Submitted:
10 September 2024
Posted:
11 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. General environmental Patterns in the Region
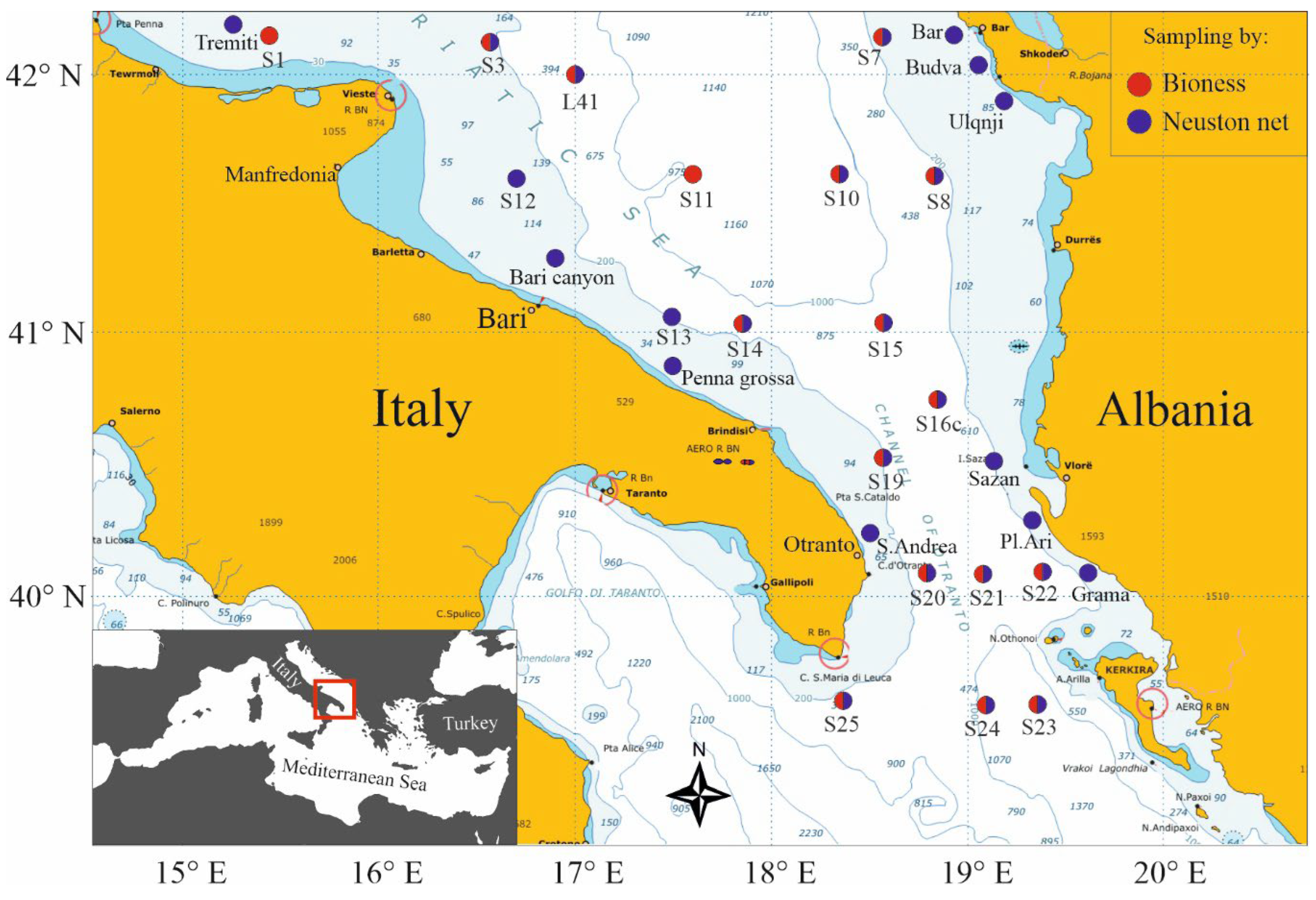
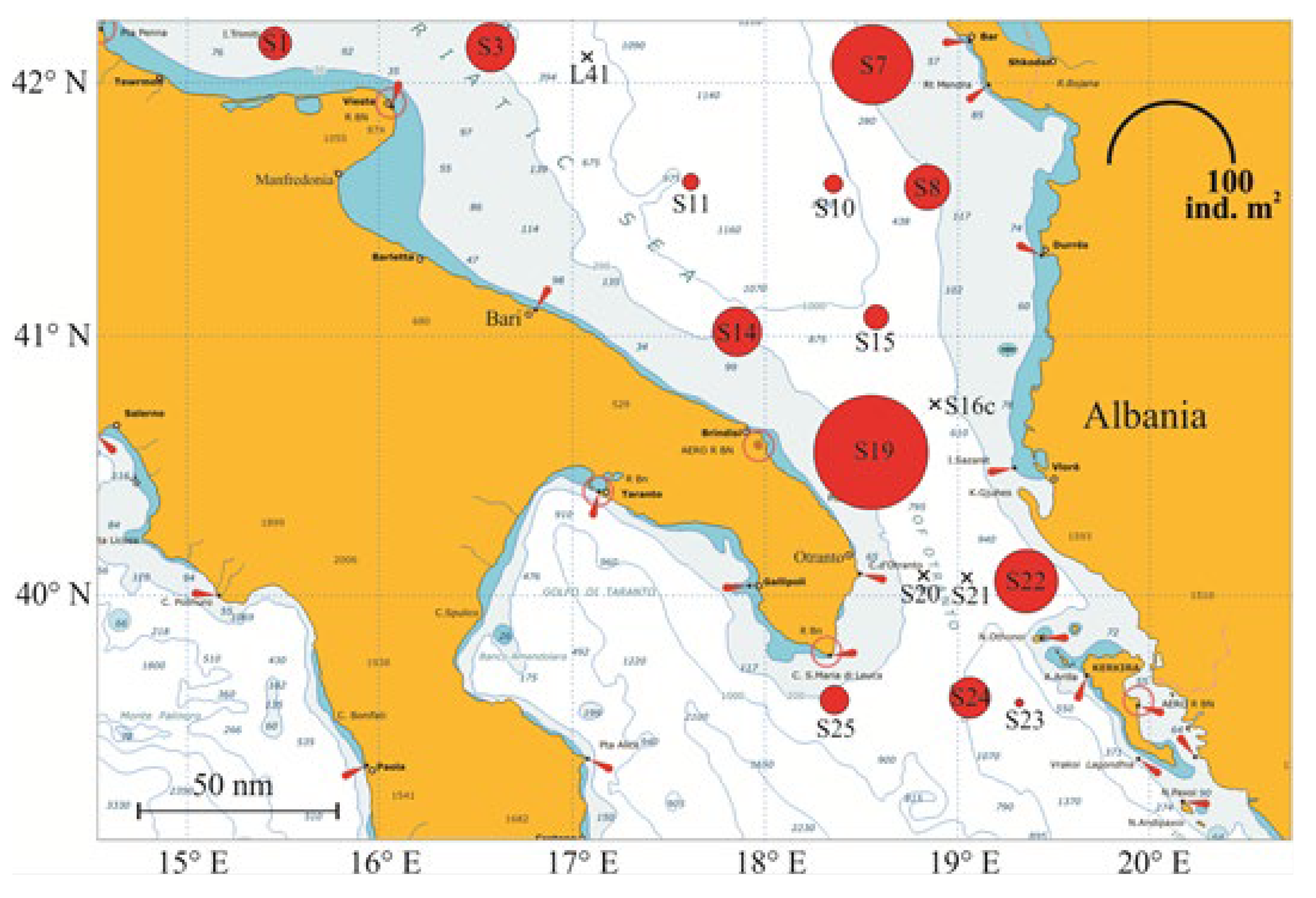
3. Materials and Methods
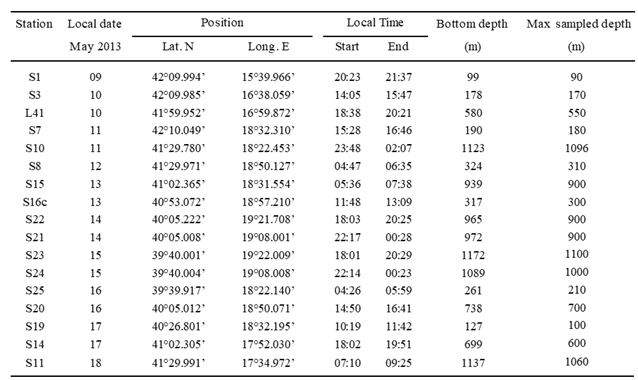
3.1. BIONESS Sampling
3.2. Neuston Collection
3.3. Laboratory Analysis
3.4. Statistical Analysis
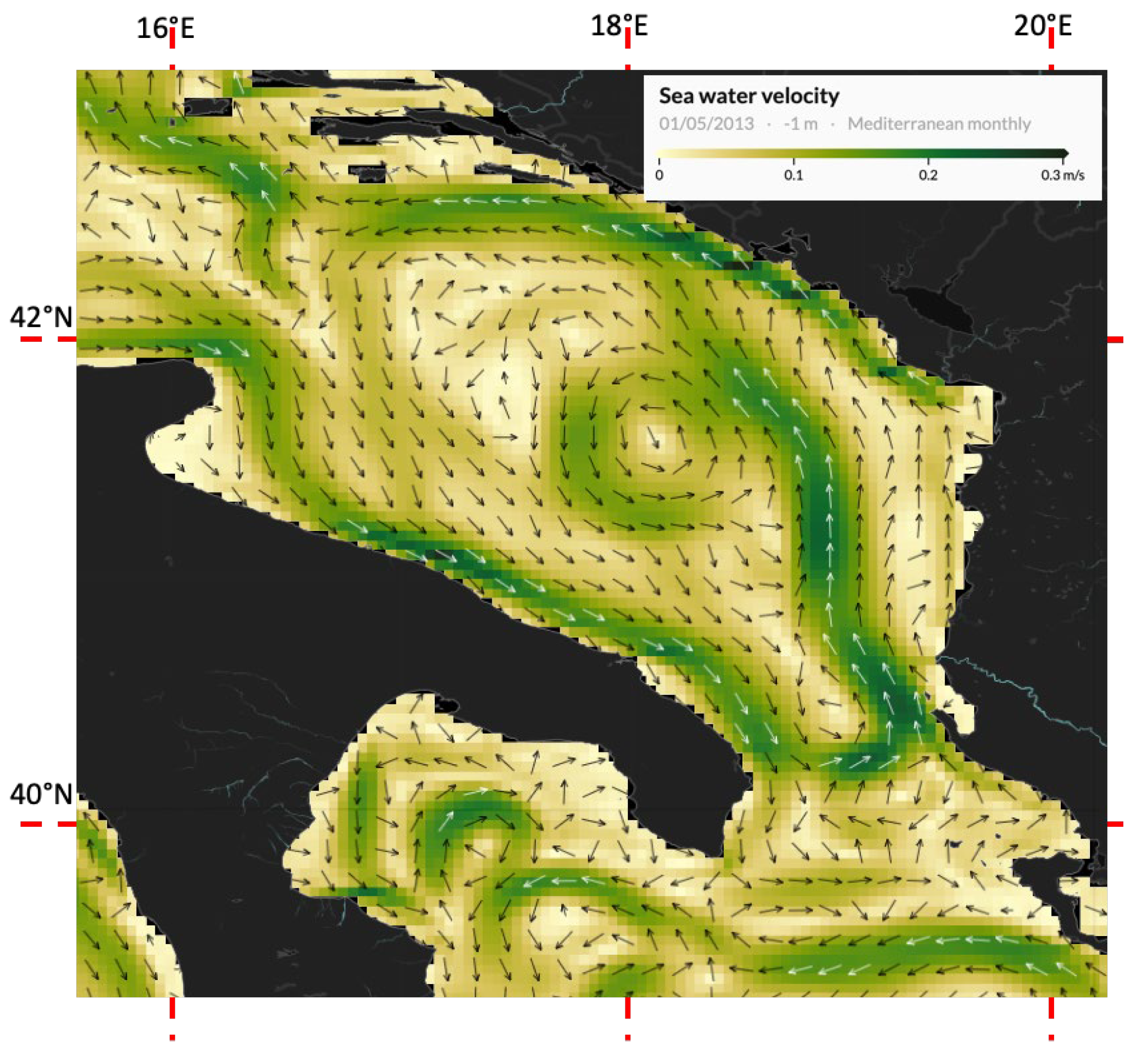
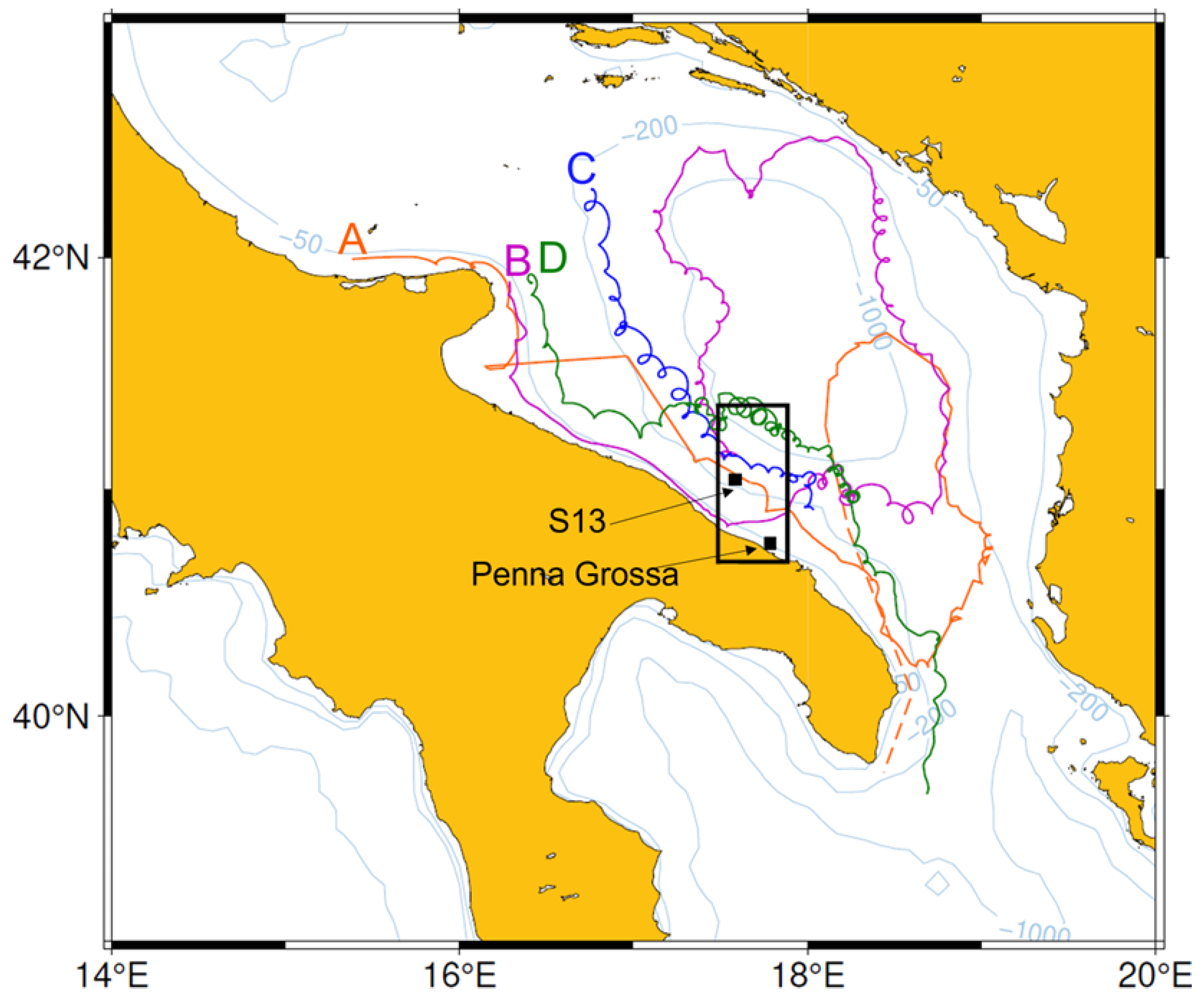
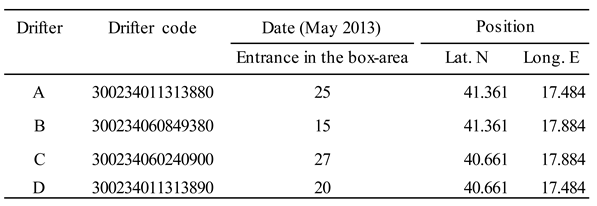
3.5. Surface Drifters
4. Results
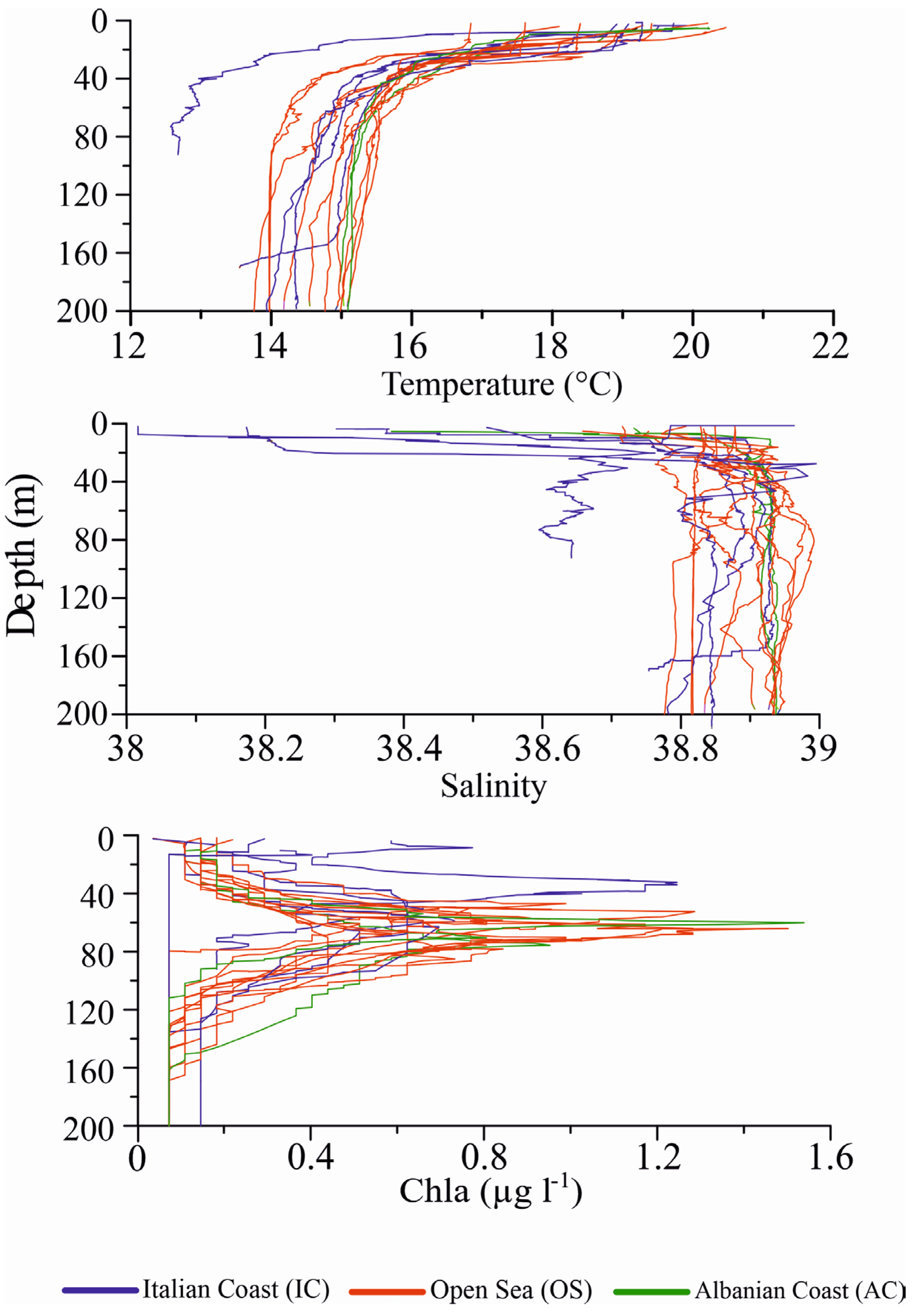
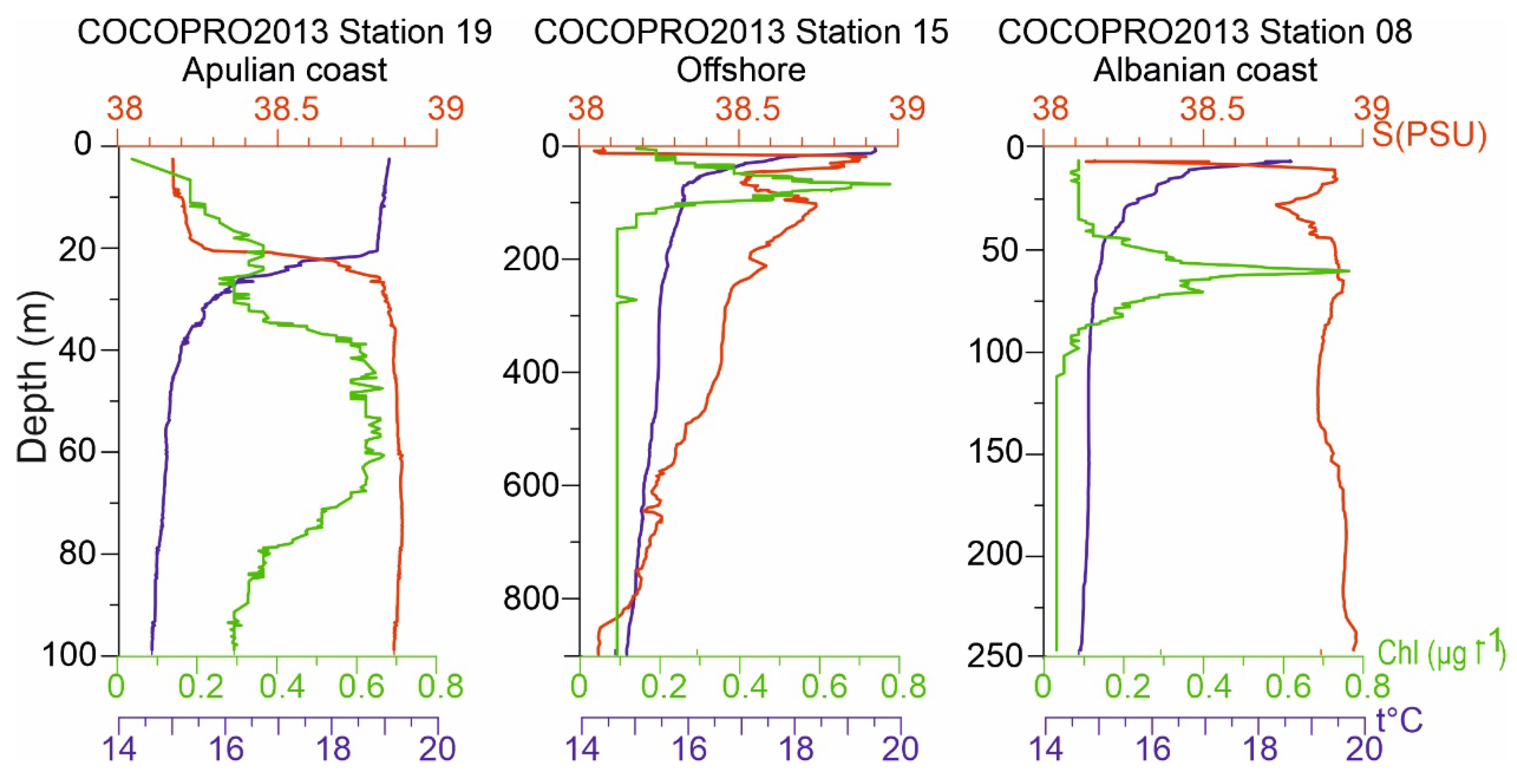
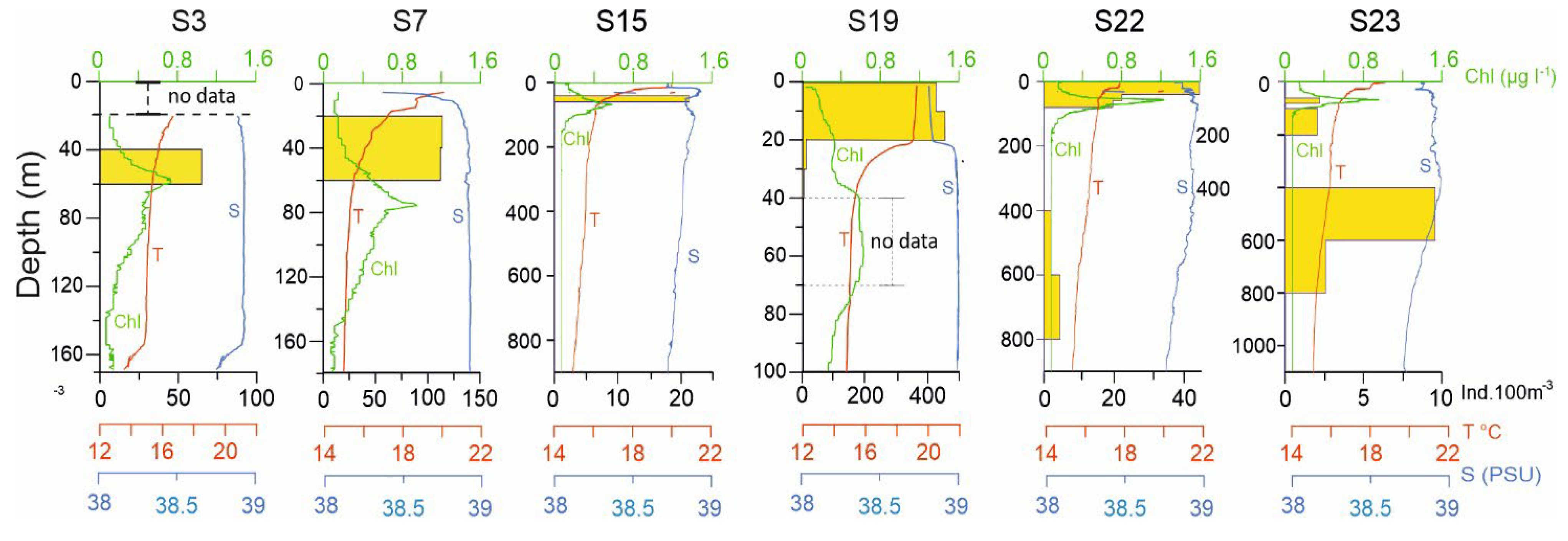
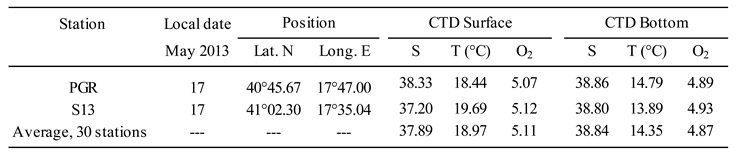
4.1. Hydrographic conditions
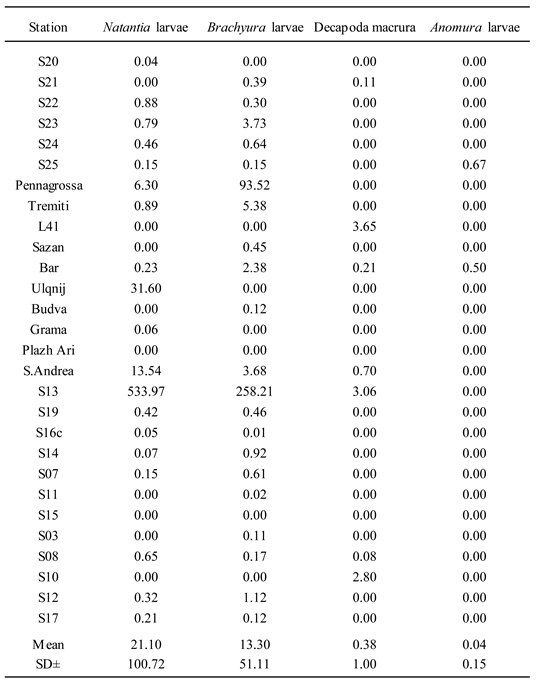
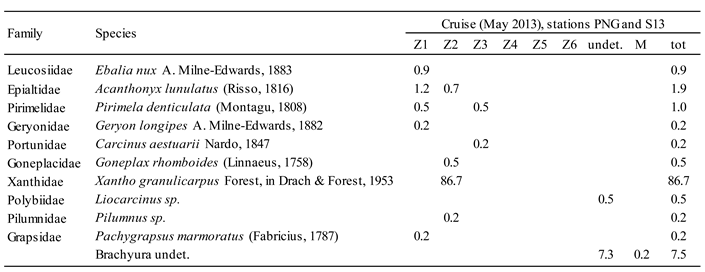
4.2. Zooplankton and Decapod Larvae Spatial Distribution
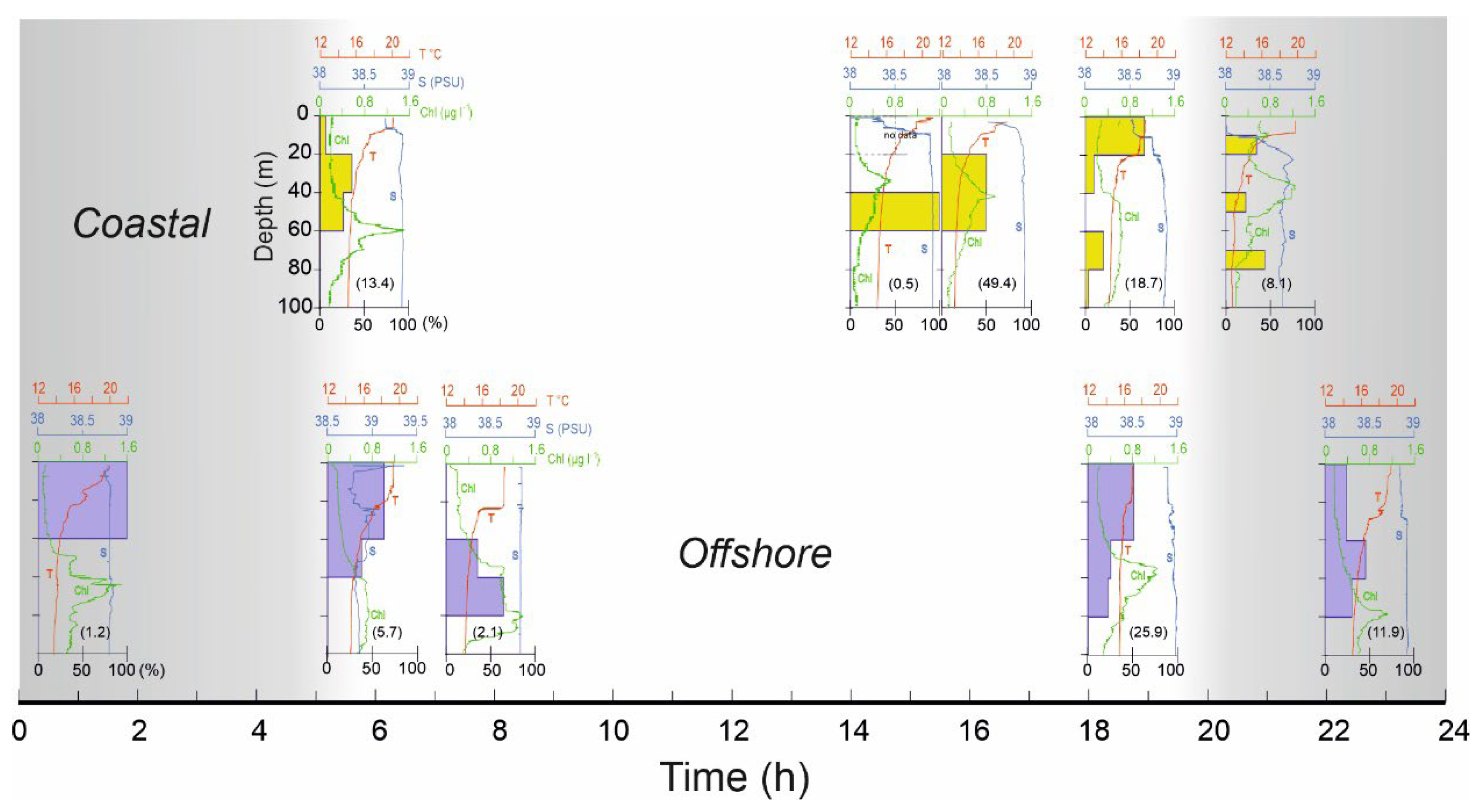
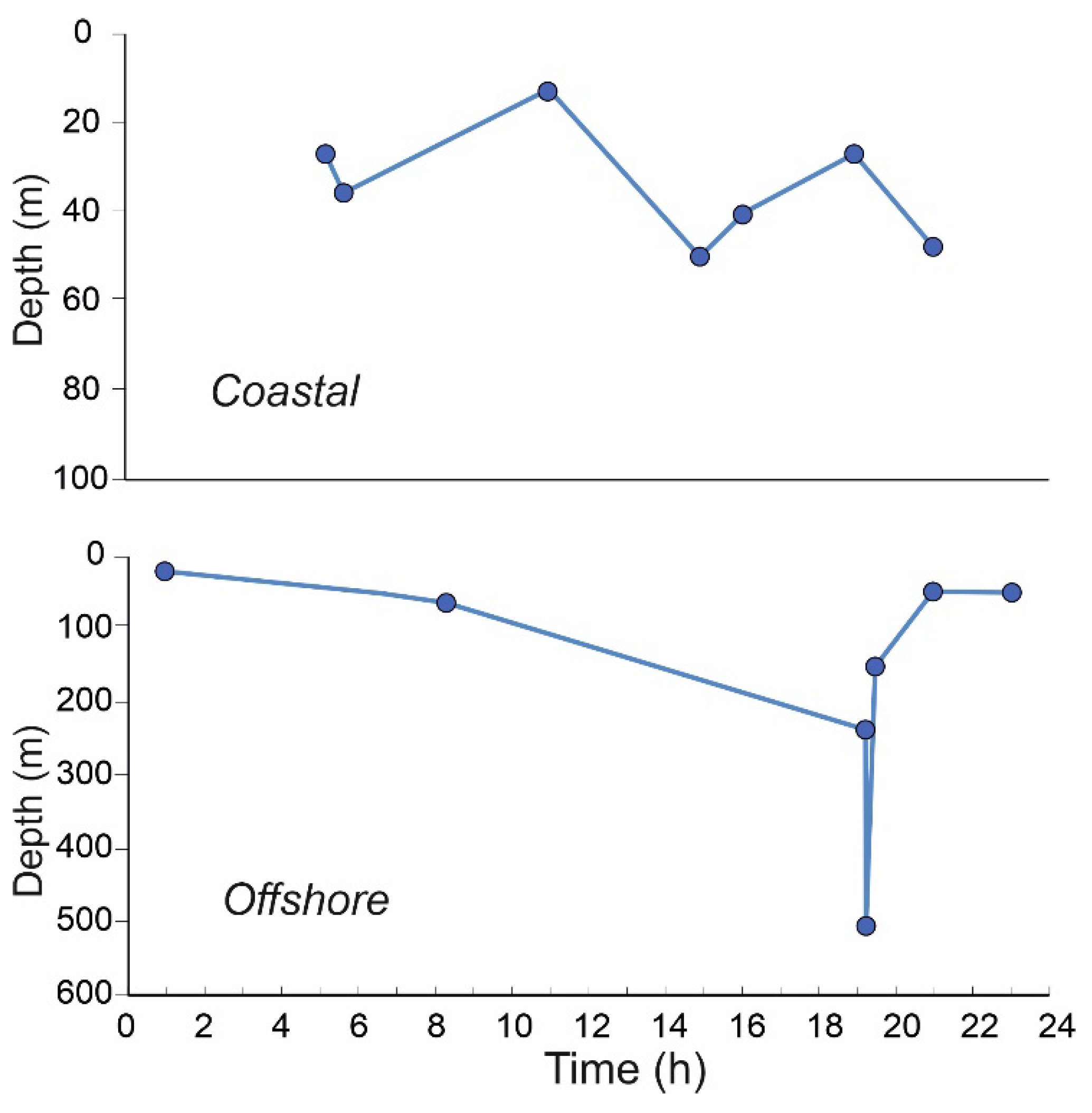
4.3. Diel Vertical Migration
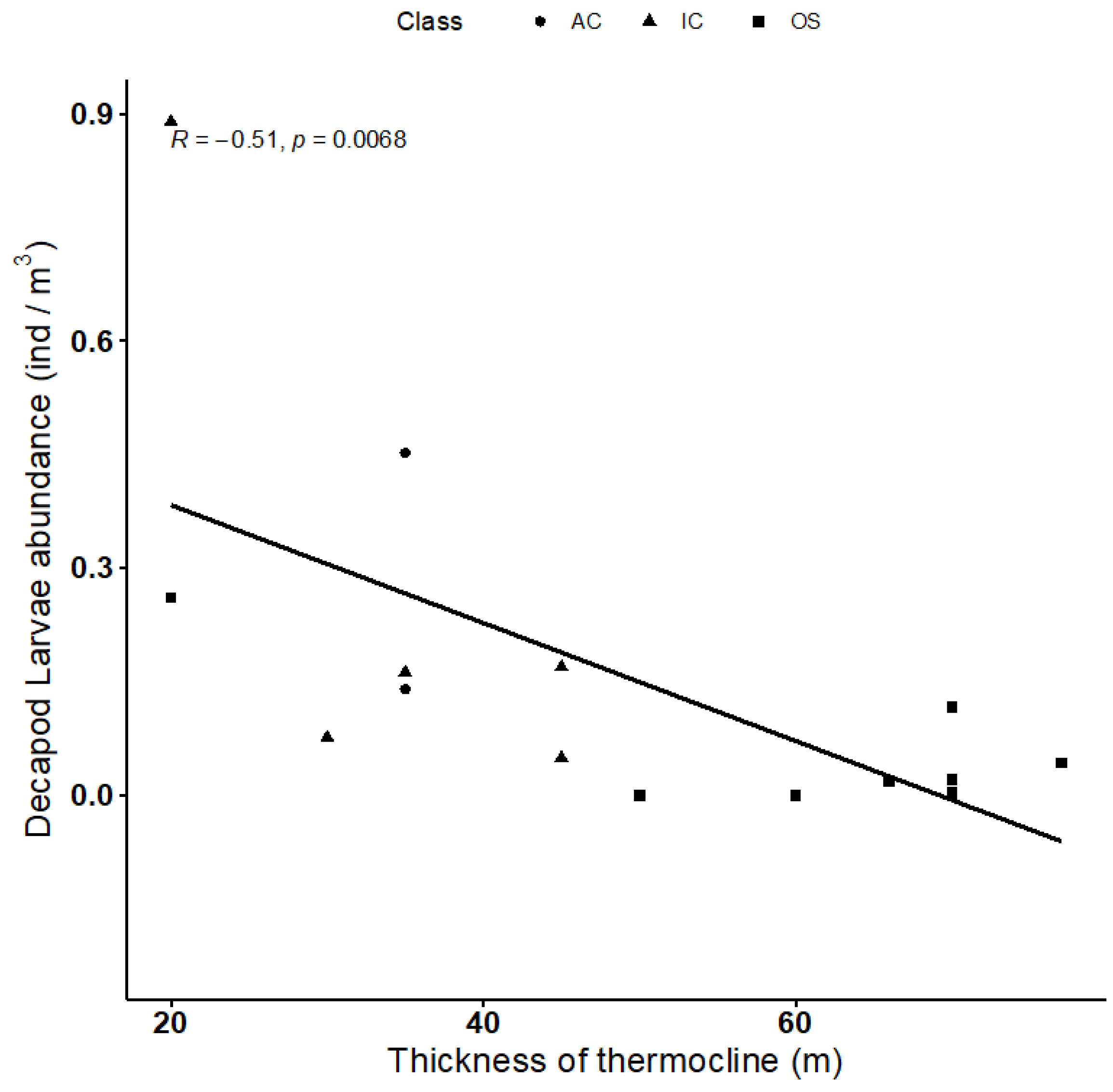
4.4. Vertical Larvae Distribution in Relation to Environmental Variables
4.5. Neuston Collection
4.6. Transport by Surface Currents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheltema, R.S. Passive dispersal of planktonic larvae and the biogeography of tropical sublittoral invertebrate species. In Marine Eutrophication and Population Dynamics; Colombo, G., Ferrari, I., Ceccherelli, V.U., Rossi, R., Eds.; Olsen & Olsen: Fredensborg, Denmark, 1992; pp. 195–202. [Google Scholar]
- Giangrande, A.; Geraci, S.; Belmonte, G. Life-cycle and life-history diversity in marine invertebrates and the implications in community dynamics. Oceanography and Marine Biology, Annual Review 1994, 32, 305–333. [Google Scholar]
- Gaines, S.; Roughgarden, J. Larval settlement rate: a leading determinant of structure in an ecological community of the marine intertidal zone. Proceedings of the National Academy of Sciences 1985, 83, 3707–3711. [Google Scholar] [CrossRef] [PubMed]
- Lewin, R. Supply-side ecology. Science 1986, 234, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Scheltema, R.S. On dispersal and planktonic larvae of benthic invertebrates: an eclectic overview and summary of problems. Bulletin of Marine Science 1986, 39, 290–322. [Google Scholar]
- Sulkin, S.D.; Mckeen, G.L. Laboratory study of survival and duration of individual zoeal stages as a function of temperature in the brachyuran crab Cancer magister. Marine Biology 1989, 103, 31–37. [Google Scholar] [CrossRef]
- Sastry, A. Culture of brachyuran crab larvae using a re-circulating sea-water system in the laboratory. Helgolander wiss. Meeresunters 1970, 20, 406–416. [Google Scholar] [CrossRef]
- Bliss, D.E.; Vernberg, F.; Vernberg, W. Biology of the Crustacea. 7: Behaviour and Ecology; Academic Press: London, UK, 1983. [Google Scholar]
- Nagaraj, M. Combined effects of temperature and salinity on the zoeal development of then green crab, Carcinus maenas (Linnaeus, 1758) (Decapoda: Portunidae). Scientia Marina 1993, 57, 1–8. [Google Scholar]
- Lough, R.G. Dynamics of crab larvae (Anomura, Brachyura) off the central Oregon coast, 1969-1971. Thesis of the Oregon State University 1975.
- Miller, S.H. Larval behavior and natural trace element signatures as indicators of crustacean population connectivity. Thesis of the University of California 2011.
- Kinlan, B.P.; Gaines, S.D.; Lester, S.E. Propagule dispersal and the scales of marine community process. Diversity and Distributions 2005, 11, 139–148. [Google Scholar] [CrossRef]
- Cowen, R.K.; Paris, C.B.; Olson, D.B.; Fortuna, J.L. The role of long distance dispersal versus local retention in replenishing marine populations. Gulf and Caribbean Research 2003, 14, 129–137. [Google Scholar] [CrossRef]
- Sotka, E.E.; Wares, J.P.; Barth, J.A.; Grosberg, R.K.; Palumbi, S.R. Strong genetic clines and geographical variation in gene flow in the rocky intertidal barnacle Balanus glandula. Molecular Ecology 2004, 13, 2143–2156. [Google Scholar]
- Scheltema, R.S. Larval dispersal as a means of genetic exchange between geographically separated populations of shallow-water benthic marine gastropods. Biological Bulletin 1971, 140, 284–322. [Google Scholar] [CrossRef]
- Roughgarden, J.; Gaines, S.; Possingham, H.P. Recruitment dynamics in complex life cycles. Science 1988, 241, 1460–1466. [Google Scholar] [CrossRef]
- Botsford, L.W.; Moloney, C.L.; Hastings, A.; Largier, J.L.; Powell, T.M.; Higgins, K.; Quinn, J.F. The influence of spatially and temporally varying oceanographic conditions on meroplanktonic metapopulations. Deep Sea Research II 1994, 41, 107–145. [Google Scholar] [CrossRef]
- Cowen, R.K.; Lwiza, K.M.M.; Sponaugle, S.; Paris, C.B.; Olson, D.B. Connectivity of Marine Populations: Open or Closed? Science 2000, 287, 857–859. [Google Scholar] [CrossRef]
- Kinlan, B.P.; Gaines, S.D. Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 2003, 84, 2007–2020. [Google Scholar] [CrossRef]
- Shanks, A.L.; Grantham, B.A.; Carr, M. Propagule dispersal distance and the size and spacing of marine reserves. Ecological Applications 2003, 13, S159–S169. [Google Scholar] [CrossRef]
- Shanks, A. L. Pelagic larval duration and dispersal distance revisited. Biological Bulletin 2009, 216, 373–385. [Google Scholar] [CrossRef]
- Siegel, D. A.; Kinlan B., P.; Gaylord, D.; Gaines S., D. Lagrangian descriptions of marine larval dispersion. Marine Ecology Progress Series 2003, 260, 83–96. [Google Scholar] [CrossRef]
- Pires, R.F.T.; Pan, M.; Santos, A.M.P.; Peliz, Á.; Boutov, D.; dos Santos, A. Modelling the variation in larval dispersal of estuarine and coastal ghost shrimp: Upogebia congeners in the Gulf of Cadiz. Marine Ecology Progress Series 2013, 492, 153–168. [Google Scholar] [CrossRef]
- Bray, L.; Kassis, D.; Hall-Spencer, J.M. Assessing larval connectivity for marine spatial planning in the Adriatic. Marine Environmental Research 2017, 125, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, Y. P. Neuston of Seas and Oceans. In The sea surface and the global change; Liss, P.S.; Duce, R.A., Eds.; Cambridge, University Press, 1997; pp. 371-382.
- dos Santos, A.; Santos, A.M.P.; Conway, D.V.P.; Bartilotti, C.; Lourenço, P.; Queiroga, H. Diel vertical migration of decapod larvae in the Portuguese coastal upwelling ecosystem: implications for offshore transport. Marine Ecology Progress Series 2008, 359, 171–183. [Google Scholar] [CrossRef]
- Pochelon, P.N.; Pires, R.F.T.; Dubert, J.; Nolasco, R.; Santos, A.M.P.; Queiroga, H. , dos Santos, A. Decapod larvae distribution and species composition off the southern Portuguese coast. Continental Shelf Research 2017, 151, 53–61. [Google Scholar] [CrossRef]
- Torres, A.P.; Dos Santos, A. Balbín, R.; Alemany, F.; Massutí, E.; Reglero, P. Decapod crustacean larval communities in the Balearic Sea (western Mediterranean): seasonal composition, horizontal and vertical distribution patterns. Journal of Marine Systems 2014, 138, 112–126. [Google Scholar] [CrossRef]
- Torres, A.P.; Reglero, P.; Hidalgo, M.; Abello, P.; Simao, D.S.; Alemany, F.; Massutí, E.; Dos Santos, A. Contrasting patterns in the vertical distribution of decapod crustaceans throughout ontogeny. Hydrobiologia 2018, 808, 1 37–152. [Google Scholar] [CrossRef]
- Bartilotti, C.; dos Santos, A.; Castro, M.; Peliz Santos, A.M.P. Decapod larval retention within distributional bands in a coastal upwelling ecosystem. Marine Ecology Progress Series 2014, 507, 233–247. [Google Scholar] [CrossRef]
- de Santana, C.S.; Schwamborn, R.; Neumann-Leitão, S.; de Jesus Flores Montes, M.; de Albuquerque Lira, S.M. Spatio-temporal variation of planktonic decapods along the leeward coast of the Fernando de Noronha archipelago Brazil. Brazilian Journal of Oceanography 2018, 66, 1–14. [Google Scholar] [CrossRef]
- de Lima, F.A.; Butturi-Gomes, D.; das Neves Pantoja, M.H.; Martinelli-Lemos, J.M. Larval dispersal of Brachyura in one of the largest estuarine/marine systems in the world. PLOS ONE 2022, 17, e0252695. [Google Scholar] [CrossRef]
- Landeira, J.M.; Brochier, T.; Mason, E.; Lozano-Soldevilla, F.; Hernández-León, S.; Barton, E.D. Transport pathways of decapod larvae under intense mesoscale activity in the Canary-African coastal transition zone: implications for population connectivity. Scientia Marina 2017, 81. [Google Scholar] [CrossRef]
- Carrettone, M.; Company, J. B.; Boné, A.; Rotllant, G.; Guerao, G.; Bahamon, N.; et al. Decapod crustacean larval community structure of the submarine canyon off Blanes (NW Mediterranean Sea). Scientia Marina 2020, 84, 71–82. [Google Scholar] [CrossRef]
- Carreton, M.; Rotllant, G.; Castejòn, D.; Bahamòn, N.; Company, J.B. Summer decapod crustacean larval communities along the eastern Spanish Mediterranean coast. PLoS ONE 2022, 17, e0275892. [Google Scholar] [CrossRef]
- Hidalgo, M.; Reglero, P.; Álvarez-Berastegui, D.; Torres, A.P.; Álvarez, I.; Rodriguez, J.M.; Carbonell, A.; Zaragoza, N.; Tor, A.; Goñi, R.; Mallol, S.; Balbín, R. , Alemany, F. Hydrographic and biological components of the seascape structure the meroplankton community in a frontal system. Marine Ecology Progress Series 2014, 505, 65–80. [Google Scholar] [CrossRef]
- Mallol, S.; Mateo-Ramírez, A.; Alemany, F.; Álvarez-Berastegui, D.; Díaz, D.; López-Jurado, J.L.; Goñi, R. Abundance and distribution of scyllarid phyllosoma larvae (decapoda: scyllaridae) in the Balearic Sea (Western Mediterranean). Journal of Crustacean Biology 2014, 34, 442–452. [Google Scholar] [CrossRef]
- Carbonell, A.; Aparicio-González, A.; Papiol, V.; Cartes, J.E. Composition and distribution of the larval decapod community in the deep sea of the Western Mediterranean Sea Balearic Sub-basin. Fisheries Oceanography. 2021, 30, 205–218. [Google Scholar] [CrossRef]
- Kurian, C.V. Larvae of Decapod Crustacea from the Adriatic Sea. Acta Adriatica 1956, 6 (1953-1976), 1-108.
- Lucic, D. Annual variability of the decapod larvae community in the shallow waters of the southern Adriatic. Acta Adriatica 1998, 39, 25–30. [Google Scholar]
- Di Muzio, G.; Belmonte, G.; Pessani, D. Biodiversity and distribution of crustacean decapod larvae in South Adriatic and Otranto Channel. Biologia Marina Mediterranea 2016, 23, 283–284. [Google Scholar]
- Belmonte, G.; Scirocco, T.; Denitto, F. Zooplankton composition in Lake Varano (Adriatic Sea coast, Italy). Italian Journal of Zoology 2011, 78, 370–378. [Google Scholar] [CrossRef]
- Fanelli, E.; Menicucci, S.; Malavolti, S.; De Felice, A.; Leonori, I. Spatial changes in community composition and food web structure of mesozooplankton across the Adriatic basin (Mediterranean Sea). Biogeosciences 2022, 19, 1833–1851. [Google Scholar] [CrossRef]
- Liparoto, A.; Mancinelli, G.; Belmonte, G. Spatial variation in biodiversity patterns of neuston in the Western Mediterranean and Southern Adriatic Seas, Journal of Sea Research 2016, 129, 12-21.
- Guglielmo, R.; Bergamasco, A.; Minutoli, R.; Patti, F.P.; Belmonte, G.; Spanò, N.; Zagami, G.; Bonanzinga, V.; Guglielmo, L.; Granata, A. The Otranto Channel (South Adriatic Sea), a hot spot area of plankton biodiversity: pelagic polychaetes. Scientific Reports 2019, 9, 19490. [Google Scholar] [CrossRef]
- Zambianchi, E.; Trani, M.; Falco, P. Lagrangian Transport of Marine Litter in the Mediterranean Sea. Frontiers Environmental Science 2017, 5. [Google Scholar] [CrossRef]
- Marino, I.A.M.; Schiavina, M.; Aglieri, G.; Bevilacqua, S.; Boscari, E.; Congiu, L; Faggion, S.; Kruschel, C.; Papetti, C.; Patarnello, T.; Paterno, M.; Voutsinas, E.; Zane, L.; Melià, P. Assessment of connectivity patterns of the marbled crab Pachygrapsus marmoratus in the Adriatic and Ionian seas through combination of genetic data and Lagrangian simulations. Frontiers in Marine Science 2022, 9:944851. [CrossRef]
- Artegiani, A.; Bregant, D.; Paschini, E.; Pinardi, N.; Raicich, F.; Russo, A. The Adriatic Sea general circulation. Part I: Air–sea interactions and water mass structure. Journal of Physical Oceanography 1997, 27, 1492-1514.
- Fonda-Umani, S. Successioni fitoplanctoniche, micro e mesozoplanctoniche nell'Alto Adriatico.In: Marchetti, R., Cotta Ramusino, M. (Eds.), Atti V Congresso SITE, 1992 pp. 221-246.
- Zore-Armanda, M. The system of currents in the Adriatic Sea. Stud. Rev. General Fisheries Council for the Mediterranean 1968, 34, 1–48. [Google Scholar]
- Shabrang, L.; Menna, M.; Pizzi, C.; Lavigne, H.; Civitarese, G.; Gačić, M. Long-term variability of the South Adriatic circulation and phytoplankton biomass in relation to large-scale climatic pattern, Ocean Science Discussions 2015, 12, 203.226. [CrossRef]
- Gačić, M.; et al. Extreme winter 2012 in the Adriatic: an example of climatic effect on the BiOS rhythm. Ocean Science 2014, 10, 513–522. [Google Scholar] [CrossRef]
- Specchiulli, A.; Bignami, F.; Marini, M.; Fabbrocini, A.; Scirocco, T.; Campanelli, A.; Penna, P.; Santucci, A.; D'Adamo, R. The role of forcing agents on biogeochemical variability along the southwestern Adriatic coast: The Gulf of Manfredonia case study. Estuarine, Coastal and Shelf Science 2016, 183, 136–149. [Google Scholar] [CrossRef]
- CMEMS (2024), Global Ocean Monthly Mean Sea Surface Wind and Stress from Scatterometer and Model, Product ID: WIND_GLO_PHY_CLIMATE_L4_MY_012_003. [CrossRef]
- CMEMS (2024), Mediterranean Sea Physics Reanalysis, Product ID: MEDSEA_MULTIYEAR_PHY_006_004. [CrossRef]
- Boero, F.; Foglini, F.; Fraschetti, S.; Goriup, P.; Macpherson, E.; Planes, S. COCONET CONSOTIUM. CoCoNet: Towards Coast to Coast Networks of marine protected areas (from the shore to the high and deep sea), coupled with sea-based wind energy potential. SCIRES-IT, 2016, 6(S), 1-95.
- Sameoto, D.D.; Saroszynsky, L.O.; Fraser, W.B. BIONESS, a new design in multiple net zooplankton sampler. Journal. Fishery Research. Board Canada 1980, 3, 722–724. [Google Scholar] [CrossRef]
- Pessani, D.; Burri, R.; Salton, L. A key for the identification of the known larval stages of the Mediterranean Brachyura. Invertebrate Reproduction and Development 1998, 33, 191–199. [Google Scholar] [CrossRef]
- Pessani, D.; Tirelli, T.; Flagella, S. Key for the Identification of Mediterranean Brachyuran megalopae. Mediterranean Marine Science 2004, 5, 53–64. [Google Scholar] [CrossRef]
- De Melo Dos Santos, A.M. Larvas de Crustáceos Decápodes ao largo da Costa Portuguesa. Tese apresentada à Faculdade de Ciências da Universidade de Lisboa para a obtenção do grau de Doutor, Lisboa 1999, 262 pp.
- Pohle, G.; Mantelatto, F.L.M.; Negreiros-Fransozo, M.L.; Fransozo, A. Larval Decapoda (Brachyura). In: South Atlantic Zooplankton, Vol. 2. D. Boltovskoy (Ed). Backhuys Publishers, Leiden. The Netherlands: 1999,1281-1351.
- Rodrigues dos Santos Bento, M.A. Keys and bibliography for the identification of larval stages of brachyuran crabs from the Western Indian Ocean. Mestrado em Ecologia Marinha, Universidade de Lisboa Faculdade de Ciências Departamento de Biologia Animal, 2017, 49 pp.
- Barange, M. Vertical migration and habitat partitioning of six euphausiid species in the northern Benguela upwelling system. Journal Plankton Research 1990, 12, 1223–1237. [Google Scholar] [CrossRef]
- Andersen, V.; Sardou, J. The die1 migrations and vertical distributions of zooplankton and micronekton in the Northwestern Mediterranean Sea, 1. Euphausiids, mysids, decapod and fishes. Journal Plankton Research 1992, 14, 112–1554. [Google Scholar]
- Davis, R.E. Observing the general circulation with floats. Deep Sea Research, Part A. Oceanographic Research Papers 1991, 38, S531–S571. [Google Scholar] [CrossRef]
- Corrado, R.; Lacorata, G.; Palatella, L.; Santoleri, R.; Zambianchi, E. General characteristics of relative dispersion in the ocean. Scientific Reports 2017, 7, 46291. [Google Scholar] [CrossRef]
- Pisano, A.; De Dominicis, M.; Biamino, W.; Bignami, F.; Gherardi, S.; Colao, F.; Coppini, G.; Marullo, S.; Sprovieri, M.; Trivero, P.; Zambianchi, E.; Santoleri, R. An oceanographic survey for oil spill monitoring and model forecasting validation using remote sensing and in situ data in the Mediterranean Sea. Deep Sea Research Part II: Topical Studies in Oceanography 2016, 133, 132–145. [Google Scholar] [CrossRef]
- Carlson, D. F.; Griffa, A.; Zambianchi, E.; Suaria, G.; Corgnati, L.; Magaldi, M.G.; Poulain, P.-M.; Russo, A.; Bellomo, L.; Mantovani, C.; Celentano, P.; Molchard, A.; Borghini, M. Observed and modeled surface Lagrangian transport between coastal regions in the Adriatic Sea with implications for marine protected areas. Continental Shelf Research 2016, 118, 23–48. [Google Scholar] [CrossRef]
- Sciascia, R.; Berta, M.; Carlson, D.F.; Griffa, A.; Panfili, M.; La Mesa, M.; Corgnati, L.; Mantovani, C.; Domenella, E.; Fredj, E.; Magaldi, M.G.; D’Adamo, R.; Pazienza, G.; Zambianchi, E.; Poulain, P.-M. Linking sardine recruitment in coastal areas to ocean currents using surface drifters and HF radar: a case study in the Gulf of Manfredonia, Adriatic Sea. Ocean Science 2018, 14, 1461–1482. [Google Scholar] [CrossRef]
- Batchelder, H.P. Forward-in-time-/backward-in-time-trajectory (FITT/BITT) modeling of particles and organisms in the coastal ocean. Journal of Atmospheric and Oceanic Technology 2006, 23, 727–741. [Google Scholar] [CrossRef]
- Cianelli, D.; D’Alelio, D.; Uttieri, M.; Sarno, D.; Zingone, A.; Zambianchi, E.; Ribera D’alcalà, M. Disentangling physical and biological drivers of phytoplankton dynamics in a coastal system. Scientific Reports 2017, 7, 15868. [Google Scholar] [CrossRef]
- Fratini, S.; Ragionieri, L.; Deli, T.; Harrer, A.; Marino, I.A.M.; Cannicci, S.; Zane, L.; Schubart, C.D. Unravelling population genetic structure with mitochondrial DNA in a notional panmictic coastal crab species: sample size makes the difference. BMC Evolutionary Biology 2016. [CrossRef]
- Schiavina, M.; Marino, I.A.M.; Zane, L.; Melià, P. Matching oceanography and genetics at the basin scale. Seascape connectivety of the Mediterranean shore crab in the Adriatic. Molecular Ecology 2014, 23, 5496–5507. [Google Scholar]
- Fraser, C.I.; Nikula, R.; Waters, G.M. Oceanic rafting by a coastal community. Proceedings Royal Society 2011, B 278, 649–55. [Google Scholar] [CrossRef]
- Treml, E.A.; Roberts, J.J.; Chao, Y.; Halpin, P.N.; Possingham, H.P.; Rigin, C. Reproductive output and Duration of the Pelagic Larval Stage determine seascape-wide connectivity of marine populations. Integrative and Comparative Biology 2012, 52, 525–537. [Google Scholar] [CrossRef]
- Jokiel, P.L. Rafting of reef corals and other organisms at Kwajalein Atoll. Marine Biology 1989, 101, 483–493. [Google Scholar] [CrossRef]
- Boero, F.; Bouillon, J. Zoogeography and life cycle patterns of Mediterranean hydromedusae (Cnidaria). Biology Journal of the Linnean Society 1993, 48, 239–266. [Google Scholar] [CrossRef]
- Pati, A.C.; Belmonte, G.; Fanelli, G.; Giangrande, A.; Gravili, C.; Saracino, O.; Boero, F. Interkingdom convergence in the architecture of asexual propagules. Biologia Marina Mediterranea 1998, 5, 365–366. [Google Scholar]
- Moscatello, S.; Belmonte, G. The plankton of a shallow submarine cave (‘Grotta di Ciolo’, Salento Peninsula, SE Italy). Marine Ecology 2007, 28 Suppl.1, 47–59. [Google Scholar] [CrossRef]
- Pati, A.C.; Belmonte, G. Asexually Generated Propagules from Subtidal Sessile Benthic Organisms. Progress in Aqua Farming and Marine Biology 2018, 1, 180002. [Google Scholar]
- Belmonte, G.; Rubino, F. Resting cysts from coastal marine plankton. Oceanography and Marine Biology Annual Review 2019, 57, 1–88. [Google Scholar]
- Weersing, K.; Toonen, R.J. Population genetics, larval dispersal, and connectivity in marine systems. Marine Ecology Progress Series 2009, 393, 1–12. [Google Scholar] [CrossRef]
 |
 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





