1. Introduction
Creating new apple varieties is based on improving biological characteristics adapted to environment conditions, processing technologies and user requirements. The development of new varieties resistant to damages during collecting, transport and storage processes contributes to reducing losses and production demand thereby contributing to reducing financial and environmental costs [
1,
2,
3,
4,
5]. Nevertheless, it is necessary to determine the micromechanical properties of this apple in order to compare it with other varieties.
Apples are composite materials consisting of various structural elements with different mechanical properties, but the majority of them comprise edible parenchymal tissue (flesh) [
6,
7]. The mechanical properties of flesh depend on the condition of cell walls and the turgor pressure of cells. From a mechanical standpoint, the fruit cell wall constitutes a robust network with a fibrous structure, providing each cell with its stable shape: a network resistant to stretching (cellulosic), a network resistant to shearing (hemicellulosic), and a network resistant to compression (pectic). Pectin is also responsible for cell adhesion strength. It is a type of polysaccharide that, in the case of apples, constitutes over half of the dry mass of the cell wall, thus primarily contributing to the mechanical properties of fruit. Its degradation is caused by enzymatic reactions naturally occurring in plants but can also be induced by pathogenic fungi and bacteria. Pectin enzymatic reaction affects the rheological properties, porosity, and ionic status of the cell wall. Pectins are also the main component of the middle lamella which binds adjacent cells, therefore they are considered to be the main determinant of the mechanical properties of the cell wall and plant tissue. Therefore, accurate determination of the stage of fruit ripeness and carrying out tests for different stages of fruit ripeness are so important in all fruit strength tests [
8,
9,
10,
11,
12,
13,
14,
15,
16].
Unfortunately, most of the available studies on the mechanical properties of apples do not include all the results necessary for the construction and validation of FEM models of the apples. Scientists focus either on studying the flesh and epidermis or the whole fruit, often neglecting the maturity stage of the examined fruit. They frequently report only firmness or the starch index as indicators of the maturity stage. Moreover, research is very often conducted on store-bought apples, which is meaningless for practical applications in the industry, as at this stage, we only see the consequences of the treatments the fruit underwent before reaching the shelf [
17,
18,
19,
20,
21,
22].
Of course, some research is aimed at showing new methodology and not at creating a database about the strength properties of a specific variety. Among the methods for measuring the mechanical properties of apple fruit, various tests can be distinguished, including uniaxial tests, multiaxial tests, tests on non-uniform samples, in situ tests, dynamic tests, as well as micro- and nanomechanical tests. Each of these methods has its advantages and disadvantages, and the choice depends on the research goal and available resources. In addition to the test method itself, it is also important to establish measurement parameters such as force, deformation, strain, modulus of elasticity, Poisson’s ratio, absorbed energy, acoustic response, surface pressures, and destructive stresses. The study of the mechanical properties of fruit tissues is an area of intense scientific research, crucial in assessing the quality and durability of fruit in harvesting, transportation, and storage processes. It is also worth noting that these methods are constantly being improved. In recent years, much research has focused on the use of modern technologies enabling both destructive and non-destructive analyses. These studies can also be divided into macro, micro, nano scales, and those conducted at the atomic level [
9,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34]. It opens the new field of fruit mechanical research: non-destructive Micro-CT imaging, combined with strength tests and numerical modeling forms the basis for reverse engineering research on apple fruit tissues.
Therefore, the article presents the results of mechanical and Micro-CT tests for specific and verified apple ripeness stages, constituting a set of complete data for building FE models.
2. Materials and Methods
2.1. Research Material
The Chopin variety apples, serving as the research material, were bred and harvested by the Experimental Orchards of the Warsaw University of Life Sciences in Wilanów. Harvest dates, established by the producer based on predictions for collective, consumption, and physiological ripening stages, fell on October 13th, October 26th, and November 14th, 2022, respectively. The harvest date for development stage of ripeness was determined using the ethylene-induced method, but additionally ethylene concentration in seed chambers was also tested for the remaining stages of maturity. The ethylene content in the seed chambers (µL/L) was assessed according to a commonly used method [
35], using a gas chromatograph (HP 5890, Hewlett Packard, Palo Alto, CA, USA).
To obtain representative samples for testing, apples have been selected based on diameter, height, and weight so that these parameters fall within the average values for the variety. Height and diameter were measured using the DC-1 caliper (Ovibell GmbH & Co. KG, Mulheim, Germany) with a measurement accuracy of 0.01 mm, while weight was measured using the laboratory scale WTC 600 (RADWAG, Psary, Poland) with a measurement accuracy of 0.001 g. The apples were tested within 24 hours after harvest.
2.2. Determination of Basic Physicochemical Properties of Fruit
In order to confirm the ripening stages, and determine the well know parameters for the apple characterization, a measurement of firmness (F), soluble solids content (SSC), and starch index (SI) were conducted to calculate the Streif Index (IS) based on these three parameters. Firmness was measured using a manual penetrometer (Facchini FT 327, Alfonsine, Italy) equipped with a cylindrical penetrating attachment with a diameter of 11.1 mm. Prior to firmness testing, the apple skin was removed. Soluble solids content was determined using a manual refractometer (RMR 200, Hanna Instruments, Woonsocket, Rhode Island, USA) [
36]. Starch index (SI) was determined by immersing apple slices in an iodine solution for one minute, followed by drying and comparison with standards [
37].
The Streif index (IS) was calculated using Equation 1:
Where: F – firmness [kG], SSC – Soluble sugar content [%], SI – starch index [–].
Water content was determined using a gravimetric method. Freeze-drying was performed using a Labconco 4.5L lyophilizer (Kansas City, MO, USA) under the following parameters: pressure 50 Pa, collector temperature -48ºC, drying time 24 hours.
Pectins were precipitated from the solution of the tested sample using 100% acetone. Results were obtained using gravimetric methods: filtration, drying, and weighing the resulting precipitate [
38].
Malic acid content was determined based on acidimetric analysis - 5 ml of juice and 100 ml of distilled water were titrated with 0.1 N NaOH to pH 8.1 (neutralization of the solution) [
4].
2.3. Determination of Strength Properties of Apple Flesh and Skin
2.3.1. Apple Flesh
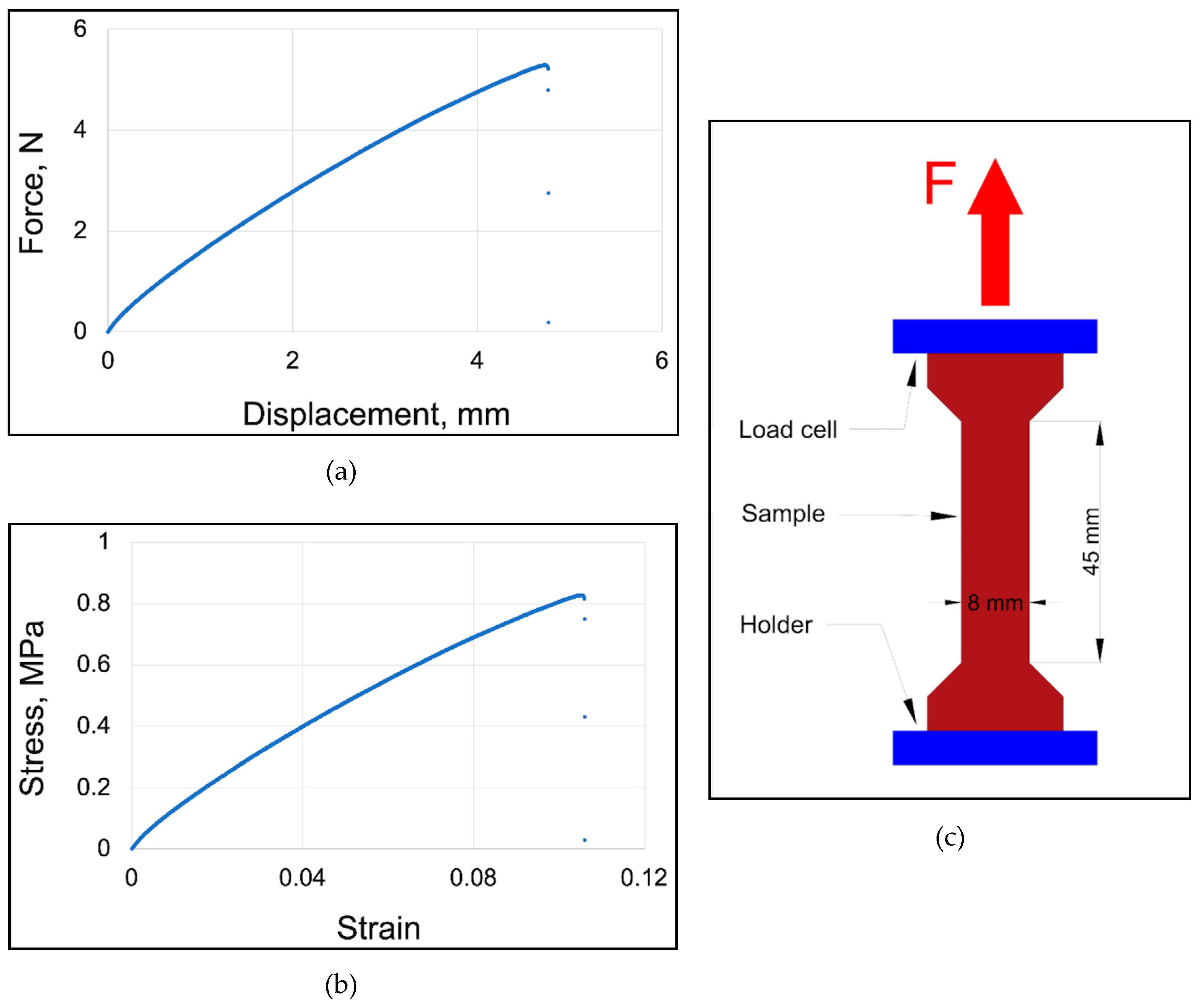
The strength properties of flesh, for the studied stages of maturity, were determined in a compression test. For this purpose, cuboid samples of flesh were tested. Their height was no more than 1.5 times the base edge to avoid the lack of a uniaxial stress state, thus meeting the assumptions of Saint-Venant’s principle (
Figure 1a). The samples were loaded using an Instron 5566 testing machine (Instron, Norwood, Massachusetts, USA) under quasi-static conditions at a loading speed of 1 mm·min-1. The accuracy class of the force measuring head was 0.5 according to ISO 7500-1.
Figure 1b presents the average results of the conversion of force-displacement plots to a stress-strain plot, for all ripening stages of apples. The Young’s Modulus values were calculated based on the quotient of the yield stress versus strain results. Poisson’s ratio values were determined based on the quotient of transverse strain to axial strain, averaged to each sample. During the tests, Poisson’s ratio was also determined by measuring the strain in two mutually perpendicular directions of the sample in a compression test (
Figure 1c). For this purpose, a ME45 optical extensometer (Messphysik, ZwickRoell Testing Systems, Fürstenfeld, Austria) was used and the standard equation for determining the Poisson’s ratio
v =
ε1·
ε2-1 was adopted, where
v – Poisson’s ratio,
ε1 - transverse strain,
ε2 - axial strain. This value was calculated, for each sample, throughout the entire loading range until the yield point was reached. Then the collected values were averaged for all loading range of each sample. The tests were carried out in 10 repetitions.
2.3.2. Apple Skin
The strength properties of the epidermis were determined in a tensile test. The sample preparation method is shown in
Figure 2c. Similarly, to the compression test of the flesh, the samples were loaded using testing machine under quasi-static conditions at a loading speed of 1 mm·min-1. The Young’s Modulus values, were calculated based on the quotient of the failure stress and the strain (the maximum stress shown on
Figure 2b).
Figure 2a presents an example of force-displacement plot used to calculate the above-mentioned stress-strain relationship. The tests were carried out in 10 repetitions.
2.4. Determination of Strength Properties of Apple Fruit
The load process was measured as a function of displacement using a testing machine, under quasi-static conditions at a loading speed of 1 mm·min-1. In addition, the distribution of the surface pressure exerted by the sample in the compression test was measured with the Tekscan I-Scan VHS System (Tekscan, Boston, Massachusetts, U.S.A). The tests were carried out in 5 repetitions.
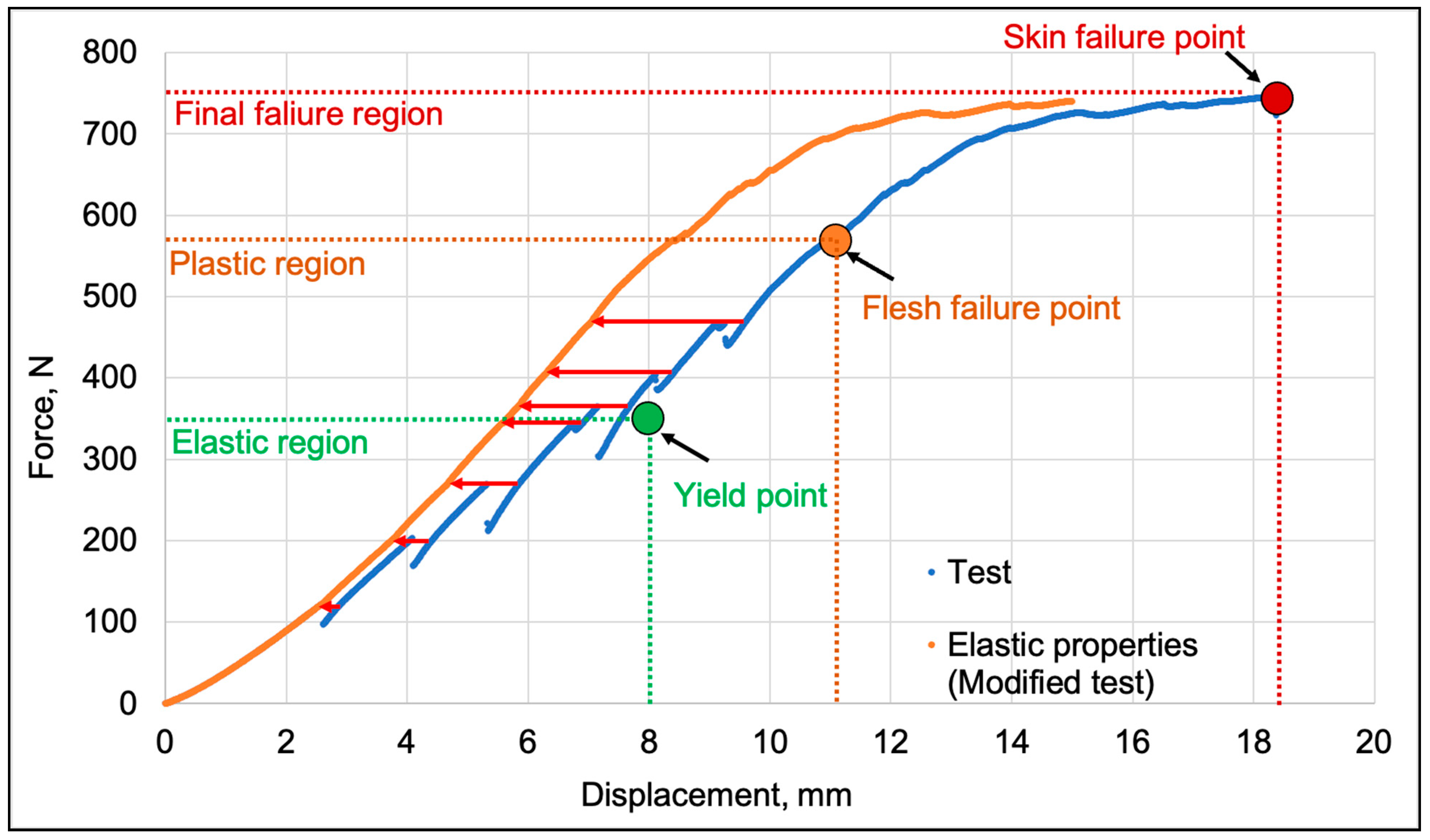
The process of flesh cracking characteristic of fresh apple fruit (characterized by local drops in force) prevented the interpretation of the elastic properties of whole fruit. Therefore, the test data (marked in blue) were modified by removing these local drops (marked in red) as shown on
Figure 3. This resulted in graphs appropriate for elastic material (marked in orange), and this modification made it possible to divide the compression process into three phases: elastic, plastic, and the moment when the flesh tissue along the compression axis is already completely destroyed, followed by a crack as the skin’s structural continuity is broken. This procedure required identifying the maximum force before the drop and then removing the forces leading to the sample’s re-strengthening and continuing the graph with the force increasing further. Consequently, the displacement does not correspond to the actual values occurring during the test, so the graph was used only to visualize elastic behaviors and critical points of the process, while the results were described according to the actual graphs. The yield point and flesh failure were determined using MatLab software, which detected changes in the slope angle of the graph indicating a transition from elastic to plastic behavior. The modification is, of course, arbitrary, as it concerns the force-displacement graph, not stress-strain, and has been presented in this way to allow for the description of phenomena occurring in whole fruit compression tests in the results section. This graph was also created to validate elastic FEM models in other studies.
2.5. Determination of the Bruise Geometry and Volume Using Micro-Computed Tomography
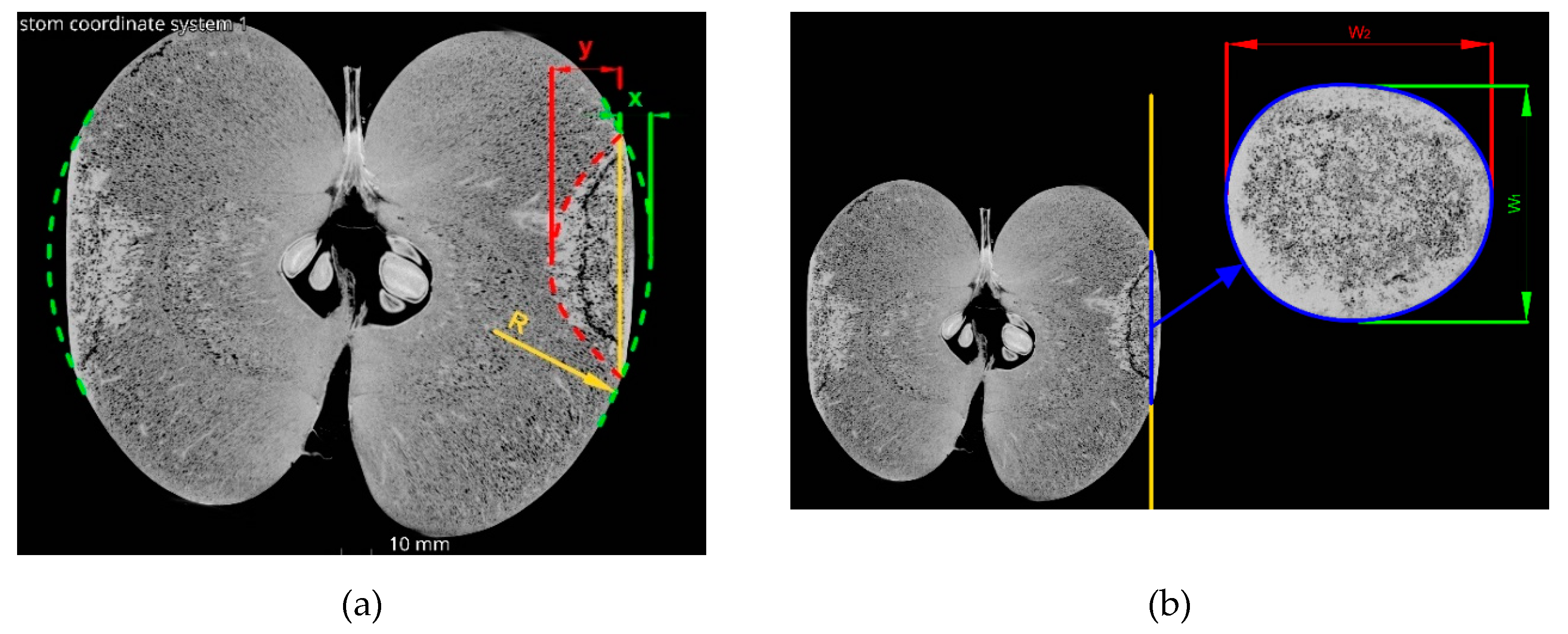
Based on the results of apple compression tests (0) at the stage of development, the average value of the fruit failure force was determined. This value was used to determine the extent of damage to fruit at all stages of ripeness, which were subjected to loads of 20%, 50% and 80% of the average failure force. The value of the forces was 146 N, 365 N and 585 N, respectively. Immediately after loading, the fruit was scanned using a computer microtomograph GE phoenix v tome x (General Electrics, North Carolina, USA). Scans were performed with the following parameters: voltage 100 kV, current 400 µA, scanning time of one frame 0.33 ms, averaging every 2 frames and skipping 1 frame. As a result, a voxel resolution of 0.1 mm was achieved.
Figure 4 presents the original Micro-CT pictures from VG Studio MAX software (Volume Graphics GmbH, Heidelberg, Germany), along with a description of the sizing of the fruit bruise geometry used to calculate the fruit bruise volume, according to the Equations 2,3 and 4:
Where: V1 – volume of bruise above the contact plane [mm3], V2 – volume of bruise below the contact plane [mm3], V – total bruise volume [mm3], W1 – width of the shorter axis of the bruise ellipse [mm], W2 – width of the longer axis of the bruise ellipse [mm], x– bruise depth above the contact plane [mm], y – bruise depth below the contact plane [mm].
3. Results
3.1. Characteristics of the Material Selected for Testing
Table 1 shows the results of testing the density, water content and geometric parameters of the fruit. In addition, the average dimensions of fruit cores were measured, the average value of the diameter was 32 mm with a standard deviation of ±2.1 mm, while the average value of the height was 39 mm with a standard deviation of ±3.06 mm.
The water content in the fruit decreased with time with their physiological development, ranging from 83.42 to 85.57%.
3.1. Basic Physicochemical Properties of Fruit
Table 2 presents the results of tests for firmness, SSC content, starch index and the Streif index determined on their basis, as well as ethylene concentration, malic acid and pectin content at individual fruit harvest dates.
Based on
Table 2, it can be concluded that firmness decreased with the stage of physiological development, ranging from 5.8 kG to 7.4 kG. The Soluble Solids Content (SSC), as well as the starch index, increased with fruit ripeness. For development and ripening maturity, the values were similar and amounted to 14.00% and 14.06%, while for senescence maturity the SSC was 11.6%. The values of the Streif index were 0.12 for the stage of development maturity, 0.07 for the stage of ripening maturity and 0.04 for the stage of senescence maturity. Fruit at the senescence maturity stage exhibited the lowest malic acid content, measuring at 66%, whereas those at the development maturity stage had the highest content, reaching 83%. For fruit at the ripening maturity stage, the malic acid content stood at 0.78%. The pectin content in fruit decreased with their maturity, reaching the following values: 9.53% for development; 7.2% for ripening and 4.07% for senescence maturity stage.
3.1. Strength Properties of Apple Flesh and Skin
Table 3 presents the results for mechanical properties of apple skin and flesh which constitute the basis for defining the material’s behavior under load.
The value of skin elasticity modulus decreased with the stage of apple ripeness from 10.78 to 5.23 MPa. Modulus od elasticity for the flesh in development stage of ripeness was 5.04 MPa, for the ripening stage it slightly increased to 5.12 MPa, and then significantly decreased to a value of 3.85 MPa for the senescence stage of ripeness. The lowest Poisson’s ratio value for apple flesh, 0.21, was achieved by the fruit at the ripening maturity stage. Fruit at the development maturity stage were slightly more prone to transverse deformations, with a Poisson’s ratio of 0.25. The highest Poisson’s ratio was observed in fruit at the senescence maturity stage, with a value of 0.35.
3.1. Strength Properties of Apple Fruit
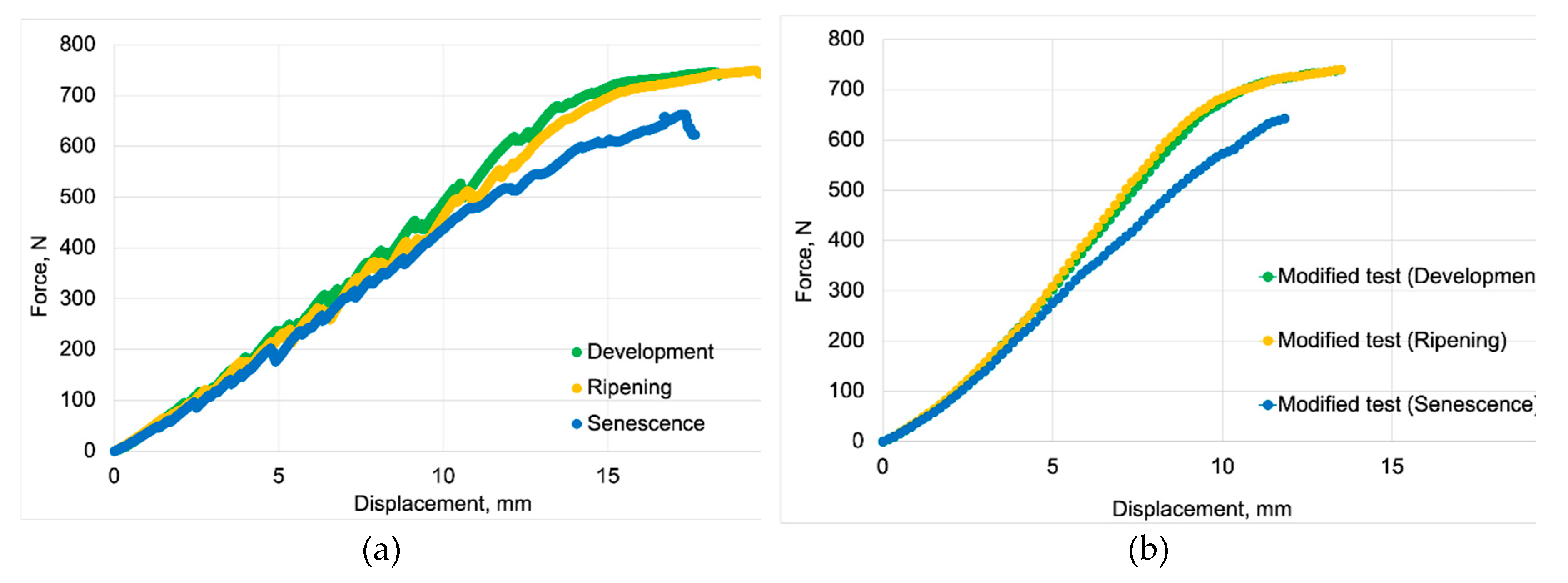
Figure 5 and
Figure 6 present the averaged results of compression tests for three maturity stages. The original test data of force-displacement curves are shown in
Figure 5a, while
Figure 5b presents curves modified according to the methodology outlined in
Figure 3 in subsection 2.4.
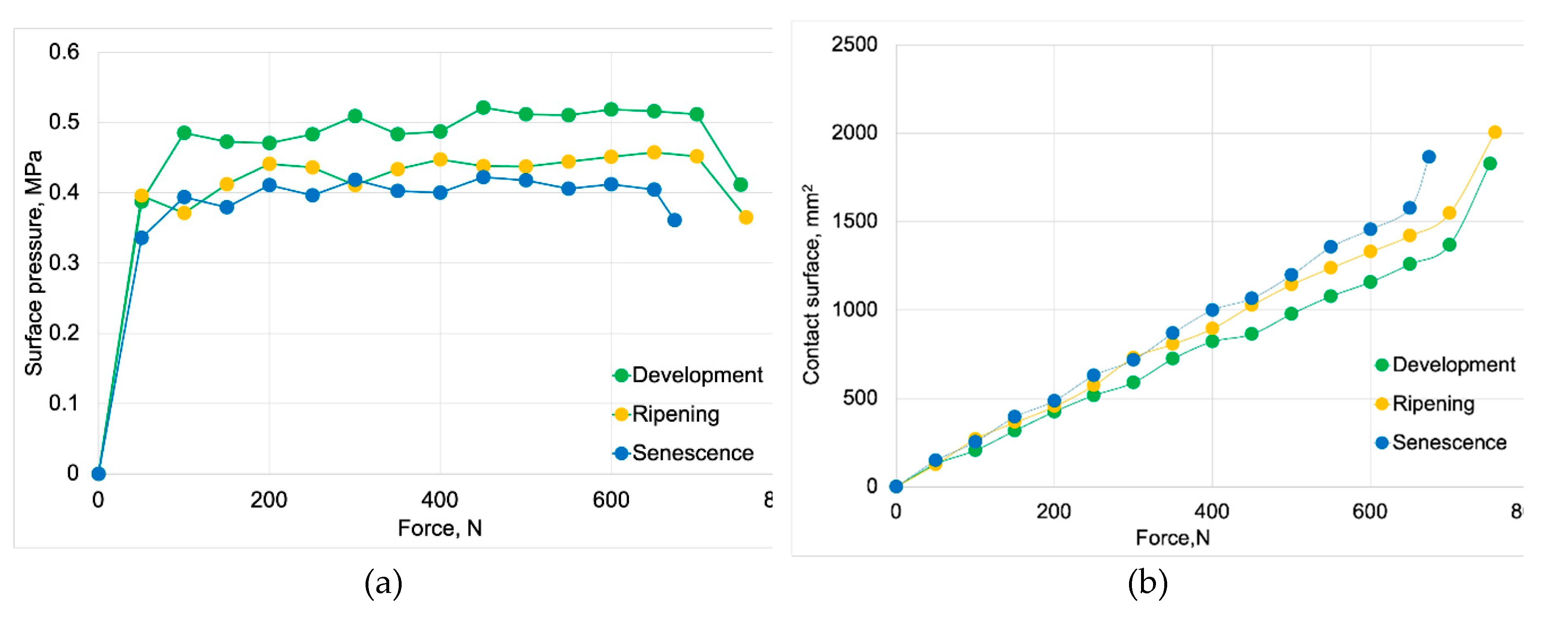
Figure 6a shows the surface pressure as a function of force, while
Figure 6b presents the contact surface as a function of force. The results presented in both figures are included in
Table 4, divided into elastic region, plastic region and the region of a total flesh failure, for all stages of fruit ripeness.
Comparing
Figure 5a,b, it can be seen that after removing local force drops, we see that fruit at the stage of ripening maturity were characterized by slightly greater strength in the plastic region. This also means that these drops in force were either more frequent or had greater values.
Based on
Table 4, it can be observed that as apples progressed through the stages of development, plastic deformations began at lower force values. Similarly, the force causing the disruption of the flesh tissue structure also decreased with ripening stage. However, when the flesh in the compression axis ceased to transmit loads due to the disruption of its cohesive connection with the seed nest, it was found that fruit at the ripening stage were the last to crack, at the highest force and displacement value. The discussion and conclusion drawn from this phenomenon are described later in the paper.
From the surface pressure results presented in
Figure 6 and
Table 4, it is evident that as the fruit matures, the contact surface increases (
Figure 6b), leading to a decrease in surface pressures. Throughout the entire compression process, it can also be observed that they remain at a consistent level within the range corresponding to their maturity stage (
Figure 6a).
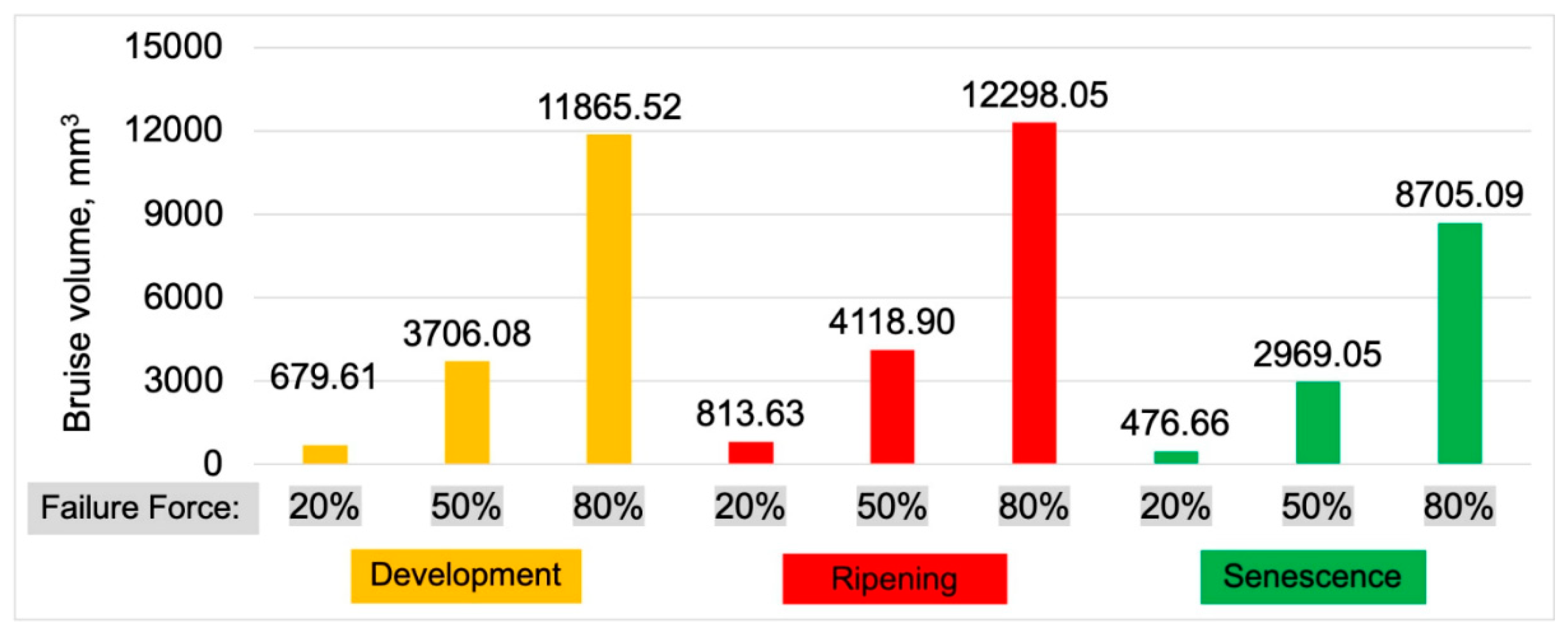
3.1. Bruises Volume
Figure 7 presents the results of apple bruise volumes determined based on tomographic images. The graph is divided into load ranges (20%, 50%, and 80% of Failure Force) and maturity stages. Maturity stages are indicated by colors: red represents the ripening stage, where the extent of bruising was the highest among the other maturity stages. The development stage is marked in orange, with bruise volumes lower than those for the ripening stage. The senescence stage is represented in green, showing the lowest bruise volumes.
These results, especially for the ripening maturity, have allowed us to formulate research hypothesis discussed in detail in the discussion section.
3. Discussion
The new variety of apples, Chopin, is highly regarded by researchers. The fruit has been studied in terms of: The effect of storage conditions on the content of molecules [
3] and storability and nutritional value [
2], Bioactive compounds, antioxidant potential and cultivar functional properties [
39]; The effect of mycorrhizal fungi and PGPR on Chopin tree nutritional status and growth [
40]; Quality and nutritional value in relation to popular apples growing in Poland [
4]. This article explores different issues than those previously addressed by researchers, thereby providing a good complement to the current state of knowledge. Now the practical application of mechanical research on fruit is even more evident, especially since robotic fruit harvesting is becoming increasingly popular, and designing devices for this purpose requires defining the mechanical properties of the fruit [
41,
42].
Fruit at the stage of ripening maturity were characterized by the highest elastic modulus of the flesh tissue, higher strength of whole fruit in plastic region (based on modified charts), the highest value of the final apple destructive force, but not the highest strength in a significant part of the whole apple compression process, and not the highest resistance to bruising. Results of Micro-Ct and the results of modified and real compression tests of whole fruit have allowed us to formulate the research hypothesis regarding the influence of flesh cracking (characterized by local drops of force), influencing the bruise visibility and detection.
It’s important to note that bruises visible in microtomographic images are apparent due to the leakage of cell sap and the tearing of flesh tissue to such an extent that the detection of discontinuity in the foam structure of the apple flesh is possible. Therefore, delving into the cellular structure and understanding the mechanism of apple fruit behavior is so crucial for a comprehensive description of the phenomena occurring in the tissue at different stages of loading, with particular emphasis on their ripening stages. According to Stopa, 2010) process of gas diffusion, rupture of intercellular connections, cracking of cell walls, and migration of cell sap throughout the tissue should be considered to fully define the behavior of the apple flesh under load. Creating a coherent and uniform theory of mechanics for biological materials would lead to a system of equilibrium equations with a complex structure with many variables. Such a system would have to include solutions in the field of biochemistry, biomechanics, and biophysics [
44].
4. Conclusions
During the research, a significant difference was found between fruit at the stage of senescence ripeness and fruit at the stage of development and ripening maturity.
The results of mechanical and Micro-CT tests for specific and verified stages of apple ripeness were determined, providing a complete dataset for building and validating elastic and elastic-plastic FE models. Further conclusions on the superiority of Elastic-Plastic models over Viscoelastic models (for tissue models covering the full range of fruit loading) will be presented in the next article, which will focus exclusively on modeling.
Results of Micro-Ct and the results of modified and real compression tests of whole fruit have allowed us to formulate the research hypothesis regarding the influence of flesh cracking (characterized by local drops of force), influencing the bruise visibility and detection.
The research confirmed the need to carefully take into account the maturity stage in mechanical research, and in particular in the construction of MES models for food industry.
Author Contributions
Conceptualization, M.S. and R.S.; methodology, M.S.; software, M.S.; validation, M.S.; formal analysis, M.S.; investigation, M.S.; resources, M.S.; data curation, M.S.; writing—original draft preparation, M.S.; writing—review and editing, M.S.; visualization, M.S.; supervision, R.S.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Polish National Science Centre [2021/41/N/NZ9/02874].
Data Availability Statement
Data will be made available on request.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Komarnicki, P.; Stopa, R.; Szyjewicz, D.; Młotek, M. Evaluation of Bruise Resistance of Pears to Impact Load. Postharvest Biol Technol 2016, 114, 36–44. [Google Scholar] [CrossRef]
- Kistechok, A.; Wrona, D.; Krupa, T. Effect of Storage Conditions on the Storability and Nutritional Value of New Polish Apples Grown in Central Poland. Agriculture (Switzerland) 2024, 14, 59. [Google Scholar] [CrossRef]
- Ponder, A.; Jariené, E.; Hallmann, E. The Effect of Storage Conditions on the Content of Molecules in Malus Domestica ‘Chopin’ Cv. and Their In Vitro Antioxidant Activity. Molecules 2022, 27, 6979. [Google Scholar] [CrossRef] [PubMed]
- Kistechok, A.; Wrona, D.; Krupa, T. Quality and Nutritional Value of ‘Chopin’ and Clone ‘JB’ in Relation to Popular Apples Growing in Poland. Agriculture (Switzerland) 2022, 12, 1876. [Google Scholar] [CrossRef]
- Noiton, D.A.M.; Alspach, P.A. Founding Clones, Inbreeding, Coancestry, and Status Number of Modern Apple Cultivars; 1996; Vol. 121.
- Zygmunt Hejnowicz Anatomia i Histogeneza Roślin Naczyniowych; Wydawnictwo Naukowe PWN: Warszawa, 2002; Vol. 1.
- Pratt, C. Apple Flower and Fruit: Morphology and Anatomy. 1988.
- Mccann1, M.C.; Roberts, K. Changes in Cell Wall Architecture during Cell Elongation; 1994; Vol. 45.
- Zdunek, A.; Kozioł, A.; Cybulska, J.; Lekka, M.; Pieczywek, P.M. The Stiffening of the Cell Walls Observed during Physiological Softening of Pears. Planta 2016, 243, 519–529. [Google Scholar] [CrossRef]
- Billy, L.; Mehinagic, E.; Royer, G.; Renard, C.M.G.C.; Arvisenet, G.; Prost, C.; Jourjon, F. Relationship between Texture and Pectin Composition of Two Apple Cultivars during Storage. Postharvest Biol Technol 2008. [CrossRef]
- Willats, W.G.T.; Mccartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell Biology and Prospects for Functional Analysis; 2001; Vol. 47;
- Videcoq, P.; Barbacci, A.; Assor, C.; Magnenet, V.; Arnould, O.; Le Gall, S.; Lahaye, M. Examining the Contribution of Cell Wall Polysaccharides to the Mechanical Properties of Apple Parenchyma Tissue Using Exogenous Enzymes. J Exp Bot 2017, 68, 5137–5146. [Google Scholar] [CrossRef]
- Bich, L.; Pradeu, T.; Moreau, J.F. Understanding Multicellularity: The Functional Organization of the Intercellular Space. Front Physiol 2019, 10, 1170. [Google Scholar] [CrossRef]
- Wei, J.; Ma, F.; Shi, S.; Qi, X.; Zhu, X.; Yuan, J. Changes and Postharvest Regulation of Activity and Gene Expression of Enzymes Related to Cell Wall Degradation in Ripening Apple Fruit. Postharvest Biol Technol 2010, 56, 147–154. [Google Scholar] [CrossRef]
- Abbott, J.A. Quality Measurement of Fruits and Vegetables; 1999; Vol. 15;
- Oey, M.; Vanstreels, E.; Debaerdemaeker, J.; Tijskens, B.; Ramon, H.; Nicolaï, B.M. Influence of Turgor on Micromechanical and Structural Properties of Apple Tissue. In Proceedings of the 13th World Congress of Food Science & Technology; EDP Sciences: Les Ulis, France, 2006. [Google Scholar]
- Juxia, W.; Qingliang, C.; Hongbo, L.; Yaping, L. Experimental Research on Mechanical Properties of Apple Peels. Journal of Engineering and Technological Sciences 2015, 47, 688–705. [Google Scholar] [CrossRef]
- Grotte, M.; Duprat, F.; Loonis, D.; Piétri, E. Mechanical Properties of the Skin and the Flesh of Apples. Int J Food Prop 2001, 4, 149–161. [Google Scholar] [CrossRef]
- Ruiz-Altisent, M.; Moreda, G.P. Fruits, Mechanical Properties and Bruise Susceptibility. In Encyclopedia of Earth Sciences Series; 2011; Vol. Part 4.
- Ekrami-Rad, N.; Khazaei, J.; Khoshtaghaza, M.H. Selected Mechanical Properties of Pomegranate Peel and Fruit. Int J Food Prop 2011, 14, 570–582. [Google Scholar] [CrossRef]
- Li, Z.; Thomas, C. Quantitative Evaluation of Mechanical Damage to Fresh Fruits. Trends Food Sci Technol 2014, 35, 138–150. [Google Scholar] [CrossRef]
- Farkas, C.; Petróczki, K.; Fenyvesi, L. Method for Measuring Fruit Failure Caused by Different Mechanical Loads. Hungarian Agricultural Engineering 2016, 51–54. [Google Scholar] [CrossRef]
- Thielen, M.; Speck, T.; Seidel, R. Viscoelasticity and Compaction Behaviour of the Foam-like Pomelo (Citrus Maxima) Peel. J Mater Sci 2013, 48, 3469–3478. [Google Scholar] [CrossRef]
- Fathizadeh, Z.; Aboonajmi, M.; Beygi, S.R.H. Nondestructive Firmness Prediction of Apple Fruit Using Acoustic Vibration Response. Sci Hortic 2020, 262, 109073. [Google Scholar] [CrossRef]
- Opara, U.L.; Pathare, P.B. Bruise Damage Measurement and Analysis of Fresh Horticultural Produce-A Review. Postharvest Biol Technol 2014, 91, 9–24. [Google Scholar] [CrossRef]
- Fadiji, T.; Coetzee, C.; Chen, L.; Chukwu, O.; Opara, U.L. Susceptibility of Apples to Bruising inside Ventilated Corrugated Paperboard Packages during Simulated Transport Damage. Postharvest Biol Technol 2016, 118, 111–119. [Google Scholar] [CrossRef]
- Fadiji, T.; Coetzee, C.; Pathare, P.; Opara, U.L. Susceptibility to Impact Damage of Apples inside Ventilated Corrugated Paperboard Packages: Effects of Package Design. Postharvest Biol Technol 2016, 111, 286–296. [Google Scholar] [CrossRef]
- Stropek, Z.; Gołacki, K. A New Method for Measuring Impact Related Bruises in Fruits. Postharvest Biol Technol 2015, 110, 131–139. [Google Scholar] [CrossRef]
- Diels, E.; van Dael, M.; Keresztes, J.; Vanmaercke, S.; Verboven, P.; Nicolai, B.; Saeys, W.; Ramon, H.; Smeets, B. Assessment of Bruise Volumes in Apples Using X-Ray Computed Tomography. Postharvest Biol Technol 2017, 128, 24–32. [Google Scholar] [CrossRef]
- Van De Looverbosch, T.; Vandenbussche, B.; Verboven, P.; Nicolaï, B. Nondestructive High-Throughput Sugar Beet Fruit Analysis Using X-Ray CT and Deep Learning. Comput Electron Agric 2022, 200, 107228. [Google Scholar] [CrossRef]
- Alamar, M.C.; Vanstreels, E.; Oey, M.L.; Moltó, E.; Nicolaï, B.M. Micromechanical Behaviour of Apple Tissue in Tensile and Compression Tests: Storage Conditions and Cultivar Effect. J Food Eng 2008, 86, 324–333. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive Measurement of Fruit and Vegetable Quality by Means of NIR Spectroscopy: A Review. Postharvest Biol Technol 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Herremans, E.; Melado-Herreros, A.; Defraeye, T.; Verlinden, B.; Hertog, M.; Verboven, P.; Val, J.; Fernández-Valle, M.E.; Bongaers, E.; Estrade, P.; et al. Comparison of X-Ray CT and MRI of Watercore Disorder of Different Apple Cultivars. Postharvest Biol Technol 2014, 87, 42–50. [Google Scholar] [CrossRef]
- Wang, L.J.; Zhang, Q.; Song, H.; Wang, Z.W. Mechanical Damage of ‘Huangguan’ Pear Using Different Packaging under Random Vibration. Postharvest Biol Technol 2022, 187, 111847. [Google Scholar] [CrossRef]
- Bejaei, M.; Stanich, K.; Cliff, M.A. Modelling and Classification of Apple Textural Attributes Using Sensory, Instrumental and Compositional Analyses. Foods 2021, 10, 384. [Google Scholar] [CrossRef]
- Stopa, R.; Szyjewicz, D.; Komarnicki, P.; Kuta, Ł. Determining the Resistance to Mechanical Damage of Apples under Impact Loads. Postharvest Biol Technol 2018, 146, 79–89. [Google Scholar] [CrossRef]
- Tomala, K.; Grzęda, M.; Guzek, D.; Głąbska, D.; Gutkowska, K. The Effects of Preharvest 1-Methylcyclopropene (1-MCP) Treatment on the Fruit Quality Parameters of Cold-Stored ‘Szampion’ Cultivar Apples. Agriculture (Switzerland) 2020, 10, 80. [Google Scholar] [CrossRef]
- Pijanowski, E.; Mrożewski, S.; Horubała, A.; Jarczyk, A. Technologia Produktów Owocowych i Warzywnych; PWRiL: Warszawa, 1973. [Google Scholar]
- Sawicka, M.; Latocha, P.; Łata, B. Peel to Flesh Bioactive Compounds Ratio Affect Apple Antioxidant Potential and Cultivar Functional Properties. Agriculture (Switzerland) 2023, 13, 478. [Google Scholar] [CrossRef]
- Przybyłko, S.; Kowalczyk, W.; Wrona, D. Article the Effect of Mycorrhizal Fungi and Pgpr on Tree Nutritional Status and Growth in Organic Apple Production. Agronomy 2021, 11, 1402. [Google Scholar] [CrossRef]
- Ji, W.; Qian, Z.; Xu, B.; Tang, W.; Li, J.; Zhao, D. Grasping Damage Analysis of Apple by End-Effector in Harvesting Robot. J Food Process Eng 2017, 40, e12589. [Google Scholar] [CrossRef]
- Jia, W.; Zhang, Y.; Lian, J.; Zheng, Y.; Zhao, D.; Li, C. Apple Harvesting Robot under Information Technology: A Review. Int J Adv Robot Syst 2020, 17, 1729881420925310. [Google Scholar] [CrossRef]
- Roman Stopa Modelowanie Deformacji Korzenia Marchwi w Warunkach Obciążeń Skupionych Metodą Elementów Skończonych. Monografia, Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu: Wrocław, 2010.
- Murase, H.; Merva, G.E.; Segerlind, L.J.; Member Member Member, A.; Asae, A.; Abstract, A. Variation of Young’s Modulus of Potato as A Function of Water Potential, 1980.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).