Submitted:
10 September 2024
Posted:
11 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
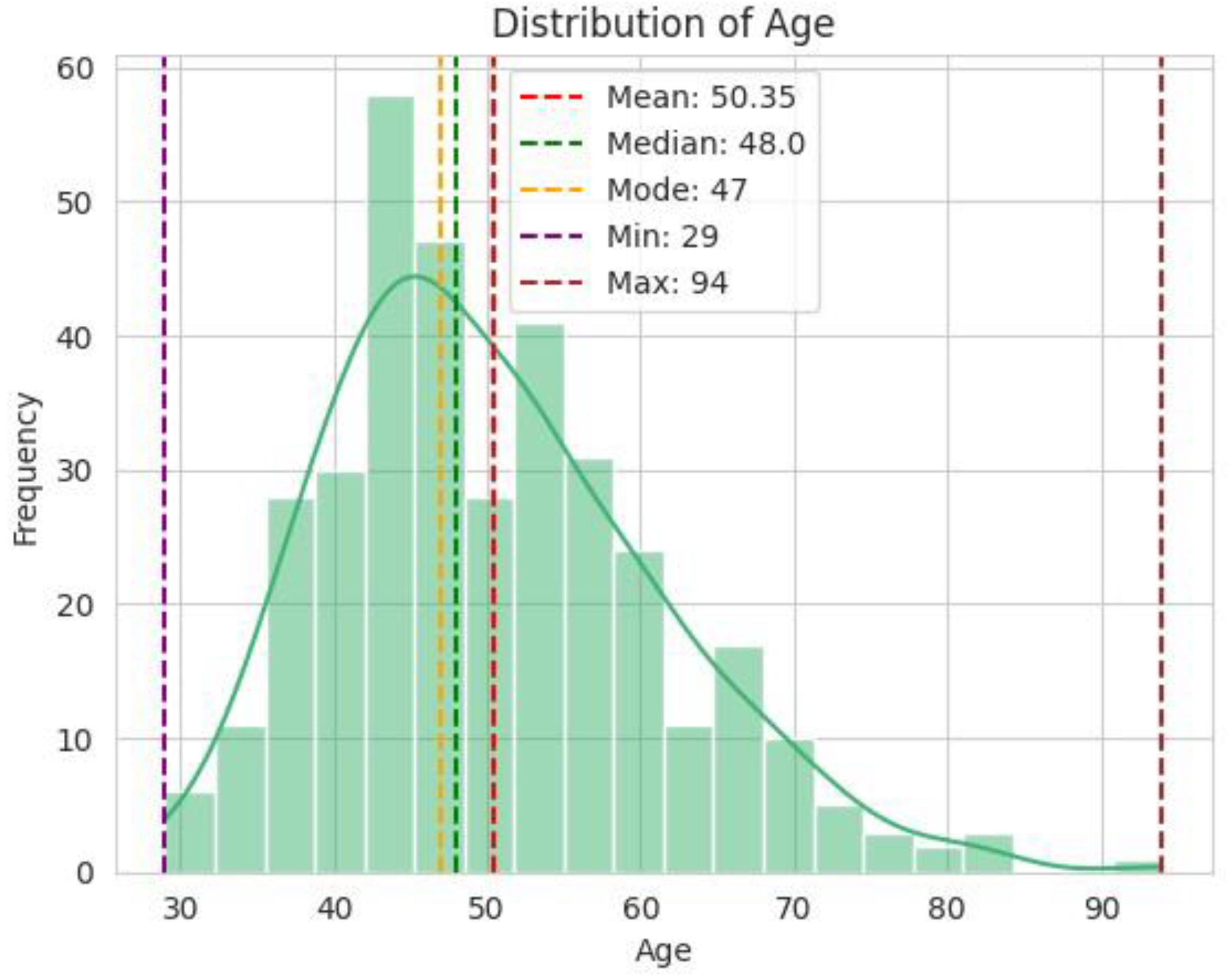
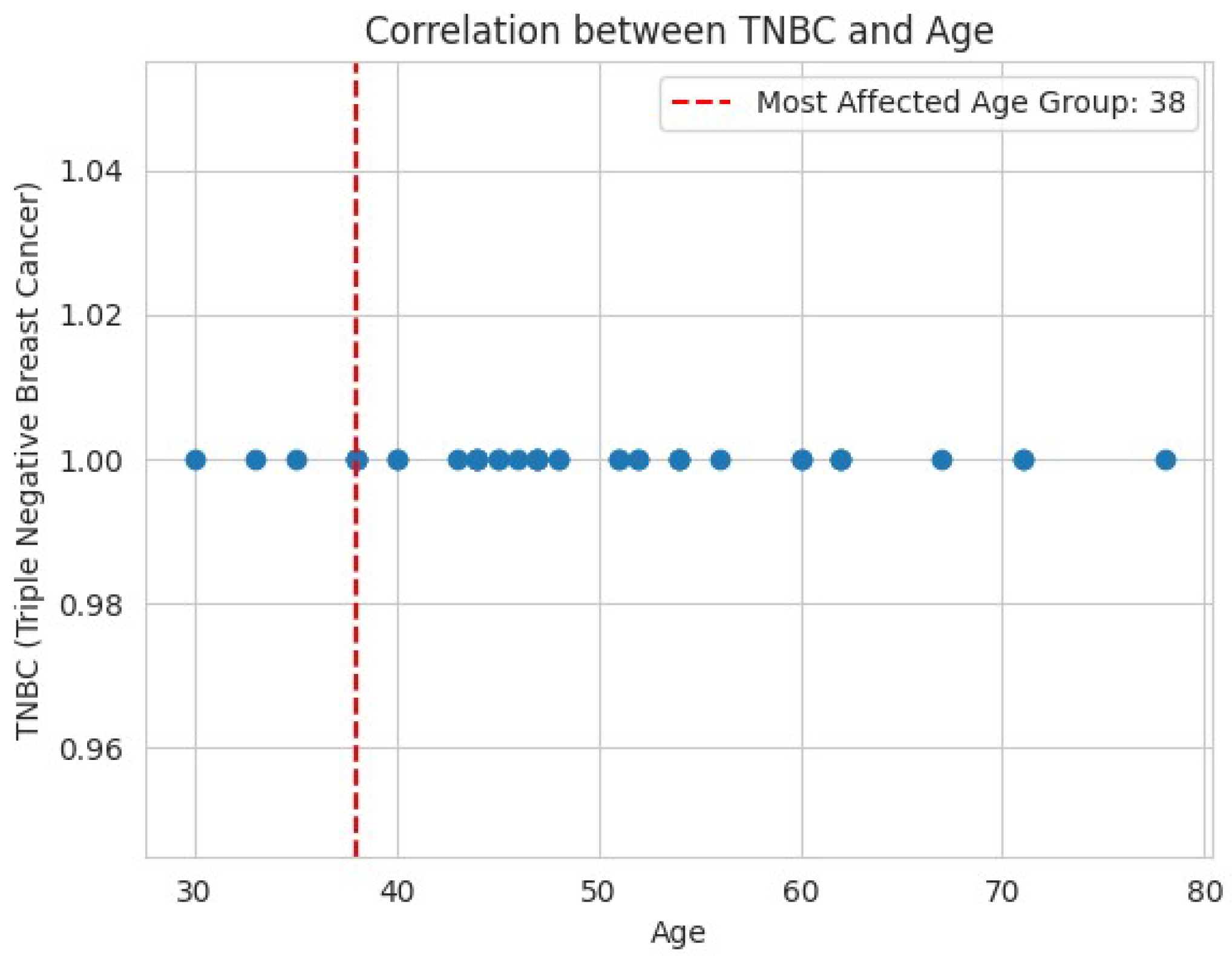
3. Results and Discussions
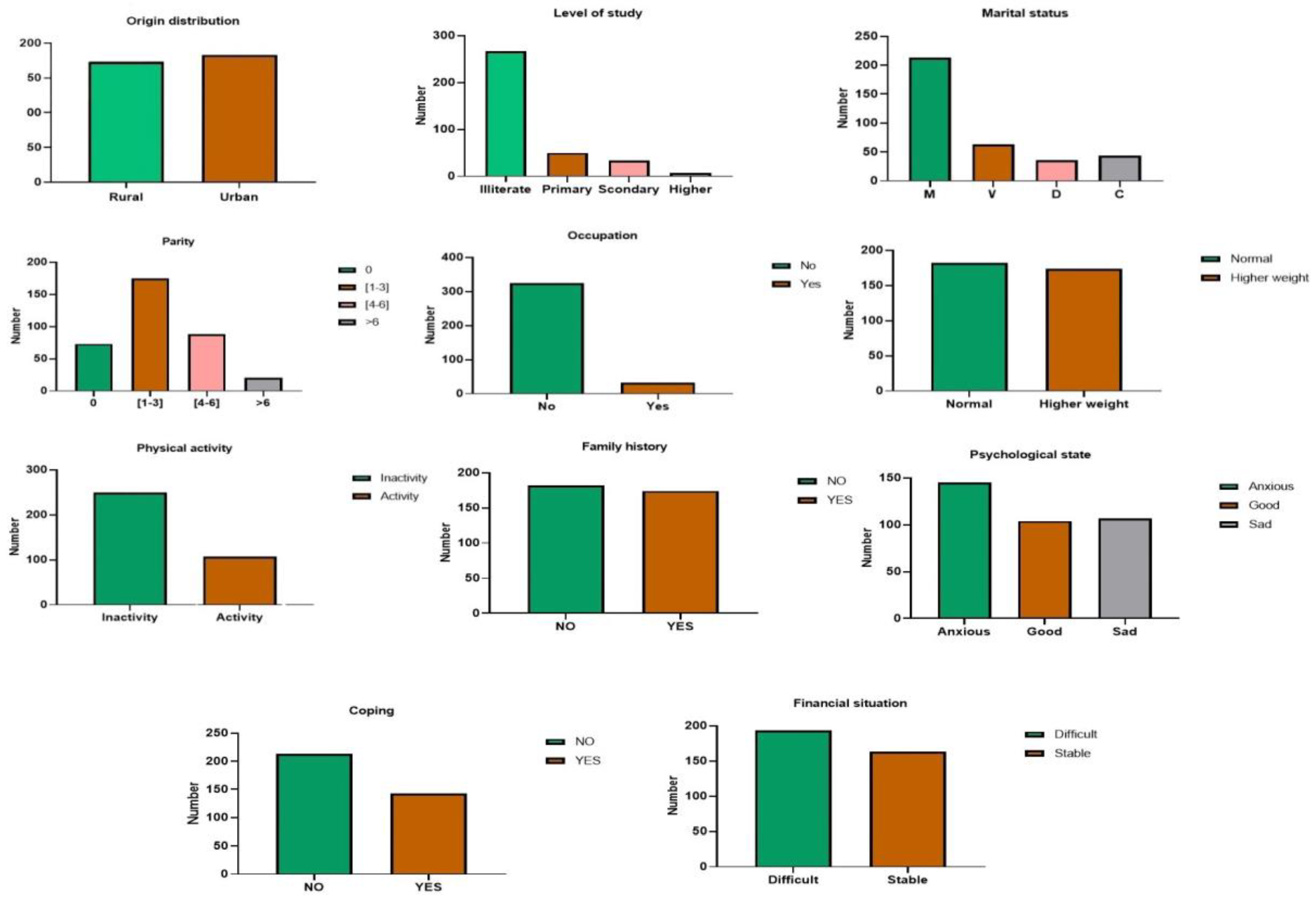
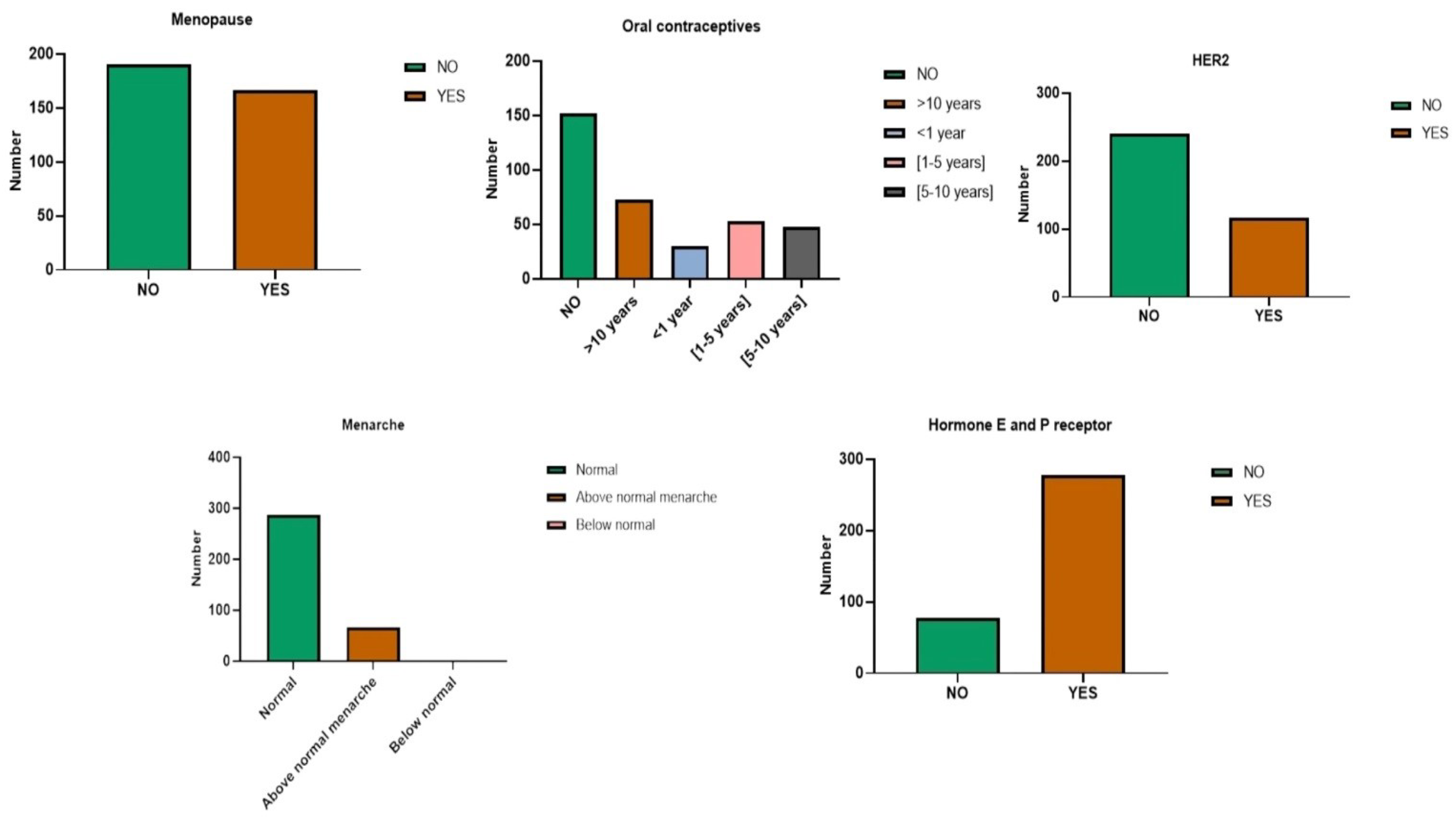
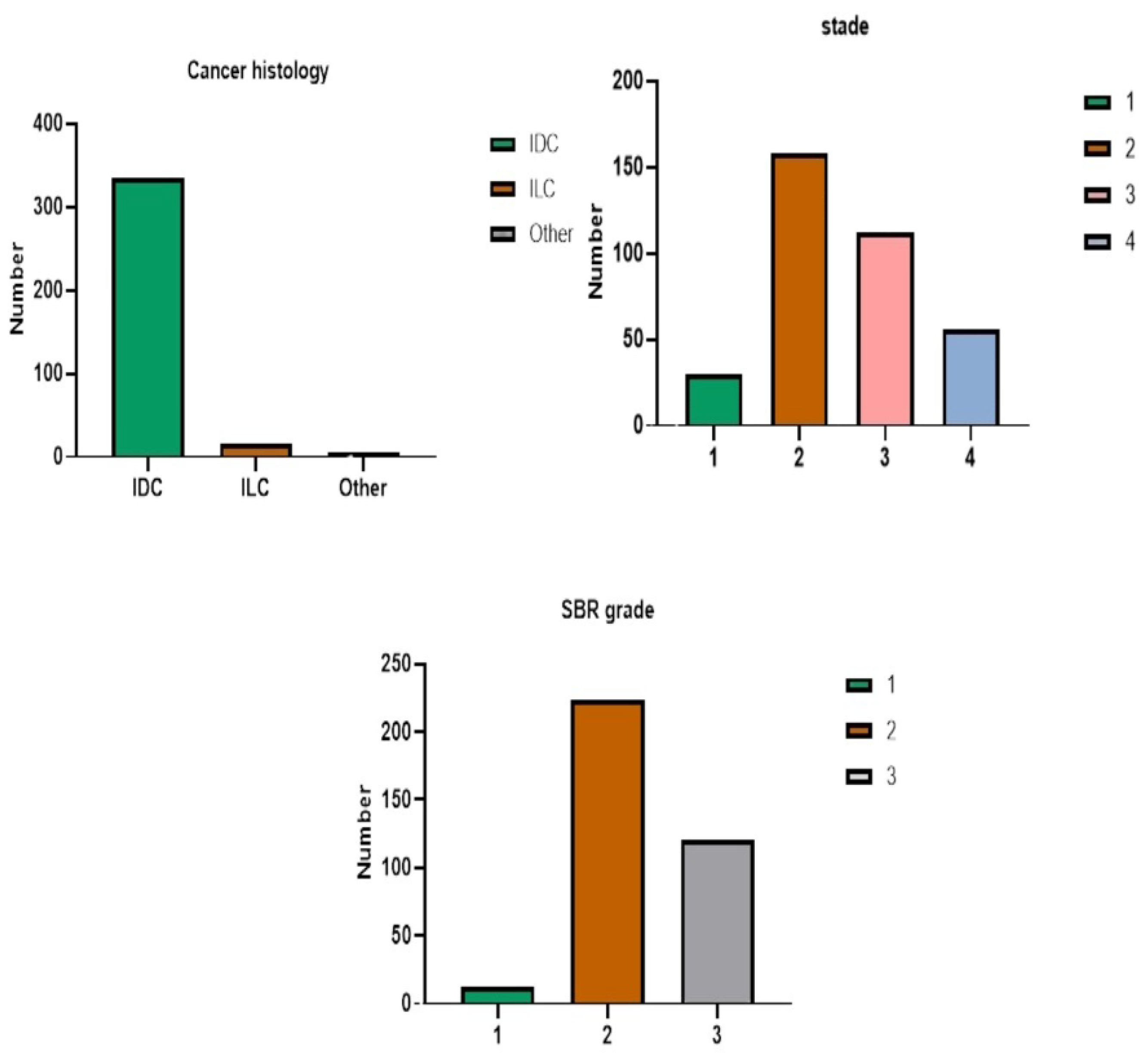
3.1. Impact of Socio-Economic, Clinical, and Hormonal Variables
3.1.1. Socio-Economic Factors
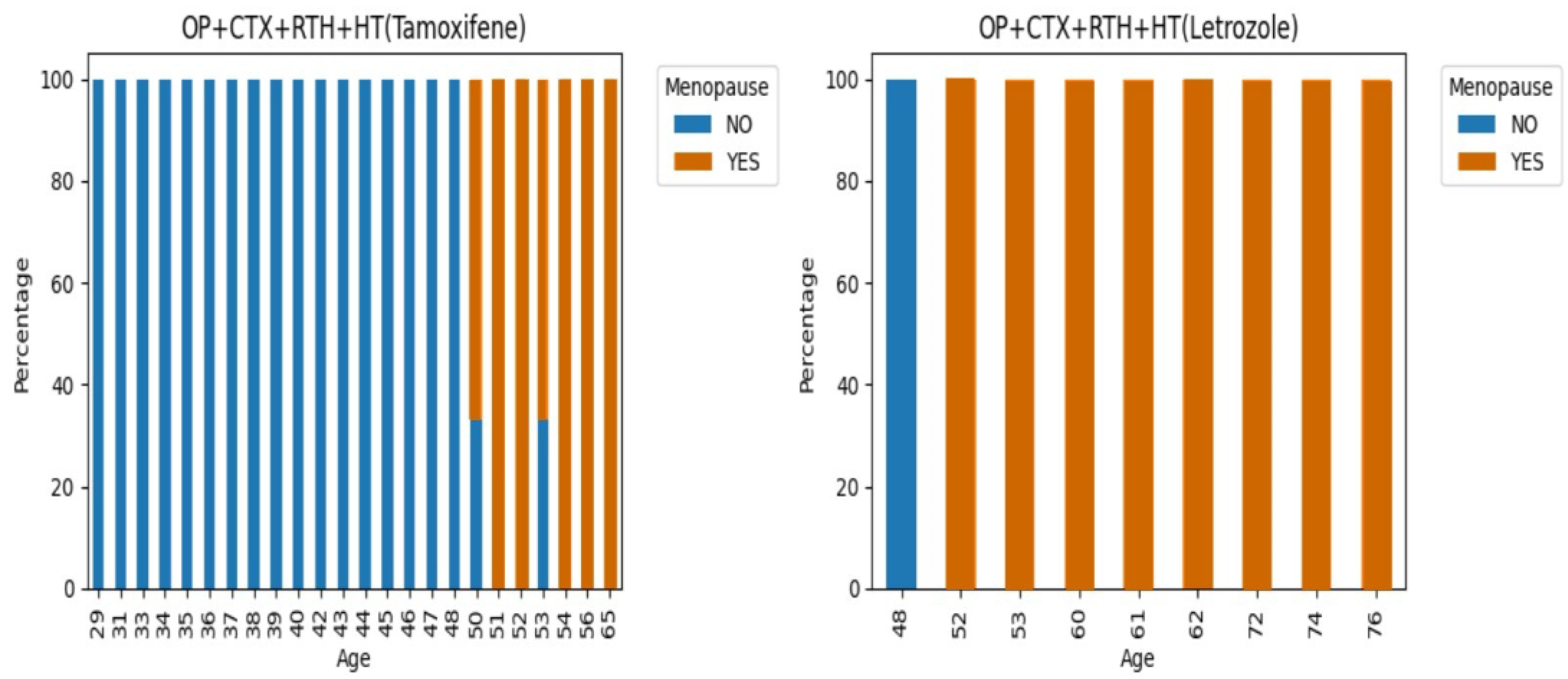
3.1.2. Hormonal Factors
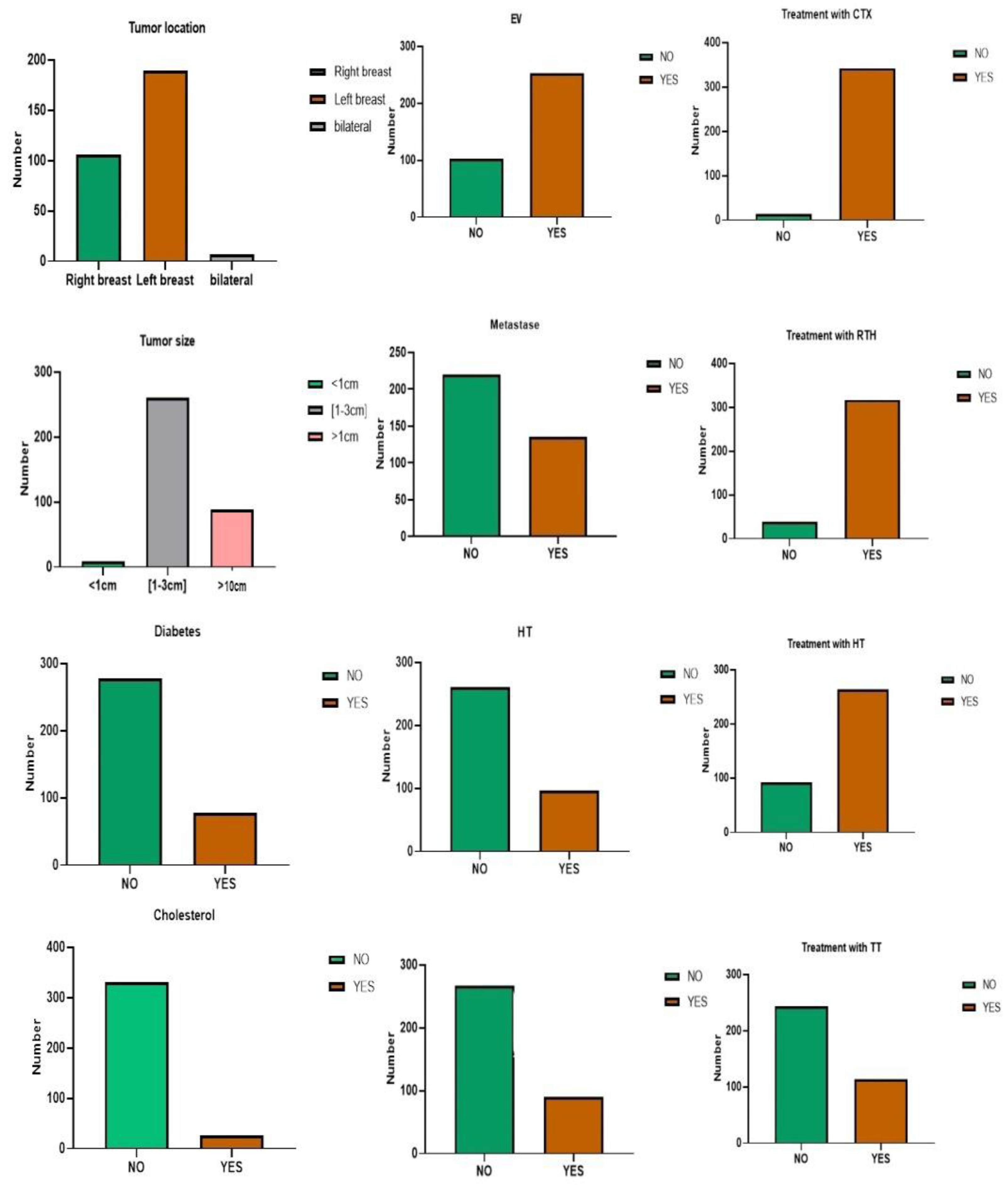
3.1.3. Clinical Factors
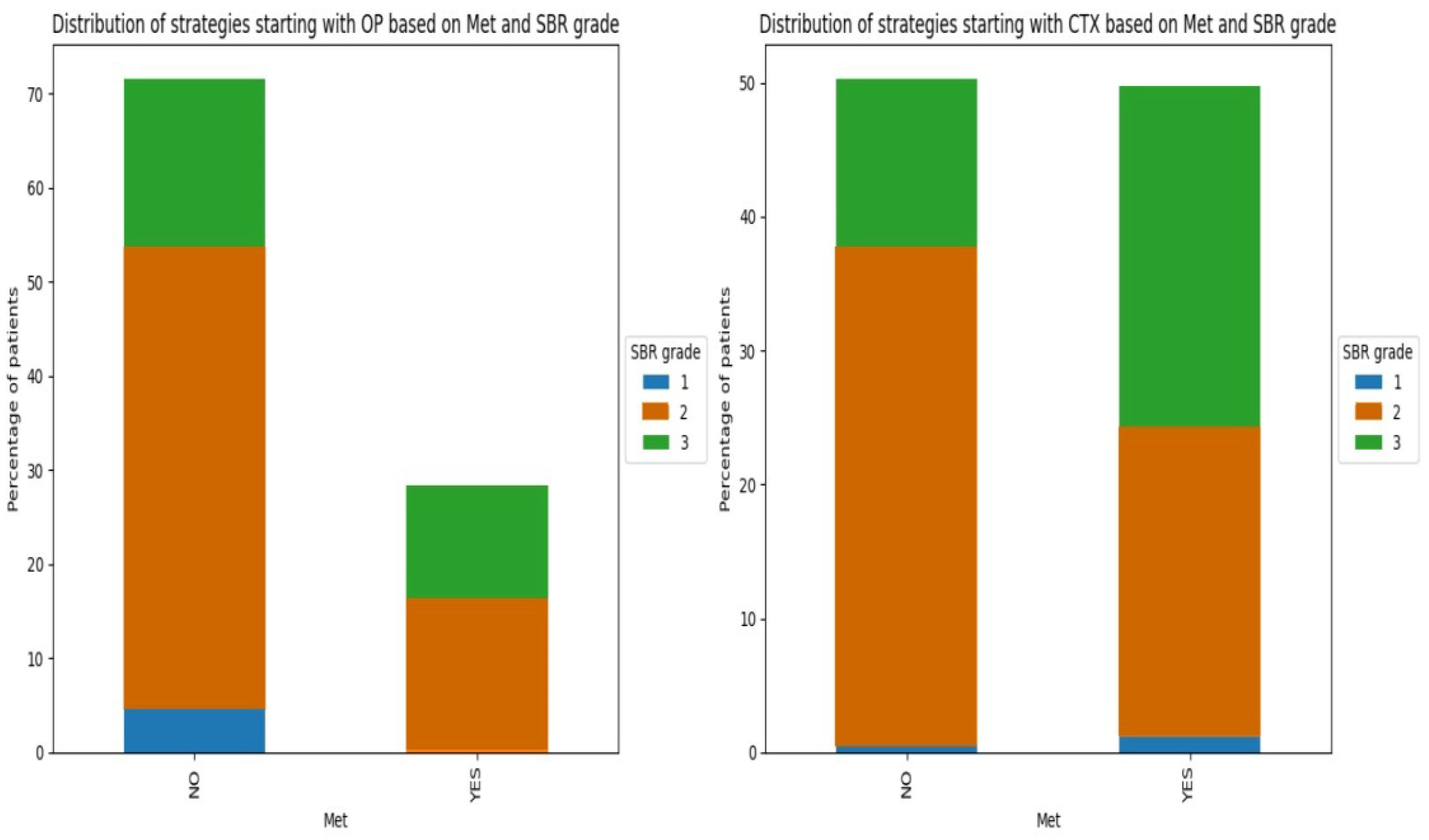
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jafarian, A.H.; Kooshki forooshani, M.; Rasoliostadi, A.; Mohamadian roshan, N. Vascular Mimicry Expression in Invasive Ductal Carcinoma; A New Technique for Prospect of Aggressiveness. Iran J Pathol 2019, 14, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Chouchane, L.; Boussen, H.; Sastry, K.S.R. Breast Cancer in Arab Populations: Molecular Characteristics and Disease Management Implications. The Lancet Oncology 2013, 14, e417–e424. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA A Cancer J Clinicians 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- McGuire, A.; Brown, J.; Malone, C.; McLaughlin, R.; Kerin, M. Effects of Age on the Detection and Management of Breast Cancer. Cancers 2015, 7, 908–929. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Gradishar, W.; O’Regan, R.; Gadi, V. Risk of Recurrence in Patients With HER2+ Early-Stage Breast Cancer: Literature Analysis of Patient and Disease Characteristics. Clinical Breast Cancer 2023, 23, 350–362. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Azubuike, S.O. Relationship between Parity and Breast Cancer Risk: A Critical Review of Evidence (with Focus on Sub-Saharan Africa). International Journal of Noncommunicable Diseases 2023, 8, 66–74. [Google Scholar] [CrossRef]
- Veisi, P.; Nikouei, M.; Cheraghi, M.; Shahgheibi, S.; Moradi, Y. The Association between the Multiple Birth and Breast Cancer Incidence: An Update of a Systematic Review and Meta-Analysis from 1983 to 2022. Arch Public Health 2023, 81, 76. [Google Scholar] [CrossRef] [PubMed]
- Ak, N.; Tuz, Z.; Aydin, E.; Ferhatoğlu, F.; Sari, M.; Paksoy, N.; Doğan, İ.; Yildiz, A.; Di̇Şçi̇, R.; Sai̇P, P.M. The Effect of Parity, Breastfeeding History, and Duration on Clinical and Pathological Characteristics of Breast Cancer Patients. Turkish Journal of Medical Sciences 2024, 54, 229–238. [Google Scholar] [CrossRef]
- LeVee, A.; Mortimer, J. The Challenges of Treating Patients with Breast Cancer and Obesity. Cancers 2023, 15, 2526. [Google Scholar] [CrossRef]
- Nikolaos Tzenios OBESITY AND BREAST CANCER: THE ROLE OF ADIPOSE TISSUES AND HORMONES. EPRA 2023, 178–180. [CrossRef]
- Ajabnoor, G.M.A. The Molecular and Genetic Interactions between Obesity and Breast Cancer Risk. Medicina 2023, 59, 1338. [Google Scholar] [CrossRef] [PubMed]
- Hajji-Louati, M.; Cordina-Duverger, E.; Laouali, N.; Mancini, F.-R.; Guénel, P. A Case–Control Study in France Showing That a pro-Inflammatory Diet Is Associated with a Higher Risk of Breast Cancer. Sci Rep 2021, 11, 17019. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.; Yan, C.H.; Ko, N.Y.; Nabulsi, N.A.; Hoskins, K.F.; Chiu, B.C.-H.; Calip, G.S. Physical Functioning, Frailty and Risks of Locally-Advanced Breast Cancer among Older Women. The Breast 2022, 64, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Frikha, N.; Chlif, M. Un aperçu des facteurs de risque du cancer du sein. Bulletin de l’Académie Nationale de Médecine 2021, 205, 519–527. [Google Scholar] [CrossRef]
- Obeagu, E.I.; Obeagu, G.U. Breast Cancer: A Review of Risk Factors and Diagnosis. Medicine 2024, 103, e36905. [Google Scholar] [CrossRef]
- Wang, H.; MacInnis, R.J.; Li, S. Family History and Breast Cancer Risk for Asian Women: A Systematic Review and Meta-Analysis. BMC Med 2023, 21, 239. [Google Scholar] [CrossRef]
- Ponce-Chazarri, L.; Ponce-Blandón, J.A.; Immordino, P.; Giordano, A.; Morales, F. Barriers to Breast Cancer-Screening Adherence in Vulnerable Populations. Cancers 2023, 15, 604. [Google Scholar] [CrossRef]
- Henderson, L.M.; O’Meara, E.S.; Haas, J.S.; Lee, C.I.; Kerlikowske, K.; Sprague, B.L.; Alford-Teaster, J.; Onega, T. The Role of Social Determinants of Health in Self-Reported Access to Health Care Among Women Undergoing Screening Mammography. Journal of Women’s Health 2020, 29, 1437–1446. [Google Scholar] [CrossRef]
- Krebber, A.M.H.; Buffart, L.M.; Kleijn, G.; Riepma, I.C.; de Bree, R.; Leemans, C.R.; Becker, A.; Brug, J.; van Straten, A.; Cuijpers, P.; et al. Prevalence of Depression in Cancer Patients: A Meta-analysis of Diagnostic Interviews and Self-report Instruments. Psycho-Oncology 2014, 23, 121–130. [Google Scholar] [CrossRef]
- Linden, W.; Vodermaier, A.; MacKenzie, R.; Greig, D. Anxiety and Depression after Cancer Diagnosis: Prevalence Rates by Cancer Type, Gender, and Age. Journal of Affective Disorders 2012, 141, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Li, C.; Li, J.; Balluz, L.S. Physical Activity, Psychological Distress, and Receipt of Mental Healthcare Services among Cancer Survivors. J Cancer Surviv 2013, 7, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Schaab, M.; Wijlens, K.A.E.; Bode, C. Psychological Coping Factors Associated With Breast Cancer-Related Fatigue: A Systematic Review of Recent Evidence for Stages 0 to III. Clinical Breast Cancer 2023, 23, e401–e411. [Google Scholar] [CrossRef]
- Fraumeni JF Jr,; Lloyd JW,; Smith EM,; Wagoner JK. Cancer Mortality Among Nuns: Role of Marital Status in Etiology of Neoplastic Disease in Women. JNCI: Journal of the National Cancer Institute 1969. [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat Rev Dis Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Satish, S.; Moore, J.F.; Littlefield, J.M.; Bishop, I.J.; Rojas, K.E. Re-Evaluating the Association Between Hormonal Contraception and Breast Cancer Risk. BCTT 2023, Volume 15, 227–235. [Google Scholar] [CrossRef]
- Torres-de la Roche, L.A.; Acevedo-Mesa, A.; Lizarazo, I.L.; Devassy, R.; Becker, S.; Krentel, H.; De Wilde, R.L. Hormonal Contraception and the Risk of Breast Cancer in Women of Reproductive Age: A Meta-Analysis. Cancers 2023, 15, 5624. [Google Scholar] [CrossRef]
- Maurya, A.P.; Brahmachari, S. Association of Hormonal and Reproductive Risk Factors with Breast Cancer in Indian Women: A Systematic Review of Case–Control Studies. Indian Journal of Cancer 2023, 60, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Wang, A.; Hu, D.; Wang, Y. Molecular Mechanisms of Breast Cancer Metastasis by Gene Expression Profile Analysis. Molecular Medicine Reports 2017, 16, 4671–4677. [Google Scholar] [CrossRef]
- Kuksis, M.; Gao, Y.; Tran, W.; Hoey, C.; Kiss, A.; Komorowski, A.S.; Dhaliwal, A.J.; Sahgal, A.; Das, S.; Chan, K.K.; et al. The Incidence of Brain Metastases among Patients with Metastatic Breast Cancer: A Systematic Review and Meta-Analysis. Neuro-Oncology 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Purdie, C.A.; Baker, L.; Ashfield, A.; Chatterjee, S.; Jordan, L.B.; Quinlan, P.; Adamson, D.J.A.; Dewar, J.A.; Thompson, A.M. Increased Mortality in HER2 Positive, Oestrogen Receptor Positive Invasive Breast Cancer: A Population-Based Study. Br J Cancer 2010, 103, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, Y.-R.; Ji, P.; Hu, X.; Shao, Z.-M. Impact of Molecular Subtypes on Metastatic Breast Cancer Patients: A SEER Population-Based Study. Sci Rep 2017, 7, 45411. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Roman, L.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.-A.; et al. Efficacy and Safety of Neoadjuvant Pertuzumab and Trastuzumab in Women with Locally Advanced, Inflammatory, or Early HER2-Positive Breast Cancer (NeoSphere): A Randomised Multicentre, Open-Label, Phase 2 Trial. The Lancet Oncology 2012, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Elston, C.W.; Ellis, I.O. Pathological Prognostic Factors in Breast Cancer. I. The Value of Histological Grade in Breast Cancer: Experience from a Large Study with Long-term Follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Gu, K. ; C C Cowie; M I Harris Diabetes and Decline in Heart Disease Mortality in US Adults. JAMA 1999, 281, 1291. [Google Scholar] [CrossRef]
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Rohan, T.E.; Manson, J.E.; Li, J.; Ho, G.Y.F.; Xue, X.; Anderson, G.L.; et al. Insulin, Insulin-Like Growth Factor-I, and Risk of Breast Cancer in Postmenopausal Women. JNCI Journal of the National Cancer Institute 2009, 101, 48–60. [Google Scholar] [CrossRef]
- Lu, Y.; Hajjar, A.; Cryns, V.L.; Trentham-Dietz, A.; Gangnon, R.E.; Heckman-Stoddard, B.M.; Alagoz, O. Breast Cancer Risk for Women with Diabetes and the Impact of Metformin: A Meta-analysis. Cancer Medicine 2023, 12, 11703–11718. [Google Scholar] [CrossRef]
- Guidelines for Diagnosis and Treatment of Advanced Breast Cancer in China (2022 Edition). Journal of the National Cancer Center 2024, 4, 107–127. [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Annals of Oncology 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Weiss, A.; King, T.A. What Is the Role of Neoadjuvant Endocrine Therapy for Breast Cancer? Advances in Surgery 2022, 56, 275–286. [Google Scholar] [CrossRef]
- Cucciniello, L.; Gerratana, L.; Del Mastro, L.; Puglisi, F. Tailoring Adjuvant Endocrine Therapy in Early Breast Cancer: When, How, and How Long? Cancer Treatment Reviews 2022, 110, 102445. [Google Scholar] [CrossRef] [PubMed]
- Rouzier, R.; Perou, C.M.; Symmans, W.F.; Ibrahim, N.; Cristofanilli, M.; Anderson, K.; Hess, K.R.; Stec, J.; Ayers, M.; Wagner, P.; et al. Breast Cancer Molecular Subtypes Respond Differently to Preoperative Chemotherapy. Clinical Cancer Research 2005, 11, 5678–5685. [Google Scholar] [CrossRef]
- Tsimberidou, A.-M. Targeted Therapy in Cancer. Cancer Chemother Pharmacol 2015, 76, 1113–1132. [Google Scholar] [CrossRef]
- Stanowicka-Grada, M.; Senkus, E. Anti-HER2 Drugs for the Treatment of Advanced HER2 Positive Breast Cancer. Curr. Treat. Options in Oncol. 2023, 24, 1633–1650. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; du Bois, A.; Schmidt, M.; Maass, N.; Cufer, T.; de Jongh, F.E.; Maartense, E.; Zielinski, C.; Kaufmann, M.; Bauer, W.; et al. Trastuzumab Beyond Progression in Human Epidermal Growth Factor Receptor 2–Positive Advanced Breast Cancer: A German Breast Group 26/Breast International Group 03-05 Study. JCO 2009, 27, 1999–2006. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N Engl J Med 2015, 372, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, V.; Dieci, M.V.; Griguolo, G.; Miglietta, F.; Girardi, F.; Bisagni, G.; Generali, D.G.; Cagossi, K.; Sarti, S.; Frassoldati, A.; et al. Trastuzumab-Lapatinib as Neoadjuvant Therapy for HER2-Positive Early Breast Cancer: Survival Analyses of the CHER-Lob Trial. European Journal of Cancer 2021, 153, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wu, Y.; Song, B.; Yi, M.; Yan, Y.; Mei, Q.; Wu, K. Recent Advances in Targeted Strategies for Triple-Negative Breast Cancer. J Hematol Oncol 2023, 16, 100. [Google Scholar] [CrossRef]
- Geyer, C.E.; Sikov, W.M.; Huober, J.; Rugo, H.S.; Wolmark, N.; O’Shaughnessy, J.; Maag, D.; Untch, M.; Golshan, M.; Lorenzo, J.P.; et al. Long-Term Efficacy and Safety of Addition of Carboplatin with or without Veliparib to Standard Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer: 4-Year Follow-up Data from BrighTNess, a Randomized Phase III Trial. Annals of Oncology 2022, 33, 384–394. [Google Scholar] [CrossRef]
- Braal, C.L.; Jongbloed, E.M.; Wilting, S.M.; Mathijssen, R.H.J.; Koolen, S.L.W.; Jager, A. Inhibiting CDK4/6 in Breast Cancer with Palbociclib, Ribociclib, and Abemaciclib: Similarities and Differences. Drugs 2021, 81, 317–331. [Google Scholar] [CrossRef]
- Abdelmalak, M.; Singh, R.; Anwer, M.; Ivanchenko, P.; Randhawa, A.; Ahmed, M.; Ashton, A.W.; Du, Y.; Jiao, X.; Pestell, R. The Renaissance of CDK Inhibitors in Breast Cancer Therapy: An Update on Clinical Trials and Therapy Resistance. Cancers 2022, 14, 5388. [Google Scholar] [CrossRef] [PubMed]
- Sobande, F.; Dušek, L.; Matějková, A.; Rozkoš, T.; Laco, J.; Ryška, A. EGFR in Triple Negative Breast Carcinoma: Significance of Protein Expression and High Gene Copy Number. Cesk Patol 2015, 51, 80–86. [Google Scholar] [PubMed]
- Sabbah, D.A.; Hajjo, R.; Sweidan, K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. CTMC 2020, 20, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Nnorom, S.O.; Akinyemi, O.; Tran, J.; Baig, H.; Cornwell, E.E.; Frederick, W.A.; Wilson, L.L. Color or Money?: The Impact of Socioeconomic Status and Race/Ethnicity on Breast Cancer Mortality. The American Journal of Surgery 2022, 224, 1403–1408. [Google Scholar] [CrossRef]
- Azin, A.; Tahmasebi, H.; Brar, A.; Azin, S.; Ko, G.; Covelli, A.; Cil, T. Racial, Ethnic and Socioeconomic Disparities in Diagnosis, Treatment, and Survival of Patients with Breast Cancer. The American Journal of Surgery 2023, 225, 154–161. [Google Scholar] [CrossRef]
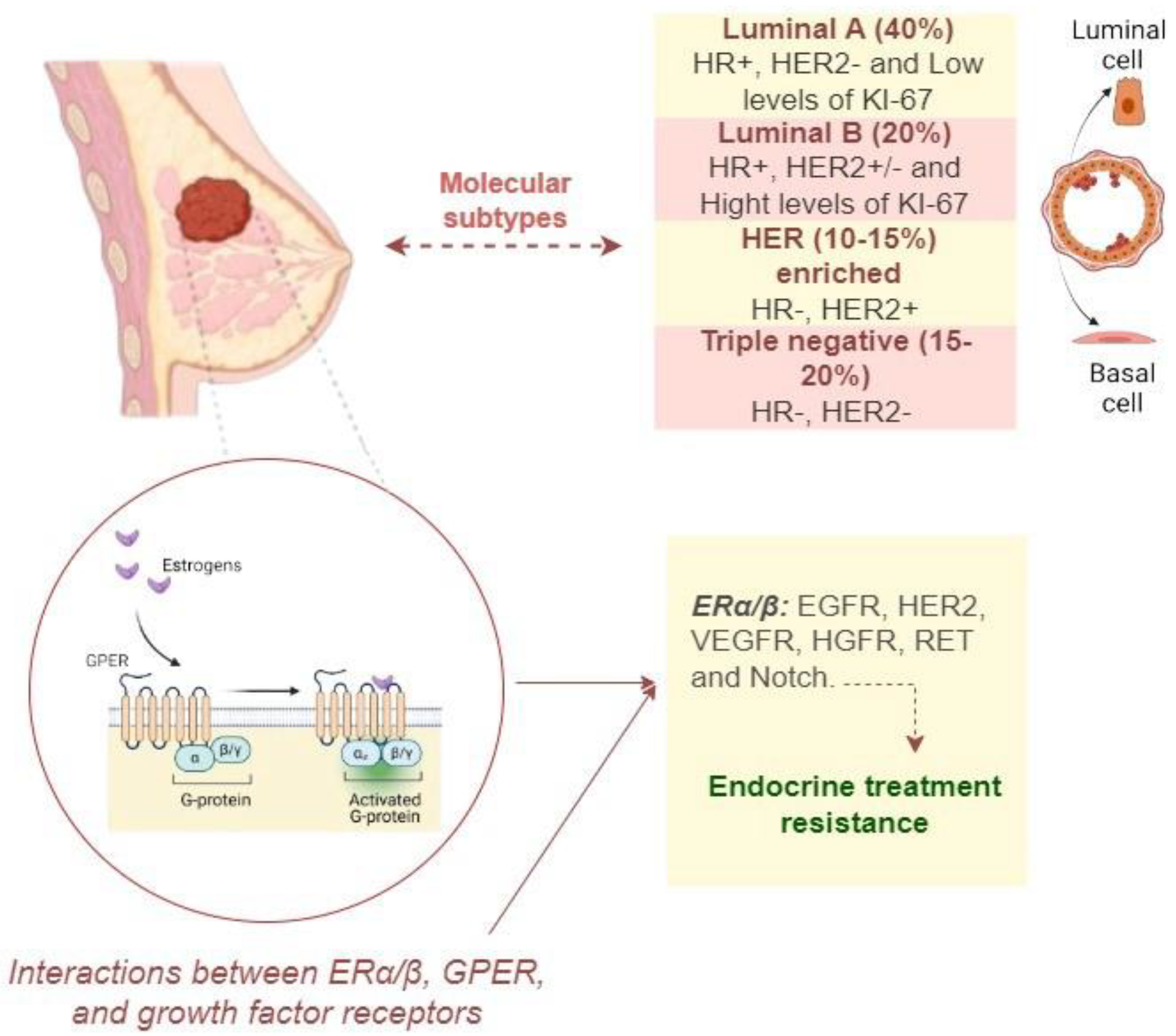
- Yan, S.; Ji, J.; Zhang, Z.; Imam, M.; Chen, H.; Zhang, D.; Wang, J. Targeting the Crosstalk between Estrogen Receptors and Membrane Growth Factor Receptors in Breast Cancer Treatment: Advances and Opportunities. Biomedicine & Pharmacotherapy 2024, 175, 116615. [Google Scholar] [CrossRef]
- Tavčar Kunstič, T.; Debeljak, N.; Fon Tacer, K. Heterogeneity in Hormone-Dependent Breast Cancer and Therapy: Steroid Hormones, HER2, Melanoma Antigens, and Cannabinoid Receptors. Advances in Cancer Biology - Metastasis 2023, 7, 100086. [Google Scholar] [CrossRef]
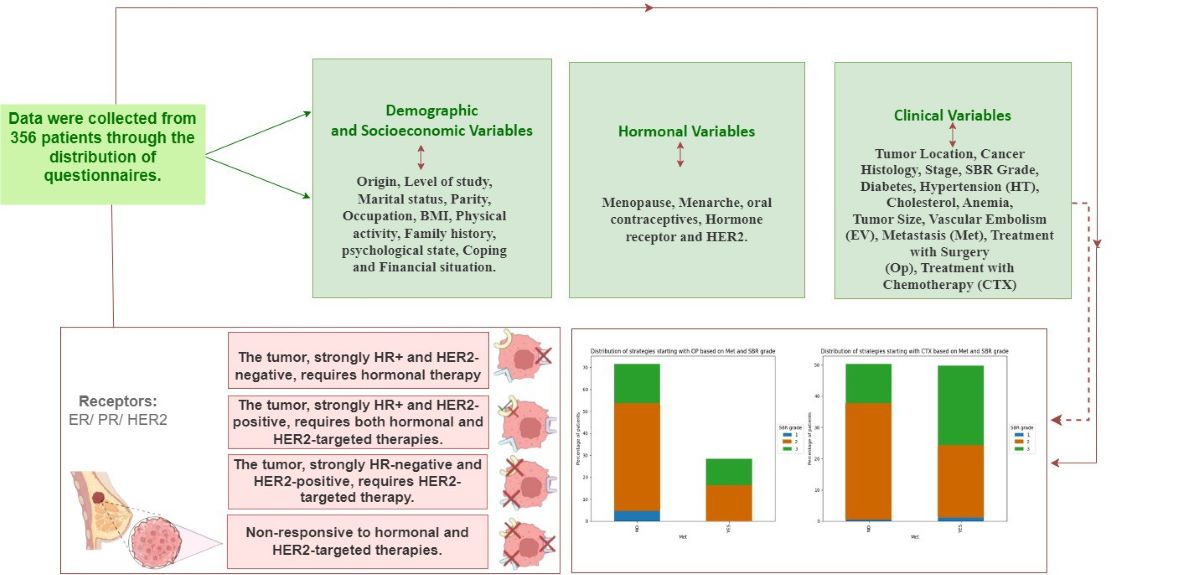
| Field | Variables |
|---|---|
| Socio-economic and demographic aspects | Origin, Level of study, Marital status, Parity, Occupation, BMI, Physical activity, Family history, psychological state, Coping and Financial situation. |
| Hormonal aspects | Menopause, Menarche, oral contraceptives, Hormone receptor and HER2. |
| Clinical aspects | Tumor Location, Cancer Histology, Stage, SBR Grade, Diabetes, Hypertension (HT), Cholesterol, Anemia, Tumor Size, Vascular Embolism (EV), Metastasis (Met), Treatment with Surgery (Op), Treatment with Chemotherapy (CTX), Treatment with Hormone Therapy (HT), Treatment with Radiotherapy (RT), Treatment with Targeted Therapy (TT). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





