1. Introduction
Within a year, up to 3 % of patients with episodic migraine evolve into a chronic migraine (CM) [
1]. One of the causative factors of transformation is the overuse of symptomatic drugs, which can lead to the diagnosis of medication overuse headache (MOH) [
2]. All drugs commonly used for acute treatment can induce MOH. Among the hypothesized mechanisms underlying the transformation are central sensitization and a defect in pain inhibition by the brainstem and cortical systems [
3]. Little is known about the direct effect of acute medications at the cortical level.
Electrophysiological studies showed a specific direct effect of individual drug classes at the level of the sensorimotor system, which in turn depends on the duration of the chronic and/or overuse phase [
4,
5]. In particular, the level of basal cortical excitability (sensitization) in both CM and MOH patients, as measured by the amplitude of evoked potentials, is increased in comparison to healthy subjects and then decreases late in CM (a phenomenon called transient sensitization, with a normal delayed habituation), whereas it continues to increase in MOH (called persistent sensitization, with a delayed lack of habituation). This is accompanied by changes in thalamocortical activity and the degree of lateral intracortical inhibition. In fact, while in patients with CM the level of thalamocortical activity and the degree of lateral intracortical inhibition is within normal limits [
6], both are increased in patients with MOH compared to healthy subjects [
7].
Contradictory results were obtained when analyzing cortical responses to visual stimuli. Evidence in favor of central sensitization came from the analysis of visual responses recorded using a magnetoelectroencephalography, which, similar to sensorimotor responses, were only initially transiently sensitized in CM patients [
8]. After preventive treatment with topiramate, the sensitization of the visual cortex is replaced by an electrophysiological pattern similar to that of episodic interictal migraine, characterized by a tendency to decrease initial evoked responses followed by a delayed habituation deficit [
9]. Other researchers, recording visual evoked potentials with an electroencephalograph in a small cohort of patients with CM and with CM+MOH patients, observed no between groups differences in the levels of sensitization and amplitude habituation [
10,
11]. Therefore, further research in a larger cohort of patients is needed to verify whether visual responses are different in patients with MOH and CM compared to healthy subjects.
The aim of this study is to record visual pattern evoked potentials (VEPs) in a group of patients with MOH and to compare them with a group of patients with CM and healthy controls (HCs). The primary outcome of this study is to test whether there are differences between the groups with regard to both the degree of initial sensitization and that of delayed habituation; the secondary outcome is to test whether these electrophysiological variables change depending on the type of medication the patient is overusing, and after 3-weeks off acute medication without the simultaneous start of preventive therapy. Based on previous results with somatosensory potentials, we hypothesize that MOH patients show persistent sensitization patterns, which are maximal in patients with excessive drug combination use.
2. Materials and Methods
Study Participants
Among consecutive patients attending the authors’ headache clinic, 100 provided informed consent to participate in the study, of whom 19 were excluded because they did not fulfil the inclusion criteria. Participants were included if they were between 18 and 65 years of age and had at least a 1-year clinical history of migraine. Participants were excluded from the study if they were regularly taking medication (e.g., antibiotics, corticosteroids, antidepressants, benzodiazepines, or prophylactic migraine medication) during the 3 months preceding the study, except for contraceptive pills (taken by 5 HCs, 8 MOH, and 4 CM). All the participants underwent an ophthalmological assessment that involved determining the best-corrected visual acuity, examining the eye with a slit-lamp biomicroscope, measuring intraocular pressure, and conducting indirect ophthalmoscopy.
Ophthalmological Exclusion Criteria:
- -
The presence of central scotoma, square-wave jerks, saccadic intrusions, and nystagmus in the primary point of gaze that can affect the capacity to maintain a stable fixation throughout the VEP recordings;
- -
Coexistence of various systemic diseases (such as diabetes, systemic hypertension, and rheumatologic disorders) that could impact retinal function;
- -
The presence of glaucoma or other illnesses affecting the cornea, lens (LOCS III stage < 1), uvea, or retina.
General Exclusion Criteria:
Individuals with a history of other neurological disorders, systemic hypertension, diabetes, or other metabolic or autoimmune disease, or any other type of primary or secondary headache, were also excluded.
Patients did not always experience the headaches on the same side. All participants received a complete description of the study and provided written informed consent. The study was approved by the local ethics review board and was conducted in accordance with the Helsinki Declaration.
According to the inclusion/exclusion criteria, the final dataset comprised 81 patients (
Table 1), of whom 16 were diagnosed with
de novo MOH (IHCD-III code 8.2), and 24, with
de novo CM, with no history of medication overuse (ICHD-III code 1.3). MOH patients never underwent a detoxification program. The sample of patients with MOH included 21 patients overusing triptans (IHCD-III code 8.2.1), 32 non-opioid analgesic drugs (NOAs) (IHCD-III code 8.2.3), and 28 patients overusing combinations of multiple drug classes, not individually overused (IHCD-III code 8.2.6). Before progressing to MOH, all patients had a clear-cut history of episodic migraine without aura (ICHD-III code 1.1). Because of the high number of headache days experienced by these patients, we decided to accept recordings, non-exclusively during the pain-free phase, but even during a mild headache (1-5 on VAS scale). Because MOH patients tend to take acute medications compulsively and frequently during the day, it was impossible to prevent them from taking medication on the day of recordings. It was managed, however, to perform the recordings at least 3 h after the last medication intake.
Of the 81 patients with MOH studied, 33 (10 triptan, 15 NOAs, and 8 combination overusers) agreed to be re-evaluated clinically and electrophysiologically (VEP recordings) 3 weeks after withdrawing from acute medication overuse, without prophylactic medication. The post-withdrawal recording session took place at least 72 h before and after an eventual migraine attack, as checked by telephone interview.
For comparison, VEPs were recorded in 24 healthy controls (HCs) with comparable age and sex distribution (
Table 1), and no personal or familial history (first degree relatives) of migraine and no overt medical condition. To avoid variability due to hormonal changes, female participants were examined outside their pre-menstrual or menstrual cycles.
Recording of Visual Evoked Potentials (VEP)
We conducted VEP recordings using the methodology described in our previously published research [
12,
13,
14,
15].
Subjects were seated in a semi-dark, acoustically isolated room in front of the display and surrounded by a uniform field of luminance of 5 cd·m2 for monocular recordings. We used a visual stimulus of a full-screen checkerboard pattern (contrast 80%, mean luminance 110 cd/m2) generated on a monitor and reversed in contrast at the rate of 3.1 reversals per second. A small fixation target, subtending a visual angle of approximately 0.5 degrees (estimated after considering spectacle-corrected individual refractive errors) was placed at the center of the pattern stimulus. At the viewing distance of 114 cm, in the monitor screen subtending 23 degrees, the checked edges subtended 15′ of the visual angle for the VEP recordings [
16].
VEPs were derived from right monocular stimulation. To maintain stable fixation during the recording session, subjects were instructed to fixate a red dot at the center of the screen with their right eye while a patch covered the contralateral eye. VEPs were recorded from the scalp using silver/chloride cup electrodes placed at Oz (active electrode) and Fz (reference electrode, 10/20 system) [
17]. A grounding electrode was placed on the right forearm.
Signals were amplified by DigitimerTM D360 (bandwidth 0.05-2000 Hz, gain 1000) and recorded using a CEDTM power 1401 device (Cambridge Electronic Design Ltd, CED, Cambridge, UK). Six-hundred consecutive traces, each lasting 200 msec, were collected and sampled at 4000 Hz. The cortical responses were divided into 6 sequential blocks of 100, consisting of at least 95 artifact-free traces. Off-line averaging of the responses in each block ("block averages") was performed using SignalTM software version 4.11 (CED Ltd). Artifacts were automatically removed using SignalTM’s artifact rejection tool only if the signal amplitude exceeded 90 percent of the analog-to-digital conversion (ADC) range, which was further checked through visual inspection. The EP signal was corrected off-line for DC deviations, eye movements, and blinking.
VEP components were identified according to their implicit times: N1 was defined as the major negative peak between 60 and 90 msec, P1 as the major positive peak following N1 between 80 and 120 msec, and N2 as the major negative peak following P1.
We measured the N1, P1, and P2 implicit times and peak-to-peak amplitude of the N1-P1 and of P1-N2 complexes (in μV).
Sensitization was defined as an increased N1-P1 amplitude recorded during block 1 (after a low number of 100 stimuli). Habituation was defined as the slope of the linear regression line for the 6 VEP blocks. Positive values indicate a lack of amplitude habituation (delayed augmenting responses), whereas negative values indicate a more significant amplitude habituation (delayed decreasing responses).
All recordings were collected by the researchers (G.S., C.A.), who had not met the participants before the examination and were not involved in recruiting and including the subjects. All recordings were numbered anonymously and analyzed off-line blinded by a researcher, who was not blinded to the order of the blocks (G.C.).
Statistical Analysis
We used the Statistical Package for the Social Sciences (SPSS) for Windows, version 21.0 for all analyses. The Levene’s test was used to check for normal distribution, and all the considered variables displayed a normal distribution. For patients’ clinical features, variables were tested in a one-way analysis of variance (ANOVA) with group factor “subjects” (MOH, CM, and HCs). To assess behavioural changes in VEP amplitude between blocks 1 and 6 N1-P1 and P1-N2 amplitudes were tested first with a repeated-measure ANOVA with group factor “subjects” and repeated measures factor “block” then using as group factor “MOH subgroups” (MOH-triptans, MOH-NOAs, MOH-combination, and HCs). A separate repeated-measure ANOVA was carried out to compare the electrophysiological variables before and after 3-week acute medication withdrawal (factor time x group x type of overused medication). Tukey’s test was used for post hoc analyses. Paired sample t-test was used to compare clinical variables (days with headache and number of tablets taken during the month preceding and succeeding the withdrawal program) before and after acute medication withdrawal. Pearson’s correlation coefficient was calculated to test correlations between block 1 VEP amplitude or VEP habituation slope and clinical data (disease duration, days with headache, number of tablets taken per month, duration of chronic headache). P values less than 0.01 were considered reflecting statistical significance to compensate for the number of clinical variables.
3. Results
Analyzable VEP recordings were obtained from all patients and HCs participating in the study.
VEP implicit times of N1, P1, and N2 components were not different between groups (for each measure F(2,127), p > 0.05) (
Table 2).
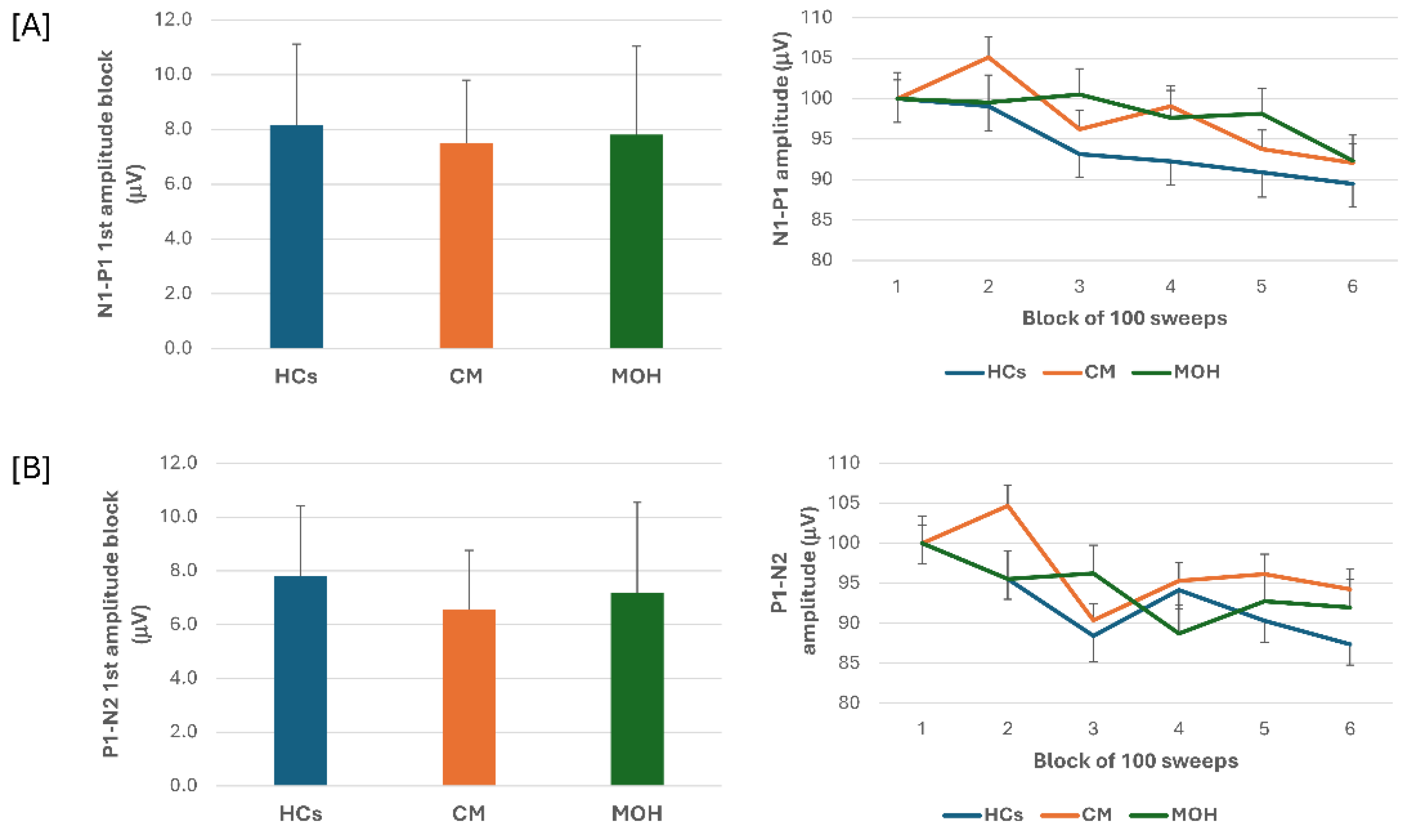
ANOVA testing N1-P1 VEP amplitude block averages disclosed a significant effect for factor block (F(5,635) = 9.64, p = 0.017), but fail to disclose an effect for factor group (F(2,127) = 0.14, p = 0.86), and for the interaction block*group (F(10,635) = 1.51, p = 0.13). Post hoc analysis did not reveal significant between groups changes in amplitude of block-1 N1-P1 VEP (F(2,127) = 0.97, p = 0.38) (
Figure 1). Habituation slope did not differ between groups (F(2,127) = 0.97, p = 0.38).
ANOVA testing P1-N2 VEP amplitude block averages fail to disclose a significant effect for factors group (F(2,127) = 0.54, p = 0.58), but disclosed a significant effect of factor block (F(5,635) = 7.06, p < 0.001), and of the interaction block*group (F(10,635) = 2.06, p = 0.026). Post hoc analysis revealed that the significance of the block*group interaction was due to a significant drop in amplitude of block-4 and block-6 as compared to block-1 in the MOH group (p < 0.0001 and p = 0.04, respectively). Post hoc analysis did not disclose significant between groups changes in amplitude of block-1 P1-N2 VEP (F = (2,127) = 0.25, p = 0.78) (
Figure 1). Habituation slope did not differ between groups (F(2, 127) = 0.25, p = 0.78).
In patients with MOH, monthly number of acute medication intake correlated positively with monthly days with headache (r = 0.397, p < 0.001) and duration of chronic phase (r = 0.611, p < 0.001). None of the VEP electrophysiological variables correlated to the patients clinical features.
3.1. VEPs in Patients Stratified According to the Overused Acute Medication
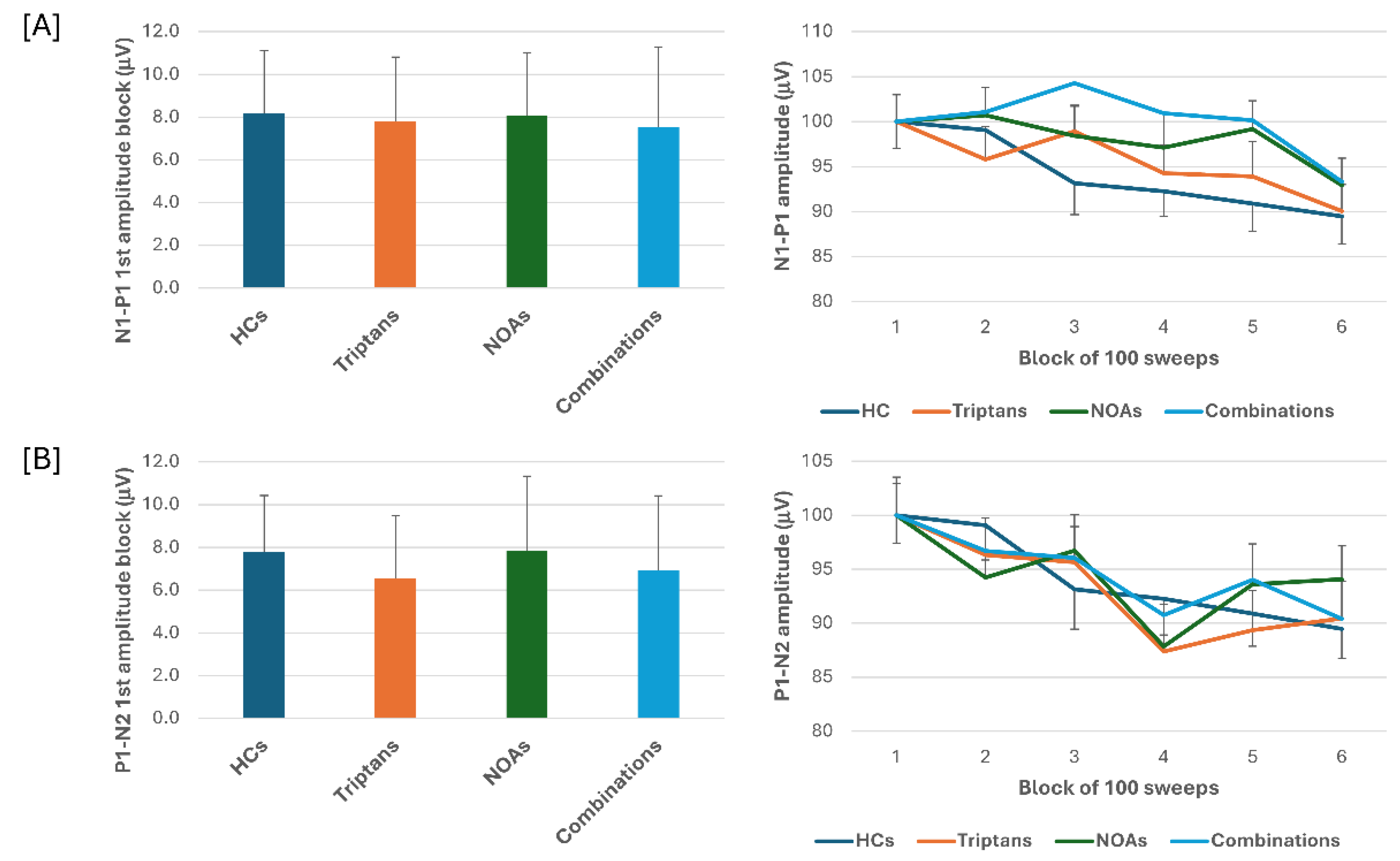
When we stratified the data for patients with MOH according to the class of drugs overused, triptans, NOAs or combinations, ANOVA for VEP N1-P1 amplitudes in the various blocks, showed a main effect for factor block (F(5,505) = 7.65, p < 0.001), but not of factor drug (F(3,101) = 0.10, p = 0.96) or their interaction (F(15,505) = 0.91, p = 0.56). ANOVA for VEP P1-N2 amplitudes in the various blocks, showed again a main effect for factor block (F(5,505) = 7.81, p < 0.001), but not of factor drug (F(3,101) = 0.86, p = 0.47) or their interaction (F(15,505) = 1.01, p = 0.44). Post hoc analysis did not reveal significant between groups changes in block-1 N1-P1 and P1-N2 VEP (F(3,101) = 0.80, p = 0.49; F(3,101) = 0.32, p = 0.81, respectively) (
Figure 2).
3.2. VEPs before and after Acute Medication Withdrawal
One-month after starting a 3-week acute medication withdrawal, we observed a significant reduction in the number of headache days (T0 = 24.6 ± 6.3; T1 = 1.8 ± 2.1; T0 vs. T1: t(1,26) = 15.6, p < 0.001) and number of acute medication intake (T0 = 30.8 ± 20.6; T1 = 1.4 ± 2.3; T0 vs. T1: t(1,26) = 6.8, p < 0.001).
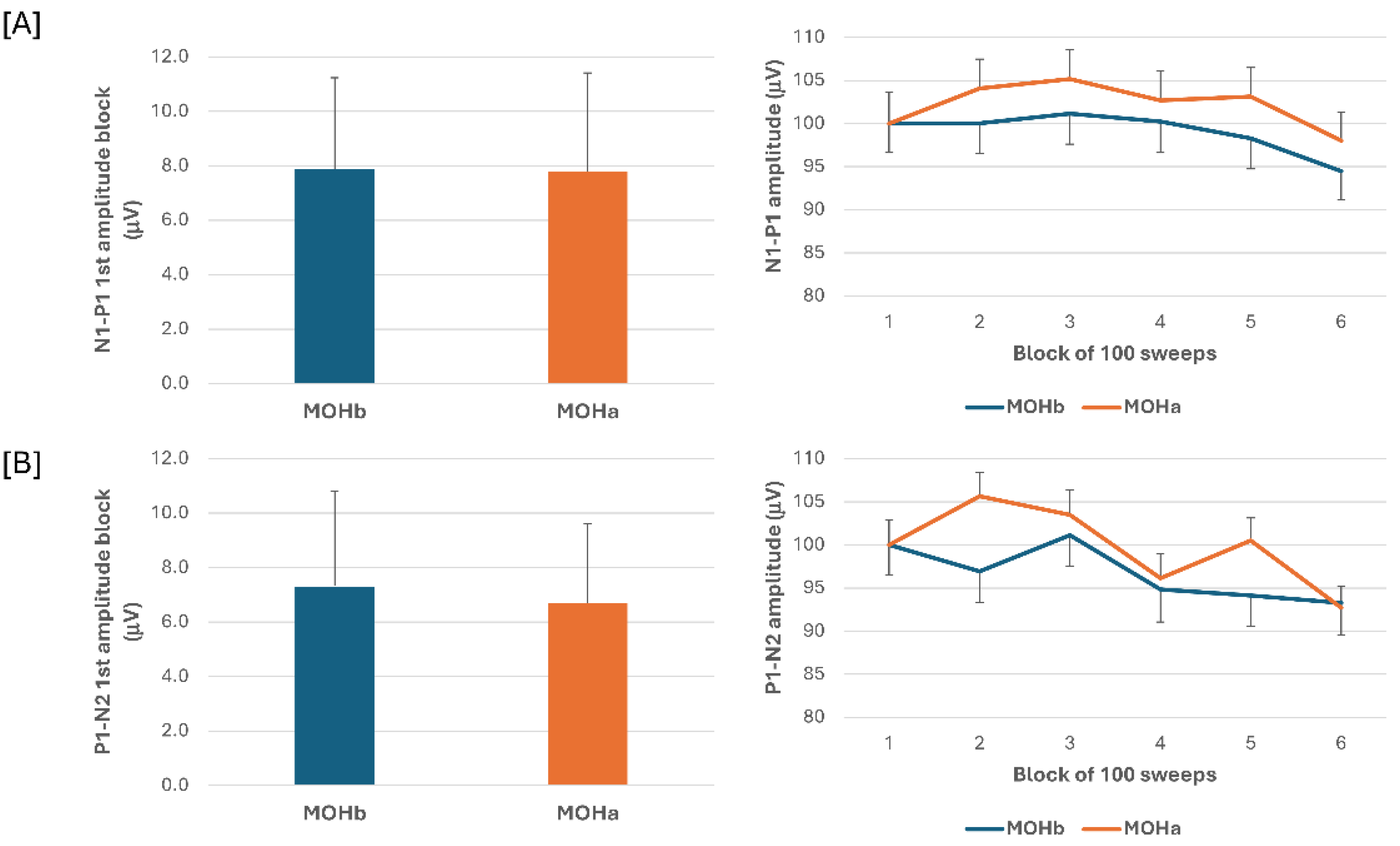
When we compared the electrophysiological data before and after 3-week acute medication withdrawal, ANOVA testing N1-P1 VEP amplitude block averages did not disclose a significant effect for factor time (F(1,60) = 0.07, p = 0.789), for factor type of medication (F(2,60) = 0.18, p = 0.84), and for the interaction time*type (F(2,60) = 0.04, p = 0.96). ANOVA testing P1-N2 VEP amplitude block averages did not disclose a significant effect for factor time (F(1,60) = 0.13, p = 0.716), for factor type (F(2,60) = 1.02, p = 0.37), and for the interaction time*type (F(2,60) = 0.10, p = 0.90) (
Figure 3).
4. Discussion
In the present study we searched for VEP abnormalities in patients with CM and in patients with MOH, before and after abrupt acute medication discontinuation, and according to the overused drugs. The major results of this electrophysiological study are summarized as follows:
- a)
In patients with CM and in patients with MOH, the VEP N1-P1 amplitude of block-1, reflecting sensitization, and the VEP N1-P1 amplitude behaviour during stimulus repetition, reflecting habituation, did not differ from those of HCs.
- b)
In patients with MOH, the VEP P1-N2 amplitude reduction during stimulus repetition was significantly more pronounced than that of both HCs and patients with CM.
- c)
N1-P1 and P1-N2 block-1 VEP amplitude and VEP delayed amplitude habituation did not differ according to the overused drug (triptans, NOAs, combination).
- d)
In patients with MOH after 3-weeks off acute medication, no difference was found in N1-P1 and P1-N2 block-1 VEP amplitude and VEP delayed amplitude habituation.
- e)
None of the VEP electrophysiological variables correlated to the patients clinical features.
This study attempted to record VEPs, an electrophysiological measure of the mass activity of visual cortical neurons, to verify the level of the initial cortical sensitization and of delayed habituation in a group of patients with CM and patients with MOH, without ongoing prophylactic medication.
Previous attempt to study visual cortical excitability in CM and MOH used both electro- and magneto- encephalography. Using standard pattern-reversal VEP, Viganò et al did not detect significant differences in patients with CM or CM+MOH as regards 1
st N1-P1 and P1-N2 amplitude block and habituation when compared with HCs [
10,
11]. In contrast to these results, others, recording visual evoked magnetic field (VEF) with a magneto-electroencephalograph, found an increase in the amplitude of the first P100m block in CM patients in comparison to healthy subjects and episodic migraineurs outside attacks [
8]. The degree of habituation of VEF amplitude in CM patients was not different from that in healthy subjects. The apparent discrepancy in the results obtained by recording VEPs and VEFs could be due to methodological differences intrinsic to the different techniques used. In fact, VEPs reflect the mass activity of the entire visual pathway, including cortical and subcortical stations. Whereas, the P100m amplitude of VEFs exclusively reflects cortical activity tangential to the scalp, with the absence of the conducted volume effect and the so-called ’paradoxical lateralisation’ effect present in VEPs [
18]. These characteristics of the VEF signal may explain why it was better at capturing alterations in cortical sensitisation levels than the VEP technique.
Our current results obtained in CM patients were in line with previous studies using the VEP method: the N1-P1 and P1-N2 amplitudes for both the early block and the late habituation were superimposable to those of healthy subjects [
10,
11]. In the group of patients with MOH, but not in that of HC and CM patients, in contrast to previous VEP studies [
11], we detected a significant decrease in P1-N2 amplitude in the later response blocks (4th and 6th) compared to the initial block. Despite this, we did not detect a significant difference between the groups in the degree of habituation as measured by the slope of the regression line calculated from the amplitude of the 6 blocks. Multichannel scalp recordings have documented a generator in the dorsal extrastriate cortex of the middle occipital gyrus for the early phase of the P1 component and a more complex genesis of the N2 component, probably deeply at the centro-parietal level [
19].
Interestingly, in a previous study using trains of transcranial magnetic stimuli (TMS) delivered to the sensorimotor cortex in groups of CM and MOH patients we found a paradoxical inhibitory cortical activity in response to an excitatory TMS paradigm in MOH patients than in CM patients and HCs [
20]. Furthermore, in a recent study Viganò and colleagues found a statistically significant modulatory effect of an inhibitory TMS paradigm only on N1-P1 and P1-N2 VEP delayed habituation levels and not on the initial response [
11]. Overall, these previous findings in MOH patients, in addition to our observation of the late drop in P1-N2 amplitude, could be due to an abnormal activation of parieto-occipital inhibitory circuits in response to repeated modulatory stimuli in patients with MOH compared to those with CM.
We further expanded the study of VEPs in patients with MOH by also analysing the responses of subgroups of patients categorised according to overuse drug and after drug withdrawal 3-week program. We did not find a drug-specific effect on VEPs (N1-P1 and P1-N2 amplitude and habituation) and furthermore did not find statistically significant differences in VEPs after 3 weeks of symptomatic drug de-addiction, without the establishment of prophylaxis. These results are apparently in contrast to our earlier observation obtained by recording somatosensory evoked potentials (SSEP). In that case, we had detected an initial sensitisation of the SSEP only in patients with overuse of NSAIDs and combination drugs, but not in those with overuse of triptans, despite the fact that all subgroups showed an equal delayed habituation deficit [
4]. This discrepancy may be explained either by a possible specific pharmacological effect on the parietal cortex of the drugs in question or by a greater involvement of the parietal cortex in pain processing than the visual cortex.
As in all previously mentioned VEP studies in patients with CM and MOH [
8,
10,
11], we found that electrophysiological parameters did not correlate with any clinical characteristics. This despite the fact that we found that in the MOH group the number of drugs consumed in a month increased progressively as the number of days with headache/month and the duration of the chronic phase increased.
A major strength of this study is that it recruited a large cohort of patients with CM and MOH, with no current prophylactic therapy and no previous attempt of drug withdrawal. A limitation of the present study is that it did not collect information on the personality trait and psychiatric comorbidity of our patients. Previous studies have in fact found that these variables can influence VEP responses [
21,
22].
5. Conclusions
This study revealed delayed visual information processing abnormalities verified by analysis of the P1-N2 component of VEPs only in MOH patients. In this group of patients, no significant differences in amplitude and habituation of VEPs were found in subgroups of overusers and after abrupt acute medication discontinuation, compared to healthy subjects. Further electrophysiological studies are needed in order to understand the pathophysiological basis of medication overuse.
Author Contributions
Conceptualization, G.C. and V.P.; methodology, M.S. and C.A.; formal analysis, A.D.R., C.A. and G.S.; investigation, C.D.L. and F.C.; resources, C.A.; writing—original draft preparation, G.C.; writing—review and editing, V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Sapienza University of Rome.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The contribution of the IRCCS -Fondazione Bietti in this paper was supported by the Italian Ministry of Health and Fondazione Roma.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scher, A.I.; Stewart, W.F.; Ricci, J.A.; Lipton, R.B. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain 2003, 106, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [CrossRef] [PubMed]
- Pozo-Rosich, P.; Coppola, G.; Pascual, J.; Schwedt, T.J. How does the brain change in chronic migraine? Developing disease biomarkers. Cephalalgia 2020, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Currà, A.; Di Lorenzo, C.; Parisi, V.; Gorini, M.; Sava, S.L.; Schoenen, J.; Pierelli, F. Abnormal cortical responses to somatosensory stimulation in medication-overuse headache. BMC Neurol. 2010, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Currà, A.; Coppola, G.; Gorini, M.; Porretta, E.; Bracaglia, M.; Di, L.; Schoenen, J.; Pierelli, F. Drug-induced changes in cortical inhibition in medication overuse headache. Cephalalgia 2011, 31, 1282–1290. [Google Scholar] [CrossRef]
- Coppola, G.; Cortese, F.; Bracaglia, M.; Di Lorenzo, C.; Serrao, M.; Magis, D.; Pierelli, F. The function of the lateral inhibitory mechanisms in the somatosensory cortex is normal in patients with chronic migraine. Clin. Neurophysiol. 2020, 131, 880–886. [Google Scholar] [CrossRef]
- Sebastianelli, G.; Casillo, F.; Abagnale, C.; Di Renzo, A.; Cioffi, E.; Parisi, V.; Di Lorenzo, C.; Fazio, F.; Petricola, F.; Mattia, C.; et al. Central sensitization mechanisms in chronic migraine with medication overuse headache: A study of thalamocortical activation and lateral cortical inhibition. Cephalalgia 2023, 43. [Google Scholar] [CrossRef]
- Chen, W.T.; Wang, S.J.; Fuh, J.L.; Lin, C.P.; Ko, Y.C.; Lin, Y.Y. Persistent ictal-like visual cortical excitability in chronic migraine. Pain 2011, 152, 254–258. [Google Scholar] [CrossRef]
- Chen, W.-T.; Wang, S.-J.; Fuh, J.-L.; Ko, Y.-C.; Lee, Y.-C.; Hämäläinen, M.S.; Lin, Y.-Y. Visual cortex excitability and plasticity associated with remission from chronic to episodic migraine. Cephalalgia 2012, 32, 537–543. [Google Scholar] [CrossRef]
- Viganò, A.; Torrieri, M.C.; Toscano, M.; Puledda, F.; Petolicchio, B.; Sasso D’Elia, T.; Verzina, A.; Ruggiero, S.; Altieri, M.; Vicenzini, E.; et al. Neurophysiological correlates of clinical improvement after greater occipital nerve (GON) block in chronic migraine: Relevance for chronic migraine pathophysiology. J. Headache Pain 2018, 19, 73. [Google Scholar] [CrossRef]
- Viganò, A.; Sasso D’Elia, T.; Sava, S.L.; Colosimo, A.; Di Piero, V.; Magis, D.; Schoenen, J. Exploring the Therapeutic Potential of Quadripulse rTMS over the Visual Cortex: A Proof-of-Concept Study in Healthy Volunteers and Chronic Migraine Patients with Medication Overuse Headache. Biomedicines 2024, 12. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Ziccardi, L.; Costanzo, E.; Tedeschi, M.; Barbano, L.; Manca, D.; Di Renzo, A.; Giorno, P.; Varano, M.; Parravano, M. Macular Functional and Morphological Changes in Intermediate Age-Related Maculopathy. Invest. Ophthalmol. Vis. Sci. 2020, 61. [Google Scholar] [CrossRef] [PubMed]
- Ziccardi, L.; Sadun, F.; De Negri, A.M.; Barboni, P.; Savini, G.; Borrelli, E.; La Morgia, C.; Carelli, V.; Parisi, V. Retinal Function and Neural Conduction Along the Visual Pathways in Affected and Unaffected Carriers With Leber’s Hereditary Optic Neuropathy. Invest. Ophthalmol. Vis. Sci. 2013, 54, 6893–6901. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Gallinaro, G.; Ziccardi, L.; Coppola, G. Electrophysiological assessment of visual function in patients with non-arteritic ischaemic optic neuropathy. Eur. J. Neurol. 2008, 839–845. [Google Scholar] [CrossRef]
- Parisi, V.; Ziccardi, L.; Sadun, F.; De Negri, A.M.; La Morgia, C.; Barbano, L.; Carelli, V.; Barboni, P. Functional Changes of Retinal Ganglion Cells and Visual Pathways in Patients with Chronic Leber’s Hereditary Optic Neuropathy during One Year of Follow-up. Ophthalmology 2019, 126, 1033–1044. [Google Scholar] [CrossRef]
- Odom, J.V.; Bach, M.; Brigell, M.; Holder, G.E.; McCulloch, D.L.; Mizota, A.; Tormene, A.P. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc. Ophthalmol. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Klem, G.H.; Lüders, H.O.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 66, 349. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Hari, R.; Ilmoniemi, R.J.; Knuutila, J.; Lounasmaa, O.V. Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev. Mod. Phys. 1993, 65, 413. [Google Scholar] [CrossRef]
- Di Russo, F.; Martínez, A.; Sereno, M.I.; Pitzalis, S.; Hillyard, S.A. Cortical sources of the early components of the visual evoked potential. Hum. Brain Mapp. 2002, 15, 95. [Google Scholar] [CrossRef]
- Cortese, F.; Pierelli, F.; Pauri, F.; Di Lorenzo, C.; Lepre, C.; Malavolta, G.; Merluzzo, C.; Parisi, V.; Serrao, M.; Coppola, G. Short-term cortical synaptic depression/potentiation mechanisms in chronic migraine patients with or without medication overuse. Cephalalgia 2019, 39, 237–244. [Google Scholar] [CrossRef]
- Buonfiglio, M.; Di Sabato, F. Lack of habituation of visual evoked potentials in migraine and healthy subjects: Correlated cognitive behavioral aspects. Cephalalgia 2018, 38, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- Casillo, F.; Abagnale, C.; Sebastianelli, G.; Di Renzo, A.; Parisi, V.; Cioffi, E.; Serrao, M.; Lorenzo, C. Di Cortical excitability in patients with migraine with aura and depressive symptoms: A visual evoked potentials study. Confin. Cephalalgica 2024, 34. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).