Submitted:
11 September 2024
Posted:
11 September 2024
You are already at the latest version
Abstract
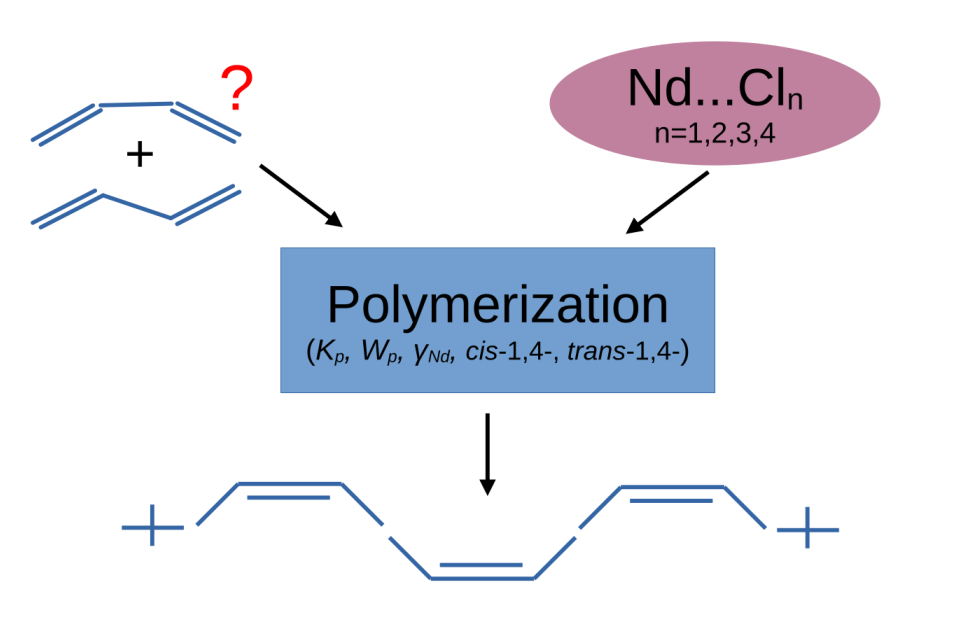
Keywords:
1. Introduction
2. Computational Details
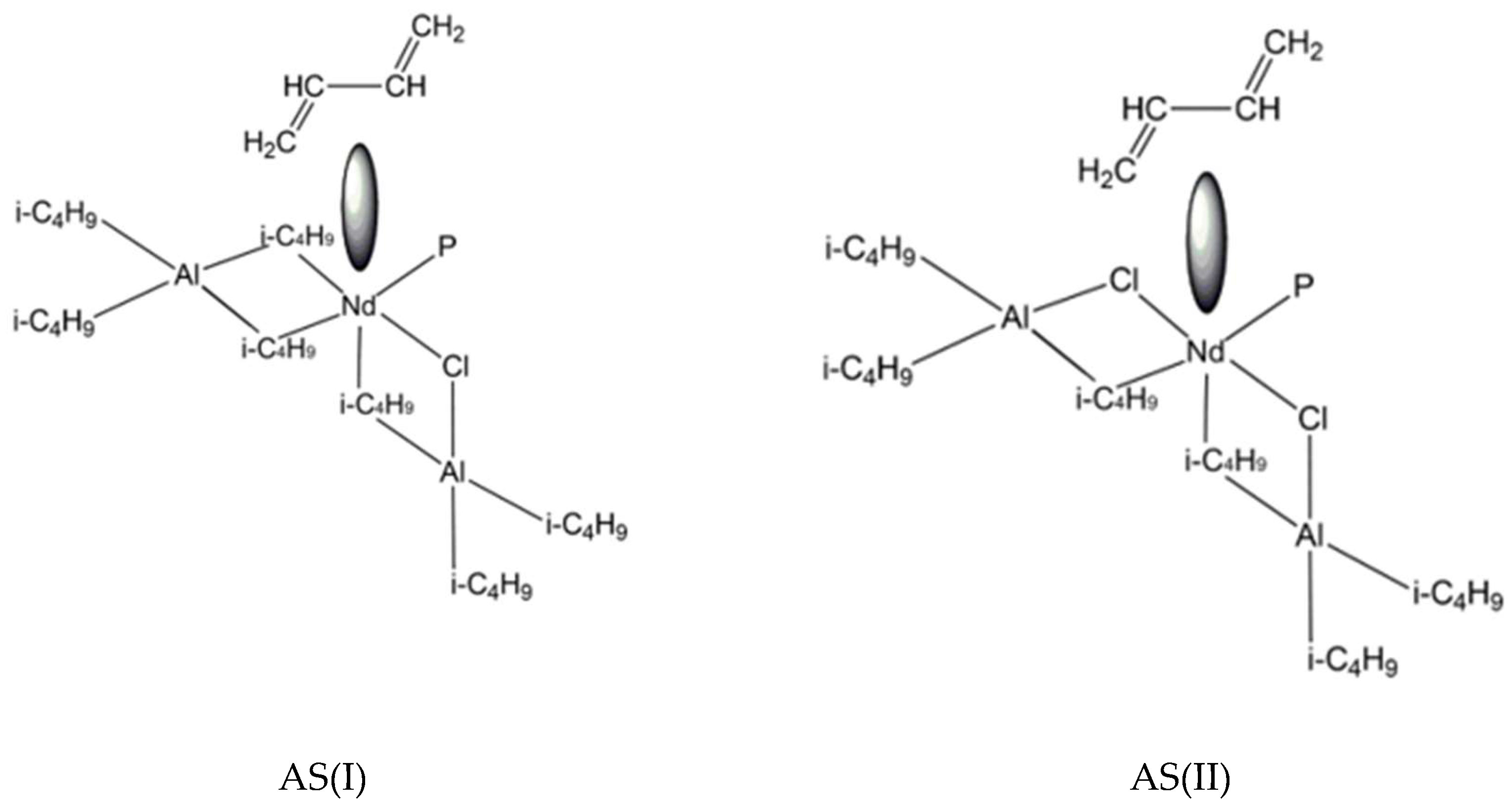
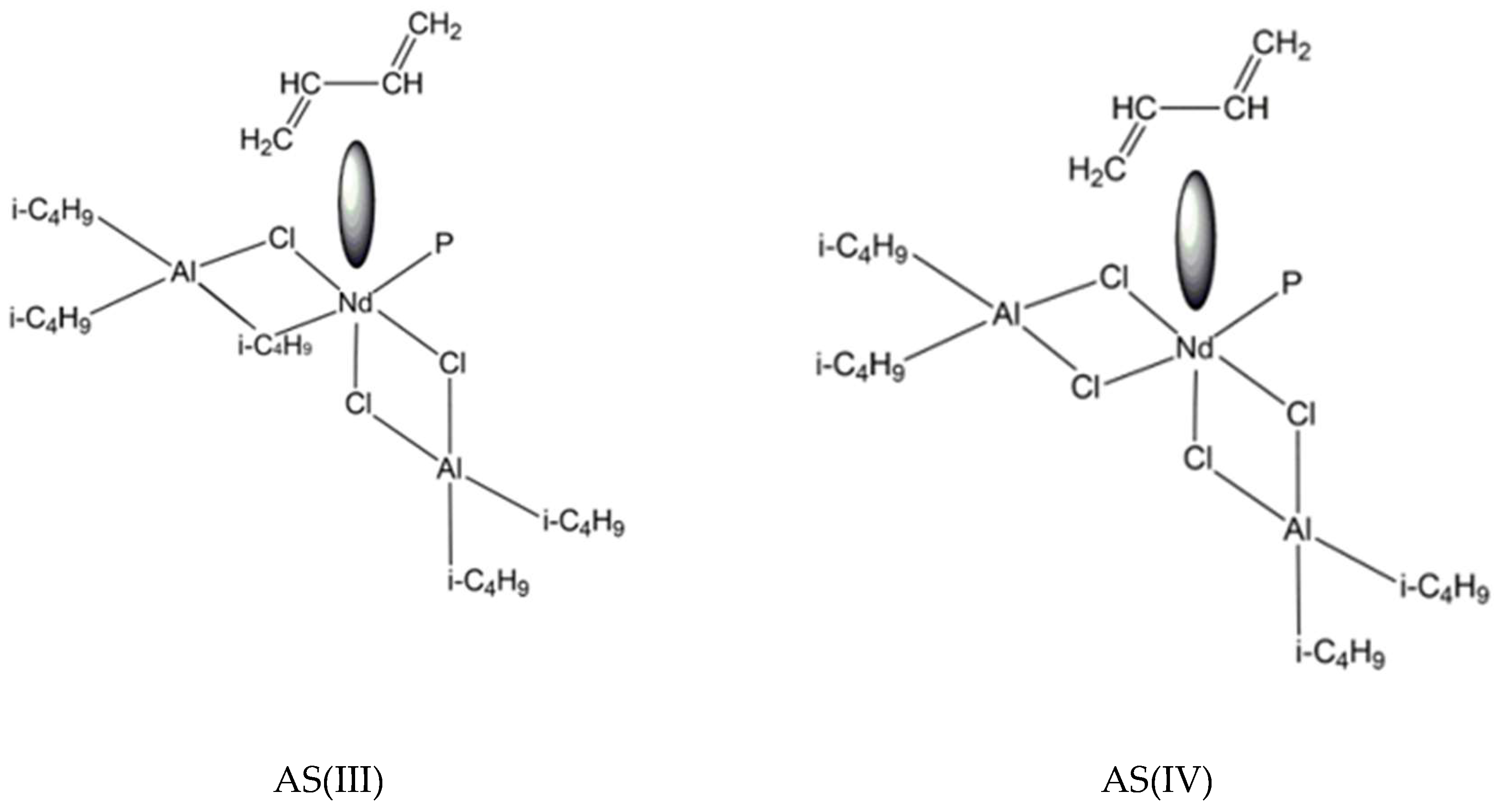
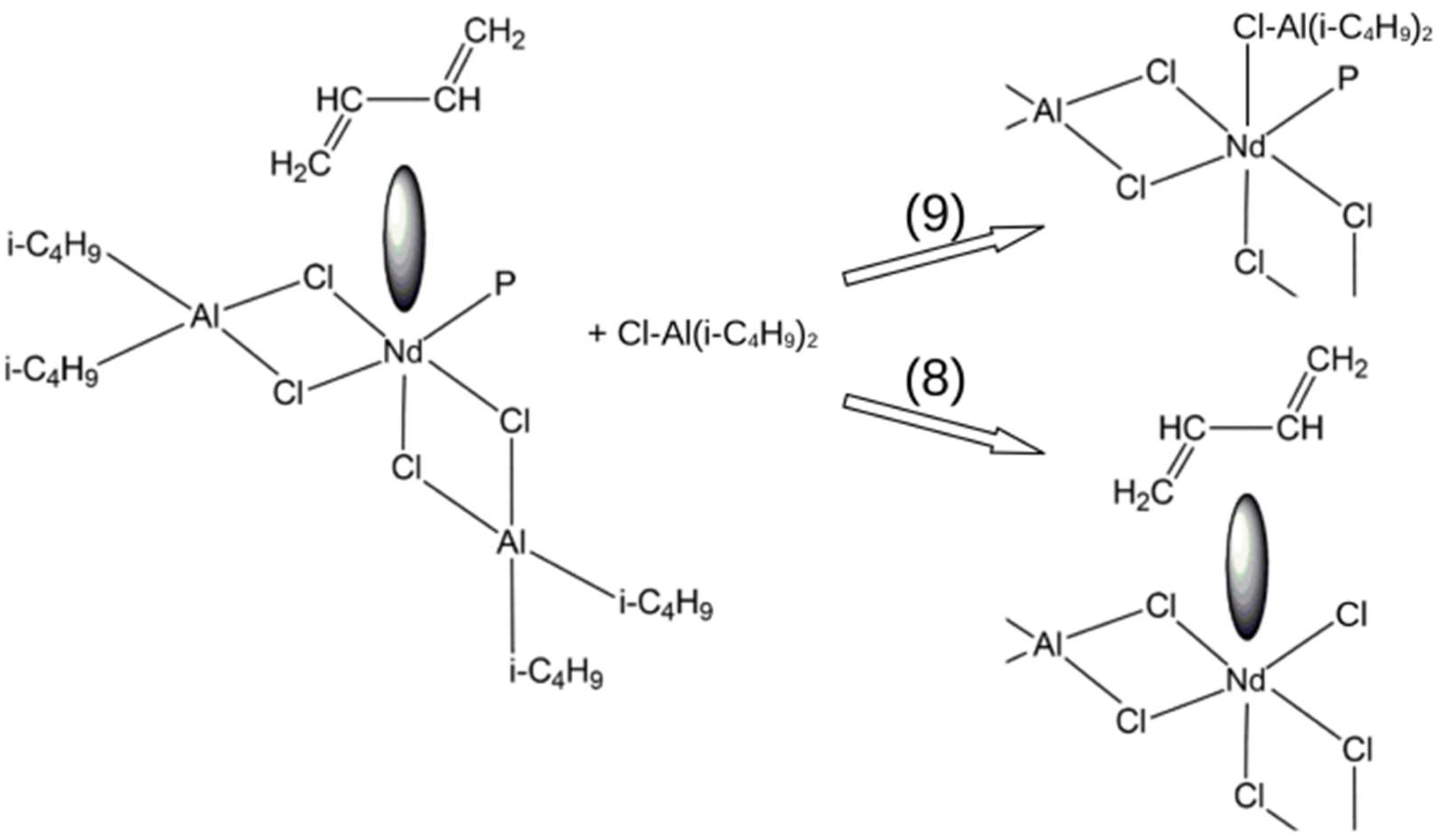
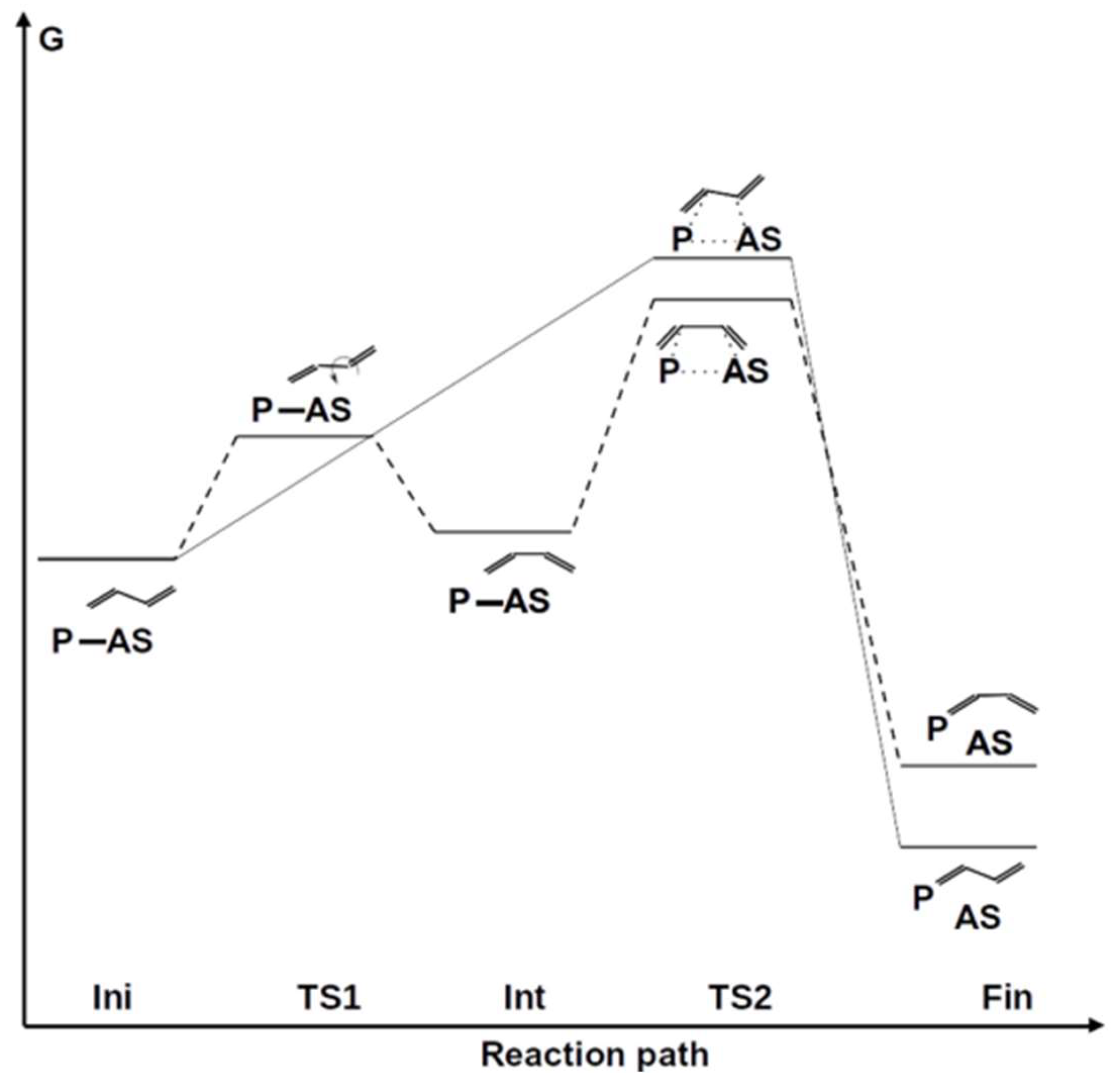
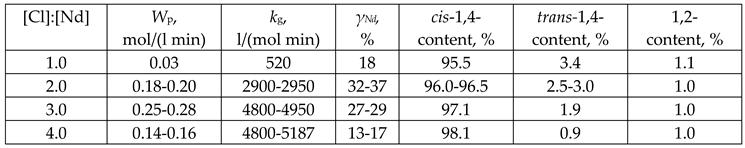
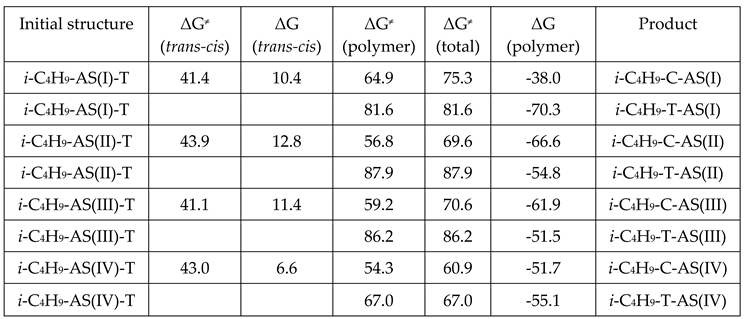
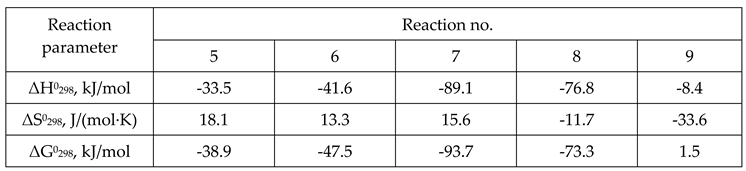
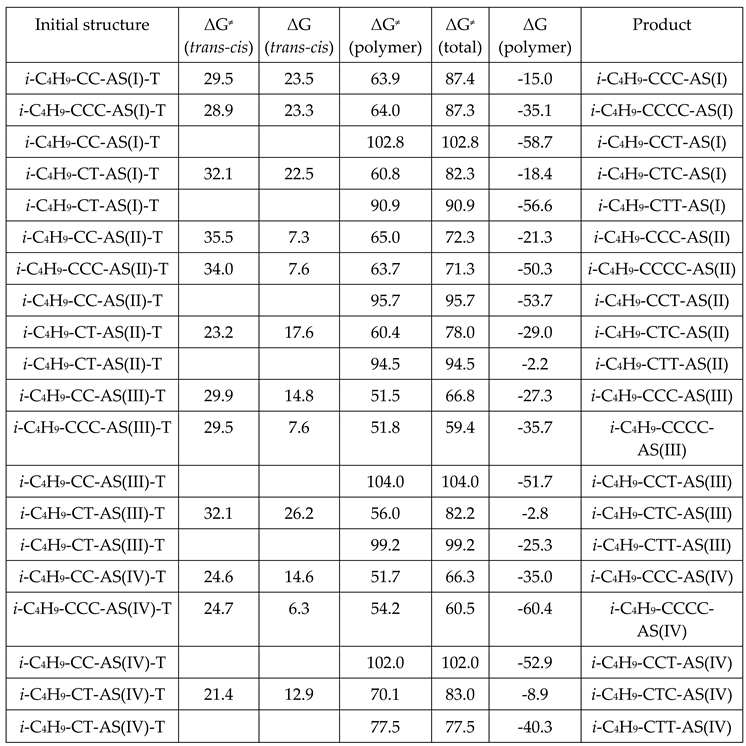
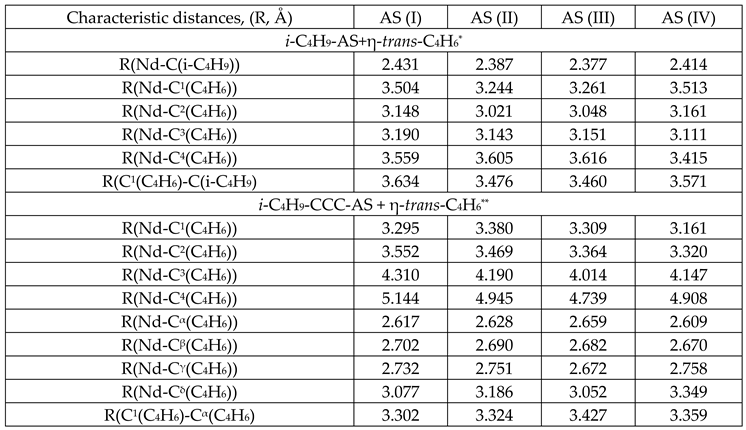
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cooper, W., & Vaughan, G. (1967). Recent developments in the polymerization of conjugated dienes. Progress in Polymer Science, 1, 91-160. [CrossRef]
- The stereo rubbers, William M. Saltman, Wiley-Interscience, New York, 1977, 897 pp. [CrossRef]
- Hsieh, H. L., & Yeh, H. C. (1985). Polymerization of butadiene and isoprene with lanthanide catalysts; characterization and properties of homopolymers and copolymers. Rubber Chemistry and Technology, 58(1), 117-145. [CrossRef]
- Marina, N. G., Monakov, Y. B., Sabirov, Z. M., & Tolstikov, G. A. (1991). Lanthanide compounds—Catalysts of stereospecific polymerization of diene monomers. Review. Polymer science USSR, 33(3), 387-417.
- Friebe, L., Nuyken, O., & Obrecht, W. (2006). Neodymium-based Ziegler/Natta catalysts and their application in diene polymerization. Neodymium Based Ziegler Catalysts–Fundamental Chemistry, 1-154. [CrossRef]
- Fischbach A, Anwander R. Neodymium Based Ziegler Catalysts Fundamental Chemistry. In: Nuyken O, ed. Berlin, Heidelberg: Springer, 2006. 204: 155–281. https://doi: 10.1007/11761013.
- Zhang, Z., Cui, D., Wang, B., Liu, B., & Yang, Y. (2010). Polymerization of 1, 3-conjugated dienes with rare-earth metal precursors. Molecular catalysis of rare-earth elements, 49-108. https://doi: 10.1007/430_2010_16.
- Wang, F., Liu, H., Hu, Y., & Zhang, X. (2018). Lanthanide complexes mediated coordinative chain transfer polymerization of conjugated dienes. Science China Technological Sciences, 61(9), 1286-1294. [CrossRef]
- Quirk, R. P., Kells, A. M., Yunlu, K., & Cuif, J. P. (2000). Butadiene polymerization using neodymium versatate-based catalysts: catalyst optimization and effects of water and excess versatic acid. Polymer, 41(15), 5903-5908. [CrossRef]
- Kwag, G. (2002). A highly reactive and monomeric neodymium catalyst. Macromolecules, 35(13), 4875-4879. [CrossRef]
- Zheng, W., Yang, Q., Dong, J., Wang, F., Luo, F., Liu, H., & Zhang, X. (2021). Neodymium-based one-precatalyst/dual-cocatalyst system for chain shuttling polymerization to access cis-1, 4/trans-1, 4 multiblock polybutadienes. Materials Today Communications, 27, 102453. [CrossRef]
- Wang, H., Cue, J. M. O., Calubaquib, E. L., Kularatne, R. N., Taslimy, S., Miller, J. T., & Stefan, M. C. (2021). Neodymium catalysts for polymerization of dienes, vinyl monomers, and ε-caprolactone. Polymer Chemistry, 12(47), 6790-6823. https://doi: 10.1039/D1PY01270C.
- Iovu, H., Hubca, G., Simionescu, E., Badea, E. G., & Dimonie, M. (1997). Polymerization of butadiene and isoprene with the NdCl3· 3TBP-TIBA catalyst system. Die Angewandte Makromolekulare Chemie: Applied Macromolecular Chemistry and Physics, 249(1), 59-77. [CrossRef]
- Srinivasa Rao, G. S., Upadhyay, V. K., & Jain, R. C. (1999). Polymerization of 1, 3-butadiene using neodymium chloride tripentanolate–triethyl aluminum catalyst systems. Journal of applied polymer science, 71(4), 595-602.
- Ren, C., Li, G., Dong, W., Jiang, L., Zhang, X., & Wang, F. (2007). Soluble neodymium chloride 2-ethylhexanol complex as a highly active catalyst for controlled isoprene polymerization. Polymer, 48(9), 2470-2474. [CrossRef]
- Hu, Y., Zhang, C., Liu, X., Gao, K., Cao, Y., Zhang, C., & Zhang, X. (2014). Methylaluminoxane-activated neodymium chloride tributylphosphate catalyst for isoprene polymerization. Journal of Applied Polymer Science, 131(8). [CrossRef]
- Kularatne, R. N., Yang, A., Nguyen, H. Q., McCandless, G. T., & Stefan, M. C. (2017). Neodymium catalyst for the polymerization of dienes and polar vinyl monomers. Macromolecular Rapid Communications, 38(19), 1700427. [CrossRef]
- Oehme, A., Gebauer, U., Gehrke, K., Beyer, P., Hartmann, B., & Lechner, M. D. (1994). The influence of the catalyst preparation on the homo-and copolymerization of butadiene and isoprene. Macromolecular Chemistry and Physics, 195(12), 3773-3781. [CrossRef]
- Boisson, C., Barbotin, F., & Spitz, R. (1999). Polymerization of butadiene with a new catalyst based on a neodymium amide precursor. Macromolecular Chemistry and Physics, 200(5), 1163-1166.
- Friebe, L., Nuyken, O., Windisch, H., & Obrecht, W. (2002). Polymerization of 1, 3-Butadiene Initiated by Neodymium Versatate/Diisobutylaluminium Hydride/Ethylaluminium Sesquichloride: Kinetics and Conclusions About the Reaction Mechanism. Macromolecular Chemistry and Physics, 203(8), 1055-1064.
- Quirk, R. P., Kells, A. M. (2000). Polymerization of butadiene using neodymium versatate-based catalyst systems: preformed catalysts with SiCl4 as halide source. Polymer International, 49(7), 751-756. [CrossRef]
- Sigaeva, N. N., Usmanov, T. S., Budtov, V. P., & Monakov, Y. B. (2001). Effect of organoaluminum compound on kinetic nonuniformity and structure of active centers of neodymium catalytic systems in butadiene polymerization. Russian journal of applied chemistry, 74(7), 1141-1146. https://doi: 10.1023/A:1013019001878.
- Monakov, Y. B., Sabirov, Z. M., Urazbaev, V. N., & Efimov, V. P. (2001). Relationship between the stereospecificity of lanthanide catalysts and the structures of active sites and dienes, the nature of a cocatalyst, and preparation conditions. Kinetics and catalysis, 42(3), 310-316. https://doi: 10.1023/A:1010453013151.
- Sigaeva, N. N., Usmanov, T. S., Budtov, V. P., Spivak, S. I., Zaikov, G. E., & Monakov, Y. B. (2003). The influence of the nature of organoaluminum compound on kinetic heterogeneity of active sites in lanthanide-based diene polymerization. Journal of applied polymer science, 87(3), 358-368. [CrossRef]
- Monakov, Y. B., Sabirov, Z. M., Urazbaev, V. N., & Efimov, V. P. (2002). Diene polymerization initiated by NdCl3. 3TBP-based catalytic systems. Multiplicity of active centers and their structure and stereospecificity distributions. Polymer science. Series A, 44(3), 228-231.
- Urazbaev, V. N., Efimov, V. P., Sabirov, Z. M., & Monakov, Y. B. (2003). Structure of active centers, their stereospecificity distribution, and multiplicity in diene polymerization initiated by NdCl3-based catalytic systems. Journal of applied polymer science, 89(3), 601-603. [CrossRef]
- Masliy, A. N., Akhmetov, I. G., Kuznetsov, A. M., & Davletbaeva, I. M. (2023). DFT and ONIOM Simulation of 1, 3-Butadiene Polymerization Catalyzed by Neodymium-Based Ziegler–Natta System. Polymers, 15(5), 1166. [CrossRef]
- Masliy, A. N., Akhmetov, I. G., Kuznetsov, A. M., & Davletbaeva, I. M. (2024). Comparative ONIOM modeling of 1, 3-butadiene polymerization using Nd (III) and Gd (III) Ziegler–Natta catalyst systems. International Journal of Quantum Chemistry, 124(1), e27297. [CrossRef]
- Akhmetov, I. G., Kozlov, V. G., Salakhov, I. I., Sakhabutdinov, A. G., & D’yakonov, G. S. (2010). Polymerisation kinetics and molecular characteristics of “neodymium” polybutadiene: influence of halogenating agent concentration. International Polymer Science and Technology, 37(3), 1-5.
- Salakhov, I. I., Akhmetov, I. G., & Kozlov, V. G. (2011). Polymerization of butadiene during the action of the catalytic system neodymium versatate-diisobutylaluminum hydride-hexachloro-p-xylene. Polymer Science Series B, 53(7), 385-390. https://doi: 10.1134/S1560090411070074.
- Akhmetov, I. G. Sintez dienovyh kauchukov s ispol’zovaniem modificirovannyh kataliticheskih sistem na osnove soedinenij neodima i litiya. Diss. dokt. chem. Nauk [Synthesis of diene rubbers using modified catalytic systems based on neodymium and lithium compounds. Dr. chem. sci. diss.]. Kazan, KNRTU Publ., 2013. 379 p.
- Neese, F. (2012). The ORCA program system. Wiley Interdisciplinary Reviews: Computational Molecular Science, 2(1), 73-78. [CrossRef]
- Neese, F. (2017). Software update: the ORCA program system, version 4.0. Wiley Interdisciplinary Reviews-Computational Molecular Science, 8(1), 73-78. [CrossRef]
- Dapprich, S., Komáromi, I., Byun, K. S., Morokuma, K., & Frisch, M. J. (1999). A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. Journal of Molecular Structure: THEOCHEM, 461, 1-21. [CrossRef]
- Becke, A. Density-functional thermochemistry. III. The role of exact exchange (1993) J. Chem. Phys, 98, 5648. [CrossRef]
- Lee, C., Yang, W., & Parr, R. G. (1988). Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physical review B, 37(2), 785. [CrossRef]
- Weigend, F., & Ahlrichs, R. (2005). Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Physical Chemistry Chemical Physics, 7(18), 3297-3305. [CrossRef]
- Stoychev, G. L., Auer, A. A., & Neese, F. (2017). Automatic generation of auxiliary basis sets. Journal of chemical theory and computation, 13(2), 554-562. [CrossRef]
- Dolg, M., Stoll, H., & Preuss, H. (1989). Energy-adjusted abinitio pseudopotentials for the rare earth elements. The Journal of chemical physics, 90(3), 1730-1734. [CrossRef]
- Grimme, S., Bannwarth, C., & Shushkov, P. (2017). A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for all spd-block elements (Z= 1–86). Journal of chemical theory and computation, 13(5), 1989-2009. [CrossRef]
- Bannwarth, C., Ehlert, S., & Grimme, S. (2019). GFN2-xTB—An accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. Journal of chemical theory and computation, 15(3), 1652-1671. [CrossRef]
- Grimme, S., Antony, J., Ehrlich, S., & Krieg, H. (2010). A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. The Journal of chemical physics, 132(15), 154104. [CrossRef]
- Ehlert, S., Stahn, M., Spicher, S., & Grimme, S. (2021). Robust and efficient implicit solvation model for fast semiempirical methods. Journal of Chemical Theory and Computation, 17(7), 4250-4261. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





