganese(III) acetate: a versatile reagent in organic chemistry. Curr. Org. Synth. 2007, 4, 321–350. (b) Mondal, M.; Bora, U. Recent advances in manganese(III) acetate mediated organic synthesis. RSC Advances. 2013, 3, 18716-18754. (c) Carney, J. R.; Dillon, B. R.; Thomas, S. P. Recent advances of manganese catalysis for organic synthesis. Eur. J. Org. Chem. 2016, 3912-3929. (d) Snider, B. B. Chapter 9. Manganese(III) acetate-mediated cyclizations. In Manganese Catalysis in Organic Synthesis. Wiley-VCH, 2022, 293-322. |
| 5 | (a) Goodwin, H. A.; Sylva, R. N. The magnetic properties of some complexes of higher-valent manganese. Aust. J. Chem. 1967, 20, 629–637. (b) Funk, H.; Kreis, H. Zur kenntnis des dreiwertigen mangans: verbindungen des mangan(III)-chlorids mit aminen und einigen Äthern. Z. Anorg. Allg. Chem. 1967, 349, 45–49. (c) Davis, T. S.; Fackler, J. P.; Weeks, M. J. spectra of manganese(III) complexes. the origin of the low-energy band. Inorg. Chem. 1968, 7, 1994–2002. (d) Nachtigall, O.; Pataki, A.; Molski, M.; Lentz, D.; Spandl, J. solvates of manganese trichloride revisited - synthesis, isolation, and crystal structure of MnCl3(THF)3. Z. Anorg. Allg. Chem. 2015, 641, 1164–1168. |
| 6 | Perlepes, S. P.; Blackman, A. G.; Huffman, J. C.; Christou, G. Complete carboxylate removal from [Mn12O12(OAc)16(H2O)4]•2HOAc•4H2O with Me3SiCl: synthesis and characterization of polymeric [MnCl3(bipy)]n and an improved Synthesis of (NEt4)2MnCl5. Inorg. Chem. 1991, 30, 1665–1668. |
| 7 | Saju, A.; Griffiths, J. R.; MacMillan, S. N.; Lacy, D. C. Synthesis of a bench-stable manganese(III) chloride compound: coordination chemistry and alkene dichlorination. J. Am. Chem. Soc. 2022, 144, 16761. |
| 8 | ConQuest (CSD version 5.45) search for transition metal complexes with Me3NO ligands produces 18 examples of the following metal ions –Re+1, Mn1+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, and Y3+. |
| 9 | Kadassery, K.J.; Dey, S.K.; Friedman, A.E.; Lacy, D.C. Exploring the role of carbonate in the formation of an organomanganese tetramer. Inorg. Chem. 2017, 56, 8748–8751. |
| 10 | (a) Uson, R.; Riera, V.; Ciriano, M.A.; Valderrama, M. Pentacoordinate neutral manganese (III) complexes. Transit. Met. Chem. 1976, 1, 122–126. (b) Contreras, E.; Riera, V.; Usón, R. Stable complexes of manganese (III) with oxides of pyridine, phosphine and arsine. Inorg. Nucl. Chem. Lett. 1972, 8, 287–291. |
| 11 | Saju, A.; Crawley, M.R.; MacMillan, S.N.; Lacy, D.C. Manganese(III) nitrate complexes as bench-Stable powerful oxidants. J. Am. Chem. Soc. 2024, 146, 11616–11621. |
| 12 | Pokhodnya, K. I.; Bonner, M.; DiPasquale, A. G.; Rheingold, A. L.; Her, J. H.; Stephens, P. W.; Park, J. W.; Kennon, B. S.; Arif, A. M.; Miller, J. S. Structural and magnetic properties of MCl2 (M = Fe, Mn, Co): acetonitrile solvates. Inorg. Chem. 2007, 46, 2471–2477. |
| 13 | Paul, S.; Saju, A.; Cohen, C.; Crawley, M. R.; MacMillan, S. N.; Lacy, D. C. Synthesis of Mn(III)X3 (X = Cl, Br, I) Compounds with Phosphine (R3P) Ligands. Inorg. Chem. 2024, 34, 15791-15803. |
| 14 | (a) Nannenga, B. L.; Gonen, T. The cryo-EM method microcrystal electron diffraction (MicroED) Nature Methods, 2019, 16, 369-379. (b) Ito, S.; White, F. J.; Okunishi, E.; Aoyama, Y.; Yamano, A.; Hiroyasu, S.; Ferrara, J. D.; Jansnowski, M.; Meyer, M. Structure determination of small molecule compounds by an electron diffractometer for 3D ED/MicroED. CrystEngComm. 2021, 23, 8622-8630. |
| 15 | (a) Caputo, R. E.; Roberts, S.; Willett, R. D.; Gerstein, B. C. Crystal structure and magnetic susceptibility of [(CH3)3NH]3Mn2Cl7. Inorg. Chem. 1976, 15, 820-823. (b) Ravindran, M.; Willey, G. R.; Drew, M. G. B. Reactions of trimethylamine with Mn(II) and Cd(II) chlorides: crystal and molecular structure of [Me3NH][MnCl3]. Inorg. Chimica Acta 1990, 175, 99-103. (c) Naito, T.; Inabe, T. Molecular hexagonal perovskite: a new type of organic-inorganic hybrid conductor. Journal of Solid State Chemistry. 2003, 176, 243-249. (d) Sun, X.-F.; Li, P.-F.; Liao, W.-Q.; Wang, Z.; Gao, J.; Ye, H.-Y.; Zhang, Y. Notable broad dielectric relaxation and highly efficient red photoluminescence in perovskite-type compound: (N-methylpyrrolidinium)MnCl3. Inorg. Chem. 2017, 56, 12193-12198. |
| 16 | (a) Sun, Q.; Kioussis, N.; Prediction of manganese trihalides as two-dimensional Dirac half-metals. Phys. Rev. B, 2018, 97, 094408. (b) Zhou, B.; Li, Z.; Theoretical investigation of nonvolatile electrical control behavior by ferroelectric polarization switching in two-dimensional MnCl3/CuInF2S6 van der Waals heterostructures. J. Mater. Chem. C. 2020, 8, 4534. (c) Guo, T.; Liu, Y.; Sun, Y.; Zhang, S.; Xu, X.; Wang, L.; Zhou, W.; Liu, Y.; Yao, X.; Zhang, X. Insight into tunable electronic and magnetic properties in 2D ferromagnetic/antiferromagnetic van der Waals heterostructure. Appl. Phys. Lett. 2023, 122, 192403. |
| 17 | Saju, A.; Gunasekera, P. S.; Morgante, P.; MacMillan, S. N.; Autschbach, J.; Lacy, D. C. Experimental and computational determination of a M−Cl homolytic bond dissociation free energy: Mn(III)Cl-mediated C−H cleavage and chlorination. J. Am. Chem. Soc. 2023, 145, 13384-13391. |
| 18 | Caputo, R.E.; Roberts, S.; Willett, R.D.; Gerstein, B.C. Crystal structure and magnetic susceptibility of heptachlorotris(trimethylammonium)dimanganese. Inorg. Chem. 1976, 15, 820–823. |
| 19 | The instability of 3, the inclusion of MnII counterions in 5, and the composite nature of 6•7 precluded similar characterization. Therefore, the magnetic properties of the other compounds were not pursued in this study. Generally, mononuclear Mn(III) centers studied by us have had S = 2 ground states. Some exceptions are strong-field six-coordinate cationic Mn(III) complexes that are S = 1 (see [7,11,13,17]). |
| 20 | (a) Mondal, P.; Pirovano, P.; Das, A.; Farquhar, E. R.; McDonald, A. R. Hydrogen atom transfer by a high-valent nickel-chloride complex. J. Am. Chem. Soc. 2018, 140, 1834-1841. (b) Mondal, P.; Lovisari, M.; Twamley, B; McDonald, A. R. Fast hydrocarbon oxidation by a high-valent nickel-fluoride complex. Angew. Chem. Int. Ed. 2020, 59, 13044-13050. (c) Kwon, Y. M.; Lee, Y.; Schmautz, A. K.; Jackson, T. A.; Wang, D. C–H bond activation by a mononuclear nickel(IV)-nitrate complex. J. Am. Chem. Soc. 2022, 144, 12072-12080. (d) Kwon, Y. M.; Lee, Y.; Evenson, G. E.; Jackson, T. A.; Wang, D. Crystal structure and C–H bond cleaving reactivity of a mononuclear CoIV-dinitrate complex. J. Am. Chem. Soc. 2020, 142, 13435-13441. (e) Bower, J. K.; Reese, M. S.; Mazin, I. M.; Zarnitsa, L. M.; Cypcar, A. D.; Moore, C. E.; Sokolov, A. Y.; Zhang, S. C(Sp3)-H cyanation by a formal copper(III) cyanide complex. Chem. Sci. 2023, 14, 1301–1307. |
| 21 | (a) Liu, W.; Huang, X.; Cheng, M.; Nielsen, R. J.; Goddard, W. A.; Groves, J. T. Oxidative Aliphatic C-H Fluorination with Fluoride Ion Catalyzed by a Manganese Porphyrin. Science 2012, 337, 1322–1325. (b) Yadav, V.; Wen, L.; Yadav, S.; Siegler, M. A.; Goldberg, D. P. Selective radical transfer in a series of nonheme iron(III) complexes. Inorg. Chem. 2023, 62, 17830–17842. |
| 22 | Kütt, A.; Rodima, T.; Saame, J.; Raamat, E.; Mäemets, V.; Kaljurand, I.; Koppel, I. A.; Garlyauskayte, R. Y.; Yagupolskii, Y. L.; Yagupolskii, L. M.; Bernhardt, E.; Willner, H.; Leito, I. Equilibrium acidities of superacids. J. Org. Chem. 2011, 76, 391. |
| 23 | Explanation for BDFEMn(II)/XH. (a) In a previous report [11], we used the reduction potential of [MnIII(NO3)3(OPPh3)2] and the pKa of the conjugate acid of dissociated [NO3]– in the Bordwell equation to arrive at a thermodynamic value and referred to it as an effective bond dissociation free energy (BDFEeff). The BDFEeff, described by Mayer [24], uses the reduction potential and pKa of oxidant/base pairs that can combine in a single entity (e.g., through H-bonded adduct) to react in bimolecular C–H bond cleavage. The BDFEeff can be used as an estimate for the upper-limit of C–H bond strength the oxidant/base pair can cleave. However, since the base (X) is coordinated to the Mn(III) center, it is more appropriate to use a {MnIIX–H} BDFE (BDFEMn(II)/XH) like the {MnIIIO–H} BDFE (BDFEO–H) reported in metal-oxo/metal-hydroxo conversions as described by Borovik and others [24]. Therefore, we use the same approach as Borovik except that the pKa of the conjugate acid of the free base is used instead of the pKa of [MnIIX2(HX)]/[MnIIX3]– and refer to it as the BDFEMn(II)/XH. Hence, the BDFEMn(II)/XH is an estimate of the upper limit of C–H bond strength that can be cleaved by a {MnIIIX} reactant. We have performed a systemic analysis of this square scheme approach to estimate C–H cleavage capability in a previous report [17]. (b) Barman, S. K.; Yang, M.-Y.; Parsell, T. H.; Green, M. T.; Borovik, A. S. Semiemperical method for examining asynchronicity in metal-oxo-mediated C–H bond activation. Proc. Natl. Acad. Sci., USA. 2021, 118, e2108648118. |
| 24 | Agarwal, R. G.; Coste, S. C.; Groff, B. D.; Heuer, A. M.; Noh, H.; Parada, G. A.; Wise, C. F.; Nichols, E. M.; Warren, J. J.; Mayer, J. M. Free energies of proton-coupled electron transfer reagents and their applications. Chem. Rev. 2022, 122, 1– 4. |
| 25 | Kadassery, K. J.; Sethi, K.; Fanara, P. M.; Lacy, D. C. CO-Photolysis-induced H-atom transfer from MnIO−H Bonds. Inorg. Chem. 2019, 58, 4679–4685. |
| 26 | Hostmann, T.; Molloy, J. J.; Bussmann, K.; Gilmour, R. Light-enabled enantiodivergence: stereospecific reduction of activated alkenes using a single organocatalyst enantiomer. Org. Lett. 2019, 21, 10164–10168. |
| 27 | Eppley, H. J.; Christou, G., Synthesis of dodecaoxohexadecacarboxylatotetraaquo-dodecamanganese [Mn12O12(O2CR)16(H2O)4] (R = Me, Et, Ph, Cr) complexes, Inorg. Syn. 2002, 33, 61. |
| 28 | CrysAlisPro; Rigaku OD, The Woodlands, TX, 2015. |
| 29 | Sheldrick, G. M., SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3. |
| 30 | Sheldrick, G.M. A Short History of SHELX. Acta Cryst. 2008, A64, 112. |
| 31 | Müller, P. Practical Suggestions for Better Crystal Structures. Crystallogr. Rev. 2009, 15, 57. |
| 32 | Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339-341. |
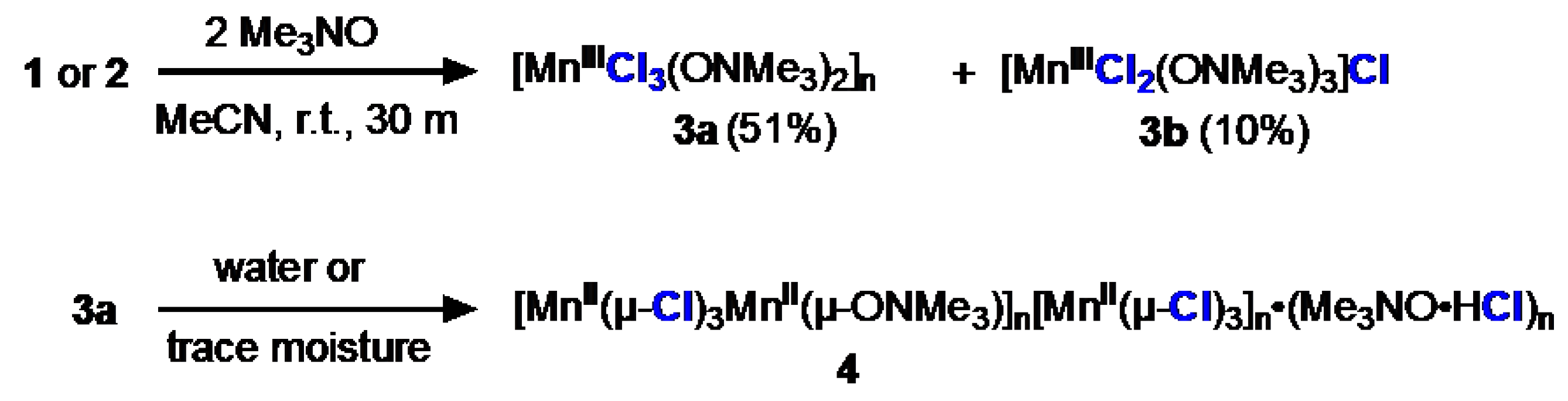
| Complex | Time | % Yielda |
|---|---|---|
| [MnIIICl3(Me3NO)2]n (3a) | 5.0 h | 45 |
| [MnIIICl(H2O)(PyNO)4][MnIICl4] (5) | 6.5 h | 86 |
| [MnIIICl3(PyNO)2] (7) | 4.0 h | 88 |
| [MnIIICl3(MeCN)x] (2) | 1.0 h at r.t.(b) | 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





