1. Introduction
The advancements in technology have led to a growing demand for energy across various aspects of human life, including the power sector, transportation, construction, and chemical industries. These energy needs are primarily met by oil and coal fossil fuels. However, relying on fossil fuels for energy production raises two significant concerns: their continuous depletion due to slow regeneration, and their environmental impact, including greenhouse gas (GHG) emissions. These emissions conflict with the goal of achieving net-zero carbon dioxide (CO
2) emissions, as outlined in the Paris Agreement (2015). The agreement aims to limit global temperature increases to 1.5 °C above pre-industrial levels, by 2040, by monitoring GHG emissions. To achieve this, CO
2 emissions must be reduced by 45 % from 2010 levels by 2030, ultimately reaching net-zero emissions by 2050. Renewable energy stands out as a promising solution for decarbonizing various sectors. Currently, a significant disparity between the supply and demand for clean renewable energy exists, while carbon-intensive fossil fuels continue to fill this gap, also contribute to increased GHG emissions [
1,
2]. Although nuclear energy from fission reactions is considered clean, it comes with safety concerns and high capital investment requirements [
3]. As a result, there is a growing emphasis on renewable energy sources such as solar, wind, hydro, geothermal, and biomass. These resources shall be harnessed and converted into other forms of energy through environmentally friendly processes.
Another approach to reducing carbon footprints involves Carbon Capture and Utilization (CCU). CCU presents an attractive pathway of chemically converting captured CO
2 into valuable products such as fuels, [including hydrogen (H
2), synthetic natural gas, gasoline, diesel oil, and heating oil], chemicals, plastics, and building materials. Experts believe that CCU will significantly contribute to reducing CO
2 emissions [
1,
2]. Although CO
2 is an abundant, non-toxic, and renewable chemical feedstock, its thermodynamic stability requires high external energy input, reactive co-reactants, and selective catalysts to fulfil high conversion and selectivity goals. Currently, CO
2 utilization is limited to specific industrial processes, such as urea, salicylic acid, and polycarbonate synthesis. However, the untapped potential lies in transforming CO
2 into various valuable chemicals and fuels, through the Power-to-X (PtX) technologies [
1,
2,
4,
5,
6].
The term PtX encompasses a range of technologies that leverage surplus (sustainable) electricity to create valuable products. The ‘X’ in PtX can represent various forms, including gas, liquid, heat, or chemicals, depending on the desired output. Note that, when there is excess electricity generated from sources like solar panels or wind turbines, PtX technologies come into play and available technologies can transform this surplus energy into products that can be stored or utilized later. One notable application of PtX is the production of synthetic fuels, commonly referred to as ‘e-fuels.’ These e-fuels are generated using electricity from renewable sources to convert CO
2 and water into hydrocarbons or other fuel molecules. E-fuels offer a potential solution for reducing greenhouse gas emissions in industries where direct electrification or conventional biofuels face challenges [
4,
5,
6]. PtX can be categorized into several types: (i) Power-to-Gas (PtG): this technology converts excess electricity into gases like hydrogen (H
2) or methane (CH
4). These e-fuel gases can be stored and later used to generate electricity or even power vehicles or used as industry’s renewable fuels [
7]; (ii) Power-to-Liquid (PtL): in this case, surplus electricity is used to produce valuable liquid e-fuels, such as methanol (MeOH), synthetic gasoline or diesel. These fuels can be utilized in existing engines or machinery [
8]; (iii) Power-to-Heat (PtH): here, surplus electricity is transformed into heat, which can be stored and used for purposes like heating buildings or generating steam for industrial processes [
6]; and (iv) Power-to-Chemicals (PtC): this involves using electrical energy to facilitate chemical reactions, resulting in valuable products like fertilizers or other industrial chemicals [
6].
By harnessing PtX technologies and e-fuels, the utilization of renewable energy sources is maximized, and waste is minimized. However, challenges remain, including energy efficiency considerations, higher costs compared to conventional fuels, catalysts performance and deactivation, and the need for a consistent and abundant supply of renewable energy. The successful integration of PtX into energy systems relies heavily on technical demonstrations and seamless system integration. As of June 2020, Europe has seen a total of 220 PtX research and demonstration projects, some realized, completed, or currently in planning stages [
9,
10,
11]. Over time, the focus of PtX applications has evolved. Initially focussed on fuel production (e.g., H
2 buses, combined heat and power generation, and natural gas grid injection), it now extends to industrial uses. While synthetic gaseous fuels dominate fuel production, liquid fuel production remains underrepresented.
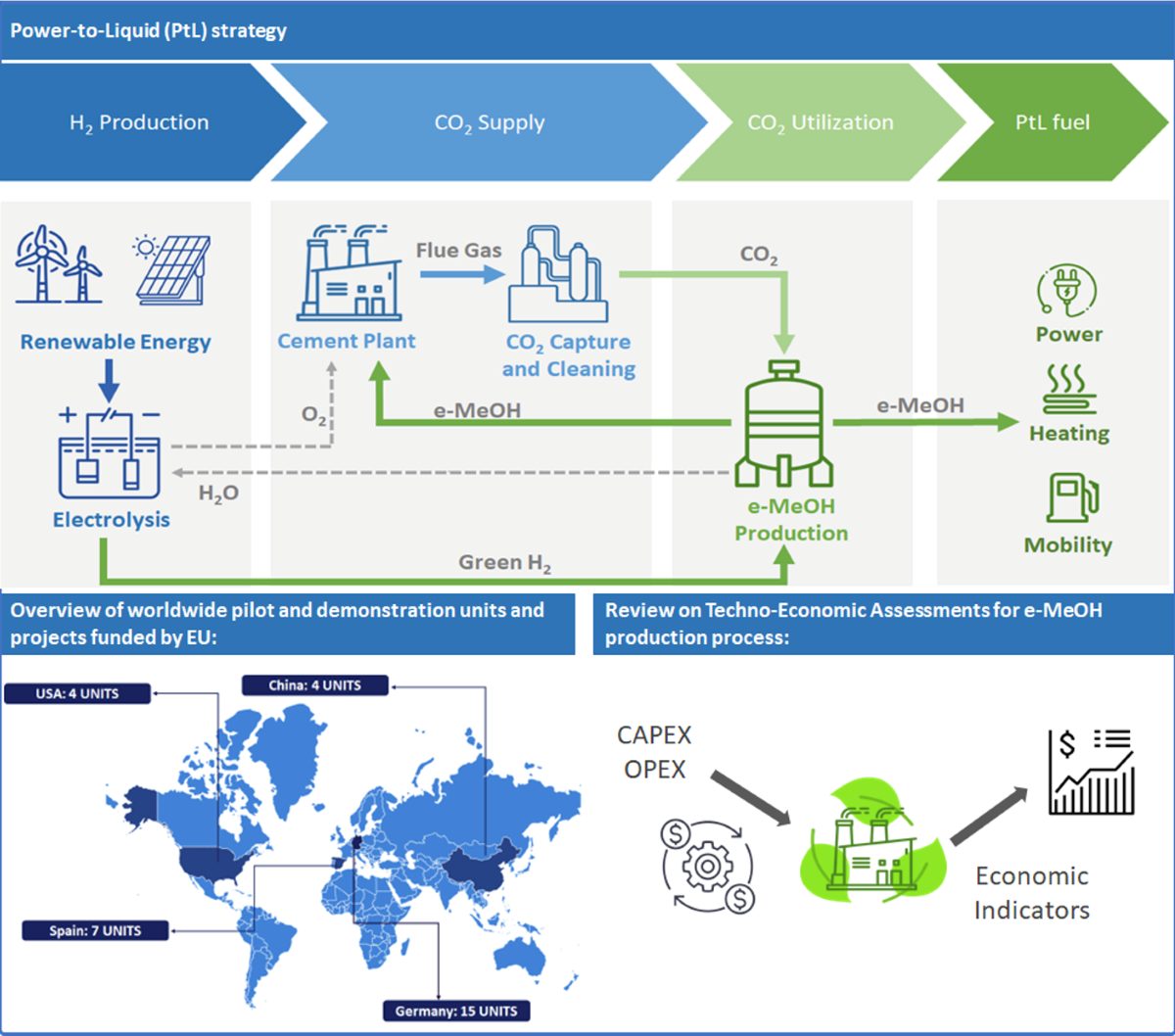
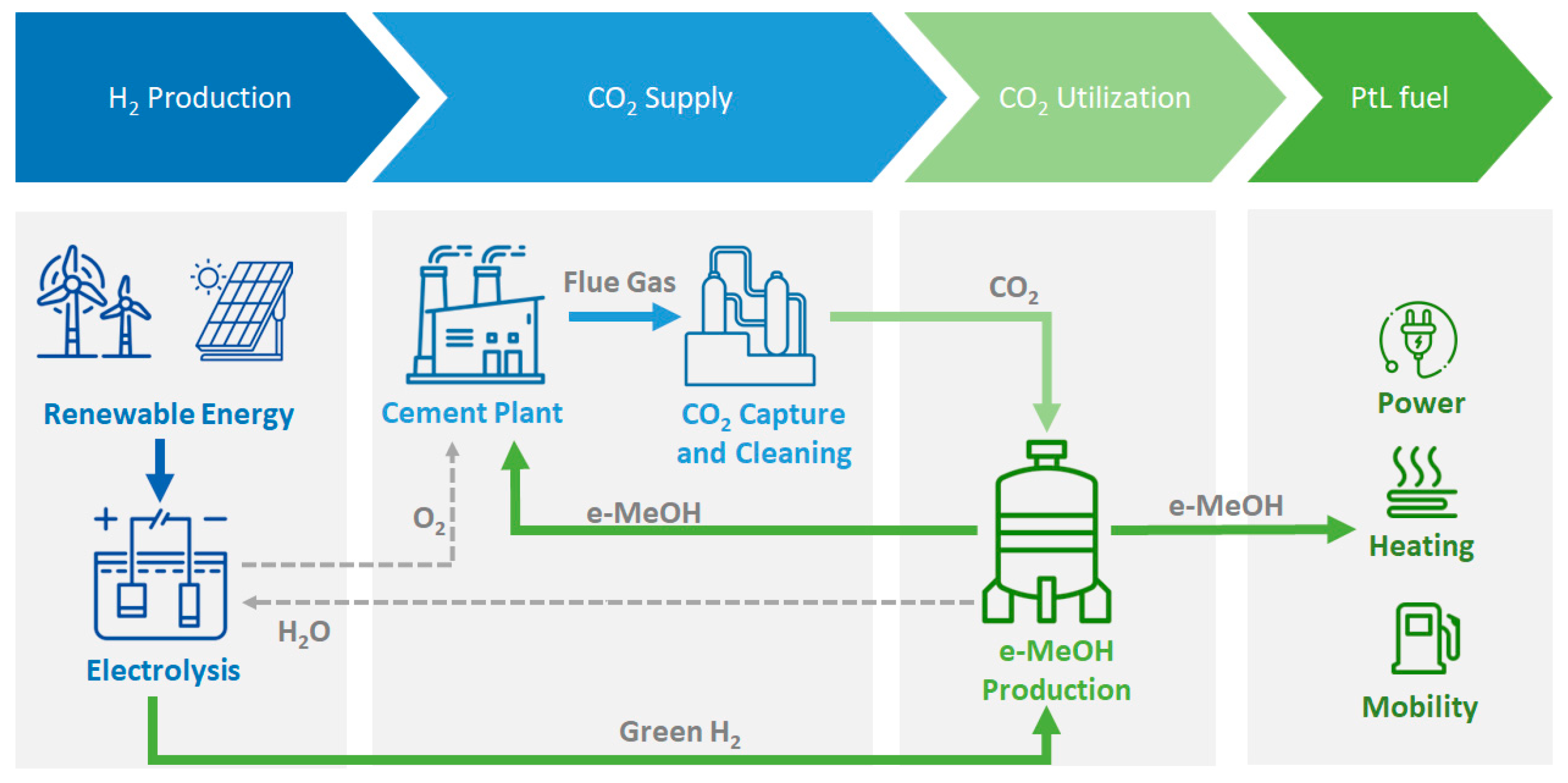
In the actual context of the decarbonisation of the industry (particularly in the cement industry), one of the promising approaches involves converting CO₂ into methanol (MeOH) through the power-to-liquid technologies (PtL) (see
Figure 1). Even with all the efforts to decarbonise the energy intensive industries, some chemical processes (such as the reaction to produce clinker) have inherent production of CO
2, that will subsist as long as the material is produced.
The CO
2 captured from cement industry flue gas reacts with hydrogen (H
2) generated from renewable sources through electrolysis of water (Green Hydrogen), and it is catalytically transformed into renewable methanol (e-MeOH), offering a sustainable solution to the geogenic/process GHG emission [
8]. Presently, around 65 % of the world’s MeOH is produced from natural gas. The remaining 35 % is mainly produced from coal, with only 0.2 % coming from biomass or green H
2 and captured CO
2.
PtL also addresses the intermittent challenge of renewable energy. By storing surplus renewable energy (whether in the form of electricity or heat) in liquid carriers, PtL provides a solution to the variability of renewable energy sources. Many reviews have been published so far in reference to the development of CO2 conversion in e-MeOH. However, none of them is focused on pilot and demonstration units in the world with special focus on cement context. Therefore, the main objective of the first section of the article is to compile research projects related to pilot and demonstration plants producing e-MeOH following PtL pathways. Additionally, this section will specifically highlight projects funded by EU and practical applications within the cement sector.
For the decision makers, even with all the technology in place, the decision is based on economic indicators provided by techno-economic assessments (TEA). The second section provides an overview of the studies existent related to e-MeOH production processes.
2. Pilot and Demonstration Units of e-MeOH (from Captured CO2 and Green H2)
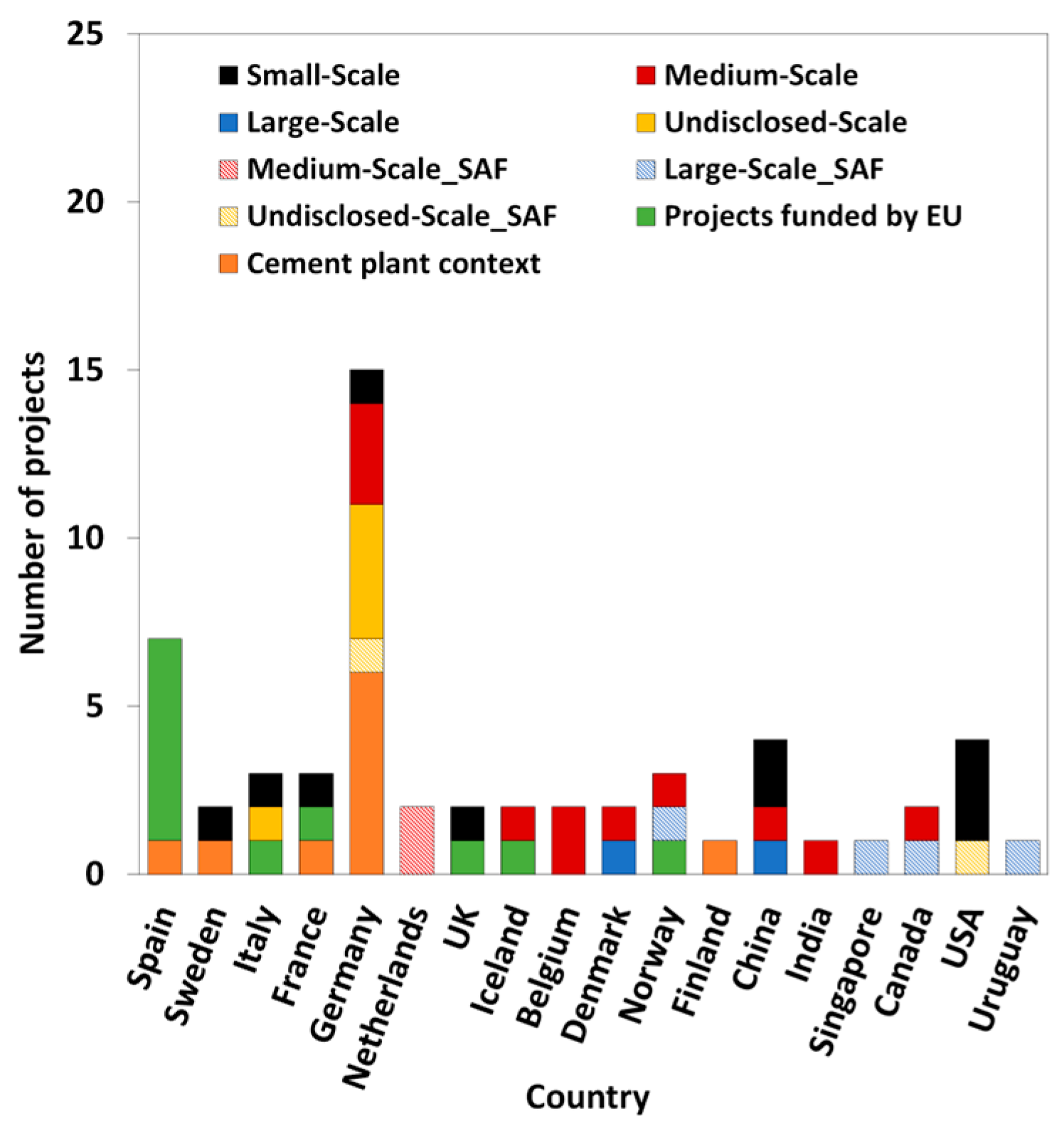
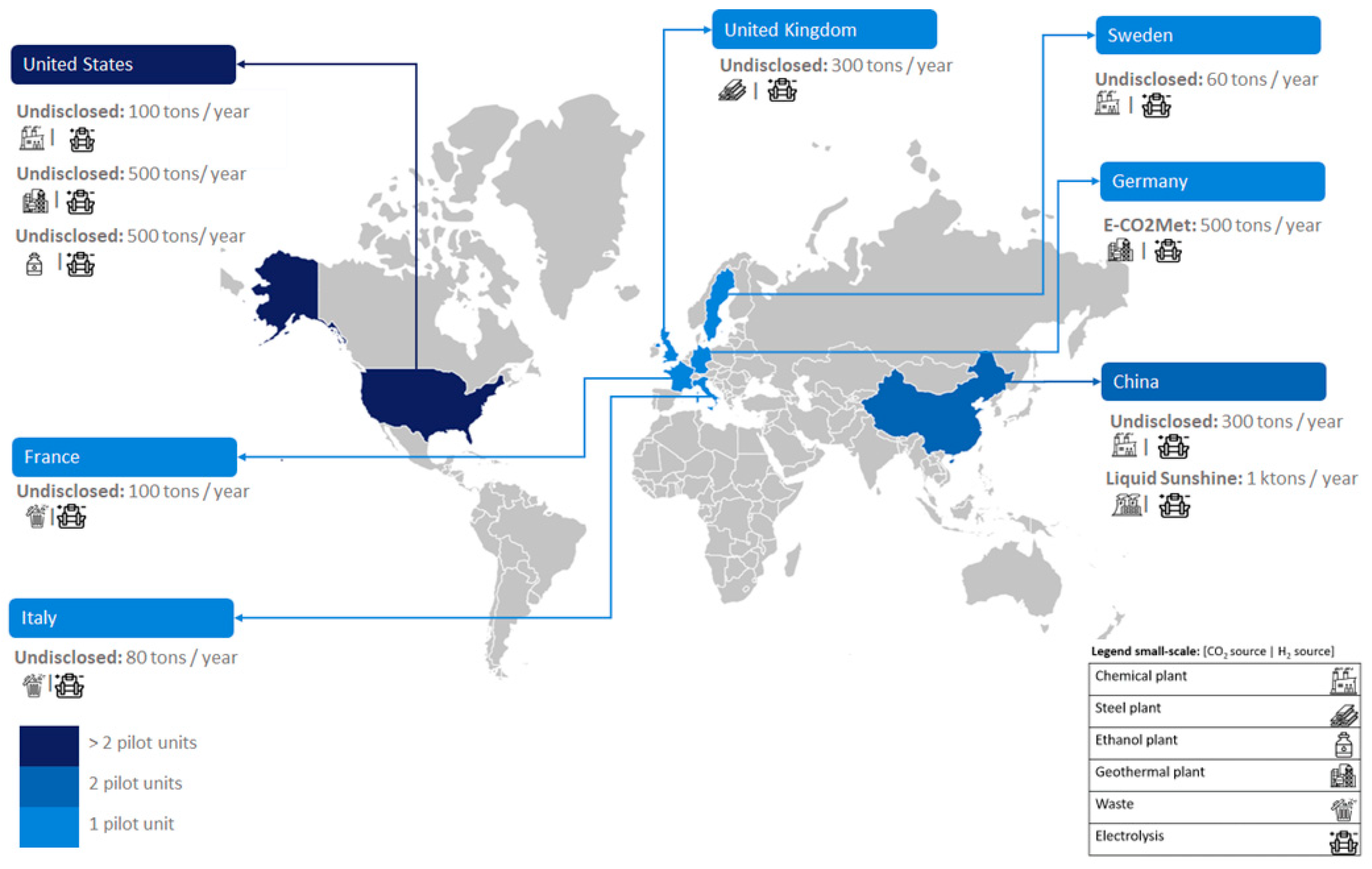
There are presently ten small-scale (≤ 1 kton/year) e-MeOH manufacturing plants, active or under construction, in the world; all of these are pilot-scale facilities.
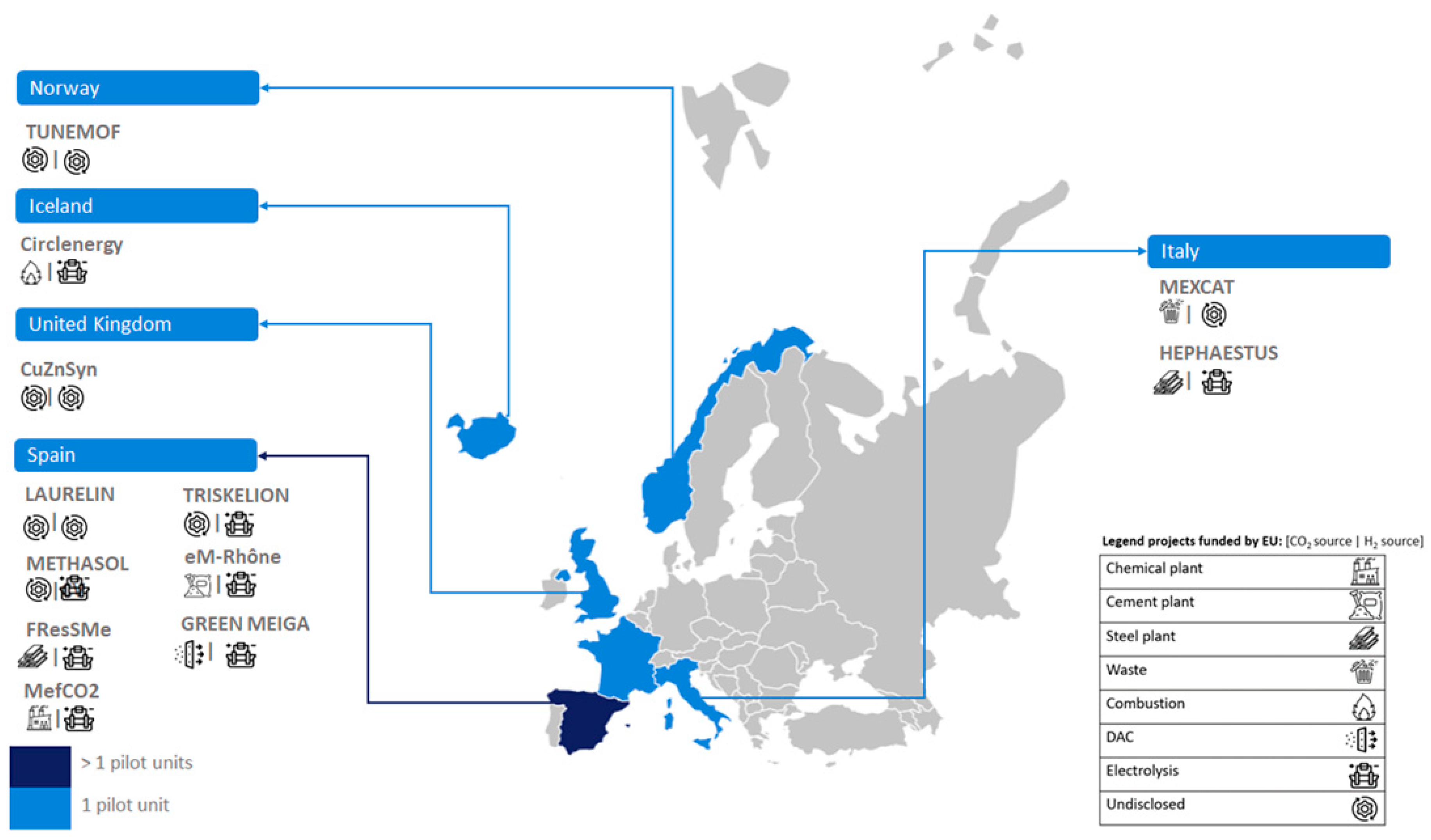
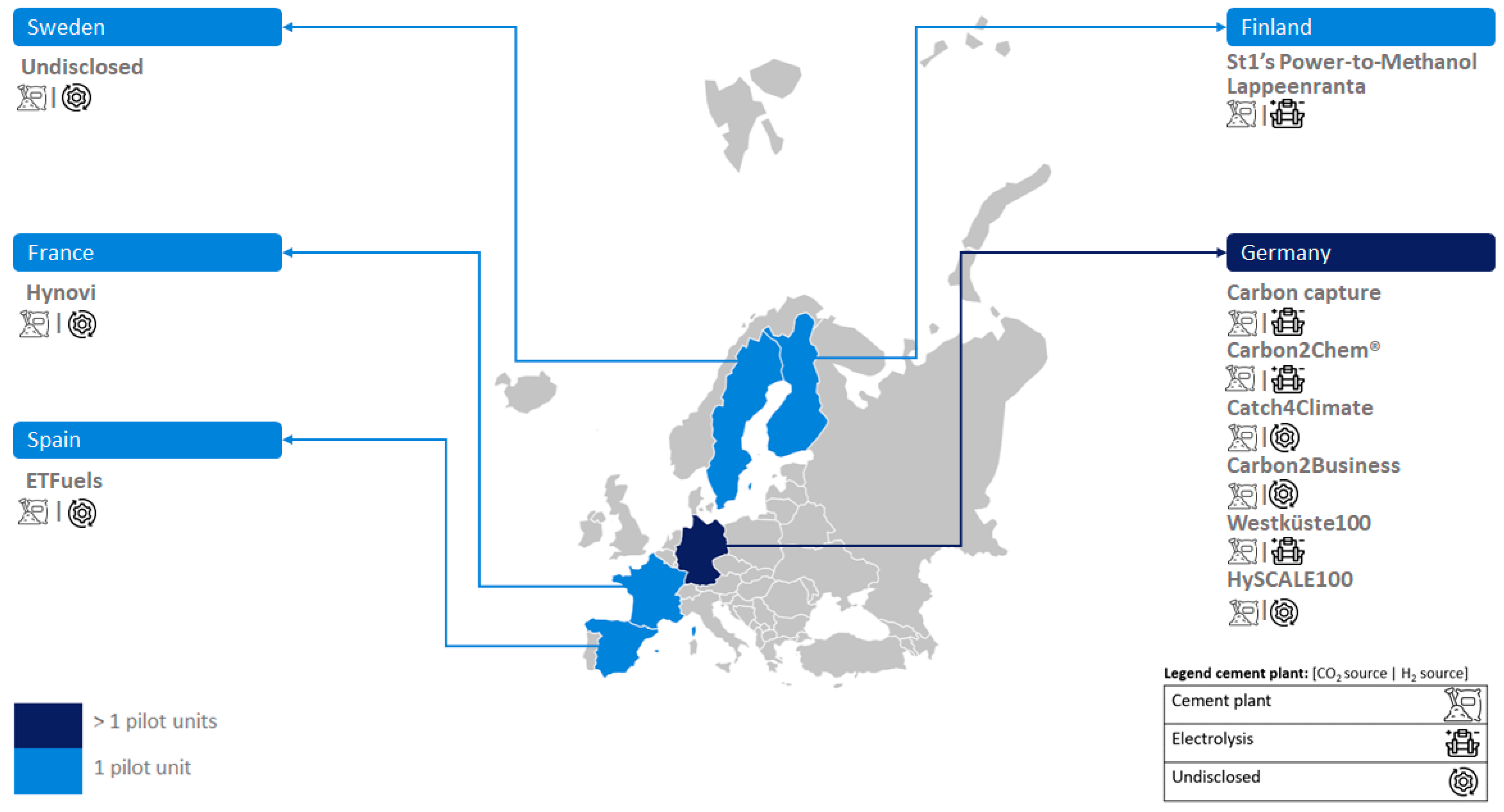
Figure 2 illustrates the development of small-scale PtL units in various European countries, including Sweden, Italy, France, Germany, and the UK. In this case, each country has only one pilot-scale facility. Also, small-scale PtL units are being developed in Asia (China, with two units) and America (USA, with three units).
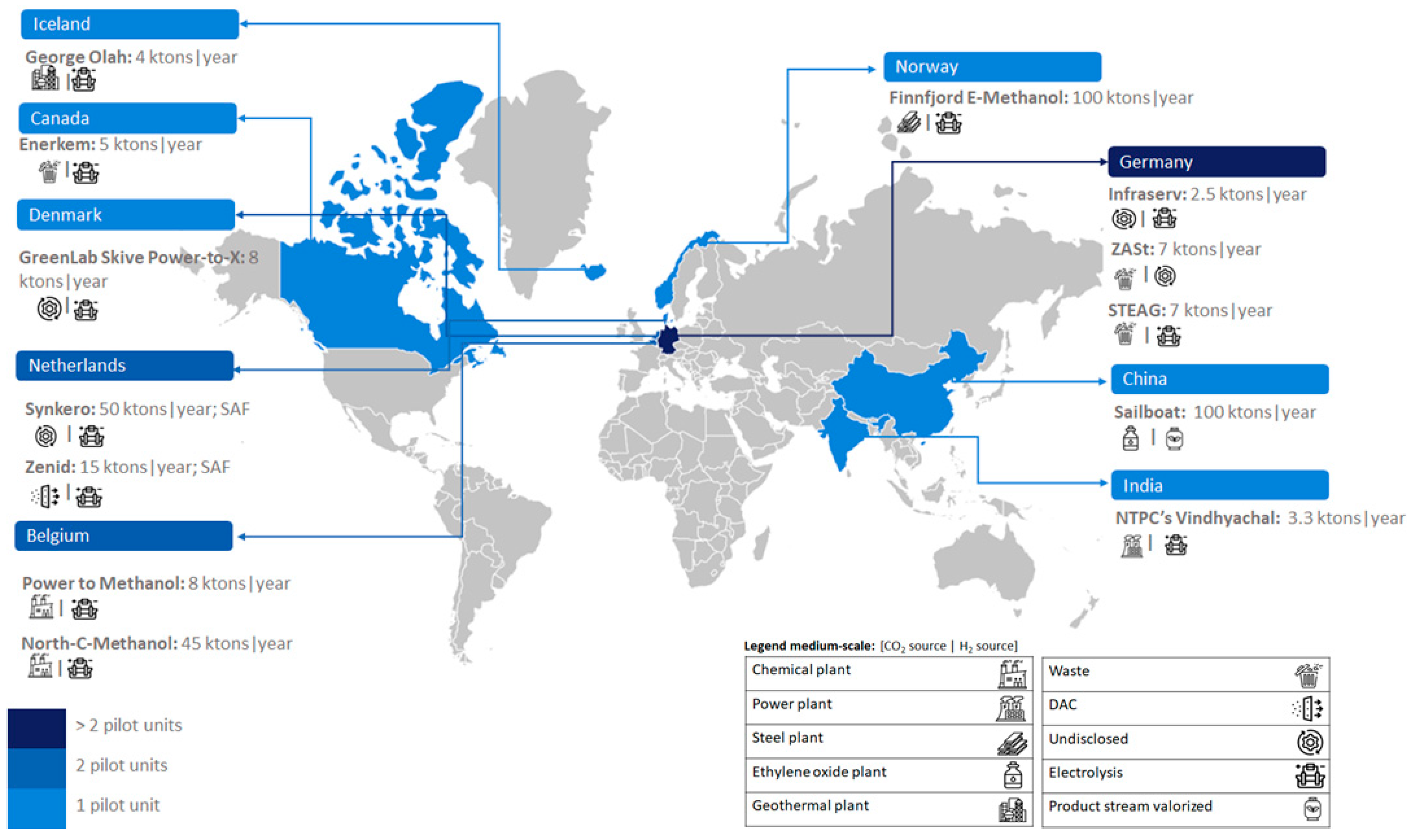
The number of e-MeOH medium-scale (1–100 kton/year) production facilities is similar (eleven units). However, the number of e-MeOH larger-scale (> 100 kton/year) production installations is globally low (only two units).
Figure 2 shows that medium-scale units are more numerous in Europe. The location is at different countries such as Germany (three units), Belgium (two units), Iceland, Denmark and Norway (with one unit each). PtL medium-scale units are also under development in Asia (China and India, with one unit each) and America (Canada, with one unit).
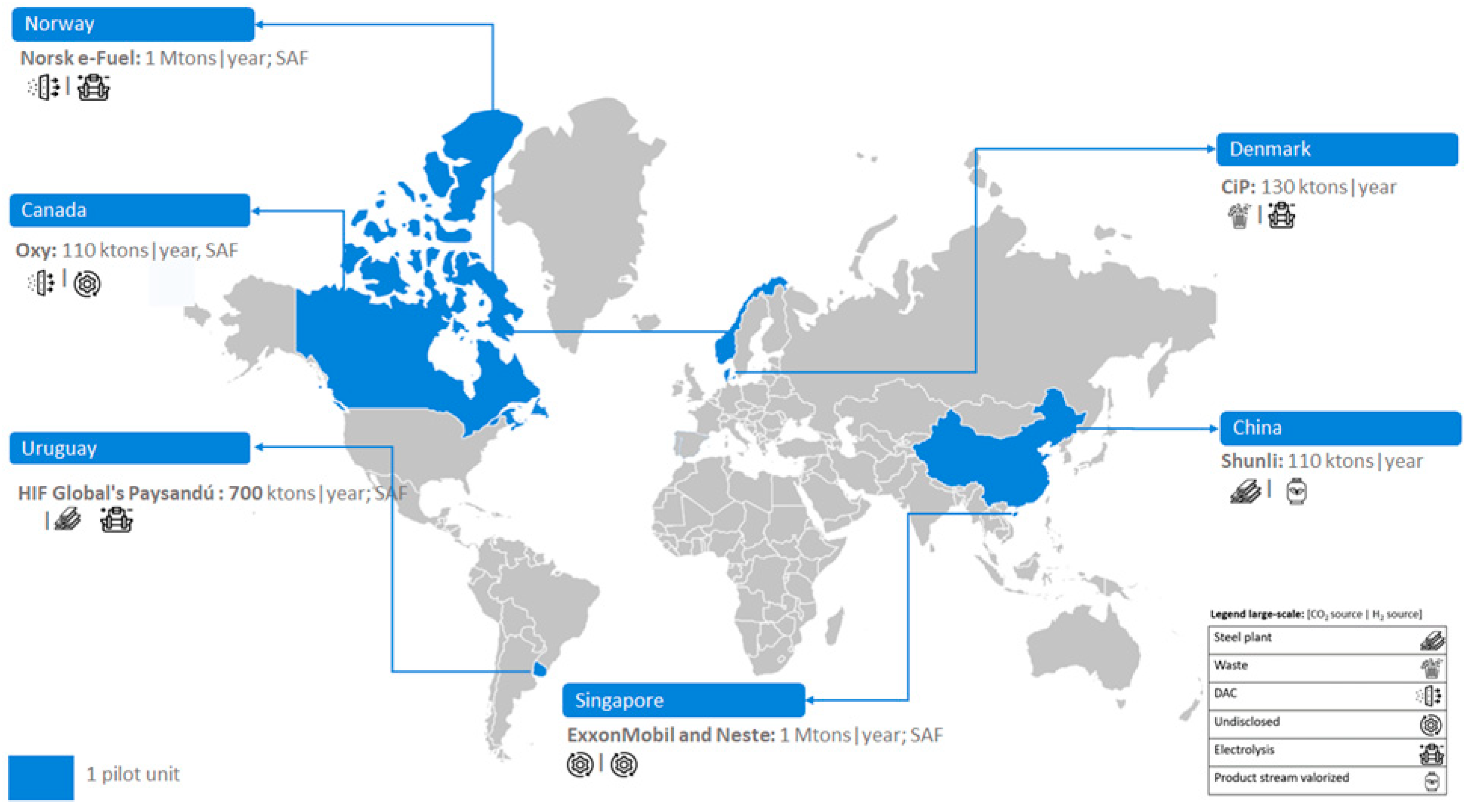
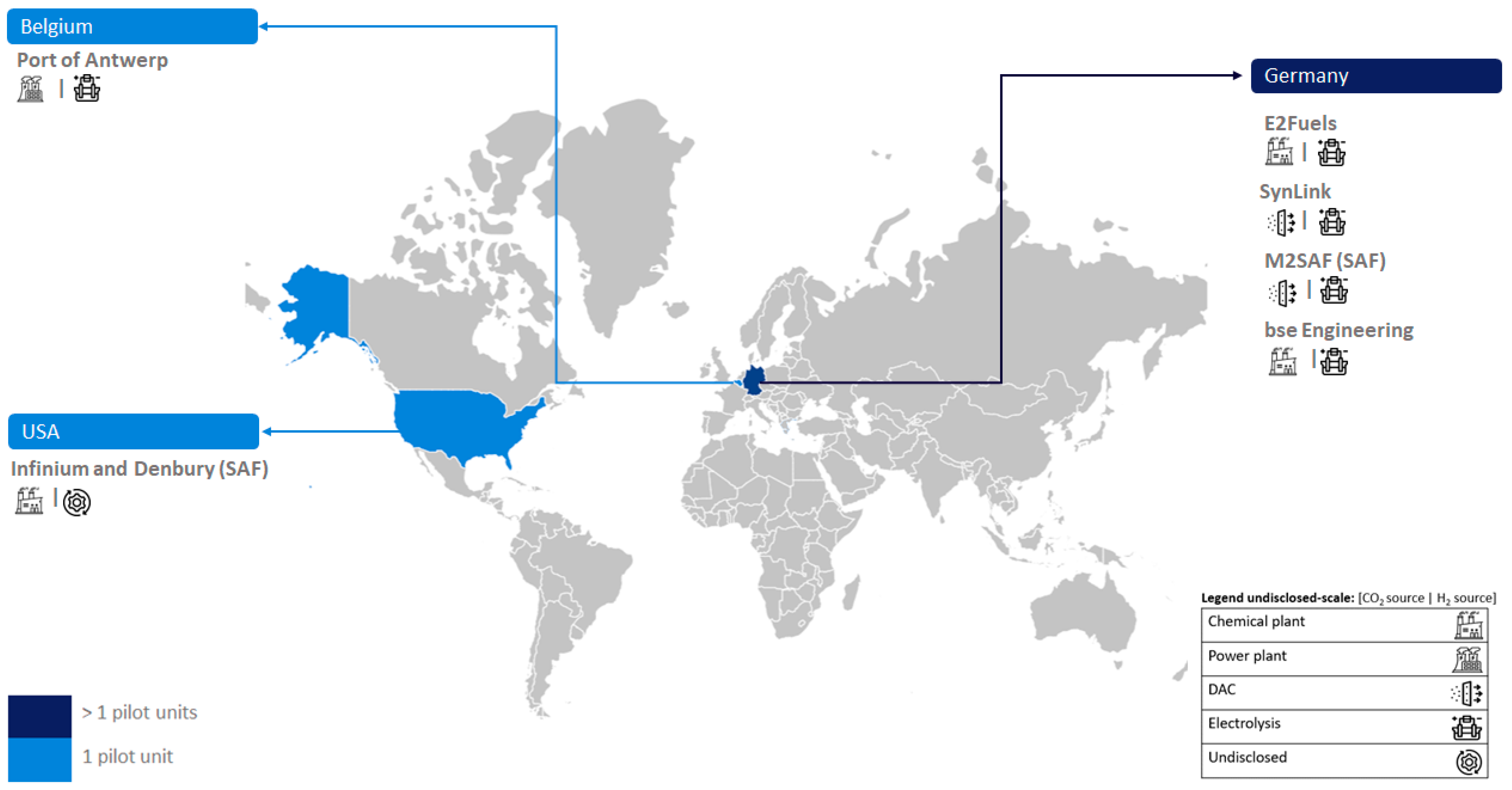
Figure 2 reveals that large-scale PtL units have been established in Europe (specifically Denmark) and Asia (China), each with one unit. However, undisclosed-scale e-MeOH production installations (four units) are exclusively developed in Europe (Germany).
The importance of e-MeOH as the basis for the synthesis of several chemicals is unquestionable [
12]. It constitutes one of the most relevant routes for the production of Sustainable Aviation Fuels (SAFs) due to the inherent difficulties in decarbonize the air transportation. To meet future demand, and at the same time reach net-zero emissions, SAFs need to incorporate more abundant feedstocks such as municipal solid waste, forestry and agricultural residues, biogenic CO
2 and even atmospheric CO
2 [
13]. In the cement plant context, the SAFs production would make use of CO
2 captured from cement industry flue gas with H
2 generated from renewable sources. Additional advantages are that e-MeOH-derived SAF can be blended with existing jet fuels without requiring significant modifications to aircraft engines or infrastructure; it can be produced globally in areas with abundant renewable energy sources; and it allows an efficient distribution using existing infrastructures. PtL technology shows promise for the long term, but challenges related to costs, technology readiness, and feedstock availability need to be addressed for widespread adoption in SAF production. Currently, eight demonstration units of SAFs have been identified on medium-scale (two units), large-scale (four units) and undisclosed-scale (two units). From
Figure 2 also can be seen that the production of SAF with PtL technology is distributed across different regions. In Europe, there are PtL units in the Netherlands (two units), Germany (one unit), and Norway (one unit). Additionally, Asia has a PtL unit in Singapore, South America (Uruguay) has a PtL unit, while North America (USA and Canada) each has one PtL unit.
In the Cordis European research database, eleven research projects (based on CO
2 conversion) were identified between 2011-2028 (four projects are finished and eight are ongoing). As can be observed in
Figure 2, Spain leads in the development of PtL projects funded by the European Union, with a total of six projects.
In the context of cement production, ten PtL demonstration units have been identified, distributed across different scales: three at medium scale, two at large scale, and five at an undisclosed scale.
Figure 2 reveals that Germany leads in the development of PtL units, with a total of six projects. Other countries, such as Finland, Sweden, France, and Spain, have only one identified unit each. Notably, all PtL demonstration units in cement plant contexts are located exclusively in European countries.
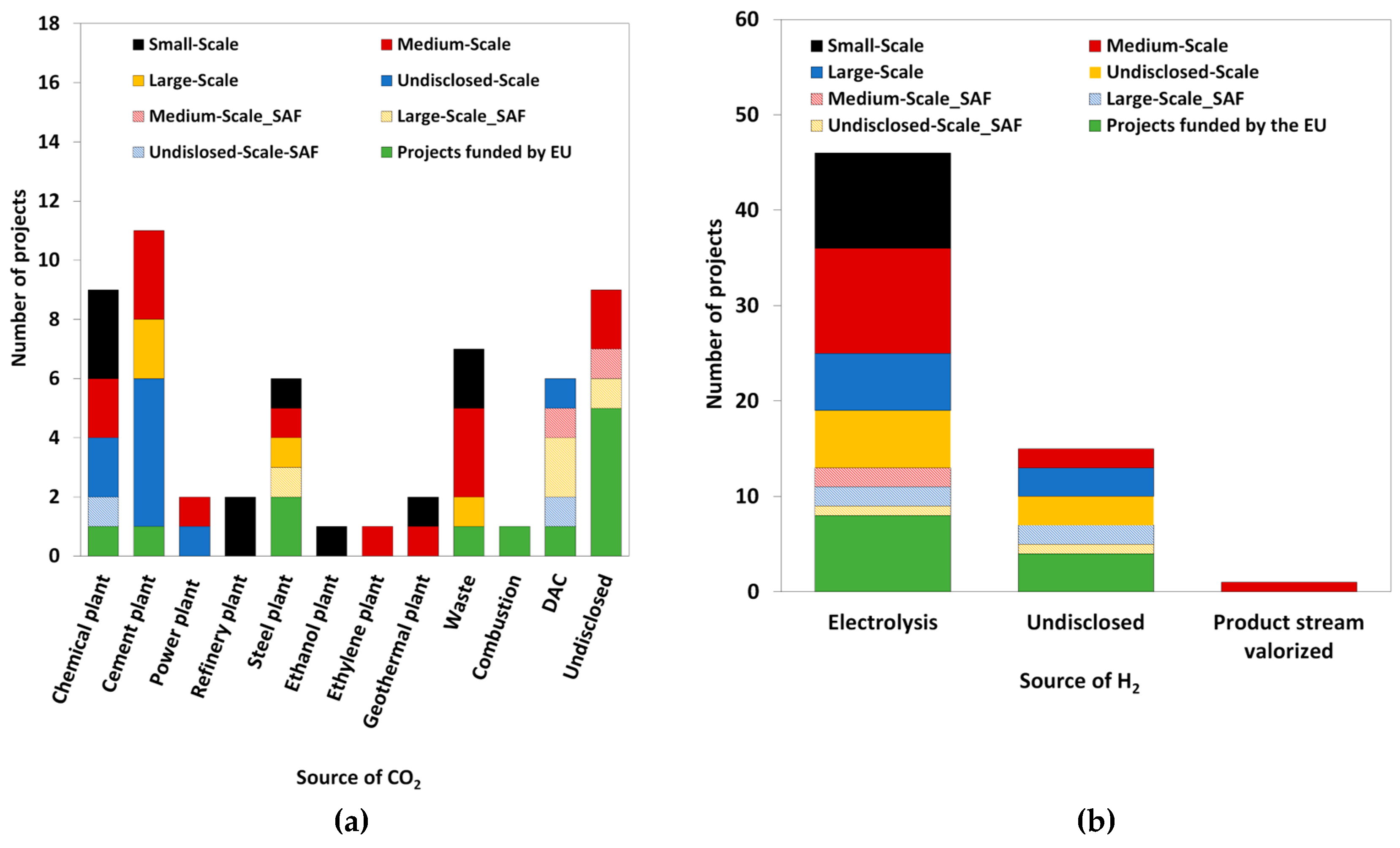
All the mentioned projects involve capturing the necessary CO
2 and producing green H
2. However, the specific sources for each of these components vary across different units and projects [see
Figure 3 (a)]. Projects in which the CO
2 sources are used to produce the so-called bio-methanol, such as the CO
2 originated from processes involving biomass, are not contemplated. Some projects emphasize non-fossil carbon sources, including two that generate CO
2 from geothermal facilities—a specific option for China and Iceland. CO
2 emissions originated from waste incineration are contemplated, being identified six units and one project funded by EU that include such CO
2 source. Several units obtain CO
2 from nearby industrial sites, such as industrial flue gas of various chemical plant, cement plant, power plant, refinery plant, steel plant, ethanol plant and ethylene oxide plant. Direct Air Capture (DAC) allows an industry-independent CO
2 source, with one undisclosed-scale unit and four SAF projects utilizing it. EU-funded projects obtain CO
2 from industrial flue gas (three projects), waste (one project), combustion processes (one project), and DAC (one project). Undisclosed CO
2 sources were identified in medium-scale units (two units) and EU-funded projects (five projects).
The sources for green H
2 [see
Figure 3 (b)] also vary. Numerous units generate green H
2 through electrolysis of water from renewable sources: small-scale (ten units), medium-scale (eleven units), large-scale (one unit), undisclosed-scale (six units) and EU-funded projects (seven units). An undisclosed green H
2 source was identified in medium-scale, large-scale units (two units for each), undisclosed-scale (three units) and EU-funded projects (four projects). A minority of two projects obtain green H
2 from valorised product stream (medium-scale and large-scale, with one unit each). Regarding SAF production, medium-scale (two units), large-scale (one unit), and undisclosed-scale (one unit) rely on green H
2 from renewable electrolysis. However, two large-scale units and one undisclosed-scale unit use undisclosed green H
2 sources.
2.1. Small-Scale Units
Ten small-scale facilities, including the E-CO2Met project in Germany and Liquid Sunshine in China, are producing e-Methanol. Additionally, there are undisclosed projects in Sweden, Italy, France, USA, China, and UK.
Figure 4 indicates the installed capacities in the European, Asian, and American countries.
2.1.1. E-CO2Met
Coordinated by TotalEnergies, the E-CO2Met (“Electricity & CO2 to Methanol”) project (2021-2024) targets the production of e-MeOH at a rate of 500 tons per year. The process utilizes CO
2 emissions from a refinery plant and green H
2 generated through water electrolysis, powered by renewable energies, such as photovoltaics or wind energy. TotalEnergies collaborates with several German organizations, including Fraunhofer CBP, Sunfire GmbH, Dresden, and Fraunhofer IWES. Sunfire´s contribution involves developing a high-temperature solid oxide electrolyser specifically designed for industrial applications [
14].
2.1.2. Liquid Sunshine
The Liquid Sunshine project operated by Dalian Institute of Chemical Physics (China) demonstrates the use of a photovoltaic system to produce 1 ktons of e-MeOH annually. The process utilizes CO
2 emissions from geothermal plant and green H
2 generate through water electrolysis. The selectivity of e-MeOH can reach 98 % and its purity can reach 99.5 % [
15].
2.1.3. Undisclosed
Besides these existing facilities, several other projects are under planning and/or development around the world [
16]. Veolia, for instance, is developing four pilot-scale e-MeOH plants in Sweden, Italy, France, and China, with capacities of 60, 80, 100 and 300 tons per year, respectively. These plants will use green H
2 and CO
2 emissions from industrial flue gas (Sweden and China) and waste (Italy and France), showcasing the technology's feedstock flexibility [
16]. Renewable energies, such as hydropower (Sweden) and solar (Italy, France and China) play a crucial role. In the USA, Carbon Clean investigates using industrial-scale demonstration plants (100 and 500 tons per year) targeting CO
2 capture from industrial flue gas and ethanol production using green H
2, from solar and wind energy sources [
16]. Lastly, an undisclosed UK project led by INEOS aims to build a pilot-scale (300 tons per year) e-MeOH plant utilizing CO
2 emissions from a steel factory and H
2 derived from syngas production [
16].
2.2. Medium-Scale Units
Eleven medium-scale facilities (refer to
Figure 5) producing e-MeOH are discussed below: Infraserv project in Germany, NTPC’s Vindhyachal project in India, George Olah Renewable Methanol project in Iceland, Enerkem in Canada, ZASt project in Germany, STEAG project in Germany, Power to Methanol project in Belgium, GreenLab Skive Power-to-X project in Denmark, North-C-Methanol project in Belgium, Sailboat project China, and Finnfjord E-Methanol project in Norway. Additionally, two SAF medium-scale plants are discussed below: Zenid project and Synkero project, both in Netherlands.
Figure 5 illustrates the installed capacities of European, Asian and American countries over the last seventy years and next three years.
2.2.1. Infraserv
The e-MeOH facility under construction by Infraserv Höchst (Germany) is projected to have an annual production capacity of 2.5 ktons. It utilizes captured CO
2 and green H
2 as feedstocks. The consortium responsible for the project includes Infraserv Höchst Group and INERATEC, both based in Germany [
17].
2.2.2. NTPC's Vindhyachal
The project coordinated by Carbon Clean (from the UK) focuses on e-MeOH production at the Tuticorin Alkali Chemicals & Fertilizers Ltd. plant in India. The consortium includes NTPC Energy Technology Research Alliance, Green Power International Pvt. Ltd (both from India), and Tecnimont (a subsidiary of Italy's Maire Tecnimont Group) [
18]. Notably, the carbon capture technology is adaptable to point source gases with CO
2 concentrations between 3 % and 25 % by volume. The plant is designed to capture 3.33 ktons of CO
2 annually (from coal-fired power plants flue gas) using a modified tertiary amine process. The captured CO
2 undergoes catalytic hydrogenation to produce e-MeOH (with purities greater than 99 %) along with H
2 [
18].
2.2.3. George Olah Renewable Methanol
Operational since April 2012, by Carbon Recycling International (CRI), this project in Iceland produces 4 ktons of e-MeOH annually. It captures CO
2 from the flue gas emissions of a geothermal power plant. The captured CO
2 combines with H
2 produced via an alkaline electrolyser powered by renewable grid electricity [
19].
2.2.4. Enerkem
This industrial-scale facility, at the Enerkem Alberta Biofuels plant, uses a thermochemical conversion process to produce e-MeOH from captured CO
2 and H
2 generated by an electrolyser. The facility has been operational since 2003, in Canada, with an annual capacity of 5 ktons, utilizing a four-step process involving gasification and catalytic synthesis. The process relies on a bubbling fluidized bed reactor with a front-end feeding system for waste. Air, or oxygen-enriched air, acts as the partial oxidation agent during gasification. The enrichment level is adjusted to control the composition of the resulting syngas, which undergoes sequential catalytic conversion to produce e-MeOH. The project is a consortium effort involving Shell, Proman, and Suncor Energy, with support from the Québec and Canadian governments [
20].
2.2.5. ZASt
This project, coordinated by Zella-Mehlis energy company (Germany), is in the planning stages. It aims to establish a production facility for e-MeOH with a projected annual capacity of approximately 7 ktons by the end of 2023. The plant will utilize CO
2 captured from residual waste streams and H
2 from water electrolysis. [
21].
2.2.6. STEAG
Led by the Essen-based energy company in Germany, this project aims to produce 5 to 7 ktons of e-MeOH annually. The process involves capturing CO
2 emissions from combustion and using sustainably generated H2 from renewable electricity. The project consortium includes both the Essen-based energy company and Zweckverband für Abfallwirtschaft Südwestthüringen (Germany) [
22].
2.2.7. Power to Methanol
The INEOS group (UK) led this project from 2020 to 2023, demonstrating e-MeOH production at an 8 kton capacity per year. The process utilized H
2 from renewable sources (solar and wind) and captured CO
2 emissions from a Belgian chemical plant’s flue gas. The consortium comprised ENGIE, Indaver, Fluxys, Participatie Maatschappij Vlaanderen, Port of Antwerp-Bruges (all from Belgium), Oiltanking (Germany), and INOVYN (UK) [
23].
2.2.8. GreenLab Skive Power-to-X
Operated by GreenLab in Denmark, this project targets an annual e-MeOH production capacity of 8 ktons. The consortium, led by GreenLab A/S, includes Danish partners such as EA Energy Analyses, DTU Energy, E.on Danmark A/S, Everfuel Europe A/S, Danish Gas Technology Center, Energinet Electricity System Operator A/S, RE:Integrate ApS, Norlys Holding A/S, GreenHydrogen A/S, and Eurowind Energy A/S. The project aims to establish a dedicated e-MeOH plant, and an electrolysis plant powered by local hybrid wind and photovoltaic renewable energy [
24].
2.2.9. North-C-Methanol
The North-C-Methanol project, coordinated by North CCY HUB in Belgium, utilizes an electrolyser powered by renewable wind energy for water electrolysis into green H₂ and O₂. The green H₂ is combined with captured CO₂ from industrial point sources within a catalytic e-MeOH synthesis plant. This plant’s production capacity is 44 ktons of e-MeOH per year. The consortium includes North Sea Port (Netherlands/Belgium), POM Oost-Vlaanderen (Belgium), Oiltanking (Germany), Fluxys (Belgium), ENGIE (France), ArcelorMittal (Luxembourg), Alco Bio Fuel (Belgium), PMV (Belgium), Mitsubishi Power (Japan), and Proman (Switzerland) [
25].
2.2.10. Sailboat
CRI, in Iceland, completed commissioning of an e-MeOH production facility in September 2023. Located in the Shenghong petrochemical industrial park in Lianyungang, Jiangsu, China, it targets an annual production capacity of 100 ktons. The process uses captured CO
2 emissions from the ethylene oxide production oven gas treatment facility, with H
2 generated through water electrolysis powered by surplus renewable energy. The collaborative effort involves Jiangsu Sailboat Petrochemicals Co. Ltd and Shenghong Petrochemical (China) [
26].
2.2.11. Finnfjord E-Methanol
CRI is also leading the Finnfjord E-Methanol project in Norway, anticipated for completion by the end of 2024. This project targets to produce 100 ktons of e-MeOH annually. It utilizes CO
2 emissions captured from ferrosilicon production unavoidable by-products. Green H
2 will be generated through a water electrolysis plant powered by local hydropower. The project is undertaken in collaboration with Finnfjord company (China) [
26].
2.2.12. SAF
Led by Carbon Clean, the Zenid project is currently in the investigative stage (2021-2026) with plans to construct an industrial-scale demonstration plant in the Netherland. The project´s focus is on producing SAF with a targeted annual capacity of 15 ktons. The production process involves using H
2 generated through an electrolysis unit and capturing CO
2 from the air via a modular Fischer-Tropsch reactor. The project consortium includes Rotterdam airports (RTHA, RHIA) in the Netherlands, SkyNRG (a sustainable air fuel company based in the Netherlands), Climeworks (Switzerland), and a Direct Air Capture manufacturing company [
27]. Additionally, Synkero is developing a commercial-scale facility to produce SAF at the Port of Amsterdam. This project targets a production capacity of 50 ktons of SAF per year upon completion in 2027. The production process utilizes green H
2 and captured CO
2. The entire plant will be powered by renewable wind energy [
28].
2.3. Large-Scale Units
Two e-MeOH large-scale plants (refer to
Figure 6) are discussed: Shunli project in China and CIP in Denmark. Also, three SAF on large-scale plants are discussed below: ExxonMobil and Neste in Singapore, Norsk e-Fuel project in Norway, HIF Global's Paysandú in Uruguay and Oxy project in Canada.
Figure 6 illustrate the installed capacities of European, Asian and American countries.
2.3.1. Shunli
Commissioned in October 2022 by CRI (from Iceland) in collaboration with Henan Shuncheng Group (China), the Shunli project is an industrial-scale e-MeOH facility with a production capacity of 110 ktons per year. The facility is located adjacent to a metallurgical coke-oven plant operated by Henan Schuncheng Group in Anyang City, China. Notably, the project utilizes green H
2 generated through water electrolysis powered by surplus renewable energy [
26].
2.3.2. CIP
Led by Copenhagen Infrastructure Partners (CIP) from Denmark, this project (2021-2028) aims to produce 130 Ktons of e-MeOH per year. It achieves this by capturing CO
2 emissions (recycling 180 ktons of waste-based CO
2 annually) from waste incineration and combining them with green H
2 generated using renewable energy sources. The project consortium includes Reno-Nord (a waste company) and Aalborg Forsyning, both from Denmark [
29].
2.3.3. SAF
ExxonMobil and Neste, in Singapore, produce 1 Mtons/year of SAF, blended with conventional fossil-based jet fuel to feed Changi Airport annually [
28]. Norsk e-Fuel, in Norway, is building a SAF plant, which will use renewable electricity for H
2 production through electrolysis and capture CO
2 from the air. The plant’s capacity will be 10 Mtons by 2024 and 20 Mtons by 2026. The consortium includes Sunfire and Climeworks from Germany [
28]. HIF Global's Paysandú has selected Johnson Matthey’s eMERALD™ technology for its Paysandú e-fuels facility in Uruguay. This facility will be the largest SAF plant in South America, with an expected production capacity of 700 ktons per year of SAF. Construction is planned for 2025 and it will be produced SAF using green H
2 and CO
2 from an ethanol plant [
30]. Oxy plant, operated by Oxy company (from Canada), uses Direct Air Capture technology to capture CO
2, with a production capacity of 110 Mtons per year [
28].
2.4. Undisclosed-Scale Units
The installed capacities of several undisclosed plants of European, and America countries are presented in
Figure 7. Four of those projects are undisclosed e-MeOH projects, namely E2fuelS, SynLink and bse Engineering projects in Germany and Port of Antwerp project in Belgium. Other two are SAF projects: M2SAF in Germany, and Infinium and Denburg in the USA.
2.4.1. E2Fuels
Coordinated by the Energy Systems Department of the Technical University of Munich, the E2Fuels project investigates a novel process for synthesizing e-MeOH. By focusing on renewable H₂ and captured CO₂ from various sources (such as industrial exhaust gases) as feedstocks, this project has the potential to significantly reduce greenhouse gas emissions associated with traditional fuel production and consumption. Leading German companies and research institutions in the energy and automotive sectors collaborate on this initiative. Key participants include Audi AG, Robert Bosch GmbH, MAN Energy Solutions SE, MAN Truck & Bus AG, Siemens AG, Stadtwerke Haßfurt GmbH, Volkswagen AG, Fraunhofer Institute for Manufacturing Technology and Advanced Materials, Center for Solar Energy and Hydrogen Research Baden-Württemberg, Friedrich-Alexander University Erlangen-Nuremberg, Darmstadt University of Applied Sciences, RWTH Aachen University, Technical University of Darmstadt, Technical University of Hamburg, and Technical University of Kaiserslautern [
31].
2.4.2. SynLink
The SynLink project, operated by Fraunhofer Center for Chemical-Biotechnological Processes, employs a co-electrolyser based on solid oxide cell to produce clean synthesis gas. The synthesis gas is converted via e-MeOH synthesis or Fischer-Tropsch synthesis. The consortium includes EIFER, Fraunhofer, GETEC, HGM, FZ Jülich, Kerafol, Lufthansa, Universität Bayreuth, Universität Stuttgart, KIT, Ineratec and ZSW from Germany, and Climeworks from Switzerland [
32].
2.4.3. Bse Engineering
The project proposes a method for e-MeOH production utilizing excess electricity and captured CO₂. The approach leverages water electrolysis to generate clean H₂ using surplus electrical current and CO₂ from off-gas sources, serving as feedstock for the e-MeOH synthesis. This method offers a potential solution for energy storage through chemical conversion while promoting resource recovery and mitigating greenhouse gas mitigation [
33].
2.4.4. Port of Antwerp
Focusing on renewable fuel production, this initiative partners with Belgian entities such as Indaver, ENGIE, INOVYN, Fluxys, Advario, and Participatie Maatschappij Vlaanderen. The project aims to develop two key categories of renewable fuels: a) Renewable Fuels of Non-Biological Origin – these fuels are synthesized entirely from renewable sources, excluding any biological feedstocks, and b) Fuels Based on Captured CO₂ and Sustainable H₂ - this category leverages captured CO₂ and H₂ generated through renewable electricity for e-fuel production. The initiative explores various pathways for sustainable fuel production, potentially reducing reliance on fossil fuels and promoting a more environmentally friendly transportation sector [
34].
2.4.5. M2SAF
Coordinated by the German Aerospace Center, the M2SAF project aims to develop a novel process technology for SAF production. Partnering with German entities like BASF Process Catalysts, OMV Germany, DLR, ASG, and ThyssenKrupp Uhde, the focus is on achieving 100 % drop-in capability for the target SAF. A unique approach is taken by utilizing e-MeOH as the feedstock, sustainably produced from captured CO₂ and green H₂ generated through water electrolysis. This pathway offers promise for reducing reliance on fossil fuels and advancing environmentally friendly aviation fuels [
35]. Infinium and Denbury colaborate to build a facility in Texas that converts captured industrial CO₂ and green H₂ from renewable sources into SAF [
28].
2.5. Research Projects Funded by the EU
In this section, a comprehensive review of laboratory scale projects funded by the EU will be presented, specifically focusing on PtL processes for e-MeOH production (refer to
Figure 8). According to data from the Cordis database, there are currently eight ongoing projects and 4 completed projects in this domain.
Figure 8 provides an overview of EU-funded projects over the last ten years.
2.5.1. HEPHAESTUS (“Heavy and Extractive industry Wastes PHAsing Out through ESG Tailings Upcycling Synergy”)
The HEPHAESTUS project (June 2022 to November 2026), funded by the European Union, aims to address dusty waste from metallurgical processes, creating a circular economy. This project focuses on creating a modular system for waste treatment, allowing for customization based on specific needs, through the implementation of units for the catalytic conversion of captured CO₂ into e-MeOH. The HEPHAESTUS consortium includes research institutions and industrial partners across Europe, with coordination led by RINA Consulting SPA (Italy) [
36].
2.5.2. TUNEMOF (“Metallolinker-Functionalized MOF Catalysts for CO2 Hydrogenation”)
This European research project, spanning from August 2022 to February 2025, focuses on developing novel catalysts for converting CO
2 into MeOH using H
2. The project takes a unique approach by combining organometallic chemistry, porous materials science, and expertise in testing homogeneous catalytic systems. Specifically, the strategy involves synthesizing metal-organic frameworks (MOFs) embedded with specific organometallic molecules that efficiently facilitate the conversion of CO
2 into e-MeOH. The TUNEMOF consortium includes research institutions and industrial partners across Europe, with coordination led by Universitete i Oslo in Norway [
37].
2.5.3. LAURELIN [“Selective CO2 Conversion to Renewable Methanol through Innovative Heterogeneous Catalyst Systems Optimized for Advanced Hydrogenation Technologies (Microwave, Plasma and Magnetic Induction)”]
Funded by the European Union and Japan (May 2021-April 2025), LAURELIN explores innovative catalyst systems alongside with advanced reaction technologies. Specifically, it focuses on three non-traditional heating methods: microwave, magnetic induction, and non-thermal plasma. These methods offer the potential for more efficient energy transfer and potentially improved reaction control compared to traditional heating. LAURELIN utilizes a new generation of catalysts (based on Pd-In
2O
3-Al
2O
3) specifically designed to achieve high selectivity towards e-MeOH production and minimize energy consumption. Schiaroli et al. (2023) [
38] reported that the Pd-In
2O
3-Al
2O
3 catalyst achieved CO
2 conversion in range of 5-8 % and MeOH selectivity of 45-70 % at 300
◦C and 30 bar. The project consortium, coordinated by Aimplas-Asociacion de Investigacion de Materiales Plastios Y Conexas in Spain, brings together researchers and industry partners from Europe and Japan, fostering knowledge sharing and expertise in catalyst development, reaction engineering, and large-scale process optimization [
39].
2.5.4. MEXCAT (“Metal EXsolved CATalysts for the CO2 Valorisation to Methanol: Design, Synthesis, and Characterisation of Next-Generation Catalysts, Unravelling Their Structure-Activity Relationship”)
This EU-funded project (Nov 2022-Oct 2024) aims to develop a new generation of catalysts for CO
2 conversion to MeOH. Specifically, the project focuses on “Metal Exsolved Catalysts” to increase MeOH yield. The consortium will create stable, active and selective catalytic nanoparticles using nanoparticle exsolution, a one-step synthesis method. The MEXCAT consortium includes research institutions and industrial partners across Europe, with coordination led by Politecnico di Torino in Italy. [
40].
2.5.5. METHASOL (“International Cooperation for Selective Conversion of CO2 into METHAnol under SOLar Light”)
The project spanning from July 2021 to Dec 2024, tackles the challenge of producing e-MeOH using solar energy. METHASOL employs photocatalysis, aiming to develop a highly efficient process that captures sunlight and drives CO
2 conversion (with 5 % efficiency) at ambient pressure and temperature – an advantage for industrial applications. METHASOL emphasizes cost-effective and selective catalysts (based on Metal-Organic Framework and appropriate Cu-based catalysts), readily obtainable and capable of achieving desired CO
2 to e-MeOH conversion while minimizing unwanted byproducts. The project consortium, coordinated by Universitat Politecnica de Valencia in Spain, brings together expertise in material science, photocatalysis, and reactor design for scalable and efficient solar to e-MeOH conversion [
41].
2.5.6. CuZnSyn (“Understanding Nano-Interactions Could Catalyse Optimised Methanol Production from CO2”)
The CuZnSyn project (Oct 2020-Sep 2022) focuses on bimetallic catalysts for MeOH production. Specifically, it aims to optimize catalyst design by arranging Cu and Zn atoms in a specific electronic structure for maximum CO
2 conversion. The goal is to create efficient Cu-Zn interfaces within a well-defined and highly tuneable ligand framework. After isolating these centres, the project will analyse binding, activation, and interconversion of intermediates during CO
2 hydrogenation. The consortium includes European research institutions and industrial partners, with coordination led by Imperial College of Science, Technology, and Medicine in the United Kingdom [
42].
2.5.7. Circlenergy (“Production of Renewable Methanol from Captured Emissions and Renewable Energy Sources, for Its Utilisation for Clean Fuel Production and Green Consumer Goods”)
Circlenergy, funded by the European Union (Jan 2019-Jun 2021), takes a novel approach to producing e-MeOH that emphasizes sustainability and resource efficiency. Instead of relying on fossil fuels, it utilizes captured CO
2 and renewable electricity to create a closed-loop system for e-MeOH generation. The project aims for a total e-MeOH production capacity of 1 Mtons per year by 2022. Sustainability and life cycle assessment are key focuses, ensuring environmental impact considerations. The consortium involves European research institutions and industrial partners, coordinated by Carbon Recycling International in Iceland [
43].
2.5.8. FreSMe (“From Residual Steel Gases to Methanol”)
The FreSMe project (Jan 2019-Jun 2021), funded by the European Commission, addresses sustainable e-MeOH production (333 tons per year) using residual steel gases and H
2 generation from renewable sources. By integrating existing technologies, it aims to reduce CO
2 emissions and create valuable clean fuel. Notably, the Stena Germanica ship used e-MeOH as a low-emission fuel in June 2021. Researchers explored novel Cu/perovskite catalysts, multi-scale process optimization, and analysed impurities and N
2 impact [
44]. The consortium includes European research institutions and industrial partners, with coordination led by I-Deals Innovation & Technology Venturing Services SL in Spain [
45].
2.5.9. MefCO2 (“Synthesis of Methanol from Captured Carbon Dioxide Using Surplus Electricity”)
The MefCO2 project (Dec 2014-Jun 2019), funded by the European Union under the SPIRE (Sustainable Process Industry through Resource and Energy Efficiency) program, pioneered in demonstrating the viability of sustainable e-MeOH production. Coordinated by Carbon Recycling International, this facility in Germany, has a production capacity of 333 tons per year. It captures CO
2 from a lignite-fired power plant and combines it with H
2 generated by a renewable-powered electrolyser. Researchers explored novel catalysts (Pd/Zn bimetallic alloys on TiO
2 and Al
2O
3 supports) for CO
2 hydrogenation. Bahruji et al. [
46] reported CO
2 conversion and MeOH selectivity in the range of 6.7-10.1 % and 38-53 %, for Pd/ZnO/TiO
2, and in the range 2.4-8.6 % and 4-19 %, for Pd/ZnO/Al
2O
3, respectively, at 250
◦C and 20 bar. Pd/Zn alloy on TiO
2 exhibited significantly higher catalytic activity for e-MeOH production due to its well-dispersed structure. The project involves collaboration among European research institutions and industrial partners [
47].
2.5.10. GREEN MEIGA (“Green Methanol in Galicia”)
The GREEN MEIGA project (Oct 2023-Jun 2027) aims to produce 100 ktons/year of e-MeOH in Spain. It focuses on enhancing production performance, operational flexibility, and competitive costs. The project integrates novel technologies, including (i) a hybridized H
2 production system (combining alkaline, proton-exchange membrane, and solid oxide electrolysis), (ii) an integrated e-MeOH production system, and (iii) an advanced CO
2 capture system (using enzyme-based and direct air-capture technologies). GREEN MEIGA contributes to climate neutrality, aligns with the REPowerEU Plan, and reduces fossil fuel dependency by producing e-MeOH on-site [
48].
2.5.11. TRISKELION (“Green Methanol Manufacturing from CO2”)
The Triskelion project (Jan 2024-Dec 2027) aims to annually produce 40 ktons tons of e-MeOH using a 50 MW electrolyzer. Located in Mugardos, Spain, it plans to achieve commercial quality comparable to fossil-based MeOH. The technology involves renewable energy (wind or solar) powering electrolysis [
49]. Forestal del Atlántico leads this initiative.
2.5.12. eM-Rhône (“electroMeThanol- Rhône”)
The eM-Rhône project (Jan 2024-Dec 2025, expected to begin operation in May 2028), is situated within the Roches-Roussillon chemical platform in Salaise-sur-Sanne, Isère. Administered by the Economic Interest Group (EIG) OSIRIS, this platform serves as a hub for industry and chemistry leaders in the Rhône Valley. Notably, it plays a significant role in MeOH consumption within metropolitan France. The eM-Rhône project aims to produce 150 ktons tons per year of e-MeOH in the Rhône Valley. This innovative initiative provides a decarbonization solution for the chemical industry and maritime operators. The process involves using renewable H
2 production and carbon capture and utilization. Specifically, green H
2 is produced through an electrolyser powered by renewable energy. Additionally, the project plans to deploy Cryocap™ technology to capture CO₂ emissions from Holcim’s Le Teil cement plant [
50]
2.6. Cement Plant Context
Currently, the cement industry remains a significant contributor to CO
2 emissions. Approximately 60 % of these emissions arise from the calcination of limestone, a crucial step in clinker production. Given that limestone serves as the primary raw material for cement, reducing these emissions poses substantial challenges. To address this, the production of e-MeOH within cement factories aims to utilize the otherwise hard-to-abate CO
2 emissions from the raw limestone. By doing so, it significantly reduces the overall emissions from cement plants. Presently, the number of e-MeOH production projects associated with cement plants worldwide is on the rise (as depicted in
Figure 9).
Figure 9 shows the capacities of the units that are being installed and those planned over the next three years in European countries. An undisclosed project, in Sweden, led by Carbon Clean Solutions, is constructing a demonstration unit e-MeOH plant with a capacity of 5 ktons per year. This plant will use CO
2 emissions from a local cement factory as its feedstock [
16]. The remaining projects included in
Figure 9 are described in the following sub-sections.
2.6.1. St1 Power-to-Methanol Lappeenranta
Coordinated by St1 Nordic Oy (Finland), the project focuses on producing e-MeOH with a capacity of 25 ktons per year. The facility is expected to begin operating in 2026, pending successful project execution. The production process utilizes green H
2 generated through electrolysis, powered by renewable wind energy sources. Captured CO
2 emissions from the limestone raw material used by the Finnsementti cement factory will serve as a carbon source. The project consortium includes Finnish partners LUT University, Finnsementti cement factory, Vantaa Energy, and Sweden’s Vattenfall [
51].
2.6.2. Carbon Capture
Led by Carbon Clean, this facility in Rüdersdorf, Germany, aims to be completed by the end of 2026. Carbon Clean collaborates with CEMEX (Europe) to explore large-scale e-MeOH production feasibility, with CO
2 capture. The project is structured in phases: Phase 1: Initial CO
2 capture targets 100 tons per day from the plant itself; Phase 2: Scaling up CO
2 capture to an additional 300 tons per day, using H
2 delivered through a dedicated pipeline; Phase 3: A feasibility study for further scaling up to capture 2 ktons of CO
2 daily. The project leverages CO
2 emissions from a nearby cement factory as feedstock, aiming to produce 100 ktons of e-MeOH annually [
18].
2.6.3. Hynovi
Coordinated by Vicat (France), the project is expected to complete by the end of 2025. It represents a significant step towards CCUS technology in the cement industry. The project targets capturing 40 % of the CO
2 emissions from Vicat's cement plant in Montalieu-Vercieu, France. The captured carbon will be combined with H
2 produced through an electrolyser (scheduled for installation in 2025) to enable the annual production of over 200 ktons of e-MeOH. This process effectively prevents the release of half a million tons of CO
2 emissions into the atmosphere. The consortium responsible for this endeavour includes Vicat and Hynamics [
52].
2.6.4. ETFuels
Operated by ETFuels (Spain), this project targets the capture and utilization of CO
2 emissions from CEMEX's Alicante cement plant. The captured CO
2 will be combined with green H
2 to produce 300 ktons per year of e-MeOH specifically for the shipping industry [
53].
2.6.5. Carbon2Chem®
Coordinated by the Max Planck Institute for Chemical Energy Conversion, the project offers a potential solution for mitigating CO₂ emissions in the German steel industry. If everything goes according to plan, the plant will be operational in 2025. This large-scale initiative, funded by a consortium comprised of AkzoNobel, BASF, Clariant, Covestro, Evonik Industries, Fraunhofer Institute for Solar Energy Systems, Fraunhofer Institute for Environmental, Safety, and Energy Technology, Karlsruhe Institute of Technology, Linde, Max Planck Institute for Coal Research, RWTH Aachen University, Ruhr University Bochum, Siemens, Technische Universität Kaiserslautern, ThyssenKrupp, Volkswagen, and Center for Fuel Cell Technology (all from Germany), aims to achieve an annual reduction of 20 Mtons of CO₂ emissions. The project has two separate phases. Phase1: Focuses on utilizing emissions from steel production as a feedstock for e-MeOH production. Green H₂ generated from renewable energy sources is employed. Phase 2: Aims to broaden the concept’s applicability by investigating the utilization of CO₂ from other sources, such as waste incineration plants and cement factories [
54].
2.6.6. Catch4Climate
HeidelbergCement, a German company, leads a project to capture CO₂ emissions from a cement plant for e-MeOH production for airplanes. The project involves other cement companies from France and Germany, including Vicat S.A, SCHWENK Zement KG and Buzzi Unicem SpA- Dyckerhoff GmbH.
2.6.7. Carbon2Business
The project (scheduled for 2023-2028) coordinated by German company Holcim Gmbh collaborates with Holcim Technology Ltd to capture and reuse CO₂ within the cement industry. The captured CO₂, using oxyfuel technology, can either be processed into e-MeOH or repurposed as a raw material [
55].
2.6.8. Westküste100
This large-scale German project involves several companies collaborating to produce clean fuels for airplanes. The plan is to capture CO₂ emissions from a cement plant and use it to create fuels. This project shares similarities with the recently funded Carbon2Business project, both focusing on producing e-MeOH from cement plant CO₂ emissions. The consortium includes hüga Aktiengesellschaft, Thyssenkrupp, tadtwerke Heide GmbH, Entwicklungsagentur Region Heide, Raffinerie Heide GmbH, Ørsted, Open Grid Europe GmbH, Holcim Deutschlant, Fachhochschule Westküste and Hynamics Deutschland GmbH [
56].
2.6.9. HySCALE100
Hynamics, a German company, partners with Holcim and the refinery Heide GmbH to produce e-MeOH. This project is connected to the larger Westküste100 project, which aims to make both H
2 and e-MeOH to reduce pollution in the cement and petrochemical industries [
57]
3. Techno-Economic Assessment
In the pilot units, demonstration units and projects funded by the EU, CO
2 conversion technology for e-MeOH production has been successfully demonstrated. Lab-scale assemblies, pilot units and demonstration units do not have as primary goal to be a profitable production unit but will be essential in the process of increasing the technology readiness levels (TRLs) of the technology under consideration. Nonetheless, several studies on techno-economic assessments (TEA) of e-MeOH production processes have been published, within different production scales, using different renewable energy sources, technologies of electrolysers, reactors, energetic integration approaches among others. The definition of the system to be considered for the different techno-economic studies is of utmost relevance since an analysis and comparison of studies needs to be under similar premisses. Some published studies do not consider the installation and operation of the electrolysers, other do; some consider a mixture of renewable energy sources, others just consider one type with a combination with the grid. In this article, no mention or comparison will be made with the production of biofuels, and its challenges, that are associated with biomass feedstock such as the scarcity of resources, extensive land usage, or potential threats to biodiversity reduction [
58].
An important strategy to reach the maximum potential of decarbonisation is to prioritise waste heat integration analysis (with the use of pinch analyses and heat pump integration, all available waste heat is utilized in this integration, leaving no waste heat to be recovered from exhausts and effluents), which result in an immediate reduction of CO
2 emissions and fossil-based consumption technologies. Note that, in the absence of this integration, some of the waste heat will have to be used for CO
2 capture [
59]. Such a strategy applied to 2018 scenario values, in the USA, would result in a 30 % reduction (250 Mton/year) of CO
2 conversion to e-MeOH, which in turn would lower the H
2 demand in 40 % (40 Mton/year), and a corresponding decrease of 33 % in renewable electricity (9 EJ/year). The result would be a deficit in 100 Mton/year (17 % less) in the production of e-MeOH. Adding to this forecast in the USA, the Methanol Institute has identified 152 e-MeOH projects worldwide, with an expected production of 11.6 Mtons by 2027, and 15.0 Mtons by 2029 [
60].
The process of directly converting CO
2 to e-MeOH involves several safety considerations due to the operation at moderate temperature and pressure conditions. All the R&D efforts needed to reach a mature state of operation is still underway and most of the times the associated cost to safety measures is not presented in the techno-economic analysis, or it is used a traditional percentual factor for the CAPEX (Capital Expenditures) or OPEX (Operating Expenditures) calculations. Feng et al. (2024) [
61] stress the reality of posing risks of accidents if contra measures are not taken. Nonetheless, it is more advantageous than the indirect route which require higher pressures and higher energy consumption. In the direct route, the reactants, intermediaries and products involved are flammable (class A – H
2 or class B – CO and CH
3OH), constituting a threat of combustion and detonation, since in the process there are several potential ignition sources (thermal radiation, adiabatic compression, impact friction, and electrostatic sparks). This requires consideration of several safety optimization mechanisms during the design, material choice and construction of compressors, and its main components. Additionally, to the handling of chemicals, the global process of CO
2 hydrogenation to e-MeOH primarily relies on the MeOH synthesis unit. Considering safety aspects of exothermic catalytic reactions, the synthesis unit needs to be resistant to high temperatures, leaks, and explosions. The choice of the material and type of reactor is of utmost importance, and will prevent issues caused by, e.g., H
2 embrittlement fracture or accidental release of energy. Another challenge is the deactivation or sintering of catalysts during reaction lowering the needed heat transfer, and eventually leading to reactor tube walls overheating. Finally, the authors stress that ensuring the safety of the production process, especially when scaling from laboratory to industrial applications, is crucial. This makes necessary the safety optimization design of equipment, consideration of safety concerns, and implementation of adequate countermeasures, being all an additional cost that needs to be considered in the techno-economic analysis.
One research paper by Nieminen et al. (2019) [
62] deals with the comparison of gas-phase (the focus of this paper) and liquid-phase (butanol) processes and presents several advantages that are strategic towards the establishment of a safer process, even if this topic was not approached. Nonetheless, aspects such as operation at lower reaction temperatures (leading to higher equilibrium conversion, reduced energy consumption and reduced costs associated with heat management) and the possibility for innovative advanced reactor designs (such as reactive distillation) with better temperature control, catalyst stability, and overall process efficiency, allied with continued research and breakthroughs in process integration, optimization, and catalysis would be an alternative to the more problematic gas-phase reactions. Besides other limitations related to limited experimental data, energy consumption, heat efficiency and production costs, the authors highlight the fact that liquid-phase processes involve complicated and energy-intensive separation stages, introducing complexity and increasing separation costs.
The analysed articles, summarised in
Table 1, are restricted to industrial punctual sources of CO
2. Each study provides insights into its financial, operational, and environmental metrics, including CAPEX, OPEX, CO
2 capture, H
2 production, and revenues. Note that, the CAPEX and OPEX, vary significantly across the studies, reflecting differences in scale, technology, and operational efficiency. Observe that, the CAPEX and OPEX were adjusted according to the Chemical Engineering Plant Cost Index(CEPCI) [
63] and values were normalized for the year 2024.
Li et al. (2024) [
64] reports a CAPEX of 2333 €/ton, focusing on a synthesis process involving copper (Cu)-based catalysts at 200 - 300 °C and 80 bar, achieving a 72 % efficiency, to produce 83 kton e-MeOH/year, which requires around 12 kton/year of H
2. In comparison, Nyári et al. (2020) [
65] shows a substantially lower CAPEX of 282 €/ton but also produces a much larger amount of e-MeOH (1825 kton/year) and produces 350 kton/year of H
2. The efficiency and economic viability of these projects hinge on various factors, including energy consumption, raw material costs, and the efficiency of CO
2 and H
2 conversion processes, as also reported in other projects reported by Pratschner et al. (2023) [
66], Sollai et al. (2023) [
67], Yang et al. (2022) [
68], Su et al. (2022) [
69] and Vaquerizo et al. (2023) [
70]. This indicates that higher investments can lead to higher production capacities.
Similar to CAPEX, the OPEX also show considerable variation. For example, Li et al. (2024) [
64] and Bellotti et al. (2021) [
71] reports an OPEX of 526 €/year and 90 €/year, respectively, while Bellotti et al. (2019) [
72] and Nyári et al. (2020) [
65] reports a much higher OPEX of 1080 and 1107 €/year, respectively. This indicates that the ongoing costs of operation can also vary widely, as also reported in other projects reported by Pratschner et al. (2023) [
66], Sollai et al. (2023) [
67], Yang et al. (2022) [
68], Su et al. (2022) [
69] and Vaquerizo et al. (2023) [
70].
Projects utilizing renewable energy sources, such as wind or solar, tend to have higher initial CAPEX but benefit from lower OPEX and reduced GHG emissions over time. For example, Alsayegh et al. (2020) [
73], which uses ultrahigh concentrated solar power, achieves an energy efficiency of 31 % and has a relatively high CAPEX of 6626 €/ton reflecting the costs associated with advanced renewable technologies. Projects like Cormos et al. (2023) [
74] and Meunier et al. (2020) [
75] emphasize the impact of catalyst costs and lifetime on overall efficiency. Both projects use Cu-based catalysts but differ in operational parameters and economic outcomes. Cormos et al. (2023) [
74], with a catalyst cost of 129 €/kg and a three-year lifetime, achieves a carbon emission of just 0.04 kgCO
2/kg e-MeOH, highlighting the potential for low-emission e-MeOH production.
The amount of CO
2 captured varies across the different references, which could be due to differences in the scale of the projects or the efficiency of the capture technologies used. For instance, Li et al. (2024) [
64] reports a CO
2 capture of 86.4 kton/year, while Cormos et al. (2023) reports a much higher capture of around 141 kton/year. The amount of H
2 produced also varies across the different references. For example, Li et al. (2024) reports an H
2 production of around 12 kton/year, while Cormos et al. (2023) [
74] reports a higher production of around 19 kton/year. This could be due to differences in the efficiency of the H
2 production technologies used.
The levelized cost of MeOH (LCOM) varies across projects, influenced by factors like CO
2 capture efficiency, H
2 production methods, and overall process integration. Nyári et al. (2020) [
65], for instance, has an LCOM of 700 €/ton, benefiting from high CO
2 capture (2550 kton/year) and substantial H
2 production (350 kton/year). This project demonstrates the importance of scale and integration in achieving cost-effective and environmentally sustainable MeOH production. As the use of renewable energy grows and starts to replace thermal power generation, industrial CO
2 emissions will make up a progressively larger share of the emissions. Such emissions are categorized into two types: those related to combustion (fossil-fuel, biofuel, or e-fuel) and those associated with chemical reactions that are required to produce a desired product (process or geogenic). Remember that in the cement industry, combustion and process emissions are mixed, increasing the percentage of CO
2 in the flue gas (20 - 25 %). Zuberi et al. (2024) [
59] highlights that the higher the concentration, the lower the energy demands and costs for the CO
2 capture [
59]. In the future, industrial hubs of CO
2 with simultaneous supply from small, non-electrifiable combustion or due to process reactions, will also be a reality [
58]. Nonetheless, studies with small CO
2 emitters and studies with DAC were not considered.
Three energy-intensive industries (cement, iron and steel sectors) stand out for their low estimated CO
2 capture costs, ranging from 62 - 112 €/tons of CO
2, due to the flue gases’ high CO
2 concentration and larger emission volumes [
59]. This same article presented other sources with CO
2 capture costs in the same range. Additionally, to the capture it is necessary to handle the gas, and the estimations for the average CO
2 compression and transportation costs are 11 - 12 €/tons of CO
2 [
59].
The cost of the electrolysers to produce H
2 is given as one of the major responsible to the total equipment cost, and therefore for the CAPEX [
67,
76,
77]. During operation, the electricity cost to operate the electrolyser is the major fixed cost, and all TEAs conclude that only at lower values of the electricity price the levelized cost of e-MeOH (LCOeM) is competitive with the market values. Note that, the cost of producing green H
2 from renewable electricity is expected to decline significantly in the near future [
78]. Additionally, the cost of the electrolyser equipment itself will have its installation costs reduced. By 2030, the cost of alkaline electrolyser (AE) stacks is expected to decrease from 242 - 388 €/kW to 52 - 79 €/kW, while proton exchange membrane (PEM) stacks will decrease from 384 - 1071 €/kW to 63 - 234 €/kW [
79]. These cost reductions are driven by increased current density and the use of more affordable materials. Additionally, mass manufacturing at a gigawatt scale will contribute to cost savings. Notably, AE experiences a smaller cost decrease compared to PEM due to its maturity. The uncertainty range for PEM stacks arises from the low technology readiness level (TRL) associated with advanced design PEM stacks. Note that, as of July 2024, the average cost of green H
2 production stands at approximately 2.59 €/kg. However, this figure was influenced by a single day of extreme negative pricing due to favorable conditions. By 2030, costs are expected to decrease significantly. In an average scenario, the cost could be around 1.93 €/kg, and in an optimistic scenario, it might drop as low as 1.18 €/kg. However, these estimates are subject to change based on technology advancements and market dynamics [
80].
Considering the CO
2 tax is crucial as it can be accounted as a profit. Li et al. (2024) [
64] describes a change in the LCOM from 776.79 €/ton, when considering two profits (CO
2 tax and O
2 sales price), to 877.48 €/ton. The sensitivity of poor and optimistic scenarios to the CO
2 tax showed a difference of 46 €/ton relative to the base scenario, when considering a carbon tax of 0 €/ton and 92 €/ton, respectively. The uncertainty regarding the future value of the carbon tax, but the actual certainty that it will be higher, does not bring good news for economical evaluations. For 2030, prices recommended by the High-Level Commission on Carbon Prices to limit temperature rise to well below 2 °C range between 58 and 117 €/ton; while carbon price levels consistent with limiting temperature rises to 1.5 °C are between 209 to 356 €/ton [
81]. The mentioned values represent much worst-case scenarios in the techno-economical evaluations than the initial one presented.
Scenarios with different renewable energy type and availability has been analysed, most of the times backed up by a connection to the grid [
82], and/or with the use of batteries [
83]. Rivarolo et. al. [
82] conclude that the best economic scenario is attained when using hydroelectricity, since it is possible to produce energy for 3000 equivalent operative hours, compared to 1300 hours for wind and 1100 h for solar energies [
82]. However, on the assumption of future price increases for e-MeOH, wind and solar become also viable options. Other study state that geographical factors significantly influence solar energy production [
84]. Since a location with higher daily solar irradiation can achieve similar results even with the current panel efficiency, choosing a proper location can enhance energy generation greatly and shorten the Payback Period considerably.
Bellotti et al. (2019) [
84] considers three distinct economic scenarios (Germany, Italy, and China) to evaluate the viability of a large-scale e-MeOH production plant. To evaluate the viability, the authors consider an average cost for electrical energy to supply the plant (Italy - 53.95 €/MWh, Germany - 33.31 €/MWh, and China - 10 €/MWh) and analyse the impact of various parameters (such as MeOH selling price, electrical energy cost, and O
2 selling). Authors calculated a Payback Period (PBP) for the current selling price of e-MeOH (400 €/ton) for each of the countries, resulting in >20 years, 6 years, and 3 years for Italy, Germany, and China, respectively. For each scenario the MeOH production cost is 650 €/ton, 433 €/ton, and 186 €/ton, respectively. These calculations indicate an ideal scenario for the plant installation in China.
The relative geographic location between the three main process units (capture, H2 production and conversion) poses several challenges. The closer the carbon capture unit is from the large point sources, the more efficient and cost-effective is the process. The location of the electrolysis unit should be close to a reliable renewable source of electricity, ideally close to several sources, due to the intermittent characteristic of wind and solar, two of the mostly used. Finally, the conversion unit should be close to the previous ones to minimize transportation costs and energy losses. The CO2 emitter location is defined, what would place the other two units (capture and conversion) nearby. In a situation where several emitters could benefit, simultaneously, from the CCU strategy, an analysis of the best locations would need to be made. Note that, consideration of socio-economic factors is due, since the creation of new infrastructures might have implications for land use and could potentially lead to conflicts with other societal objectives.
The techno-economic viability of a CCU unit is a complex issue that depends on a wide range of variables, from technical and economic factors to regulatory and socio-economic considerations. The stage of development of the technology (being quantified via TechnologyRL, IntegrationRL or SystemRL) can significantly impact its economic viability. Emerging technologies typically experience a drastic increase in performance and decrease in cost. While such events do not happen, investors proceed with caution until mass-market deployment is reached, even more if the amount of capital at stake is of the order of M€ - kM€. These units (with more emphasis in the electrolyser unit due to the required elevated amount of energy) are highly dependent on the volatility of the energy market, both in availability and cost of energy, especially from renewable sources. The forecasted high demand leading to high pressure to install future renewable energy production units will encounter factors such as public acceptance, potential impact on local communities, and conflicts with other societal objectives that will influence the feasibility and thus the economic viability of a CCU unit.
Other factors that will affect the economic viability are the type and value of the products generated from CO
2 utilization, and the policies, regulations, and incentives related to carbon capture and utilization. Sensitivity analysis made in recent studies indicate that the dependence on the market dynamics is decisive: evolution of the price of H
2, energy, equipments, CO
2, utilities, and catalyst; decisions on CO
2 taxation; production scale; evolution of sales price of e-fuels, and O
2 [
62,
65,
66,
72,
73,
83,
85,
86,
87].
Gu et al. (2022) [
83] conduct a sensitivity analysis on the CO
2 capture cost, O
2 selling price, cost of system components, and carbon tax variations, achieving levelized LCOM reductions from 10 to 30 % depending on the assumed conditions. Additionally, Nyari et. al. (2022) [
88] found that the selection of the kinetic model has a substantial impact on the estimated LCOM. Specifically, the difference between the three models considered can lead to a variation of up to 10 % in the calculated cost. Differences in one-pass yield are thought to be the cause. As CO
2 utilization becomes economically viable, it’s essential to understand these uncertainties for effective project planning. Consequently, these findings propose that including the kinetic models contribution in a TEA’s sensitivity analysis could enhance confidence in its robustness.
Bellotti et al. (2017) [
76] assumes an average cost for electrical energy (30 €/MWh) to feed electrolysers, and three different plant capacities for producing e-MeOH (4, 10 and 50 ktons/year, with a total capital investment of 10.10 M€, 21.70 M€ and 75 M€, respectively) in the context of the MefCO2 project. Larger plant sizes despite requiring higher total capital investment showed a lower discounted payback period, making such production scale more economically feasible.
The impact of the most important parameters (e-MeOH selling price, O
2 selling option, and electrolysers' capital cost) on the plant's profitability has been analysed by Bellotti et al. (2017) [
76]. The authors found that higher e-MeOH prices (compared with current prices of conventional MeOH, 450 or 500 €/ton) are necessary to achieve economically feasible results, making even smaller-size solutions practical. The reasoning for the assumed increase in market price is the expected greater demand for e-MeOH (in detriment to MeOH). Fasihi et al. [
89] shows that the e-MeOH could be produced for a cost range of 1200 - 1500, 600 - 680, 390 - 430 and 315 - 350 €/ton (MeOH) in 2020, 2030, 2040 and 2050, respectively. The option to sell the excess O
2 produced by the electrolysers significantly impacts the feasibility of the plant [
65,
68,
71,
90].The cost of PEM electrolysers are the most expensive components of the plant, and the sensitivity analysis indicates that only in case of selling MeOH at more than 600 €/ton, the plant would be feasible [
76]. Other authors claim that even with the decrease in the price of the electrolysers, it is the price of the renewable energy that influences the most the levelized cost of e-MeOH [
67,
77,
82].
A major part of the techno-economic analysis presented conclude that the LCOeM is higher than the LCOM. Nonetheless, despite being a price to pay for the reductions demanded by the decarbonization goals, several authors have recognized the importance of standardization and distributed production (i.e. smaller delocalized industrial units), both of which are integral to modular design principles [
58]. These standardised modules may offer substantial cost reductions (decreased engineering and construction expenses), due to automated manufacturing, involving reduced investment risk, that it is presently the major hurdle to the leverage of CCU installations. Nonetheless, the authors conclude that further studies should consider the prospects and drawbacks of simplifying complexity.
The simulations / calculations are done with the best tools available, i.e. equipment costs are provided by modelling and simulation dedicate software, but such estimates are being done based on non-similar equipment. Also, black-box models are being used to simulate such equipment. Gathering real-data to feed the software database is being hauled by the enormous investments needed to finish the entire development work (getting to TRL 9).
The political debate (even with heavy consultancy to researchers) to construct legislation that defines what can be considered short-, medium- and long-term storage options in the CCU scenario, and how to proceed with Life Cycle Assessment (LCA) accountability of CO
2 derived products is mandatory to business players to decide company strategies towards the sustainability goals imposed until 2050. The cement industry, where process/geogenic emissions are a very important part of the equation, only recently (to the best of our knowledge) had a mention to this kind of emissions in an official document from CEMBUREAU [
91].