Submitted:
11 September 2024
Posted:
13 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Structural Properties
2.2. Electronic Properties
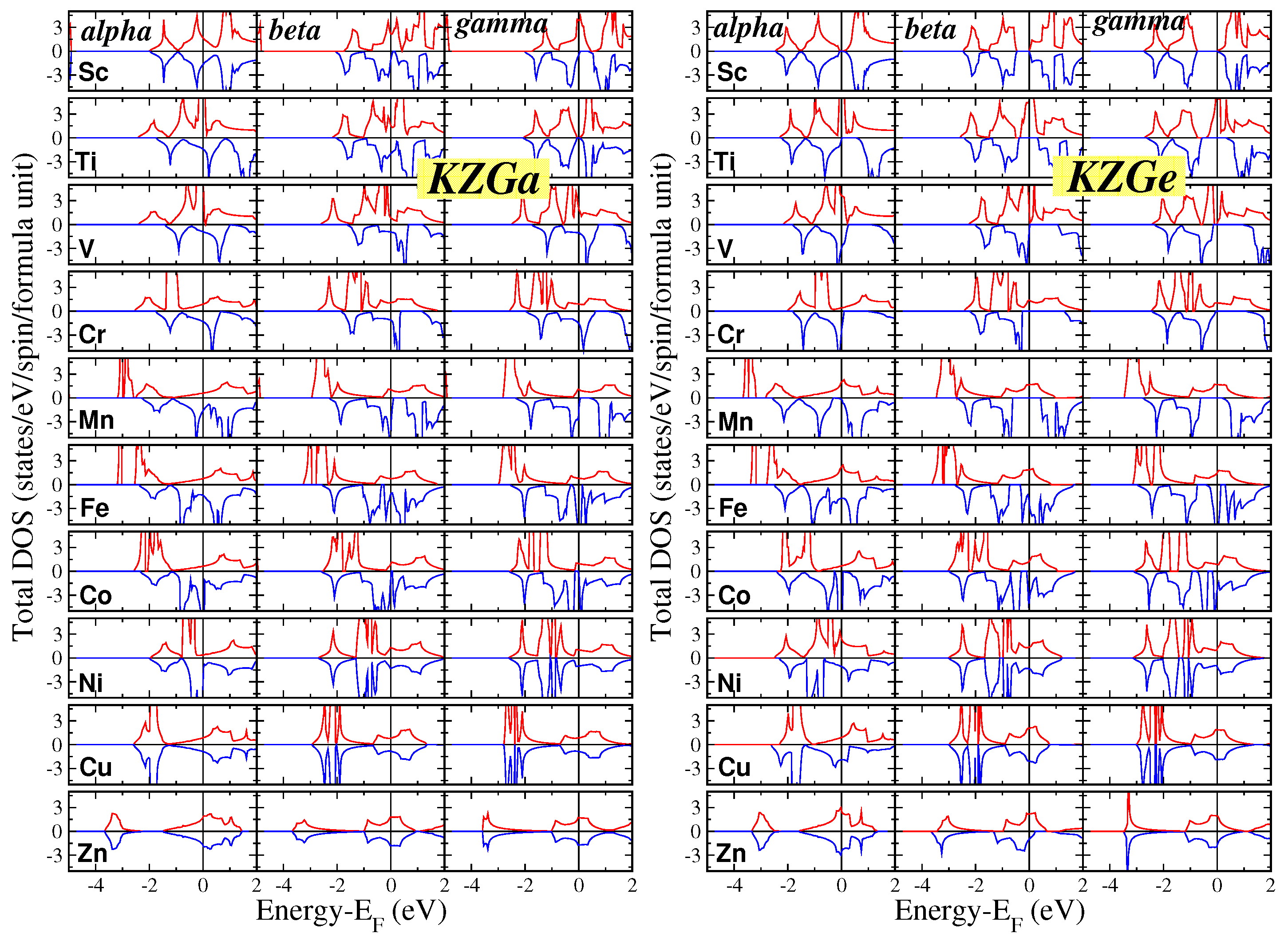
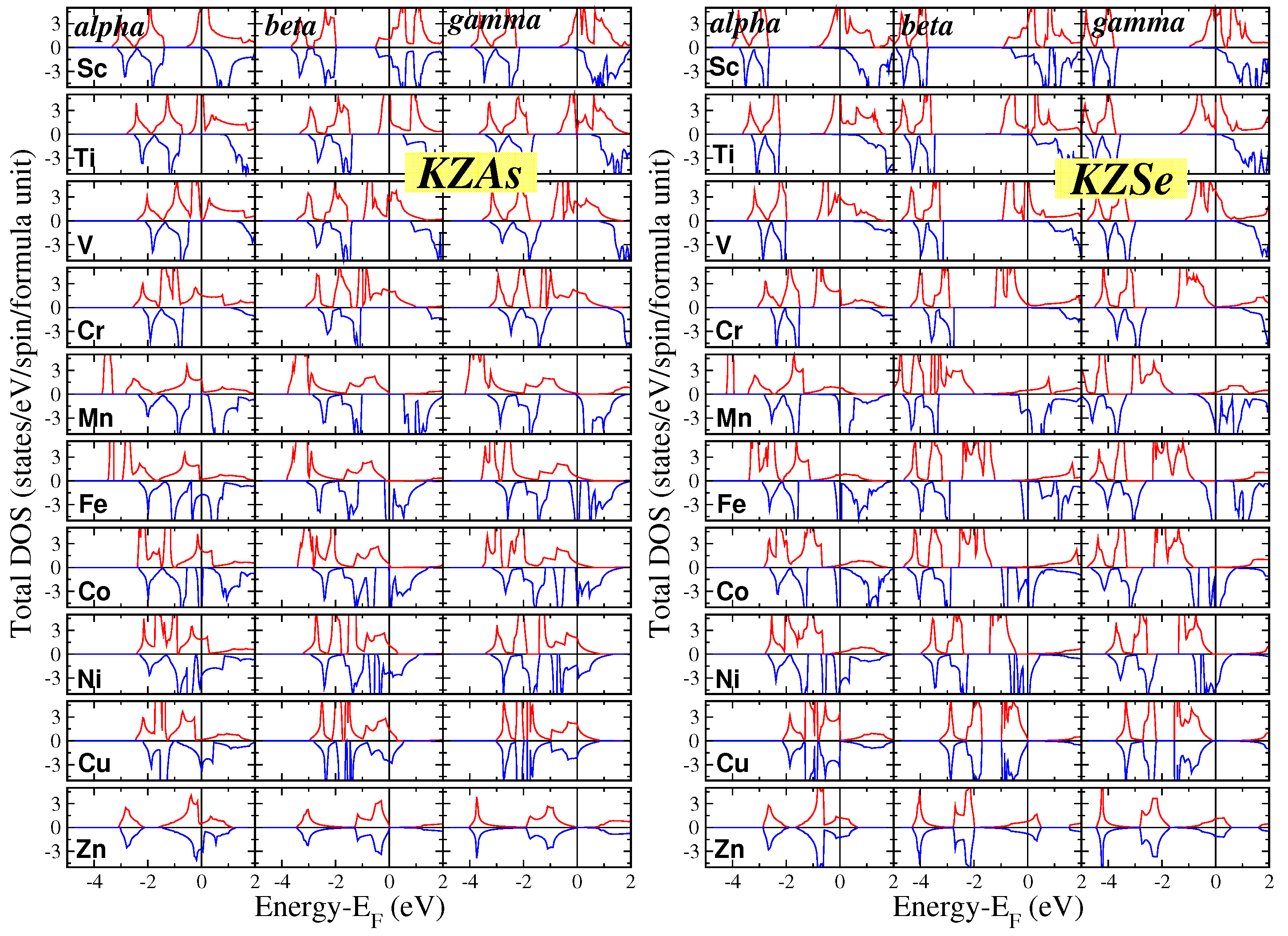
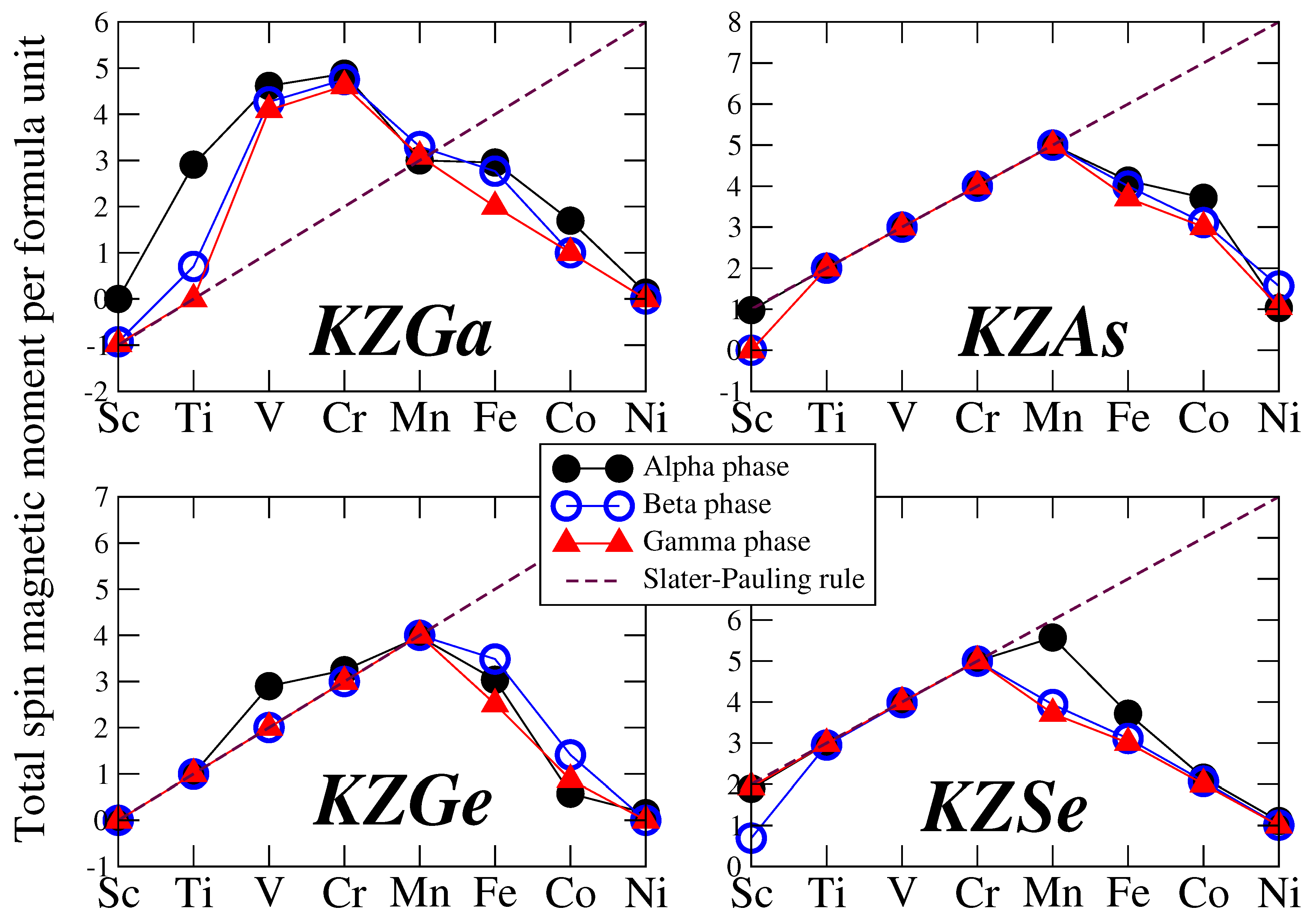
2.3. Magnetic Properties
3. Summary and conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DOS | Density of States |
| f.u. | formula unit |
| FPLO | Full-potential nonorthogonal local-orbital minimum- basis band structure approach |
| GGA | Generalized gradient approximation |
| PBE | Perdew Burke Ernzerhof |
References
- Heusler, F. Über magnetische manganlegierungen. Verh. Dtsch. Phys. Ges. 1903, 12, 219. [Google Scholar]
- Heusler, F.; Take, E. The nature of the Heusler alloys. Phys. Z. 1912, 13, 897. [Google Scholar] [CrossRef]
- Webster, P.J. ; Ziebeck., K.R.A. Alloys and Compounds of d-Elements with Main Group Elements. Part 2. In Landolt-Börnstein, New Series, Group III, vol 19c; Editor Wijn, H.R.J.; Springer: Berlin. 1988; pp. 75–184.
- Ziebeck, K.R.A.; Neumann, K.-U. Magnetic Properties of Metals. In Landolt-Börnstein, New Series, Group III, vol 32/c. Editor Wijn, H.R.J.; Springer: Berlin. 2001; pp. 64–414.
- Kawasaki, J.K.; Chatterjee, S.; Canfield, P.C. Full and half-Heusler compounds. MRS Bulletin 2022, 47, 555. [Google Scholar] [CrossRef]
- Graf, T.; Felser, C.; Parkin, S.S.P. Simple rules for the understanding of Heusler compounds. Progress in Solid State Chemistry 2011, 39, 1. [Google Scholar] [CrossRef]
- Bachagha, T; Sunol, J. -J. All-d-Metal Heusler Alloys: A Review. Metals 2023, 13, 111. [Google Scholar] [CrossRef]
- de Paula, V. G.; Reis, M. S. All-d-Metal Full Heusler Alloys: A Novel Class of Functional Materials. Chem. Mater. 2021, 33, 5483. [Google Scholar] [CrossRef]
- Tas, M; Özdoğan, K. ; Şaşıoğlu, E.; Galanakis,I. High Spin Magnetic Moments in All-3d-Metallic Co-Based Full Heusler Compounds. Materials 2023, 16, 7543. [Google Scholar] [CrossRef]
- Fortunato, N. M.; Li, X.; Schönecker, S.; Xie, R.; aubel, A.; Scheibel, F.; Opahle, I.; Gutfleisch, O.; Zhang, H. High-Throughput Screening of All-d-Metal Heusler Alloys for Magnetocaloric Applications. Chem. Mater. 2024, 36, 6765. [Google Scholar] [CrossRef]
- Galanakis, I. Slater–Pauling Behavior in Half-Metallic Heusler Compounds. Nanomaterials 2023, 13, 2010. [Google Scholar] [CrossRef]
- Katsnelson, M.I.; Irkhin, V. Yu; Chioncel, L.; Lichtenstein, A.I.; de Groot, R.A. Half-metallic ferromagnets: From band structure to many-body effects. Rev. Mod. Phys. 2008, 80, 315. [Google Scholar] [CrossRef]
- Hirohata, A.; Takanashi, K. Perspectives of Heusler compounds. J. Phys. D: Appl. Phys. 2014, 47, 193001. [Google Scholar] [CrossRef]
- Spintronics. From Materials to Devices. Editors Felser, C.; Fecher, G.H. Spintronics. From Materials to Devices. Editors Felser, C.; Fecher, G.H., Springer. 2013.
- Half-metallic Materials and Their Properties. In Series on Materials for Engineering, vol. 2. Editors Fong, C.Y.; Pask, J.E.; Yang, L.H. Imperial College Press. 2013.
- Heusler Alloys. Properties, Growth, Applications. In Springer Series in Materials Science, vol. 222. Editors Felser, C.; Hirohata, A. Springer International Publishing. 2018.
- Galanakis,I. ; Özdoğan, K.; Şaşıoğlu, E. Spin-filter and spin-gapless semiconductors: The case of Heusler compounds. AIP Adv. 2016, 6, 055606. [Google Scholar] [CrossRef]
- Gillessen, M.; Dronskowski, R. A combinatorial study of full Heusler alloys by first-principles computational methods. J. Comput. Chem. 2009, 30, 1290. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, M.; Dronskowski, R. A combinatorial study of inverse Heusler alloys by first-principles computational methods. J. Comput. Chem. 2010, 31, 612. [Google Scholar] [CrossRef]
- Ma, J.; Hegde, V.I.; Munira, K.; Xie, Y.; Keshavarz, S.; Mildebrath, D.T.; Wolverton, C.; Ghosh, A.W.; Butler, W.H. Computational investigation of half-Heusler compounds for spintronics applications. Phys. Rev. B 2017, 95, 024411. [Google Scholar] [CrossRef]
- Sanvito, S.; Oses, C.; Xue, J.; Tiwari, A.; Zic, M.; Archer, T.; Tozman, P.; Venkatesan, M.; Coey, M.; Curtarolo, S. Accelerated discovery of new magnets in the Heusler alloy family. Sci. Adv. 2017, 3, e1602241. [Google Scholar] [CrossRef]
- Faleev, S.V.; Ferrante, Y.; Jeong, J.; Samant, M.G.; Jones, B.; Parkin, S.S.P. Unified explanation of chemical ordering, the Slater-Pauling rule, and half-metallicity in full Heusler compounds. Phys. Rev. B 2017, 95, 045140. [Google Scholar] [CrossRef]
- Faleev, S.V.; Ferrante, Y.; Jeong, J.; Samant, M.G.; Jones, B.; Parkin, S.S.P. Heusler compounds with perpendicular magnetic anisotropy and large tunneling magnetoresistance. Phys. Rev. Mater. 2017, 1, 024402. [Google Scholar] [CrossRef]
- He, J.; Rabe, K. M; Wolverton, C. Computationally accelerated discovery of functional and structural Heusler materials. MRS Bulletin 2022, 47, 559. [Google Scholar] [CrossRef]
- Damewood, L.; Busemeyer, B.; Shaughnessy, M.; Fong, C. Y, ; Yang, L. H.; Felser, C. Stabilizing and increasing the magnetic moment of half-metals: The role of Li in half-Heusler LiMnZ (Z=N,P,Si). Phys. Rev. B 2015, 91, 064409. [Google Scholar] [CrossRef]
- Dehghan, A.; Davatolhagh, S. d0-d half-Heusler alloys: A potential class of advanced spintronic materials. J. All. Comp. 2019, 773, 132. [Google Scholar] [CrossRef]
- Dehghan, A.; Davatolhagh, S. First principles study of d0-d half-Heusler alloys containing group-IV, -V, and -VI sp atoms as prospective half-metals for real spintronic applications. Mat. Chem. Phys. 2021, 273, 125064. [Google Scholar] [CrossRef]
- Javari, A. R.; Davatolhagh, S; Dehghan, A. Half-metallic p0-d half-Heusler alloys with stable structure in ferromagnetic state. J. Phys. Chem. Solids 2022, 166, 110702. [Google Scholar] [CrossRef]
- Özdoğan, K.; Galanakis, I. Interplay between Structural, Electronic, and Magnetic Properties in the p0-d Semi-Heusler Compounds: The Case of Li-Based Compounds. Crystals 2024, 14, 693. [Google Scholar] [CrossRef]
- Koepernik, K.; Eschrig, H. Full-potential nonorthogonal local-orbital minimum-basis band-structure scheme. Phys. Rev. B 1999, 59, 1743. [Google Scholar] [CrossRef]
- Kopernik, K. Full Potential Local Orbital Minimum Basis Bandstructure Scheme User’s Manual (http: //www.fplo.de/download/doc.pdf).
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
| Compound | Lattice constant a in Å | Energy difference in eV | Most stable | Least stable | ||||
| -phase | -phase | -phase | phase | phase | ||||
| KScGa | 7.21 | 7.31 | 7.17 | -1.54 | -0.51 | 1.03 | ||
| KTiGa | 7.44 | 7.04 | 6.85 | -1.73 | -0.17 | 1.55 | ||
| KVGa | 7.47 | 7.14 | 7.07 | -1.47 | -0.20 | 1.27 | ||
| KCrGa | 7.58 | 7.24 | 7.17 | -1.07 | -0.07 | 1.00 | ||
| KMnGa | 7.44 | 7.12 | 6.96 | -1.23 | 0.003 | 1.23 | ||
| KFeGa | 7.39 | 6.95 | 6.80 | -1.23 | 0.01 | 1.25 | ||
| KCoGa | 7.29 | 6.85 | 6.60 | -1.42 | 0.34 | 1.77 | ||
| KNiGa | 7.12 | 6.75 | 6.63 | -1.34 | 0.44 | 1.78 | ||
| KCuGa | 7.40 | 7.02 | 6.95 | -0.86 | 0.29 | 1.15 | ||
| KZnGa | 7.64 | 7.26 | 7.23 | -0.82 | 0.11 | 0.93 | ||
| KScGe | 7.01 | 7.14 | 6.93 | -2.07 | -0.37 | 1.70 | ||
| KTiGe | 7.00 | 6.94 | 6.74 | -2.30 | -0.18 | 2.11 | ||
| KVGe | 7.12 | 6.92 | 6.66 | -2.08 | 0.0001 | 2.08 | ||
| KCrGe | 7.31 | 6.99 | 6.78 | -1.62 | 0.03 | 1.65 | ||
| KMnGe | 7.20 | 7.00 | 6.84 | -1.80 | -0.01 | 1.79 | ||
| KFeGe | 7.17 | 6.87 | 6.65 | -1.76 | 0.15 | 1.91 | ||
| KCoGe | 7.17 | 6.75 | 6.46 | -1.87 | 0.39 | 2.25 | ||
| KNiGe | 6.96 | 6.69 | 6.52 | -1.72 | 0.55 | 2.27 | ||
| KCuGe | 7.24 | 6.91 | 6.77 | -1.33 | 0.38 | 1.70 | ||
| KZnGe | 7.41 | 7.06 | 6.95 | -1.38 | 0.16 | 1.53 | ||
| KScAs | 7.03 | 6.98 | 6.88 | -2.39 | -0.71 | 1.68 | ||
| KTiAs | 6.98 | 6.92 | 6.71 | -2.48 | -0.30 | 2.19 | ||
| KVAs | 6.99 | 6.85 | 6.66 | -2.24 | -0.14 | 2.10 | ||
| KCrAs | 7.10 | 6.93 | 6.74 | -1.92 | -0.07 | 1.85 | ||
| KMnAs | 7.08 | 6.92 | 6.75 | -2.26 | -0.12 | 2.15 | ||
| KFeAs | 7.09 | 6.80 | 6.60 | -2.15 | 0.15 | 2.30 | ||
| KCoAs | 7.03 | 6.76 | 6.51 | -2.35 | 0.20 | 2.55 | ||
| KNiAs | 6.94 | 6.70 | 6.47 | -2.01 | 0.43 | 2.44 | ||
| KCuAs | 7.27 | 6.82 | 6.63 | -1.72 | 0.33 | 2.05 | ||
| KZnAs | 7.29 | 6.88 | 6.74 | -1.97 | 0.01 | 1.98 | ||
| KScSe | 7.29 | 6.99 | 7.01 | -2.00 | -0.90 | 1.10 | ||
| KTiSe | 7.21 | 6.97 | 6.84 | -2.01 | -0.62 | 1.39 | ||
| KVSe | 7.14 | 6.97 | 6.80 | -1.69 | -0.26 | 1.43 | ||
| KCrSe | 7.19 | 7.03 | 6.86 | -1.65 | -0.20 | 1.45 | ||
| KMnSe | 7.32 | 7.03 | 6.76 | -1.41 | -0.22 | 1.19 | ||
| KFeSe | 7.11 | 6.87 | 6.57 | -2.07 | 0.07 | 2.14 | ||
| KCoSe | 7.07 | 6.76 | 6.50 | -1.72 | 0.10 | 1.82 | ||
| KNiSe | 7.02 | 6.75 | 6.51 | -1.77 | 0.25 | 2.03 | ||
| KCuSe | 7.12 | 6.84 | 6.61 | -1.77 | 0.16 | 1.93 | ||
| KZnSe | 7.36 | 7.25 | 7.09 | -1.05 | -0.19 | 0.85 | ||
| Comp. | phase | Comp. | phase | ||||||||||||
| KScGa | Metal | 7 | -1 | KScGe | Gapless semiconductor | 8 | 0 | ||||||||
| 0.031 | -0.436 | -0.523 | -0.928 | 7 | -1 | Semiconductor | 8 | 0 | |||||||
| 0.013 | -0.537 | -0.448 | -0.972 | 7 | -1 | Semiconductor | 8 | 0 | |||||||
| KTiGa | 0.136 | 2.667 | 0.106 | 2.909 | 8 | 0 | KTiGe | 0.022 | 1.805 | -0.827 | 1.000 | 9 | 1 | ||
| 0.010 | 0.789 | -0.096 | 0.703 | 8 | 0 | 0.108 | 1.374 | -0.482 | 1.000 | 9 | 1 | ||||
| Gapless semiconductor | 8 | 0 | 0.032 | 1.339 | -0.371 | 1.000 | 9 | 1 | |||||||
| KVGa | 0.168 | 4.146 | 0.301 | 4.615 | 9 | 1 | KVGe | 0.062 | 3.635 | -0.797 | 2.900 | 10 | 2 | ||
| 0.058 | 3.764 | -0.448 | 4.270 | 9 | 1 | 0.091 | 2.937 | -1.025 | 2.003 | 10 | 2 | ||||
| 0.041 | 3.855 | 0.198 | 4.094 | 9 | 1 | 0.023 | 2.828 | -0.851 | 2.000 | 10 | 2 | ||||
| KCrGa | 0.052 | 5.089 | -0.261 | 4.880 | 10 | 2 | KCrGe | 0.007 | 4.991 | -1.754 | 3.244 | 11 | 3 | ||
| -0.011 | 4.814 | -0.052 | 4.751 | 10 | 2 | 0.110 | 4.438 | -1.548 | 3.000 | 11 | 3 | ||||
| -0.011 | 4.851 | -0.225 | 4.615 | 10 | 2 | 0.034 | 4.280 | -1.314 | 3.000 | 11 | 3 | ||||
| KMnGa | -0.086 | 4.624 | -0.875 | 3.663 | 11 | 3 | KMnGe | 0.010 | 4.870 | -0.912 | 3.968 | 12 | 4 | ||
| -0.078 | 4.492 | -1.113 | 3.301 | 11 | 3 | 0.120 | 4.756 | -0.876 | 4.000 | 12 | 4 | ||||
| -0.119 | 4.301 | -1.092 | 3.090 | 11 | 3 | 0.080 | 4.574 | -0.654 | 4.000 | 12 | 4 | ||||
| KFeGa | -0.029 | 3.383 | -0.399 | 2.955 | 12 | 4 | KFeGe | -0.005 | 3.526 | -0.481 | 3.040 | 13 | 5 | ||
| -0.023 | 3.311 | -0.519 | 2.769 | 12 | 4 | 0.059 | 3.573 | -0.145 | 3.487 | 13 | 5 | ||||
| -0.037 | 3.115 | -0.566 | 2.512 | 12 | 4 | 0.034 | 3.072 | -0.593 | 2.513 | 13 | 5 | ||||
| KCoGa | -0.044 | 2.022 | -0.281 | 1.697 | 13 | 5 | KCoGe | -0.051 | 2.112 | -1.484 | 0.577 | 14 | 6 | ||
| -0.060 | 1.765 | -0.705 | 1.000 | 13 | 5 | 0.043 | 2.012 | -0.643 | 1.412 | 14 | 6 | ||||
| -0.001 | 1.388 | -0.387 | 1.000 | 13 | 5 | 0.023 | 1.189 | -0.337 | 0.875 | 14 | 6 | ||||
| KNiGa | -0.020 | 0.315 | -0.159 | 0.136 | 14 | 6 | KNiGe | 0.024 | -0.460 | 0.593 | 0.157 | 15 | 7 | ||
| Metal | 14 | 6 | Metal | 15 | 7 | ||||||||||
| Metal | 14 | 6 | Metal | 15 | 7 | ||||||||||
| KCuGa | Metal | 15 | -3 | KCuGe | -0.029 | 0.036 | -1.112 | -1.105 | 16 | -2 | |||||
| Metal | 15 | -3 | Metal | 16 | -2 | ||||||||||
| Metal | 15 | -3 | Metal | 16 | -2 | ||||||||||
| KZnGa | Metal | 16 | -2 | KZnGe | Metal | 17 | -1 | ||||||||
| Metal | 16 | -2 | 0.016 | -0.062 | -0.695 | -0.741 | 17 | -1 | |||||||
| Metal | 16 | -2 | -0.002 | -0.015 | -0.096 | -0.103 | 17 | -1 | |||||||
| Comp. | phase | Comp. | phase | ||||||||||||
| KScAs | 0.050 | 1.146 | -0.199 | 0.997 | 9 | 1 | KScSe | 0.090 | 1.920 | -0.118 | 1.892 | 10 | 2 | ||
| Metal | 9 | 1 | 0.072 | 0.674 | -0.054 | 0.692 | 10 | 2 | |||||||
| 0.072 | 1.030 | -0.133 | 0.969 | 9 | 1 | 0.259 | 1.793 | -0.118 | 1.934 | 10 | 2 | ||||
| KTiAs | 0.044 | 2.371 | -0.415 | 2.000 | 10 | 2 | KTiSe | 0.096 | 3.062 | -0.174 | 2.984 | 11 | 3 | ||
| 0.140 | 2.193 | -0.333 | 2.000 | 10 | 2 | 0.294 | 2.889 | -0.230 | 2.953 | 11 | 3 | ||||
| 0.042 | 2.248 | -0.290 | 2.000 | 10 | 2 | 0.263 | 2.915 | -0.193 | 2.985 | 11 | 3 | ||||
| KVAs | 0.039 | 3.659 | -0.698 | 3.000 | 11 | 3 | KVSe | 0.090 | 4.167 | -0.698 | 4.000 | 12 | 4 | ||
| 0.150 | 3.431 | -0.581 | 3.000 | 11 | 3 | 0.252 | 4.014 | -0.581 | 3.986 | 12 | 4 | ||||
| 0.092 | 3.461 | -0.553 | 3.000 | 11 | 3 | 0.236 | 4.018 | -0.553 | 4.000 | 12 | 4 | ||||
| KCrAs | 0.028 | 4.988 | -1.016 | 4.000 | 12 | 4 | KCrse | 0.042 | 5.200 | -1.016 | 5.000 | 13 | 5 | ||
| 0.104 | 4.652 | -0.756 | 4.000 | 12 | 4 | 0.132 | 5.097 | -0.756 | 4.997 | 13 | 5 | ||||
| 0.071 | 4.582 | -0.653 | 4.000 | 12 | 4 | 0.137 | 5.060 | -0.653 | 5.000 | 13 | 5 | ||||
| KMnAs | 0.057 | 4.963 | -0.020 | 5.000 | 13 | 5 | KMnSe | 0.039 | 5.306 | -0.020 | 5.572 | 14 | 6 | ||
| 0.094 | 4.911 | -0.005 | 5.000 | 13 | 5 | -0.034 | 4.290 | -0.005 | 3.940 | 14 | 6 | ||||
| 0.123 | 4.801 | 0.064 | 4.988 | 13 | 5 | 0.089 | 4.054 | 0.035 | 3.717 | 14 | 6 | ||||
| KFeAs | 0.018 | 3.641 | 0.477 | 4.136 | 14 | 6 | KFeSe | 0.004 | 3.678 | -0.048 | 3.717 | 15 | 7 | ||
| 0.032 | 3.699 | 0.269 | 4.000 | 14 | 6 | 0.006 | 3.154 | -0.011 | 3.112 | 15 | 7 | ||||
| 0.070 | 3.486 | 0.151 | 3.707 | 14 | 6 | 0.033 | 2.985 | 0.151 | 3.007 | 15 | 7 | ||||
| KCoAs | -0.007 | 2.189 | -0.712 | 3.717 | 15 | 7 | KCoSe | -0.004 | 2.167 | -0.004 | 2.158 | 16 | 8 | ||
| 0.055 | 2.519 | 0.412 | 3.112 | 15 | 7 | -0.049 | 2.046 | 0.075 | 2.072 | 16 | 8 | ||||
| 0.078 | 2.252 | 0.371 | 3.007 | 15 | 7 | 0.005 | 1.893 | 0.106 | 2.004 | 16 | 8 | ||||
| KNiAs | 0.001 | 0.840 | 0.188 | 1.029 | 16 | 8 | KNiSe | -0.010 | 0.947 | 0.170 | 1.107 | 17 | 9 | ||
| 0.022 | 1.017 | 0.528 | 1.567 | 16 | 8 | -0.014 | 0.900 | 0.122 | 1.008 | 17 | 9 | ||||
| 0.029 | 0.645 | 0.382 | 1.056 | 16 | 8 | 0.006 | 0.827 | 0.167 | 1.000 | 17 | 9 | ||||
| KCuAs | -0.004 | 0.060 | -1.132 | -1.076 | 17 | -1 | KCuSe | Gapless semiconductor | 18 | 0 | |||||
| -0.001 | -0.081 | -0.423 | 0.505 | 17 | -1 | Semiconductor | 18 | 0 | |||||||
| Metal | 17 | -1 | Semiconductor | 18 | 0 | ||||||||||
| KZnAs | 0.001 | -0.020 | 0.324 | 0.305 | 18 | 0 | KZnSe | Metal | 19 | 1 | |||||
| Semiconductor | 18 | 0 | Metal | 19 | 1 | ||||||||||
| Semiconductor | 18 | 0 | Metal | 19 | 1 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





