Introduction
Global warming seriously threatens wheat crops worldwide because wheat is a cold-loving plant. The temperature increase directly impacts crop production activities since various climatic events have increased or altered them and threaten global food security (Kumer et al. 2022). The exposure of crops to abiotic stresses, such as heat stress, drought, salinity, cold, etc., affects the development and growth and hinders the genetic makeup, and metabolic and physiological activities of plants (Alam et al. 2018a, 2021; dos Santos et al. 2022). Climate change is expected to increase the frequency of extreme weather events such as floods, droughts, high temperatures, etc. (
Figure 1) (IPCC 2023; CRP 2024). Heat is a primary abiotic stressor that reduces crop output and degrades its quality. Heat and climate change hurt wheat production (Sánchez-Bermúdez et al. 2022).
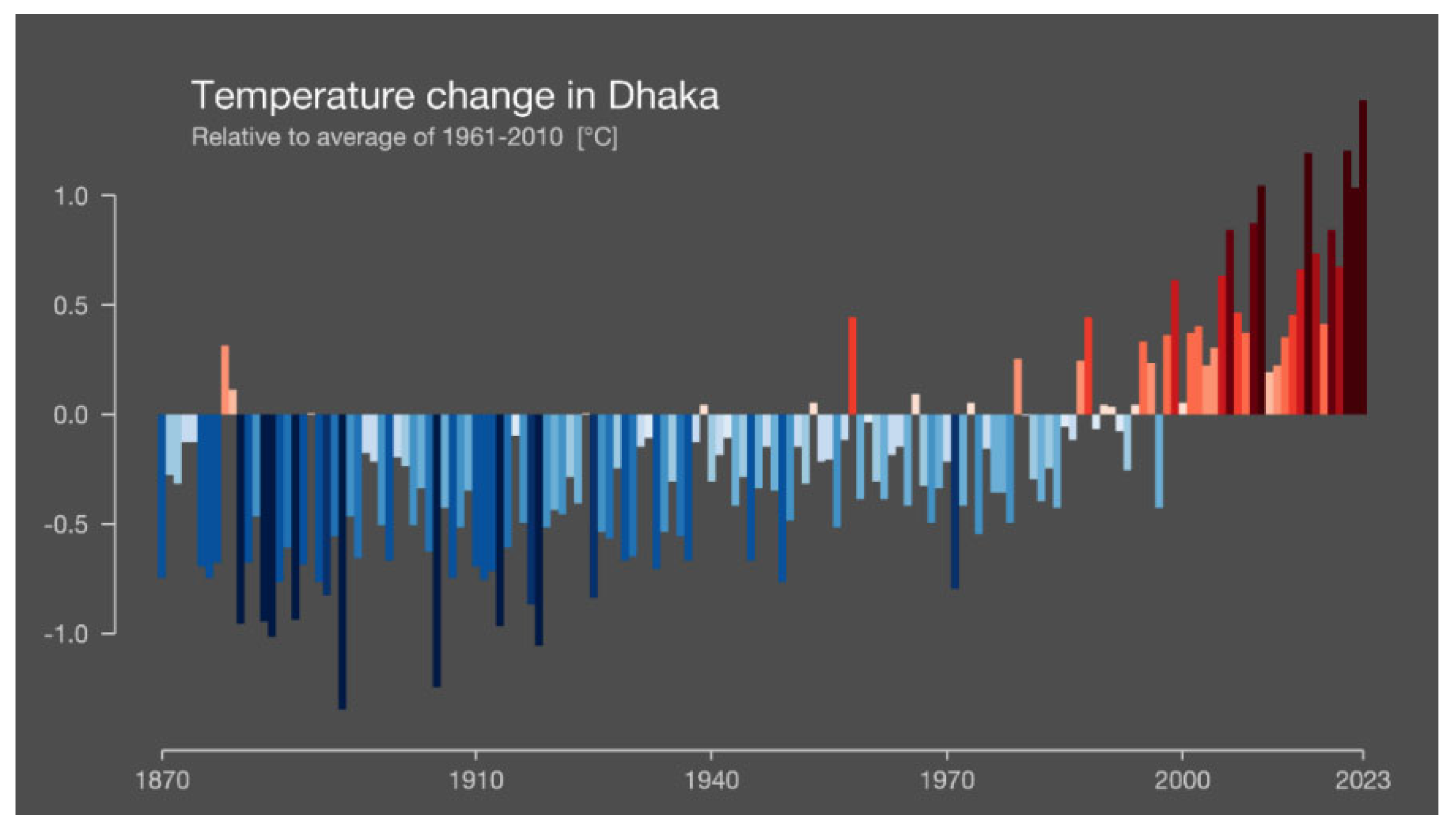
According to the IPCC (2023), human activities have caused global surface air temperature to rise from 0.8 to 1.3°C (avg. 1.07°C) between 1850 and 1900 and 2010-2019. The passage has discussed the estimated contributions of various factors to global surface temperature rises, such as well-mixed greenhouse gases, other human drivers, primarily aerosols, natural drivers (solar and volcanic), and internal variability, which range from 1.0°C to 2.0°C, 0.0°C to 0.8°C, -0.1°C to 0.1°C, and -0.2°C to 0.2°C, respectively. The worldwide surface air temperature increased by 1.09°C (range: 0.95 to 1.20°C) between 2011 and 2020 compared to 1850-1900, with more significant increases over land (1.59°C (range: 1.34 to 1.83°C) than over the ocean (0.88°C) (range: 0.68 to 1.01 °C). IPCC (2018) forecasts show a further increase in annual mean temperatures of 1.0°C by 2030, 1.4°C by 2050, and 2.4°C by 2100. The winter season (Dec, Jan, and Feb) is on a trajectory of significant temperature increases, with a rise of 1.1°C by 2030, 1.6°C by 2050, and a staggering 2.7°C by 2100. This rapid change is a cause for immediate concern. From 1949 to 2013, the annual mean, min, and max temperatures increased by 0.13˚C each decade. Temperatures increased by 0.18˚C per decade (post-monsoon), 0.18˚C per decade (winter), and 0.23˚C per decade (post-monsoon) (Zaman et al. 2013; IPCC 2023;
Figure 1). The average annual rainfall increased by 4.20 mm/year, while pre-monsoon rainfall increased by 1.35 mm/year (Alam et al. 2023). Another finding from 1981 to 2010 was that the annual maximum temperature increased compared to 1971-2000, particularly in Bangladesh's southeastern and northeastern regions, followed by the center and southern areas (
Figure 2) (Khatun et al. 2016). These forecasts warn Bangladesh of a surge in hot days, heat waves, extended dry periods, and increased drought threats. Thus, climate change is believed to be deteriorating, threatening crop output, the survival of plant and animal kingdoms, forestry, biodiversity, and global sustainability, among other issues.
In Bangladesh, wheat sowing is from 15 to 30 Nov. However, if sown in Dec, the reproductive and grain-filling stages coincide with mid-February to mid-Mar, a period of high temperatures. This terminal heat stress, with temperatures above 30°C during the reproductive development stage, severely impacts productivity (
Figure 2) (Ding and Yang 2022; Mao et al. 2023). High temperatures during the grain filling stage, combined with rising mean temperature due to global warming, significantly affect grain production, leading to a reduction in the period of grain growth, improper or shrinkage of grains, and an overall decrease in wheat yield (
Figure 2; Alam et al. 2013a; Hossain et al. 2019; 2023). Heat stress is a major cause of yield decrease, affecting around 36 million hectares of land in temperate regions globally (Elahi et al. 2022). Heat stress is a significant threat to wheat farming in South Asia. Most crops with increased yields are investigated using traditional breeding techniques and cultivated in ideal conditions at the right time of sowing. Because of this, most wheat cultivars made available for use in agriculture do not have heat stress tolerance (Hossain et al. 2023). Given the situation's urgency, biotechnological studies that genetically alter kinds with vast adaptability must proceed quickly to generate heat-tolerant genotypes that can withstand varying temperatures.
The first step in the breeding of heat-tolerant varieties involves research studies that address a range of topics, including mitigating the effects of heat, characterizing wheat varieties molecularly and biochemically, and classifying the varieties according to their resistance to heat stress (Chaudhary et al. 2020). During the 2022-2023 growing season, 1.16 million metric tons of wheat grains were produced in Bangladesh in around 0.311 million hectares of land (DAE 2023; BBS 2023). Some parts of Bangladesh are vulnerable to high temperatures, which could be impacted by climate change, despite its significance to global output. Wheat has a vast genome (~17 Gb) for bread wheat) (Walkowiak et al. 2020). Hence, heat tolerance is a complicated and quantitative feature controlled by several genes. Numerous studies on molecular processes of heat tolerance and molecular breeding for heat stress have been conducted to address these issues. Several molecular markers and quantitative trait loci (QTLs) have recently been linked to the genes that control heat signaling processes (Chaudhary et al., 2020). Significant progress has been made in identifying interest genes (Yıldırım et al. 2013; Govindaraj et al. 2018). These advancements enabled the production of future wheat crops that are heat-stress resistant. A variety of molecular markers have evolved to help achieve this goal. The most notable are DNA markers that use polymerase chain reaction (PCR). Wheat genetic characterization studies utilized PCR-based markers, such as amplified fragment length polymorphisms, sequence-tagged microsatellite site markers (SSRs) (Ateş Sönmezoğlu and Terzi 2018), and chloroplast-specific microsatellite markers (Tomar et al., 2013). Golabadi et al. (2011) employed microsatellite markers like TGW and the harvest index to discover QTLs associated with yield trait competence. In a separate study, Ramya et al. (2015) described the physiological and genetic characteristics of 24 current wheat genotypes for use in breeding studies to investigate drought and high-temperature tolerance. Previous research has shown that SSR markers can be utilized to determine heat tolerance in wheat accurately.
After several agronomic parameters were evaluated in the field, the HSI could be used to identify genotypes of wheat that yielded well under heat stress. Wheat genotypes with improved relative performance in yield components, grain yield, and HSI were quantified through high-temperature tolerance characterization (Hossain et al. 2021; Shenoda et al. 2021). The application of HSI and performance in ILS-heat-stressed conditions have also been documented in several earlier research (Bhusal et al. 2017). Genotypes with contrasting features must be found to perform genetic analyses, such as QTL investigations. This study aimed to characterize DNA as fingerprinting for future intellectual property and identify heat-responsive wheat genotypes.
Materials and Methods
Plant Materials
To evaluate the molecular diversity for terminal heat stress tolerance against 13 SSR markers associated with the trait of interest, fifteen genotypes of bread wheat (
Triticum aestivum L.) (including 13 varieties and two advanced lines) were employed (
Table 1 and
Table 2). The Bangladesh Wheat and Maize Agricultural Research Institute (BWMRI), located in Nashipur, Dinajpur, Bangladesh, provided seeds of every genotype.
Table 1 provides a summary of the genotypes' pedigrees and salient features.
Experimental Site and Sowing Date
In the Rabi season (Nov to Mar), the genotypes were evaluated at the research field of the Regional Station (RS), BWMRI, Joydebpur, Gazipur, which is located in the agro-ecological zone 28 (Madhupur Tract). This region is distinguished by intricate relief and soils that have grown on the Madhupur Clay. The terrain consists of undulating hills and splintered terraces with deep, narrow valleys. The heavy clays in the valleys are dark grey, highly acidic, and have poor fertility levels, organic matter, and moisture-holding capacity. Seeds were sown on two dates viz. Nov 21 (irrigated timely sowing or ITS) and Dec 21 (irrigated late sowing or ILS). The laboratory experiments were conducted at the Biotechnology Division, Bangladesh Agricultural Research Institute, Joydebpur, Gazipur, and the Molecular Laboratory, RS, BWMRI, Gazipur.
Extraction of DNA and SSR Analysis
Using a modified CTAB approach, genomic DNA was isolated from fresh leaves of fifteen wheat genotypes. From various chromosome sites, thirteen SSR markers (gwm291, Gwm325, Xgwm294, Gwm268, Xwmc407, Xcfa2129, gwm11, Xcfd43, Xgwm356, Xbarc137, Gwm484, Gwm293, WMC527) were chosen. Fresh leaves were ground in a mortar, then placed in a 2 ml tube, 400 µl chloroform was added, and the mixture was heated for an hour at 65°C. The supernatant was moved to a fresh tube after centrifuging the sample for ten minutes at 4ºC at 12000 rpm. Following the addition and mixing of isopropyl alcohol, the samples were kept at -20°C for two hours. The DNA pellet was centrifuged one more, cleaned with 75% ethanol, allowed to air dry for a full day, and dissolved in 100µl of 1X TE buffer. Thirteen primer pairs were utilized for SSR analysis, and a spectrophotometer was used to verify the quality and concentration of the DNA. PCR parameters that Roder et al. (1998) adhered to.
Heat Susceptibility Index (HSI)
The impact of heat stress on TGW and grain yield was assessed using the heat susceptibility index (HSI). The following formula was used to calculate the HSI in the manner outlined by Paliwal et al. (2012):
where
X represents TGW and grain yield
Xheat stress represents phenotypic values of individual genotypes for TGW and grain yield under late sowing.
Control represents phenotypic values of individual genotypes for TGW and grain yield under normal sowing conditions (control conditions).
Statistical Analysis
Only one locus was assigned to each band. The Unwaited Pair Group Method with Arthematic Mean (UPGMA), outlined for the shared allele, was used to quantify the genetic distances between the genotypes after all the scorable loci were considered to create a bivariate 1-0 data matrix. Values were designed to estimate genetic diversity using PowerMarker software, dendrograms, and polymorphism information content (PIC).
Results
The 13 SSR primers used in the study are listed in
Table 2.
Table 3 shows the primers' allele counts and sizes. The primers detected 2.0 to 8.0 alleles per genotype. Gwm293, with eight alleles, was the most polymorphic microsatellite marker, followed by Xgwm356, which had seven alleles (
Table 3;
Figure 3). Screening 15 genotypes with 13 SSR markers yielded 51 polymorphic alleles, averaging 3.92 per locus. Xcfd43 had the fewest alleles, only two alleles. The PIC values of the studied microsatellite markers ranged from 0.33 (Xwmc407) to 0.83 (Xgwm356), with an average of 0.57 (
Table 3;
Figure 3). The Xgwm294, Xcfa2129, and Gwm293 primers had the highest heterozygosity (He) values of 1.00, while the lowest values ranged from 0.33 (Xwmc407) to 0.83 (Xgwm356), with an average PIC value of 0.57. The primers gwm291, gwm325, Xwmc407, gwm11, Xcfd43, and Xbarc137 all had He values of 0.0. heterozygosity was the most commonly employed concept for evaluating population genetic variation (
Table 3). For the microsatellites included in this investigation, PIC exhibited a strong positive connection with the number of alleles (
Table 4).
Table 4 displays the highest amount of genetic diversity (0.85) found at locus Xgwm356 and the lowest level of genetic diversity (0.44) found at locus gwm11. Gene diversity was higher in markers that detected more significant alleles than in markers that detected fewer alleles.
This outcome was in line with earlier research by Herrera et al. (2008), who also found a significant genetic diversity at each SSR locus based on the number of alleles found using microsatellite markers (
Table 4). A dendrogram was drawn out based on the genetic distance between common alleles derived from 15 genotypes and 51 alleles (
Figure 4). Fifteen genotypes could easily be identified as heat tolerance or susceptible. The UPGMA cluster tree analysis drew the dendrogram with those fifteen genotypes using a distance coefficient of 0.050 (
Figure 4). Values ranging from 0.000 to 0.929 were obtained from the combined data for the 13 primers in pairwise comparisons of shared alleles and genetic distances across the variations (
Table 4).
Table 5 and
Table 6 display the mean TGW and grain yield HSI values, respectively. TGW and grain yield under ILS and ITS conditions, the mean percentage change in TGW and grain yield under ILS compared to ITS, and the values of the same parameters for each cluster member. The HSI, TGW, and grain yield under stress conditions, as well as the percent decrease in TGW and grain yield under the ILS over the ITS, were the three parameters that divided the two major groups obtained from the cluster analysis as a measure of heat tolerance under field conditions (
Table 5;
Table 6). The HSI was assessed for TGW and grain yield to determine which genotypes were heat-tolerant and heat-susceptible. The grain yield varied from 0.74 to 1.25, whereas the HSI for TGW varied from 0.772 to 1.23. Heat-tolerant genotypes were identified using these data. Heat-stress tolerance was correlated with a low HSI (HSI<1) (
Table 5; Fischer and Maurer, 1978). Different genotypes were characterized as having high heat tolerance (HSI<0.50), moderate heat tolerance (HSI = 0.50-1.00), or heat sensitivity (HSI>1.00) based on the value and direction of desire (Khanna-Chopra and Viswanathan 1999 and Singh, et al. 2011).
Table 5 displays the mean HSI values for TGW under ITS and ILS conditions, the mean percentage change in TGW and grain yield under the ILS compared to ITS, and the values of the same parameters for each cluster member. The HSI, TGW, and grain yield under stress conditions, as well as the percent decrease in TGW and grain yield under the ILS over the ITS, were the three parameters that separated the two major groups obtained from the cluster analysis as a measure of heat tolerance under field conditions (
Table 5).
Three genotypes, BARI Gom 25, BARI Gom 26, and BARI Gom 27, comprised Cluster I. Their respective HSI values for TGW and grain yield were more significant, falling between 0.885 - 0.887 and 0.903 - 0.964, respectively (
Table 5 and
Table 6). The heat tolerance of these genotypes was moderate (HSI 0.50-1.00). Under ILS, the TGW (g) and grain yield/plot (kg) varied from 38.5 to 43.8 and 1.85 to 2.31, respectively. When comparing the grain yield under the ILS to the ITS, these genotypes' TGW dropped from 10.3 to 10.5%, and their grain yield dropped from 13.7 to 14.8% (
Table 5 and
Table 6). The TGW and grain yield cluster means, and the corresponding declines in TGW and grain yield were 0.887, 0.903, 10.5%, and 13.8%, respectively. These genotypes closely matched group A's cluster II members (
Table 5 and
Table 6). The genotypes that made up Cluster II were BARI Gom 28, BARI Gom 29, BARI Gom 30, and BARI Gom 31.
The ranges of the HSIs for TGW and grain yield, as well as the decreases in TGW and yield from ITS to those under ILS, were 0.772-0.903, 0.742-0.930, 9.11-10.7%, and 11.3-14.2%, respectively. These findings open up new avenues for understanding and improving heat tolerance in wheat. The losses in TGW and grain yield, as well as the cluster means of the HSI for these variables, were 0.772, 0.742, 9.11%, and 11.3%, respectively. Additionally, these genotypes have a moderate tolerance to heat (HSI 0.50–1.00). Eight genotypes, separated into two clusters (III and IV), comprised Group B. Four genotypes made up Cluster III were BWMRI Gom 3, BAW 1290, BAW 1147, and Nadi 2. For this cluster, the mean HSIs were 1.04-1.23, 1.135-1.253, 12.3-14.4%, and 17.3-19.2% for TGW, grain yield, and the proportional decreases in TGW and grain yield under ILS compared to ITS, respectively. According to the HSI, TGW and grain yield's cluster means and the decreased percentages of TGW and grain yield were 1.04, 1.13, 12.3%, and 17.3%, respectively. There was heat susceptibility in these genotypes (HSI>1.00). Four genotypes comprised Cluster IV: BARI Gom 32, BARI Gom 33, BWMRI Gom 1, and BWMRI Gom 2. For this cluster, the mean HSIs for TGW and grain yield were 1.06-1.17 and 1.004-1.1.102, respectively; the relative decreases in TGW and grain yield under ILS compared to ITS were 12.5-13.8% and 15.4-16.9%. Additionally, some genotypes showed heat sensitivity (HSI>1.00). The losses in TGW and grain yield, as well as the cluster means of the HSI for these variables, were 1.17, 1.05, 13.8%, and 16.1%, respectively. The outcomes of Ali et al. (2013), Pinto et al. (2010), and Sadat et al. (2013) agreed with these results.
Discussion
Genetic similarity information ensures long-term productivity gains during breeding operations, which prevents elite germplasm from becoming homogenous. Numerous unique genes are probably present in cultivars with different DNA profiles. Both phenotypic and molecular data made practical assessments of genetic variation and heat-tolerant genotypes possible. SSR markers provided helpful information for DNA fingerprinting and genetic diversity estimation (Gupta et al. 2022). They produced distinct bands for heat-tolerant genotypes, indicating that they may be used to increase heat tolerance. Wheat varieties with higher yields under abiotic stress can be developed with the help of phenotypic and molecular data integration (Haliloglu et al. 2022).
The 13 SSR markers resulted in 51 polymorphic bands with an average PIC of 0.57. The number of bands per marker ranged from 2 (Xcfd43 and Xwmc407) to 7 (Xgwm356), with an average of 3.92 bands. PIC values ranged from 0.38 for Xcfd43 to 0.83 for Xgwm356, showing that some SSRs are informative. This outcome was similar to what Sharma et al. (2017) found. Variations in allele frequency may cause PIC value differences. Polymorphic bands revealed changes between genotypes, allowing researchers to analyze systematic links (Haliloglu et al. 2022). Eleven SSR markers produced distinct bands for heat-tolerant genotypes; these markers may be utilized to indicate heat tolerance, but further testing in a range of populations is required.
Table 3 shows that distinct chromosomes contain SSR markers linked to heat tolerance. With an average of 0.37 in wheat genotypes examined, low heterozygosity showed limited genetic variation, according to the SSR markers employed in this investigation. 13 loci with PIC values larger than 0.50 were deemed informative among the PIC values for the primers utilized. The PIC can be used to assess a crop's genetic variation. According to Nagy et al. (2012) and Ramadugu et al. (2015), a locus with a PIC greater than 0.5 has high diversity, while a PIC less than 0.25 indicates low diversity. The mean PIC for the SSR markers employed in this investigation was 0.57, ranging from 0.33 to 0.83 (
Table 3). As a result, most of the primers utilized in this investigation provided valuable information. The results showed that the SSRs were suitable markers for terminal heat stress tolerance selection using molecular plant breeding. Mourad et al. (2020) proposed that the number of alleles at each locus and their calculated PIC values be examined simultaneously as part of an objective assessment of genetic diversity in genotyping collections.
All four clusters had distinct traits. Cluster IV exhibited the most excellent mean HSI value (>1.00) and the most significant drop in TGW under ILS (sowing on 21 December) against ITS (sowing on 21 November) (
Table 5). In contrast, cluster III exhibited the highest mean HSI value and the most significant drop in grain output under ILS compared to ITS (
Table 6). However, BARI Gom 25 and BARI Gom 30 showed more considerable genetic potential for yield under ILS, beating the other genotypes in group A. BAW 1147 was classified as heat-sensitive due to its greater HSI for grain yield (
Table 6). Alam et al. (2013c; 2013b; 2014) reported similar outcomes. According to
Table 6, group A's genotypes BARI Gom 25, BARI Gom 26, BARI Gom 27, BARI Gom 28, BARI Gom 29, BARI Gom 30, and BARI Gom 31 were suitable for ILS circumstances. Although few discrepancies were observed, most morphological data confirmed the molecular findings. For instance, Nadi 2 in group B showed a higher HSI (1.135) and a more notable decrease in mean grain production (17.4%) under ILS compared to ITS (
Table 6). It should not be categorized as heat-sensitive because it had the highest grain yield (2.19 kg) in the ILS despite being in the heat-sensitive group. In contrast, BARI Gom 26 in group A had a higher HSI value (0.97) but the most significant drop in mean grain output (14.8%) under ILS and the lowest grain yield (1.85 kg) within the heat-tolerant group. This was comparable to the grain yield (1.63 kg) of WMRI Gom 3 in group B, indicating that it should not be categorized as a terminal heat stress-tolerant group. These variations could be attributed to the regional diversity of heat stress, which affects plants differently depending on length and timing. Heat stress has a complex impact on yield, and the genotype-environment interaction plays a crucial role in yield expression. This examination, conducted in a field with apparent weather fluctuations, is consistent with the findings of Haliloglu et al. (2022). Because temperatures above the optimum (21.3±1.27°C) during the grain-filling stage significantly impact wheat output, agricultural research prioritizes developing wheat types that can withstand heat. Genotypes that can withstand terminal heat stress or mature early without suffering appreciable yield losses must be developed or identified (Alam et al. 2014; Shenoda et al. 2021). The molecular and genetic methods used in this work demonstrated that marker-assisted breeding, cloning, and the characterization of genetic determinants for increased heat tolerance are all supported by DNA polymorphisms that confer thermotolerance. Because there were few abnormalities, the SSR markers helped classify wheat genotypes as either terminal heat stress tolerant or susceptible. Through this study, heat-tolerant and heat-sensitive wheat genotypes were identified, and the DNA fingerprinting of the genotypes was characterized to be established as future evidence of their intellectual property.
Conclusions
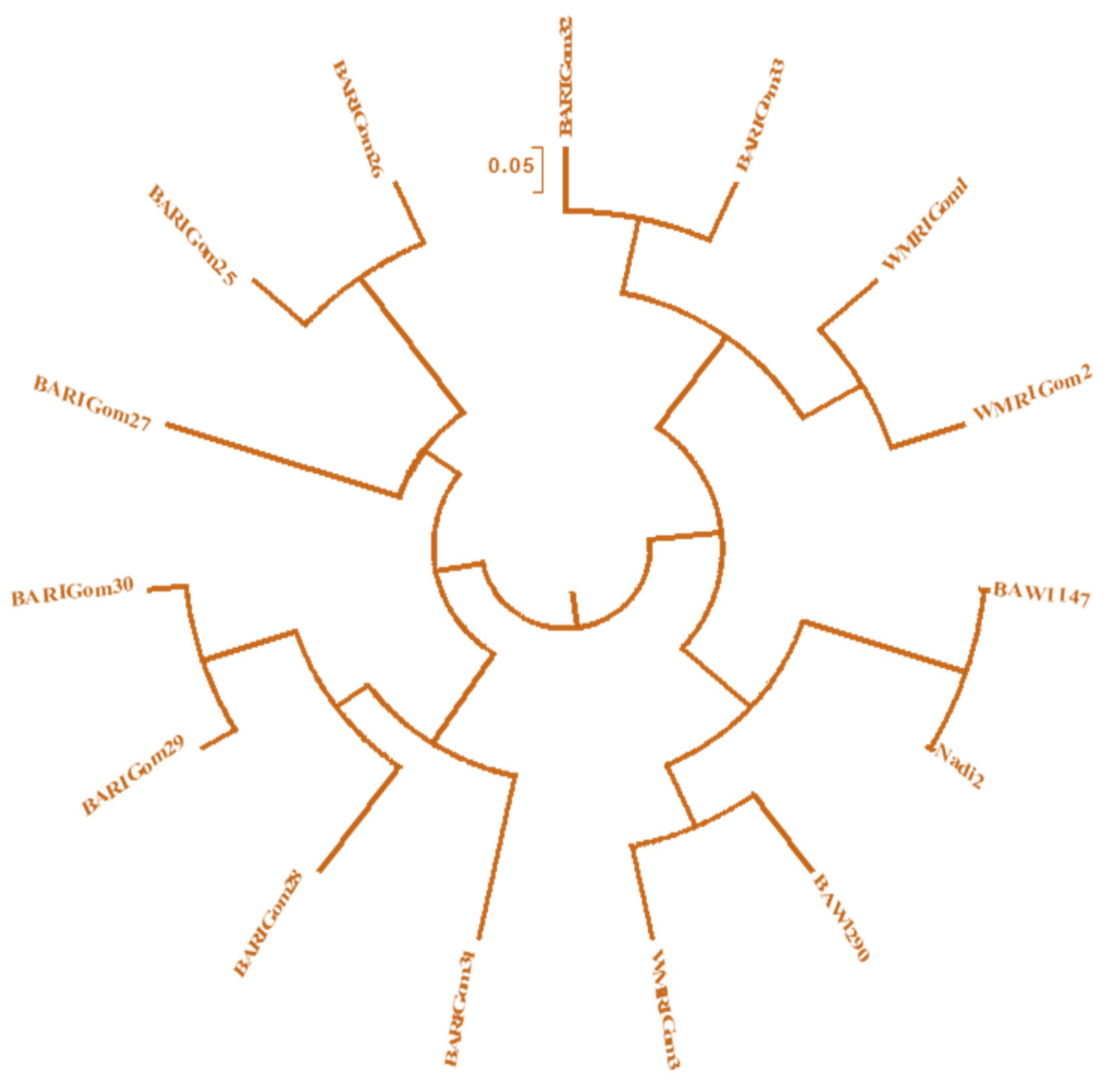
Genetic similarity data is crucial for maintaining genetic diversity and improving breeding operations. Cultivars with varied DNA profiles likely contain distinct genes, enhancing genetic variation and heat tolerance assessments. SSR markers provided valuable information for DNA fingerprinting and genetic diversity estimates, producing distinct bands for heat-tolerant genotypes. The study assessed fifteen wheat genotypes, creating a dendrogram with a distance coefficient of 0.050 and dividing them into two main categories. The most significant genetic distances were between BAW 1290 and BARI Gom 28 (0.929), followed by Nadi 2 and BARI Gom 28 (0.900), WMRI Gom 3 and BARI Gom 26 (0.889), and the lowest distance (0.000) was between Nadi 2 and BAW 1147. Cluster I genotype showed moderate heat tolerance with HSI values for the 1000-grain weight (TGW) and grain yield ranging from 0.885 to 0.964, closely matching Cluster II, which included BARI Gom 28, BARI Gom, BARI Gom 29, BARI Gom 30 and BARI Gom 31. Cluster III in Group B had higher HSIs and significant TGW and grain yield reductions under heat stress (1.038 - 1.218). Cluster IV genotypes also showed heat sensitivity (HSI > 1.00). Group A consisting of seven genotypes (Cluster I & II) (BARI Gom 25, BARI Gom 26, BARI Gom 27, BARI Gom 28, BARI Gom 29, BARI Gom 30, and BARI Gom 31 were identified as suitable for cultivation under heat-stressed conditions. This study analyzed DNA fingerprinting and identified heat-tolerant and heat-sensitive wheat genotypes, providing valuable insights for future molecular breeding programs focused on heat tolerance.
Author Contributions
GF, MNA, and MFA conceptualized and designed the experiments. MFA and GF conducted the experiments. GF collected the experiment materials. GF and MNA supervised the experiments. MNA coordinated experiments. MFA analyzed the data. MNA and MFA wrote the manuscript. GF, MMK, MMR, and MNA revised, edited, and improved it. All authors accepted the final version of the manuscript.
Funding
The project was provided to Dr. Golam Faruq, Principal Scientific Officer, Regional Station, Bangladesh Wheat and Maize Research Institute, Joydebpur, Gazipur, 1701, Bangladesh; by the Krishi Gobeshna Foundation, Farmgate, Dhaka, 1000; Project Code: BR 5-C/17; Duration: 2017-2023.
Acknowledgments
The authors are grateful to the heads of the Biotechnology Division, Bangladesh Agricultural Research Institute, Joydebpur, and Gazipur for their cooperation in providing the laboratory and greenhouse facilities to conduct the research.
Conflicts of Interest
All authors declare that they have no conflict about the research paper.
References
- Alam, E.; Hridoy, A.-E.E.; Tusher, S.M.S.H.; et al. Climate change in Bangladesh: Temperature and rainfall climatology of Bangladesh for 1949-2013 and its implication on rice yield. PLoS ONE 2023, 18, e0292668. [Google Scholar]
- Alam, M.N.; Islam, M.Z.; Mamun, M.A.A.; Md, M.S.N.; Hossain, M.M.; et al. Searching high yielding durum wheat genotype (s) through the assessment of the physiology, yield, and yield contributing attributes. International Journal of Agronomy and Agricultural Research 2023, 23, 1–9. [Google Scholar]
- Alam, M.N.; Akhter, M.M.; Hossain, M.M.; Mahbubul, S.M. Phenological changes of different wheat genotypes (Triticum aestivum L.) in high temperature imposed by late seeding. Journal of Biodiversity and Environmental Sciences 2013, 3, 83–93. [Google Scholar]
- Alam, M.N.; Akhter, M.M.; Hossain, M.M.; Rokonuzzaman. Performance of different genotypes of wheat (Triticum aestivum L.) in heat stress conditions. International Journal of Biosciences 2013, 3, 295–306. [Google Scholar] [CrossRef]
- Alam, M.N.; Bodruzzaman, M.; Hossain, M.M.; Sadekuzzaman, M. Growth performance of spring wheat under heat stress conditions. International Journal of Agronomy and Agricultural Research 2014, 4, 91–103. [Google Scholar]
- Alam, M.N.; Mannaf, M.A.; Sarker, M.A.Z.; Akhter, M.M. Effect of terminal high temperature imposed by late sowing on phenological traits of wheat (Triticum aestivum L.). International Journal of Agronomy and Agricultural Research 2013, 3, 6–10. [Google Scholar]
- Alam, M.N.; Yang, L.; Yi, X.; Wang, Q.-F.; Robin, A.H.K. Role of melatonin in inducing the physiological and biochemical processes associated with heat stress tolerance in tall fescue (Festuca arundinaceous). Journal of Plant Growth Regulation 2021, 41, 2759–2768. [Google Scholar] [CrossRef]
- Alam, M.N.; Zhang, L.; Yang, L.; et al. Transcriptomic profiling of tall fescue in response to heat stress and improved thermotolerance by melatonin and 24-epibrassinolide. BMC Genomics 2018, 19, 224. [Google Scholar] [CrossRef]
- Ali, R.A.; Kelestanie, A.; Asadi, A.; Mirfakhraei, S.R.; Abasi, A.R. Genetic Diversity in Twenty Bread Wheat Cultivars Using Microsatellite Markers. International Journal of Agronomy and Plant Production 2013, 4, 1920–1927. [Google Scholar]
- Ates Sonmezoglu, O.; Balkan, A.S. Molecular and biochemical analysis of durum wheat genotypes to examine carotenoid pigment content and lipoxygenase enzyme activity. Cereal Research Communications 2014, 42, 218–228. [Google Scholar] [CrossRef]
- Ateş Sönmezoğlu, Ö.; Terzi, B. Characterization of some bread wheat genotypes using molecular markers for drought tolerance. Physiological and Molecular Biology of Plants 2018, 24, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Asthir, B.; Bains, N.S. Effect of terminal heat stress on yield and yield attributes of wheat. Indian Journal of Applied Research 2014, 4, 1–2. [Google Scholar]
- Barrett, B.A.; Kidwell, K.K. AFLP-based genetic diversity assessment among wheat cultivars from The Pacific Northwest. Crop Science 1998, 38, 1261–1271. [Google Scholar] [CrossRef]
- BBS (Bangladesh Bureau of Statistics). Yearbook of Agricultural Statistics; Bangladesh Bureau of Statistics, Statistics and Informatics Division, Ministry of Planning, Government of the People’s Republic of Bangladesh: Agargaon, Dhaka, Bangladesh, 2023. [Google Scholar]
- Bhusal, N.; Sarial, A.K.; Sharma, P.; Sareen, S. Mapping QTLs for grain yield components in wheat under heat stress. PLoS One 2017, 12, e0189594. [Google Scholar] [CrossRef] [PubMed]
- Bita, C.; Gerats, T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Frontiers in Plant Science 2013, 4, 273. [Google Scholar] [CrossRef]
- BMD (Bangladesh Meteorological Department). Bangladesh Meteorological Department, Ministry of Defense of the Government of Bangladesh, Agargaon, Dhaka. 2024. Available online: https://dataportal.bmd.gov.bd/.
- Botstein, D.; White, R.; Skolnick, M.; Davis, R. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. American Journal of Human Genetics 1980, 32, 314–331. [Google Scholar]
- Chaudhary, S.; Devi, P.; Bhardwaj, A.; et al. Identifying and characterizing contrasting genotypes/cultivars for developing crop heat tolerance: current status and prospects. Frontiers in Plant Science 2020, 11, 587264. [Google Scholar] [CrossRef]
- CRP. Climate Risk Profile; The World Bank Group: Bangladesh, 2024. [Google Scholar]
- DAE (Department of Agricultural Extension). Annual Report 2022-23; Department of Agricultural Extension, Ministry of Planning, Government of the People’s Republic of Bangladesh: Khamarbari, Farmgate, Dhaka, 2023. [Google Scholar]
- Ding, Y.; Yang, S. Surviving and thriving: How plants perceive and respond to temperature stress. Developmental Cell 2022, 57, 947–958. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses. 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Elahi, I.; Saeed, U.; Wadood, A.; Abbas, A.; Nawaz, H.; Jabbar, S. Effect of climate change on wheat productivity. In Wheat; Ansari, M.U.R., Ed.; IntechOpen: Rijeka, 2022. [Google Scholar]
- Farhad, M.; Kumar, U.; Tomar, V.; et al. Heat stress in wheat: a global challenge to feed billions in the current era of the changing climate. Frontiers in Sustainable Food Systems 2023, 7, 2023. [Google Scholar] [CrossRef]
- Fischer, R.A.; Maurer, O. Crop temperature modification and yield potential in dwarfing spring wheat. Crop Science 1978, 16, 855–859. [Google Scholar] [CrossRef]
- Golabadi, M.; Arzani, A.; Mirmohammadi Maibody, S.A.M.; Sayed Tabatabaei, B.E.; Mohammadi, S.A. Identification of microsatellite markers linked with yield components under drought stress at terminal growth stages in durum wheat. Euphytica 2011, 177, 207–221. [Google Scholar] [CrossRef]
- Govindaraj, M.; Pattanashetti, S.K.; Patne, N.; et al. Breeding cultivars for heat stress tolerance in staple food crops. In Subsequent generation Plant Breeding; Özden, Y., Çiftçi, Y.Ö., Eds.; IntechOpen: Rijeka, 2018. [Google Scholar]
- Gupta, O.P.; Singh, A.K.; Singh, A.; et al. Wheat Biofortification: Utilizing Natural Genetic Diversity, Genome-Wide Association Mapping, Genomic Selection, and Genome Editing Technologies. Frontiers in Nutrition 2022, 9, 826131. [Google Scholar] [CrossRef] [PubMed]
- Haliloglu, K.; Turkoglu, A.; Tan, M.; Poczai, P. SSR-Based Molecular Identification and Population Structure Analysis for Forage Pea (Pisum sativum var. arvense L.) Landraces. Genetics 2022, 13, 1086. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.Y.; Zhang, X.Y.; Wang, L.F.; Dong, Y.S.; Shang, X.W.; Jia, J.Z. Genetic diversity and core collection evaluations in common wheat germplasm from the northwestern spring wheat region in China. Molecular Breeding 2006, 17, 69–77. [Google Scholar] [CrossRef]
- Hays, D.; Mason, E.; Do, J.H.; Menz, M.; Reynolds, M. Expression quantitative trait loci mapping heat tolerance during reproductive development in wheat (Triticum aestivum). In Wheat production in stressed environments; Springer: Netherlands, 2007; pp. 373–382. [Google Scholar]
- Herrera, T.G.; Duque, D.P.; Almeida, I.P.; et al. Assessment of genetic diversity in Venezuelan rice cultivars using simple sequence repeats markers. Electronic Journal of Biotechnology 2008, 11, 215–226. [Google Scholar]
- Hossain, M.M.; Rahman, M.M.; Islam, R.; et al. Evaluation of some wheat genotypes growing under heat stress conditions in two environments in Bangladesh. Journal of Multidisciplinary Science 2019, 1, 1–7. [Google Scholar] [CrossRef]
- Hossain, M.M.; Alam, M.N.; Islam, M.Z.; Ekram, M.S.B.; Islam, R.; Atiquzzaman, M. Spring wheat genotypes exhibit physiological and yield potentiality under late sowing conditions in Bangladesh. International Journal of Agronomy and Agricultural Research 2021, 18, 1–7. [Google Scholar]
- Hossain, M.M.; Alam, M.N.; Hakim, M.A.; et al. Development of short duration, tolerance to high temperature and bipolaris leaf blight, and moderately susceptible to blast disease of wheat genotype with trials in various agroecological zones in Bangladesh. International Journal of Scientific Research Management 2023, 11, 395–410. [Google Scholar]
- IPCC. Summary for Policymakers. In Global Warming of 1.5°C; An IPCC Special Report; Masson-Delmotte, V.P., Zhai, H.-O., Pörtner, D., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; pp. 3–24. [Google Scholar]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V.P., Zhai, A., Pirani, S.L., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; p. 2391. [Google Scholar]
- IPCC. Summary for Policymakers. in: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II, and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, L.H., Romero, J., Eds.; Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar]
- Joshi, A.K.; Chand, R.; Arun, B.; et al. Breeding crops for reduced-tillage management in the intensive, rice-wheat systems of South Asia. Euphytica 2007, 153, 135–151. [Google Scholar] [CrossRef]
- Khan, A.A.; Shamsuddin, A.K.M.; Barma, N.C.D.; et al. Screening for Heat Tolerance in Spring Wheat (Triticum aestivum L.). Tropical Agricultural Research and Extension 2014, 17, 26–37. [Google Scholar] [CrossRef]
- Khanna-Chopra, R.; Viswanathan, C. Evaluation of heat stress tolerance in an irrigated environment of T. aestivum and related species. I. Stability in yield and yield components. Euphytica 1999, 106, 169–180. [Google Scholar] [CrossRef]
- Khatun, M.A.; Rashid, M.B.; Hygen, O.H. “Climate of Bangladesh”; MET report, no. 08/2016; Climate, Norwegian Meteorological Institute, 2016; ISBN 2387-4201. [Google Scholar]
- Kirigwi, F.M.; van Ginkel, M.; Brown-Guedira; et al. Markers associated with a QTL for grain yield in wheat under drought. Molecular Breeding 2007, 20, 401–413. [Google Scholar] [CrossRef]
- Kirigwi, F.M.; van Ginkel, M.; Brown-Guedira; et al. Markers associated with a QTL for grain yield in wheat under drought. Molecular Breeding 2007, 20, 401–413. [Google Scholar] [CrossRef]
- Kumer, L.; Chhogyel, N.; Gopalakrishnan, T; et al. Climate change and future of agri-food production. In Future Foods; Bhat, R., Ed.; Academic Press, 2022; pp. 49–79. [Google Scholar]
- Lu; Liu, H.; Wu, Y.; Yan, G. Wheat genotypes tolerant to heat at the seedling stage tend to be also tolerant at the adult stage: The possibility of early selection for heat tolerance breeding. The Crop Journal 2022, 10, 1006–1013. [Google Scholar] [CrossRef]
- Mao, H.; Jiang, C.; Tang, C.; et al. Wheat adaptation to environmental stresses under climate change: Molecular basis and genetic improvement. Molecular Plant 2023, 16, 1564–1589. [Google Scholar] [CrossRef] [PubMed]
- Mason, E.R.; Mondal, S.; Beecher, W.F.; Hays, D.B. Genetic loci linking improved heat tolerance in wheat (Triticum aestivum L.) to lower leaf and spike temperature under controlled conditions. Euphytica 2011, 180, 181–194. [Google Scholar] [CrossRef]
- Mason, R.; Mondal, S.; Beecher, F.W.; et al. QTL associated with heat susceptibility index in wheat (Triticum aestivum L.) under short-term reproductive stage heat stress. Euphytica 2010, 174, 423–436. [Google Scholar] [CrossRef]
- Mickelbart, M.V.; Bailey-Serres, H.J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nature Review Genetics 2015, 16, 237–251. [Google Scholar] [CrossRef]
- Mohammadi, V.; Zali, A.A.; Bihamta, M.R. Mapping QTL for heat tolerance in wheat. Journal of Agricultural Science and Technology 2008, 10, 261–267. [Google Scholar]
- Mourad, A.M.I.; Belamkar, V.; Baenziger, P.S. Molecular genetic analysis of spring wheat core collection using genetic diversity, population structure, and linkage disequilibrium. BMC Genomics 2020, 21, 434. [Google Scholar] [CrossRef] [PubMed]
- Nagy, S.; Poczai, P.; Cerna, K.I.; et al. PICcalc: an online program to calculate polymorphic information content for molecular genetic studies. Biochemistry and Genetics 2012, 50, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Roder, M.S.; Kumar, U.; et al. QTL mapping of terminal heat tolerance in hexaploid wheat (Triticum aestivum L.). Theoretical Applied Genetics 2012, 125, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.C.; Rane, J.; Sareen, S.; et al. Molecular investigations on grain filling rate under terminal heat stress in bread wheat (Triticum aestivum L.). African Journal of Biotechnology 2013, 12, 4439–4445. [Google Scholar] [CrossRef]
- Pinto, S.; Chapman, S.C.; McIntyre, C.L.; et al. For canopy temperature response related to yield in both heat and drought environments. Theoretical Applied Genetics 2010, 121, 1001–1021. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Varshney, R.K.; Roym, J.K.; et al. The use of microsatellites for detecting DNA polymorphism, genotype identification and genetic diversity in wheat. Theoretical Applied Genetics 2000, 100, 584–592. [Google Scholar]
- Rahman, M.A.; Chikushi, J.; Yoshida, S.; Karim, A.J.M.S. Growth and yield components of wheat genotypes exposed to high-temperature stress under control environment. Bangladesh Journal of Agricultural Research 2009, 34, 361–372. [Google Scholar] [CrossRef]
- Ramadugu, C.; Keremane, M.L.; Hu, X.; et al. Genetic analysis of citron (Citrus medica L.) using simple sequence repeats and single nucleotide polymorphisms. Scientia Horticulture 2015, 195, 124–137. [Google Scholar] [CrossRef]
- Ramya, P.; Jain, N.; Singh, P.K.; Singh, G.P.; Prabhu, K.V. Population structure, molecular and physiological characterization of elite wheat varieties used as parents in drought and heat stress breeding in India. Indian Journal of Genetics and Plant Breeding 2015, 75, 250–252. [Google Scholar] [CrossRef]
- Rane, J.; Pannu, R.K.; Sohu, V.S.; et al. Performance of yield and stability of advanced wheat genotypes under heat-stressed environments of Indo-Gangetic Plains. Crop Science 2007, 47, 1561–1573. [Google Scholar] [CrossRef]
- Roder, M.S.; Korzun, V.; Wendehake, K.; et al. A microsatellite map of wheat. Genetics 1998, 149, 2007–2023. [Google Scholar] [CrossRef] [PubMed]
- Sadat, S.; Saeid, K.A.; Bihamta, M.R.; et al. Marker-assisted selection for heat tolerance in bread wheat. World Applied Science Journal 2013, 21, 1181–1189. [Google Scholar]
- Sánchez-Bermúdez, M.; Del Pozo, J.C.; Pernas, M. Effects of combined abiotic stresses related to climate change on root growth in crops. Frontiers in Plant Science 2022, 13, 918537. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sareen, S.; Saini, M.; Shefali. Assessing genetic variation for heat stress tolerance in Indian bread wheat genotypes using morpho-physiological traits and molecular markers. Plant Genetics Resources 2017, 15, 539–547. [Google Scholar] [CrossRef]
- Shenoda, J.E.; Sanad, M.N.M.E.; Rizkalla, A.A.; et al. Effect of long-term heat stress on grain yield, pollen grain viability and germinability in bread wheat (Triticum aestivum L.) under field conditions. Heliyon 2021, 7, e07096. [Google Scholar] [CrossRef]
- Tomar, R.S.; Deshmukh, R.K.; Naik, K.B.; Tomar, S.M.S. Development of chloroplast-specific microsatellite markers for molecular characterization of alloplasmic lines and phylogenetic analysis in wheat. Plant Breeding 2013, 133, 12–18. [Google Scholar] [CrossRef]
- Walkowiak, S.; Gao, L.; Monat, C.; et al. Multiple wheat genomes reveal global variation in modern breeding. Nature 2020, 588, 277–283. [Google Scholar] [CrossRef]
- Yıldırım, A.; Ates Sonmezoglu, O.; Sayaslan, A.; et al. Marker-assisted breeding of a durum wheat cultivar for gliadin and LMW-glutenin proteins affecting pasta quality. Turkish Journal of Agriculture and Forestry 2013, 37, 527–533. [Google Scholar] [CrossRef]
- Zaman, R.; Malaker, P.K.; Murad, K.F.I.; Sadat, M.A. TREND analysis of changing temperature in Bangladesh due to global warming. Journal of Biodiversity and Environmental Science 2013, 3, 32–38. [Google Scholar]
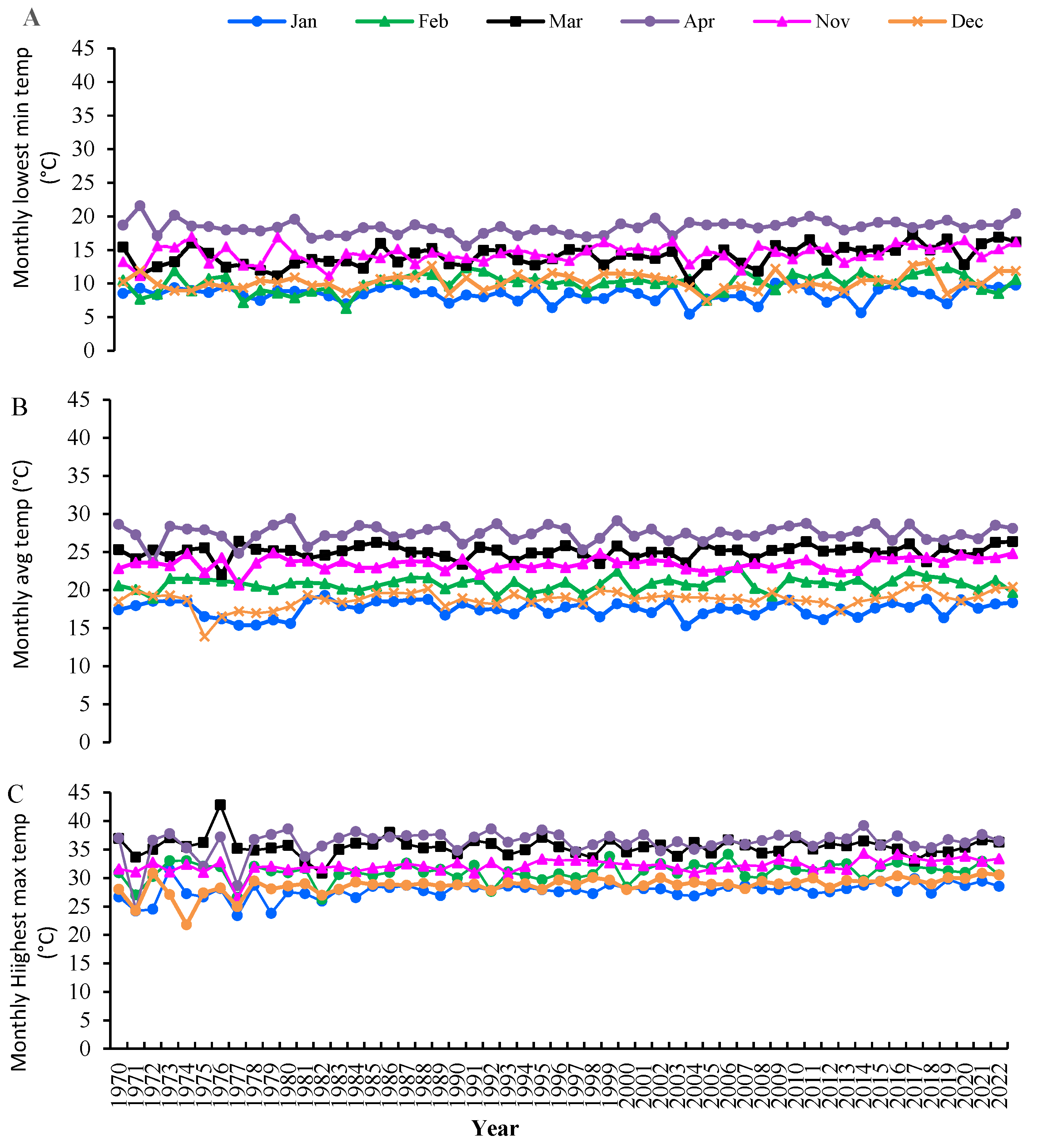
Figure 2.
The monthly minimum (A), average (B), and maximum temperature (°C) of Bangladesh for January, February, March, April, November, and December in the years 1970 to 2022. Monthly data are the average of seven divisions (Dhaka, Rajshahi, Barishal, Sylhet, Rangpur, and Mymensingh Division) and one region (Dinajpur) of Bangladesh (Average = avg, Minimum = Min, maximum = Max, Temperature = Temp) (BMD 2024).
Figure 2.
The monthly minimum (A), average (B), and maximum temperature (°C) of Bangladesh for January, February, March, April, November, and December in the years 1970 to 2022. Monthly data are the average of seven divisions (Dhaka, Rajshahi, Barishal, Sylhet, Rangpur, and Mymensingh Division) and one region (Dinajpur) of Bangladesh (Average = avg, Minimum = Min, maximum = Max, Temperature = Temp) (BMD 2024).
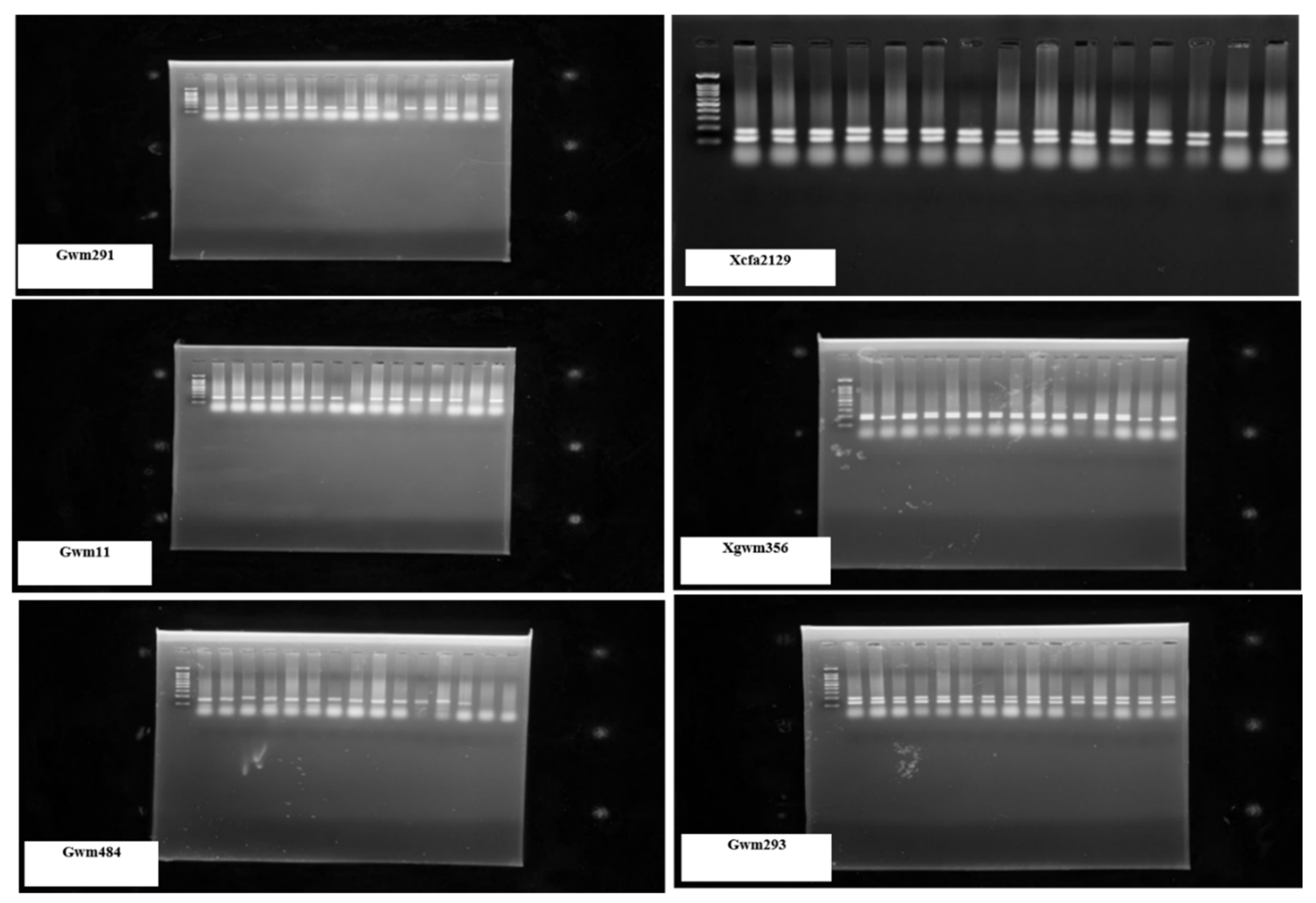
Figure 3.
SSR marker profiles of bread wheat genotypes using the SSR primers gwm291, Xcfa2129,
gwm11, Xgwm356, Gwm484, and Gwm2.
Figure 3.
SSR marker profiles of bread wheat genotypes using the SSR primers gwm291, Xcfa2129,
gwm11, Xgwm356, Gwm484, and Gwm2.
Figure 4.
Shows a Dendrogram generated through UPGMA analysis showing the genetic relationships among fifteen wheat genotypes. The names of the genotypes are given at the ends of the branches.
Figure 4.
Shows a Dendrogram generated through UPGMA analysis showing the genetic relationships among fifteen wheat genotypes. The names of the genotypes are given at the ends of the branches.
Table 1.
List of fifteen wheat genotypes with their pedigrees.
Table 1.
List of fifteen wheat genotypes with their pedigrees.
| Variety |
Pedigree |
Year of release |
Life cycle (d) |
Salient features |
| BARI Gom 25 |
ZSH 12/HLB 19//2*NL297 |
2010 |
102-110 |
Heat and salinity tolerance. It can tolerate 8.0-10 dS/m salinity at the seedling stage High yielding (3.8-5.0 t ha-1) Resistant to leaf rust (LR) and Bipolaris leaf blight (BpLB) diseases |
| BARI Gom 26 |
ICTAL 123/3/RAWAL 87//VEE/HD 2285BD(JO)9585-0JO-3JE-0JE-0JE-HRDI-RC5DI |
2010 |
104-110 |
|
| BARI Gom 27 |
WAXWING*2/VIVISTICGSS01BOOO56T-099Y-099 M-099 M-099Y-099 M-14Y-0B |
2012 |
105-110 |
|
| BARI Gom 28 |
CHIL/2*STAR/4/BOW/CROW//BUC/PVN/3/2*VEE#10CMSS95Y00624S-0100Y-0200 M-17Y-010 M-5Y-0 M |
2012 |
102-108 |
Early maturing and heat tolerance. Suitable for late sowing High yielding (4.0-5.5 t ha-1) Resistant to LR and moderately resistant to BpLB |
| BARI Gom 29 |
SOURAV/7/KLAT/SOREN//PSN/3/BOW/4/VEE#5. 10/5/CNO 67/MFD//MON/3/SERI/6/NL297BD(DI)112S-0DI-030DI-030DI-030DI-9DI |
2014 |
105-110 |
Moderately tolerant to heat stress High yielding (4.0-5.0 t ha-1) Resistant to leaf and stem rust (Ug99 race) and moderately resistant to BpLB |
| BARI Gom 30 |
BAW 677/BijoyBD(JA)1365S-0DI-15DI-3DI-HR12R3DI |
2014 |
100-105 |
Heat tolerance and suitable for late sowing High yielding (4.0-5.5 t ha-1) Resistant to leaf rust and moderately resistant to BpLB |
| BARI Gom 31 |
KAL/BB/YD/3/PASTORCMSS99M00981S-0P0M-040SY-040 M-040SY-16 M-0ZTY-0 M |
2017 |
104-109 |
|
| BARI Gom 32 |
SHATABDI/GOURABBD(DI)1686S-0DI-1DI-0DI-0DI-3DI |
2017 |
95-105 |
Early maturing, heat tolerant, short stature Resistant to LR and tolerant to spot blotch Has tolerance to wheat blast (10-12% infection at Jessore) Yield:4.6-5.0 t ha-1
|
| BARI Gom 33 |
KACHU/SOLALA |
2018 |
110-115 |
Culm robust, succulent, firm, erect, and found lodging under high wind flow Leave and stem deep green from seedling to anthesis stages. So, farmers prefer it too much Resistant to blast disease, LR, and tolerant to terminal heat stress Grain zinc enriched (50-55 ppm) |
| WMRI Gom 1 |
BARI Gom 21 (Shatabdi)/BARI Gom 24 |
2019 |
100-104 |
Short stature, tolerant to lodging. Having the shortest life cycle of all released varieties and early maturing, escaping terminal heat stress Tolerant to blast and rust diseases |
| WMRI Gom 2 |
BARI Gom 26/BARI Gom 25 |
2021 |
108-115 |
Grain in amber color Panicle tall in length, 45-48 grains/panicle, 1000-grain weight 45-50 g Resistant to LR and tolerant to heat stress Yield: 4.5-5.8 t ha-1
|
| WMRI Gom 3 |
Borlaug 100ROELFS-F-2007/4/BOBWHITE/NEELKANT//CATBIRD/3/CATBIRD/5/FRET-2/TUKURU//FRET-2 |
2021 |
108-114 |
Dwarf sized and almost no lodging attribute Resistant to LR, BpLB, and blast disease Yield: 4.0-4.5 t ha-1
|
| BAW 1290 |
BARI Gom 21/BL 3503 |
- |
- |
- |
| BAW 1147 |
OASIS/3*ANGRA//708 |
- |
- |
- |
| Nadi 2 |
- |
- |
- |
- |
Table 2.
Characteristics of 13 linked SSR markers used in the characterization.
Table 2.
Characteristics of 13 linked SSR markers used in the characterization.
| SL No. |
Marker |
QTL for |
Primers sequence Reverse (5’- 3’) |
Primers sequence Forward (5’- 3’) |
Chromosomal location |
Annealing temp (°C) |
| 1 |
gwm291 |
Leaf Curl |
AATGGTATCTATTCCGACCCG |
CATCCCTAGGCCACTCTGC |
5A |
60 |
| 2 |
Gwm325 |
HSI grain filling duration HSI kernel weight |
TTTTTACGCGTCAACGACG |
TTTCTTCTGTCGTTCTCTTCCC |
6D |
60 |
| 3 |
Xgwm294 |
HIS single kernel weight of the main spike |
GCAGAGTGATCAATGCCAGA |
GGATTGGAGTTAAGAGAGAACCG |
2A |
55 |
| 4 |
Gwm268 |
HSI kernel weight |
TTATGTGATTGCGTACGTACCC |
AGGGGATATGTTGTCACTCCA |
1B |
55 |
| 5 |
Xwmc407 |
Grain-filling duration |
CATATTTCCAAATCCCCAACTC |
GGTAATTCTAGGCTGACATATGCTC |
2A |
61 |
| 6 |
Xcfa2129 |
HIS single kernel weight of the main spike |
ATCGCTCACTCACTATCGGG |
GTTGCACGACCTACAAAGCA |
1A, 1B, 1D |
60 |
| 7 |
gwm11 |
Grain-filling duration |
GTGAATTGTGTCTTGTATGCTTCC |
GGATAGTCAGACAATTCTTGTG |
1A, 1B |
50 |
| 8 |
Xcfd43 |
Grain-filling duration |
CCAAAAACATGGTTAAAGGGG |
AACAAAAGTCGGTGCAGTCC |
2D |
60 |
| 9 |
Xgwm356 |
HSI single kernel weight of the main spike |
CCAATCAGCCTGCAACAAC |
AGCGTTCTTGGGAATTAGAGA |
2A, 6A, 7A |
55 |
| 10 |
Xbarc137 |
Waxiness |
CCAGCCCCTCTACACATTTT |
GGCCCATTTCCCACTTTCCA |
1B |
52 |
| 11 |
Gwm484 |
Waxiness |
AGTTCCGGTCATGGCTAGG |
ACATCGCTCTTCACAAACCC |
2D |
55 |
| 12 |
Gwm293 |
Grain-filling duration |
TCGCCATCACTCGTTCAAG |
TACTGGTTCACATTGGTGCG |
5A |
55 |
| 13 |
WMC527 |
HIS kernel weight of the main spike |
GCTACAGAAAACCGGAGCCTAT |
ACCCAAGATTGGTGGCAGAA |
3A, 3B |
61 |
Table 3.
Allele numbers, sizes, and PIC values for fifteen wheat genotypes for thirteen SSR markers.
Table 3.
Allele numbers, sizes, and PIC values for fifteen wheat genotypes for thirteen SSR markers.
| Marker |
Allele No |
Allele size and range |
Difference (bp) |
Major AlleleϦFrequency |
Gene Diversity |
Heterozygosity |
PIC |
| gwm291 |
3 |
150-160 |
10 |
0.46 |
0.64 |
0.00 |
0.57 |
| Gwm325 |
3 |
150-160 |
10 |
0.38 |
0.66 |
0.00 |
0.58 |
| Xgwm294 |
4 |
50-120 |
70 |
0.50 |
0.65 |
1.00 |
0.59 |
| Gwm268 |
3 |
180-285 |
105 |
0.67 |
0.48 |
0.11 |
0.40 |
| Xwmc407 |
2 |
140-145 |
5 |
0.71 |
0.41 |
0.00 |
0.33 |
| Xcfa2129 |
4 |
120-190 |
70 |
0.47 |
0.66 |
1.00 |
0.59 |
| gwm11 |
3 |
200-210 |
10 |
0.71 |
0.44 |
0.00 |
0.39 |
| Xcfd43 |
2 |
160-165 |
5 |
0.50 |
0.50 |
0.00 |
0.38 |
| Xgwm356 |
7 |
185-230 |
45 |
0.20 |
0.57 |
0.80 |
0.83 |
| Xbarc137 |
4 |
245-260 |
15 |
0.44 |
0.67 |
0.00 |
0.61 |
| Gwm484 |
4 |
90-190 |
100 |
0.36 |
0.71 |
0.92 |
0.66 |
| Gwm293 |
8 |
105-190 |
85 |
0.23 |
0.84 |
1.00 |
0.82 |
| WMC527 |
4 |
345-450 |
105 |
0.40 |
0.70 |
0.00 |
0.65 |
| Mean |
3.92 (total 51) |
- |
- |
0.46 |
0.63 |
0.37 |
0.57 |
| Range |
2.00-8.00 |
- |
2.00 - 105 |
0.200 - 0.714 |
0.49-0.85 |
0.00 - 1.00 |
0.33-0.83 |
Table 4.
Genetic distance of fifteen wheat genotypes based on thirteen SSR markers.
Table 4.
Genetic distance of fifteen wheat genotypes based on thirteen SSR markers.
| |
BARI Gom 25 |
BARI Gom 26 |
BARI Gom 27 |
BARI Gom 28 |
BARI Gom29 |
BARI Gom30 |
BARI Gom31 |
BARI Gom32 |
BARI Gom 33 |
BAW 1147 |
BAW 1290 |
Nadi 2 |
WMRI Gom 1 |
WMRI Gom2 |
WMRI Gom3 |
| BARI Gom 25 |
0.000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| BARI Gom 26 |
0.150 |
0.000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| BARI Gom 27 |
0.545 |
0.500 |
0.000 |
|
|
|
|
|
|
|
|
|
|
|
|
| BARI Gom 28 |
0.583 |
0.500 |
0.458 |
0.000 |
|
|
|
|
|
|
|
|
|
|
|
| BARI Gom 29 |
0.583 |
0.600 |
0.591 |
0.250 |
0.000 |
|
|
|
|
|
|
|
|
|
|
| BARI Gom 30 |
0.667 |
0.700 |
0.591 |
0.333 |
0.083 |
0.000 |
|
|
|
|
|
|
|
|
|
| BARI Gom 31 |
0.708 |
0.650 |
0.727 |
0.542 |
0.292 |
0.292 |
0.000 |
|
|
|
|
|
|
|
|
| BARI Gom 32 |
0.714 |
0.786 |
0.500 |
0.714 |
0.571 |
0.571 |
0.571 |
0.000 |
|
|
|
|
|
|
|
| BARI Gom 33 |
0.714 |
0.786 |
0.571 |
0.786 |
0.500 |
0.500 |
0.429 |
0.143 |
0.000 |
|
|
|
|
|
|
| BAW 1147 |
0.750 |
0.750 |
0.667 |
0.875 |
0.875 |
0.875 |
0.875 |
0.667 |
0.833 |
0.000 |
|
|
|
|
|
| BAW 1290 |
0.714 |
0.714 |
0.714 |
0.929 |
0.786 |
0.786 |
0.786 |
0.500 |
0.583 |
0.333 |
0.000 |
|
|
|
|
| Nadi 2 |
0.800 |
0.800 |
0.800 |
0.900 |
0.700 |
0.700 |
0.700 |
0.400 |
0.500 |
0.000 |
0.200 |
0.000 |
|
|
|
| WMRI Gom 1 |
0.714 |
0.786 |
0.500 |
0.786 |
0.500 |
0.500 |
0.286 |
0.333 |
0.167 |
0.875 |
0.700 |
0.625 |
0.000 |
|
|
| WMRI Gom 2 |
0.700 |
0.813 |
0.778 |
1.000 |
0.800 |
0.800 |
0.600 |
0.417 |
0.333 |
0.875 |
0.500 |
0.500 |
0.167 |
0.000 |
|
| WMRI Gom 3 |
0.800 |
0.889 |
0.667 |
0.900 |
0.700 |
0.700 |
0.600 |
0.417 |
0.333 |
0.625 |
0.214 |
0.300 |
0.333 |
0.333 |
0.000 |
Table 5.
Summary of wheat genotype clusters according to morphophysiological traits (TGW).
Table 5.
Summary of wheat genotype clusters according to morphophysiological traits (TGW).
| Cluster |
Genotypes |
HSI |
TGW |
%TGW decrease |
HSI |
TGW |
%TGW decrease |
| ITS |
ILS |
ITS |
ILS |
| Group A |
| Cluster I |
BARI Gom 25 |
0.887 |
49.00 |
43.87 |
10.47 |
0.887 |
49.00 |
43.87 |
10.47 |
| BARI Gom 26 |
0.885 |
48.05 |
43.03 |
10.45 |
| BARI Gom 27 |
0.870 |
42.85 |
38.45 |
10.27 |
| Cluster II |
BARI Gom 28 |
0.772 |
45.00 |
40.90 |
9.11 |
0.772 |
45.00 |
40.90 |
9.11 |
| BARI Gom 29 |
0.892 |
42.35 |
37.89 |
10.53 |
| BARI Gom 30 |
0.823 |
46.80 |
42.25 |
9.72 |
| BARI Gom 31 |
0.909 |
42.75 |
38.16 |
10.74 |
| Group B |
| Cluster III |
BWMRI Gom 3 |
1.038 |
43.15 |
37.86 |
12.26 |
1.038 |
43.15 |
37.86 |
12.26 |
| BAW 1290 |
1.181 |
44.30 |
38.12 |
13.95 |
| BAW 1147 |
1.218 |
45.05 |
38.57 |
14.38 |
| Nadi 2 |
1.106 |
43.55 |
37.86 |
13.07 |
| Cluster IV |
BARI Gom 32 |
1.169 |
48.55 |
41.85 |
13.80 |
1.169 |
48.55 |
41.85 |
13.80 |
| BARI Gom 33 |
1.061 |
48.85 |
42.73 |
12.53 |
| BWMRI Gom 1 |
1.129 |
48.85 |
42.34 |
13.33 |
| BWMRI Gom 2 |
1.043 |
49.30 |
43.23 |
12.31 |
Table 6.
Summary of wheat genotype clusters according to morphophysiological traits (grain yield).
Table 6.
Summary of wheat genotype clusters according to morphophysiological traits (grain yield).
| Cluster |
Genotypes |
HIS |
Yield |
% Yield decrease |
HSI |
Yield |
% Yield decrease |
| ITS |
ILS |
ITS |
ILS |
| Group A |
| Cluster I |
BARI Gom 25 |
0.903 |
2.68 |
2.31 |
13.81 |
0.903 |
2.68 |
2.31 |
13.81 |
| BARI Gom 26 |
0.964 |
2.17 |
1.85 |
14.75 |
| BARI Gom 27 |
0.960 |
2.52 |
2.15 |
14.68 |
| Cluster II |
BARI Gom 28 |
0.742 |
2.38 |
2.11 |
11.34 |
0.742 |
2.38 |
2.11 |
11.34 |
| BARI Gom 29 |
0.833 |
2.59 |
2.26 |
12.74 |
| BARI Gom 30 |
0.756 |
2.68 |
2.37 |
11.57 |
| BARI Gom 31 |
0.930 |
2.67 |
2.29 |
14.23 |
| Group B |
| Cluster III |
BWMRI Gom 3 |
1.128 |
1.97 |
1.63 |
17.26 |
1.128 |
1.97 |
1.63 |
17.26 |
| BAW 1290 |
1.189 |
2.64 |
2.16 |
18.18 |
| BAW 1147 |
1.253 |
2.66 |
2.15 |
19.17 |
| Nadi 2 |
1.135 |
2.65 |
2.19 |
17.36 |
| Cluster IV |
BARI Gom 32 |
1.050 |
2.49 |
2.09 |
16.06 |
1.050 |
2.49 |
2.09 |
16.06 |
| BARI Gom 33 |
1.004 |
2.54 |
2.15 |
15.35 |
| BWMRI Gom 1 |
1.102 |
2.61 |
2.17 |
16.86 |
| BWMRI Gom 2 |
1.056 |
2.60 |
2.18 |
16.15 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).