1. Introduction
Human papillomavirus (HPV) is the most common sexually transmitted viral infection worldwide. Its persistent infection is associated with the development of cancer of the cervix, anus, vagina, vulvar, penis and head and neck [
1,
2,
3]. The majority of the sexually experienced population will acquire HPV infection at least once in their lifetime [
4]. There are more than 200 genomic different HPV genotypes belonging to three genera, namely,
Alphapapillomavirus,
Betapapillomavirus and
Gammapapillomavirus [
5,
6]. The
Alphapapillomavirus can be divided into high-risk (HR) and low-risk (LR) HPV genotypes; the HR-HPV types are associated with the development of cancer [
6,
7].
African countries have the highest burden of HPV [
8,
9,
10]. Taku et al. reported an HR-HPV prevalence of approximately 83.0% among Eastern Cape women with high-grade cervical cancer lesions and 26.4% among women (≥30 years) attending community health clinics [
11]. Cervical cancer is a major public health problem among women worldwide, with an estimated 660,000 new cases and 350,000 deaths in 2022 [
12]. In South Africa, cervical cancer is the second most common cancer [
13] and its prevalence remains high despite the free cervical cancer screening services in health facilities of South Africa [
14,
15]. The human immunodeficiency virus (HIV) has been associated with a high rate of HPV acquisition, persistent infection and development of cervical cancer [
16,
17,
18,
19,
20]. South Africa has a high burden of HIV [
21], which contributes to the high HPV infection, HPV persistent infection and cervical cancer burden [
20]. HIV-positive women are more likely to develop cervical cancer compared to HIV-negative women [
16,
22].
South Africa introduced a national school-based HPV vaccination programme two decades ago, targeting girls aged nine years or older. However, HPV vaccine coverage rates across South African provinces are reported to vary and decrease over the years [
13,
23,
24]. The South African cervical cancer screening and control policy indicates that women should receive free cervical cytology screening starting at 30 years of age using liquid-based cytology at five-year intervals [
25]. It further stipulates that high-risk women, such as HIV-positive women, should be screened upon HIV diagnosis and at three-year intervals after that. Although widely available in private practice, HR-HPV molecular testing has not yet been fully incorporated as one of the cervical cancer screening methods in the public sector [
25,
26].
The HPV prevalence and HPV types distribution according to age and HIV status is essential in informing HPV vaccination campaigns and monitoring the impact on HPV types after vaccination. Among women of the King Sabata Dalindyebo (KSD) municipality, Eastern Cape Province of South Africa, this information is minimal. Therefore, this study investigated cervical HPV prevalence, characteristics and distribution according to age and HIV status among women attending a public community health facility in the Eastern Cape Province of South Africa.
2. Materials and Methods
2.1. Study Setting and Population
This quantitative study was conducted at the health facility in the King Sabata Dalindyelo (KSD) Department of Health sub-district, OR Tambo municipality district of the Eastern Cape province in South Africa. The health facility serves as a primary healthcare facility catering to a diverse population residing in surrounding rural, informal settlements, peri-urban and urban settings. The study population consisted of women who visited the health facility, seeking medical attention for any reason, including various conditions and collecting medication. Participants were recruited between June and July 2023 during their visits to the health facility. Participants were recruited at the health facility reception by educating all health facility attendees using posters and education leaflets on human papillomavirus and cervical cancer. Recruitment and informed consent processes were conducted in isiXhosa, a locally spoken language. Participation in the study was entirely voluntary, with no obligation or pressure placed upon the individuals to take part. Written consent was obtained from all study participants. All individuals voluntarily participated in the study, provided biological specimens, and responded to the questionnaires.
2.2. Clinical Specimen Collection and HIV Test
Study procedures were conducted in a private room; the investigator attended to all participants privately. To maintain participant confidentiality, each participant was assigned a unique study number, linking all their collected specimens and questionnaires. HIV rapid test was offered to all participants who had a negative or unknown HIV status by a qualified nurse or an HIV lay counsellor. Pre- and post-HIV counselling and rapid HIV tests were administered following the South African Department of Health HIV guidelines. The first cervical specimen was collected for cervical cancer screening purposes. It was collected using the ThinPrep Pap Test collection kit (Hologic InC., Marlborough, USA) and sent to the Nelson Mandela Academic Hospital National Health Laboratory Service cytopathology laboratory. The second cervical specimen for HPV testing was then collected using a Digene cervical sampler brush (Qiagen Inc., Gaithersburg, MD, USA) and placed into the Digene transport medium (Qiagen Inc., Gaithersburg, MD, USA). The cervical specimens were transported to the Nelson Mandela Academic Hospital NHLS/WSU Virology laboratory and stored at −20°C until nucleic acid extraction.
2.3. Nucleic Acid Extraction and Molecular Detection of HPV
Nucleic acid extraction from the cervical specimens was performed using an automated procedure, Seegene NIMBUS and a universal STARMag extraction system (Seegene Inc., Seoul, South Korea), following the manufacturer’s instructions. The extracted nucleic acid was used to detect and genotype HPV using Seegene Anyplex™ II HPV28 (Seegene Inc. Seoul, South Korea) multiplexed real-time polymerase chain reaction (PCR). The Seegene Anyplex™ II HPV28 detect, differentiate and quantify 28 different HPV genotypes (13 HR-HPV: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68; 9 LR-HPV: 6, 11, 40, 42, 43, 44, 54, 61, 70 and 6 Probable HR-HPV: 26, 53, 66, 69, 73, 82). Amplification on a Bio-Rad CFX96 real-time thermocycler (Bio-Rad, Hercules, CA, USA) was conducted according to the manufacturer’s instructions. Anyplex™ II HPV28 targets the L1 major capsid gene of the HPV types and the human housekeeping gene (β-globin) as an internal control. Data was automatically generated and interpreted by Seegene Viewer Software (Seegene Inc., Seoul, Korea) according to the manufacturer’s instructions.
Samples with negative internal control and a negative HPV result were re-analysed, and if this persisted, they were deemed invalid. If the internal control was negative, but the HPV test result was positive, the test result was considered valid. Negative HPV tests with a positive internal control were deemed negative for 28 HPV types targeted by the Seegene Anyplex™ II HPV28. A negative control (molecular biology grade water) was included for every 20 samples and taken through the extraction and HPV genotyping step to monitor contamination. In addition to HPV genotype indication, the Seegene Anyplex™ II HPV28 (Seegene Inc. Seoul, South Korea) also provide a semi-quantified HPV viral load. HPV viral load was semi-quantified as high (+++; positive signal before 31 PCR cycles), medium (++; positive signal between 31 and 39 PCR cycles), or low (+; positive signal at and after 40 PCR cycles).
2.4. Data Analysis
Single HPV infection was defined as infection with one HPV type, while multiple HPV infection was described as being infected with two or more HPV types or alpha species in the same sample. Women infected with multiple types belonging to HR-HPV and LR-HPV types will be counted more than once, similarly to those infected with types belonging to different Alphapapillomavirus. All variables were captured and coded in Microsoft Excel. GraphPad Prism Software v8.0.1.244 statistical software was used to perform. The chi-squared for trends and Fisher’s exact method were used to compare the proportions between variables. Mann-Whitney test was used to compare the median between the two groups. The 95 % confidence intervals of the proportion were calculated by the modified Wald method (GraphPad Prism). Differences were considered to be statistically significant when P-values were <0.05.
3. Results
3.1. Demographic Characteristics of the Population
A total of 325 women with a median age of 36 years (IQR: 28–45 years; range: 18–60 years) participated in the study. HIV-positive women (median age 38.5 years, IQR: 32–46 years) were older than the HIV-negative women (median age 31 years, IQR: 23–42 years, p < 0.0001,
Table 1). The majority of study participants were negative for intraepithelial lesion or malignancy (82.8%), followed by low-grade squamous intraepithelial lesion (LSIL, 4.9%), high-grade squamous intraepithelial lesion (HSIL, 4.3%), atypical squamous cells of undetermined significance (ASCUS, 2.5%), atypical squamous cells-cannot exclude high grade squamous intraepithelial lesion (ASC-H, 1.2%,
Table 1).
3.2. HPV Prevalence and Type Distribution According to HIV Status and Age
Overall HPV prevalence was 65.2% (212/325; 95% CI: 59.9–70.2%), with the highest prevalence of 80.9% (38/47; 95% CI: 67.2–89.8%) observed in the 18–25 years age group and lowest prevalence of 46.3% (37/80; 95% CI: 35.8–57.1%) in women 46–60 years of age. The overall HPV prevalence was not found to significantly differ between the HIV-positive and HIV-negative women (
Table 2). Among HIV-positive women, HPV prevalence was 67.8% (141/208; 95% CI: 61.2–73.8%), with the highest prevalence of 91.7% (11/12; 95% CI: 62.5–91.7%) observed in the 18–25 years age group and lowest prevalence of 51.8% (29/56; 95% CI: 39.0–64.3%) in women 46–60 years of age (
Table 2). Among HIV-negative women, HPV prevalence was 60.0% (69/115; 95% CI: 50.9–68.5%), with the highest prevalence of 77.1% (27/35; 95% CI: 60.7–88.2%) observed in the 18–25 years age group and lowest prevalence of 27.8% (5/18; 95% CI: 12.2–51.2%) in women 36–45 years of age. HPV prevalence was found to decrease with increasing age in all women (p for trend <0.0001), HIV-positive (p = 0.004) and HIV-negative women (p < 0.0001,
Table 2). The overall HR-HPV prevalence was found to be 53.8% (175/325; 95% CI: 48.4–59.2%), LR-HPV to be 31.1% (110/325; 95% CI: 26.3–36.3) and probable HR-HPV to be 18.2% (95% CI: 14.3–22.7%). There was no difference on HR-HPV, probable HR-HPV and LR-HPV prevalence between the HIV-positive and HIV-negative women (
Table 2).
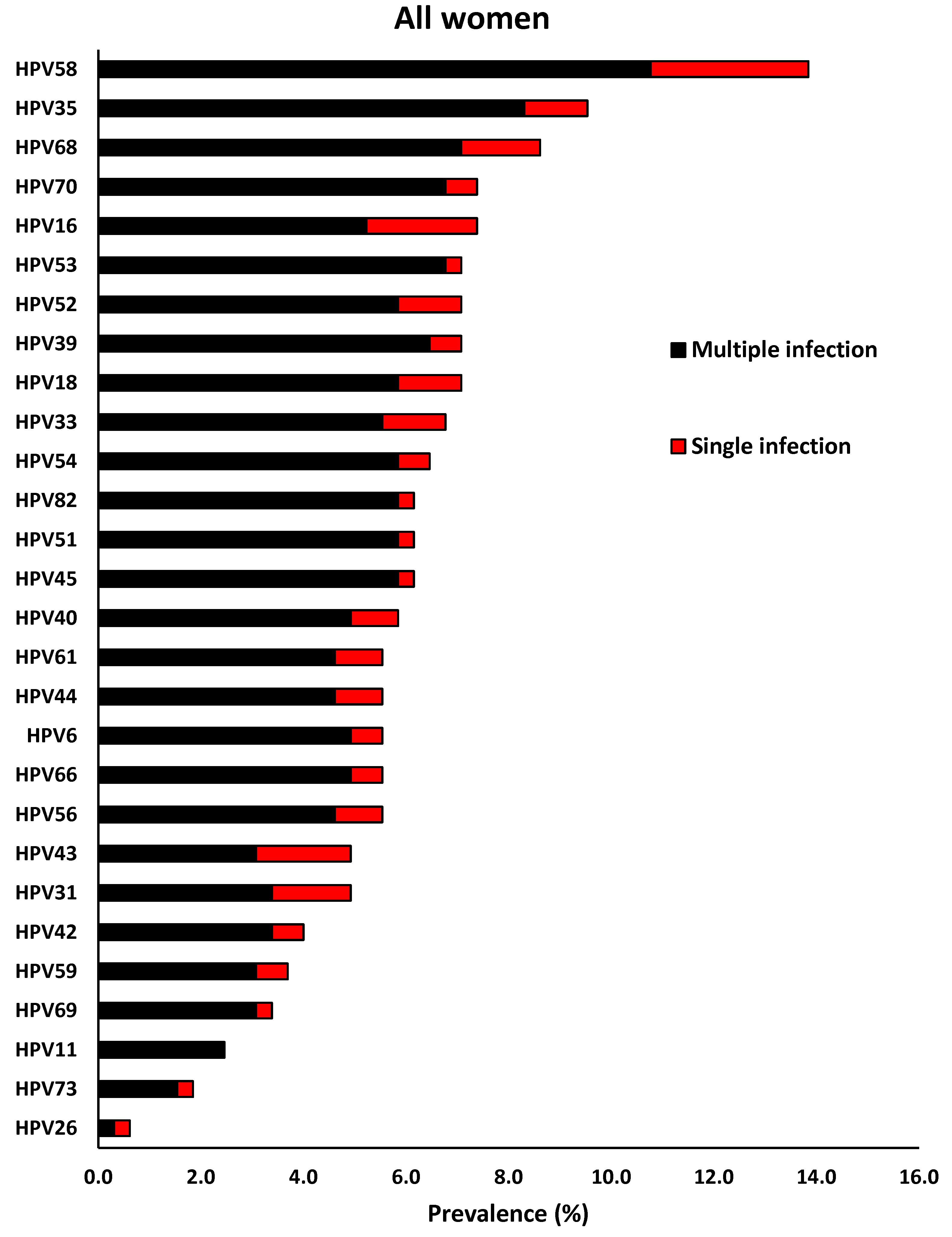
Overall, the four most common HR-HPV types were found to be HPV-58 (13.8%), HPV-35 (9.5%), HPV-68 (8.6%) and HPV-16 (7.4%). HPV-58 (3.1%), HPV-16 (2.2%), HPV-68 (1.5%) and HPV-31 (1.5%) were the most dominant HR-HPV types that presented as single HPV infection. Among the LR-HPV types, HPV-70 (7.4%), HPV-54 (6.5%), HPV-40 (5.8%), HPV-61, -44 and -6 (5.5% each) were the most dominant types. Among LR-HPV types, the most prevalent single HPV infection were HPV-43 (1.8%), HPV-40, -61, -44 (0.9%), HPV-70, -54, -42, and -6 (0.6% each,
Figure 1).
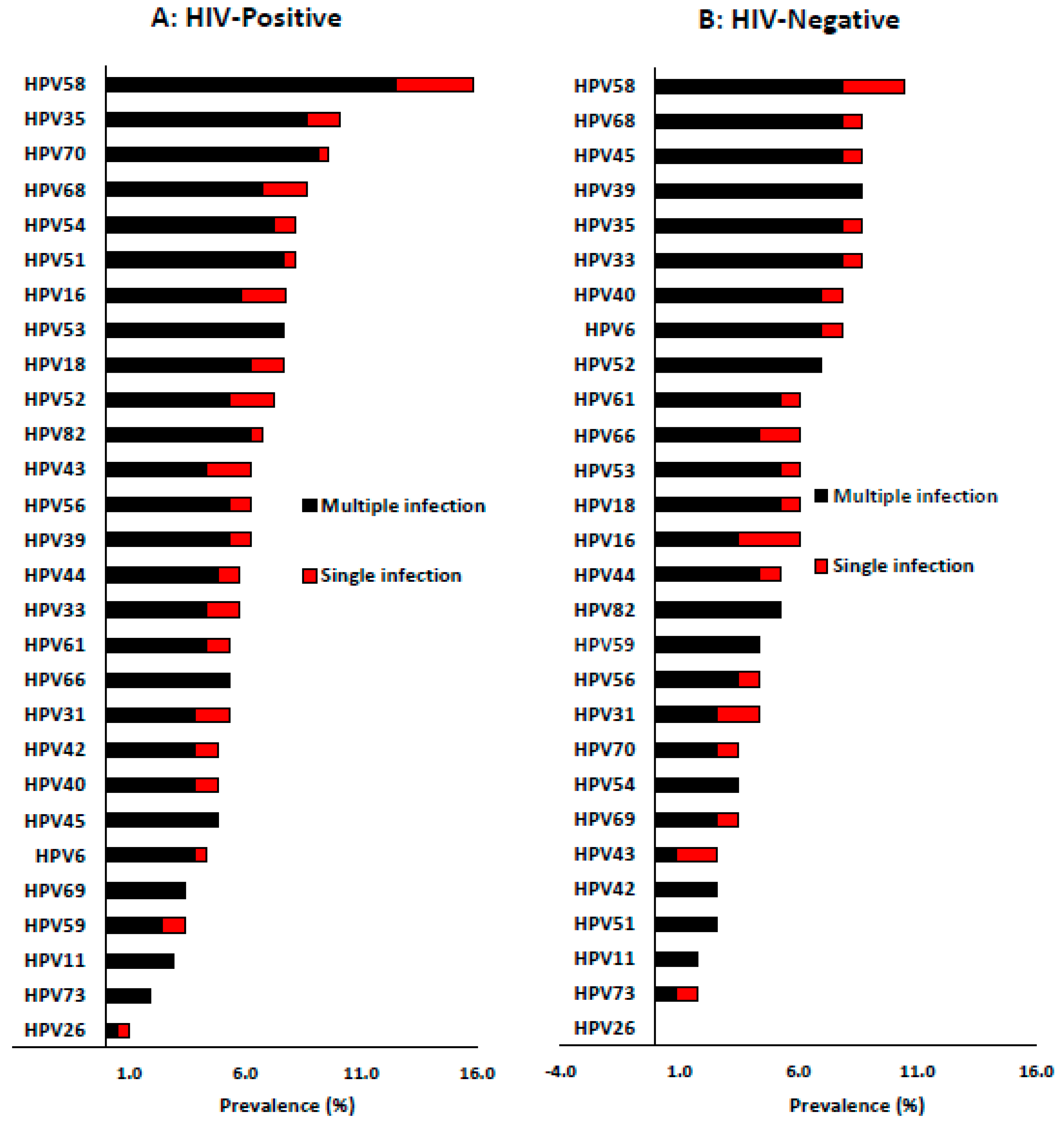
Among HIV-positive women, the four most dominant HR-HPV types were HPV-58 (15.9%), HPV-35 (10.1%), HPV-68 (8.7%) and HPV-51 (8.2%). The distribution pattern of the dominant single HR-HPV infection was HPV-58 (3.4%), HPV-68, -16, -52 (1.9% each) and HPV-35, -18, -33 and -31 (1.4%). Among the LR-HPV types, HPV-70 (9.6%), HPV-54 (8.2%), HPV-43 (6.3%) and HPV-44 (5.8%) were the most dominant types. However, the most dominant LR-HPV types presented as single HPV infection were HPV-43 (1.9%), HPV-54, -44, -61, -42, and -40 (1.0% each,
Figure 2A).
Among HIV-negative women, the most dominant HR-HPV types were HPV-58 (10.4%), HPV-68, -45, -39, -35, -33 (each 8.7%), HPV-52 (7.0%), HPV-16 and -18 (6.1%). In addition, the distribution of most common HR-HPV types presenting as single infection was slightly different from overall infection, and the most dominant single HR-HPV types were HPV-58 (2.6%), HPV-31 (1.7%), HPV-68, -45, -35, and -33 (0.9% each). HPV-40, -6 (9.0% each), HPV-61 (7.0%), HPV-44 (6.0%), HPV-70, and -54 (4.0% each) were the most common LR-HPV types among the HIV-negative women. While HPV-43 (1.7%), HPV-40, -6, -61, -44, and -70 (0.9%) were the most common types to present as single HPV infections (
Figure 2B).
3.3. The Influence of Age on Single and Multiple HPV Infection Stratified by HIV Status
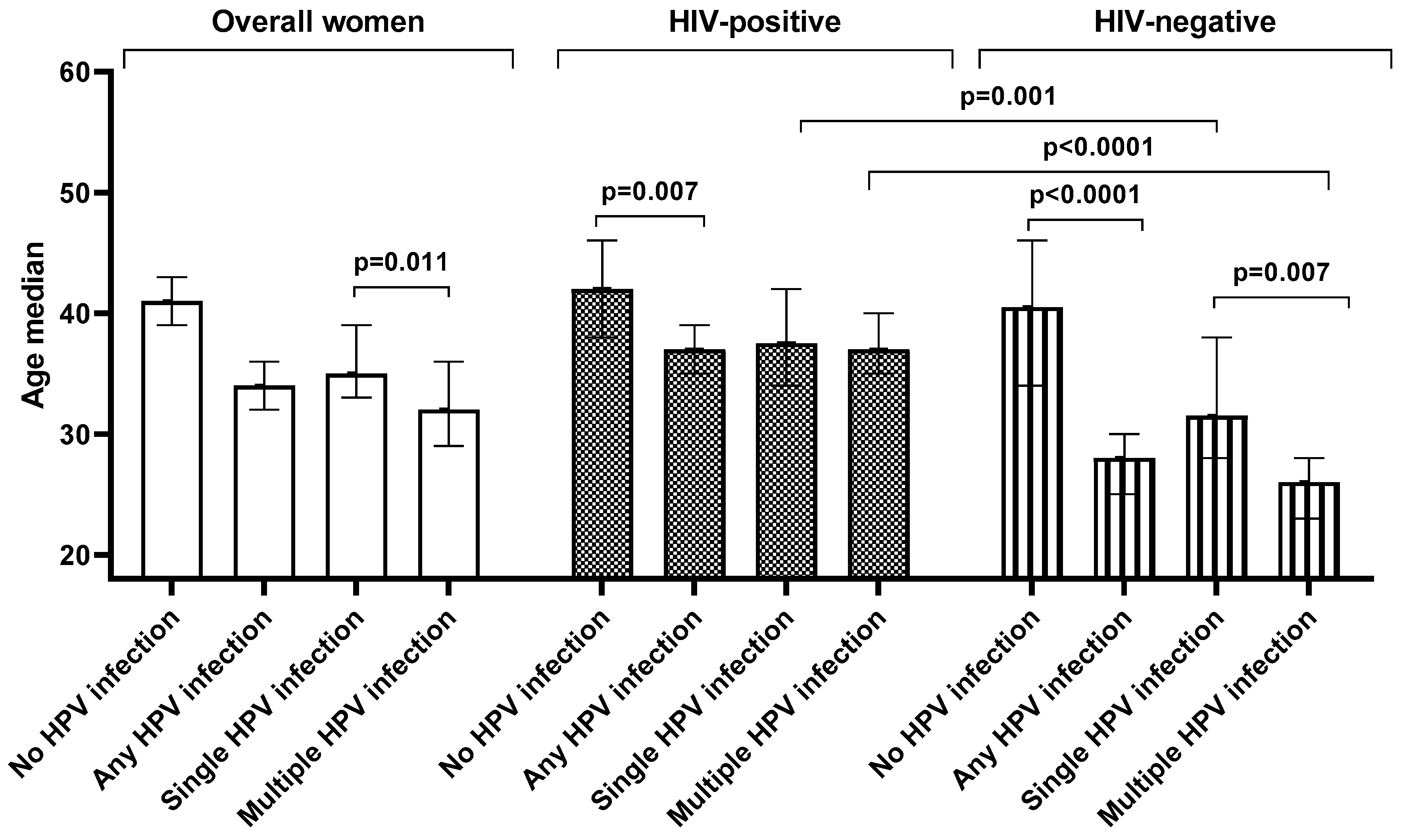
Overall, women infected with HPV (median: 34 years, IQR: 27–44 years) were significantly younger than those who were HPV negative (median: 41 years, IQR: 32–49 years, p < 0.0001). A similar trend was also observed among HIV-positive women (p = 0.007) and HIV-negative women (p < 0.0001). Similarly, in the entire sample women with single HPV infection (median: 35 years, IQR: 31–45 years) were significantly younger than the women without HPV infection (median: 41 years, IQR: 32–49 years, p = 0.013). However, this trend was further observed among HIV-negative women (p = 0.025) but not among HIV-positive (p = 0.182,
Figure 3).
Women with multiple infections (median age: 32 years, IQR: 26–40 years) were younger than women with no infection (median: 41 years, IQR: 32–49 years, p < 0.0001). A similar pattern was observed among HIV-positive women (p = 0.002) and HIV-negative (p < 0.0001). Women with single HPV infection (median: 35 years, IQR: 31–45 years) were older than women with multiple HPV infection (median: 32 years, IQR: 26–40 years, p = 0.011). When stratified according to HIV status, this pattern remains so among HIV-negative women (p = 0.007) but not among the HIV-positive women (p = 0.166,
Figure 3).
Among women with multiple HPV infection, HIV-negative women were younger (median: 26 years, IQR: 22–30 years) than the HIV-positive women (median: 37 years, IQR: 29–42 years, p < 0.0001). Similar observations were made among women with single HPV infection (median: 32 years, IQR: 28–38 years compared to median: 38 years, IQR: 33–47 years, p = 0.001) and overall HPV infection (median: 28 years, IQR: 23–33 years compared to median: 37 years, IQR: 31–44 years, p < 0.0001). However, among the HPV-negative women there was no difference in age between the HIV-positive (median: 42 years, IQR:38–46 years) and HIV-negative women (median: 41 years, IQR: 34–46 years; p = 0.601,
Figure 3).
3.4. HR and LR-HPV Prevalence by Age and HIV Status among Women Who Were Infected with HPV
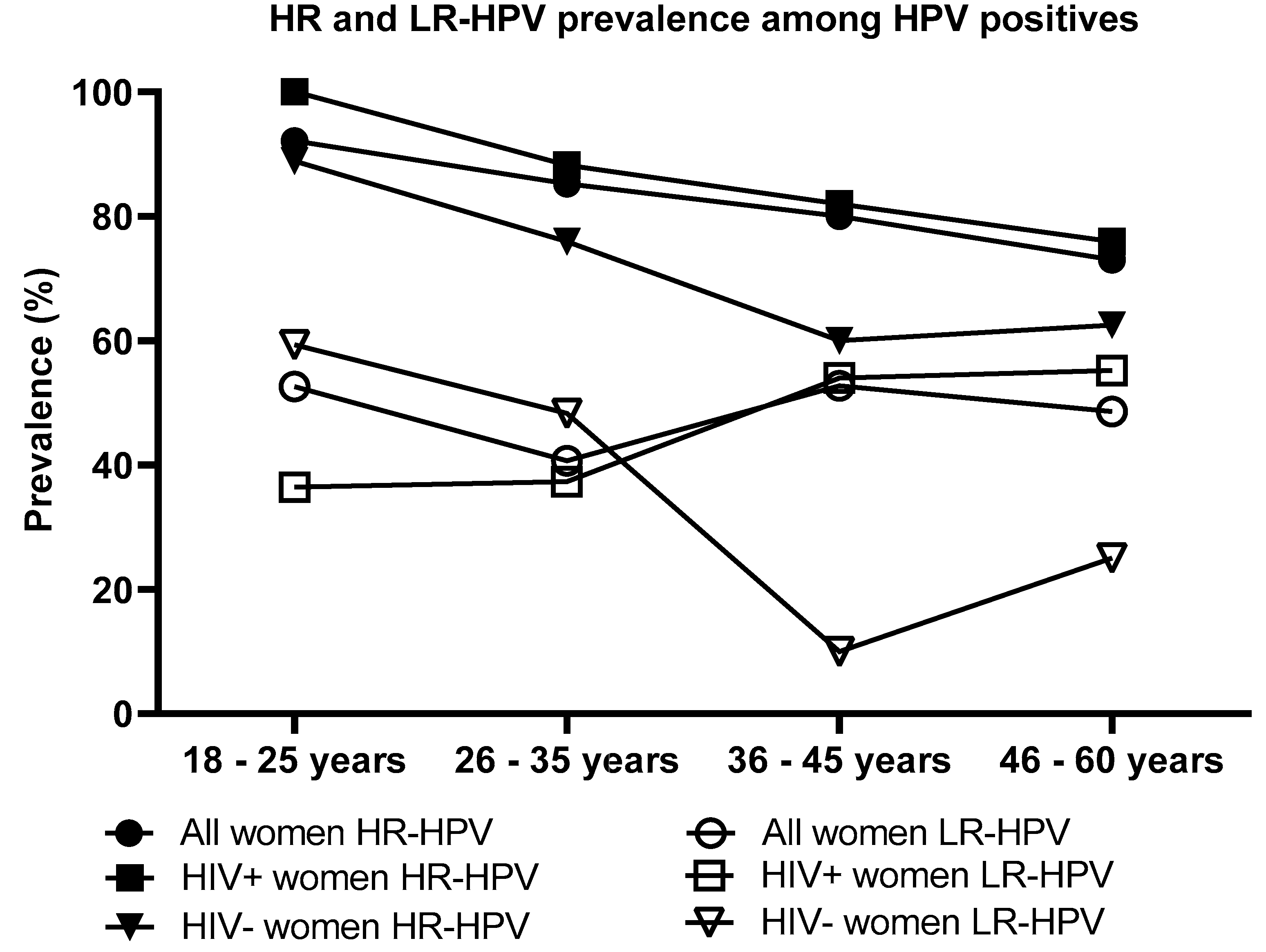
Majority of women infected with HPV had one or more HR-HPV type(s) (83.0%, 176/212), followed by LR-HPV types (47.2%, 100/212) and probable HR-HPV types (27.8%, 59/212). Between the HPV infected women that were HIV-positive or HIV-negative, there was no significant difference in the pattern of HR-HPV types (84.4%, 119/141; 82.6%, 57/69), probable HR-HPV types (26.2%, 37/141; 31.9% 22/69) and LR-HPV types (46.8%, 66/141; 49.3%, 34/69). HR-HPV infection was found to significantly decrease with increasing age among women who were HPV infected by any type (p = 0.02), but not when stratified according to HIV status (HIV-positive women p = 0.170 and HIV-negative women p = 0.192). There was no significant difference on HR or LR-HPV prevalence between the HIV-positive and HIV-negative women in each age group. Among all participants, LR-HPV infection was found not to significantly decrease with increasing age among women infected by any HPV types (overall: p = 0.792 overall; HIV-positive women: p = 0.067; and HIV-negative women: p = 0.077,
Figure 4).
3.5. Prevalence of HPV Types Targeted by Current Commercial HPV Vaccines
A proportion of 12.9% (42/325) were infected with one or more HPV types covered by the Cervarix® HPV vaccine (HPV-16 and/or -18), 18.8% (61/325) by Gardasil®4 HPV types (HPV-6, -11, -16 and/or -18) and 42.2% (137/325) by Gardasil®9 HPV types (HPV-6, -11, -16, -18, -31, -33, -45, -52 and/or -58). The HIV status did not significantly influence the prevalence for these categories (
Table 3). The prevalence of infection with types targeted by Gardasil®4 and Gardasil®9 HPV vaccine types were found to decrease with increasing age (p = 0.009 and p = 0.001) but not with Cervarix® HPV vaccine types (p = 0.074,
Table 3).
3.6. Alphapapillomavirus Species Prevalence According to HIV and Cervical Cytology
Overall, alpha-9 HPV species were the most dominant species (40.6%, 132/325) followed by alpha-7 species (29.8%, 97/325), alpha-6 (15.4%, 50/325), alpha-5 (14.8%, 48/325), alpha-10 species (12.3%, 40/325), alpha-13 (6.5%, 21/325), alpha-3 (5.5%, 18/325), alpha-1 (4.0%, 13/325) and alpha-11 (1.8%, 6/325). Among HIV-positive women, alpha-9 HPV species were the most dominant species (43.3%, 90/208) followed by alpha-7 (34.6%, 72/208), alpha-5 (16.8%, 35/208), alpha-6 (15.9%, 33/208), alpha-8 and -10 (10.8%, 22/208 each), alpha-13 (8.2%, 17/208), alpha-3 (5.3%, 11/208), alpha-1 (4.8%, 10/208) and alpha-11 species (1.9%, 4/208). While among the HIV-negative women the pattern changed, in which alpha-9 HPV species were the most dominant (35.7%, 41/115) followed by alpha-7 (20.9%, 24/115), alpha-10 (15.7%, 18/115), alpha-6 (14.8%, 17/115), alpha-5 (11.3%, 13/115), alpha-8 (10.4%, 12/115), alpha-13 (3.5%, 4/115), alpha-1 (2.6%, 3/115) and alpha-11 (1.7%, 2/115). Infection with multiple HPV alpha species infection was more common than a single infection in the overall population and when stratified according to HIV status (
Table 4). In the overall population, alpha-9 HPV species were always part of the most dominant HPV alpha species co-infection, which were alpha-9 and -7 species (17.5%, 57/325), alpha-9 and -5 species (9.8%, 32/325), alpha-9 and -5 species (9.5%, 31/325) and alpha-9 and -10 (7.4%, 24/325).
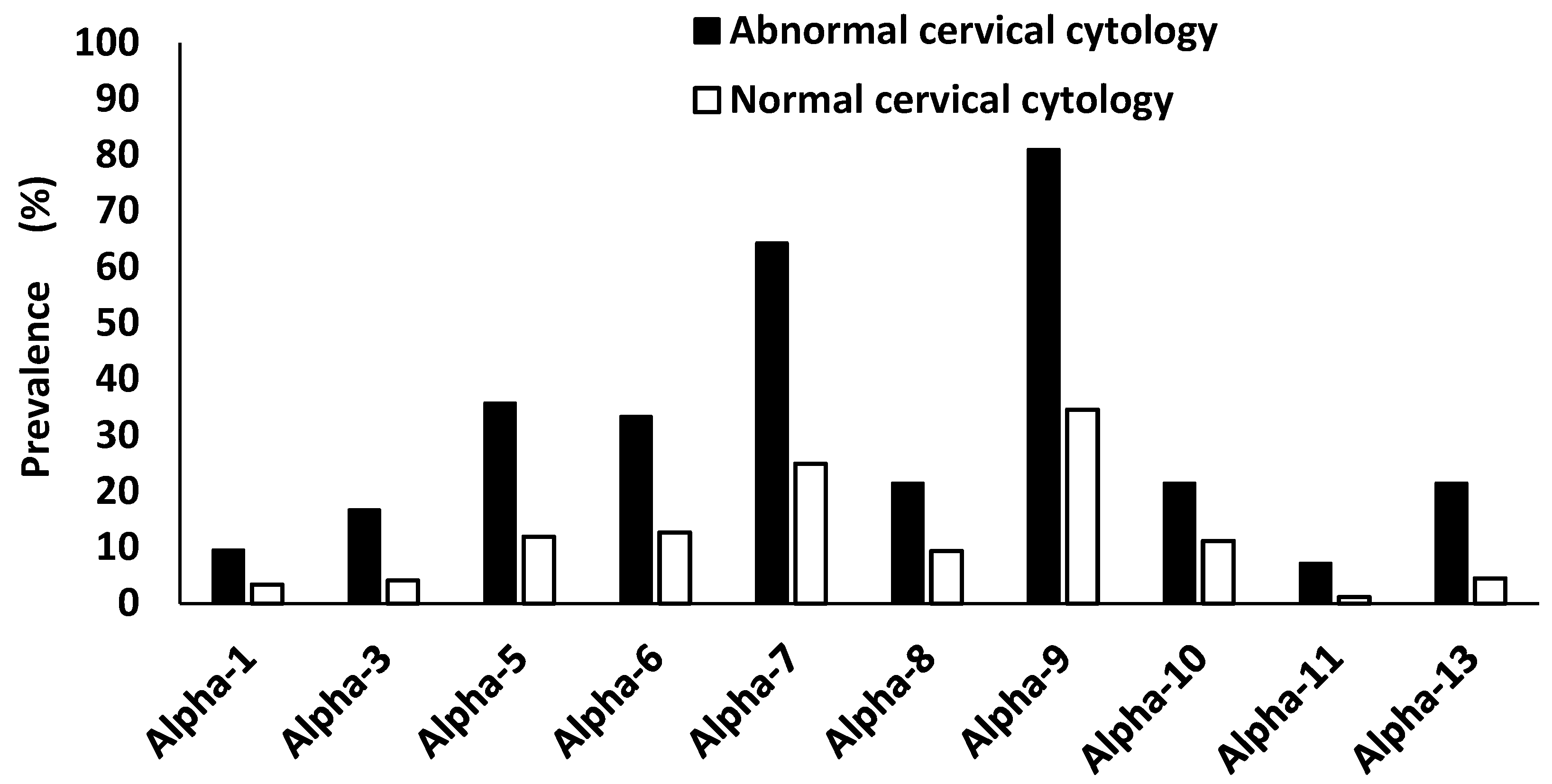
A proportion of 60.6% (163/269) women who were negative for intraepithelial lesion or malignancy (NILM) were found to be HPV positive, and 47.2% (127/269) were positive with HR-HPV types. All women who had abnormal cervical cytology (ASCUS, LSIL, ASC-H and HSIL) were HPV infected (100.0%, 42/42), and 97.6% (41/42) were infected with HR-HPV types. Women with abnormal cervical cytology were more likely to have higher semi-quantified HPV viral load than the women who were negative for intraepithelial lesion or malignancy (p < 0.0001). Due to small sample size this was not further stratified according to HIV status and age. Among women with abnormal cervical cytology, HPV alpha-9 species where the most dominant species (81.0%, 34/42) followed by alpha-7 species (64.3%, 27/42), alpha-5 species (35.7%, 15/42), alpha-6 species (33.3%, 14/42), alpha-8 species and alpha-13 species (21.4%, 9/42 each), alpha-3 species (16.7%, 7/42), alpha-3 species (16.7%, 7/42), alpha-1 species (9.5%, 4/42) and alpha-11 species (7.1% (3/42),
Figure 5).
4. Discussion
According to our knowledge, this is the first report on cervical HR, LR, probable HR-HPV and
alphapapillomavirus species prevalence, characteristics and distribution according to age and HIV among women attending public community health facility in the Eastern Cape Province of South Africa. There was a high overall HPV and HR-HPV prevalence even though 82.8% of the participants had normal cervical cytology. An HIV-positive status is commonly associated with increased HPV prevalence [
19,
27]; however, in the current study, there was no difference between the HIV-negative and HIV-positive women. This could be due to the high proportion of HIV-negative women who were significantly younger than the HIV-positives. The literature reports that the HPV prevalence tends to be higher among the young age groups [
28,
29]; similar findings were observed in the current study. In addition, in the current study, the HPV prevalence was similar between the HIV-positive and HIV-negative women of 18–25 years and 26–35 years age groups, whereas it was higher among the HIV-positive women of 36 -45 years and 46–60 years age groups than the HIV-negative women. The current study observed that younger individuals had different distributions of HPV types than older individuals. This could be due to different factors like sexual activity patterns, immune system changes, or exposure to other HPV types over time [
30].
It was interesting to note that HPV-58 was the most dominant type for both the multiple infection and single infection formats among the overall study population, HIV-positive and HIV-negative women. In Southern Africa, HPV-58 is not commonly reported as the most dominant HPV type among women with normal cervical cytology [
10,
31]. We previously reported HPV-58 as the most dominant type among the Western Cape province adolescents [
32]. However, dominant HPV-58 prevalence has been reported among black women residing in the rural community of Brazil [
33] and among Chinese women with normal cervical cytology [
34]. HPV-35 was the second most dominant type in the overall population and among HIV-positive women. HPV-35 is among the HR-HPV types that are not covered in current commercial HPV vaccines (Cervarix®, Gardasil®4 and Gardasil®9) despite being highly prevalent in populations with African ancestry origin with HSIL [
35,
36,
37,
38]. Okeke (2024) detailly explains the urgent need for HPV-35 genomic epidemiology among women of African ancestry and the justification of the necessity of commercial HPV vaccine targeting HPV-35 [
36]. Almost half of the study population were infected with HPV types targeted by the Gardasil®9 HPV vaccine, showing that South African women will greatly benefit from the currently commercial HPV vaccine targeting the high number of HPV types.
Alpha-9 species and -7 were the most dominant species among the overall population, HIV-positive women, HIV-negative women, and women with abnormal and normal cervical cytology. It was interesting to note that the alpha-9 species dominated the multiple HPV alpha-species infection. HPV types belonging to alpha-9 and -7 mostly are HR-HPV types associated with the majority of cervical cancer cases, and seven of these types (HPV-16, -18, -31, -33, -45, -52 and -58) are targeted by the Gardasil®9 HPV vaccine [
39]. Zhao et al. (2022) reported that women infected with alpha-9 HPV species had an increased incidence of HSIL compared to when infected with other alpha HPV species [
40]. A significant portion of women without evident cytological abnormalities had a high burden of alpha-9 species in the form of single and multiple HPV species infection, demonstrating an increased risk of progressing to cervical cancer [
40]. All women who had abnormal cervical cytology were infected with HPV, and almost all of them had HR-HPV types. More than 80% of these women were infected with alpha-9 HPV types which demonstrates a strong association between HR-HPV and alpha-9 HPV types infection and the high predisposition to the development of abnormal cervical cells, which are precursors to cervical cancer [
1,
3].
The cervical cancer screening uptake by South African women is also reported to be low [
14,
41]. In the South African province where the study was conducted, the knowledge about HPV and its associated cervical cancer is very limited [
41,
42]. It is of high concern that the current study observed very high overall HPV, HR-HPV and alpha-9 species prevalence among the Eastern Cape province women regardless of HIV status. These findings underscore the importance of regular cervical cancer screening not only among HIV-positive women but for all women.
Several factors could act as the limitations of the current study. First, this study is a cross-sectional study, and a longitudinal study could provide a very detailed picture of the reported HPV profile among Eastern Cape women. Secondly, the small sample size could be another limitation. Thirdly, the study was done in only one health facility. Enrolment of participants from several health centres widespread all over the Eastern Cape province will yield a sample more representative of the target population. Despite these limitations, the findings of the study remain interesting and important for Eastern Cape Province and South Africa.
5. Conclusions
High overall HPV, HR-HPV, and alpha-9 species prevalence despite the HIV status were observed among women attending the public health facility. The high prevalence of HR-HPV types among women without cervical lesions underscores the urgent need to implement cervical cancer screening using an HPV-DNA test. These findings contribute to the limited HPV distribution data among Eastern Cape women. The South African Department of Health’s policymakers could use the study’s findings to improve HPV-related policy and assess the effectiveness of the HPV vaccination.
Author Contributions
Conceptualization, Z.Z.A.M., S.T., L.M.F. and C.B.B.; methodology, Z.Z.A.M., S.K., S.T., L.M.F. and C.B.B.; formal analysis, Z.Z.A.M. and C.B.B.; investigation, Z.Z.A.M., S.K., S.T., L.M.F. and C.B.B.; resources, Z.Z.A.M., S.T., L.M.F. and C.B.B.; writing—original draft preparation, Z.Z.A.M.; writing—review and editing, Z.Z.A.M., S.K., S.T., L.M.F. and C.B.B.; visualization, Z.Z.A.M. and C.B.B.; supervision, Z.Z.A.M.; project administration, Z.Z.A.M.; funding acquisition, Z.Z.A.M. All authors reviewed, edited and approved the final manuscript.
Funding
This research was supported by the South African National Research Foundation Research Development Grant for Y-Rated Researchers, Grant number 137779.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Human Research Ethics Committee of Walter Sisulu University (HREC: 004/2022) and Eastern Cape Province Department of Health (EC_202203_011).
Informed Consent Statement
Written informed consent was obtained from all participants involved in the study.
Data Availability Statement
Data is available from the corresponding author on request.
Acknowledgments
We would like to acknowledge Prof. Teke Apalata and Dr. Sikhumbuzo Mabunda for advising during the conceptualization of the study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study’s design, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Dabán-López, P.; Fernández-Martínez, N.F.; Petrova, D.; Rodríguez-Barranco, M.; Jiménez-Moleón, J.J.; Gutierrez, J.; Sánchez, M.J. Epidemiology of human papillomavirus-associated anogenital cancers in Granada: A three-decade population-based study. Front. Public Health 2023, 11, 1205170. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, A.; Cachay, E.; Ocampo, A.; Poveda, E. Update on the epidemiological features and clinical implications of human papillomavirus infection (HPV) and human immunodeficiency virus (HIV) coinfection. Microorganisms 2022, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Pedraza-Chaverri, J. Human papillomavirus-related cancers and mitochondria. Virus research 2020, 286, 198016. [Google Scholar] [CrossRef]
- Chesson, H.W.; DUNNE, E.F.; Hariri, S.; Markowitz, L.E. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sexually Transmitted Diseases 2014, 41, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Okunade, K.S. Human papillomavirus and cervical cancer. Journal of Obstetrics and Gynaecology 2020, 40, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Skelin, J.; Tomaić, V. Comparative Analysis of Alpha and Beta HPV E6 Oncoproteins: Insights into Functional Distinctions and Divergent Mechanisms of Pathogenesis. Viruses 2023, 15, 2253. [Google Scholar] [CrossRef]
- Ozaydin-Yavuz, G.; Bilgili, S.; Guducuoglu, H.; Yavuz, I.; Elibuyuk-Aksac, S.; Karadag, A. Determinants of hgh-risk human papillomavirus infection in anogenital warts. Advances in Dermatology and Allergology/Postępy Dermatologii i Alergologii 2019, 36, 76–81. [Google Scholar] [CrossRef]
- Alrefai, E.A.; Alhejaili, R.T.; Haddad, S.A. Human Papillomavirus and Its Association With Cervical Cancer: A Review. Cureus 2024, 16. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. International Journal of Cancer. Journal International du Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Ogembo, R.K.; Gona, P.N.; Seymour, A.J.; Park, H.S.-M.; Bain, P.A.; Maranda, L.; Ogembo, J.G. Prevalence of human papillomavirus genotypes among African women with normal cervical cytology and neoplasia: A systematic review and meta-analysis. PloS ONE 2015, 10, e0122488. [Google Scholar] [CrossRef]
- Taku, O.; Businge, C.B.; Mdaka, M.L.; Phohlo, K.; Basera, W.; Garcia-Jardon, M.; Meiring, T.L.; Gyllensten, U.; Williamson, A.-L.; Mbulawa, Z.Z. Human papillomavirus prevalence and risk factors among HIV-negative and HIV-positive women residing in rural eastern cape, South Africa. International Journal of Infectious Diseases 2020, 95, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Gómez, D.; Muñoz, J.; Bosch, F.; de Sanjosé, S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in South Africa. Summary Report 2023. [Google Scholar]
- Dhokotera, T.; Asangbeh, S.; Bohlius, J.; Singh, E.; Egger, M.; Rohner, E.; Ncayiyana, J.; Clifford, G.M.; Olago, V.; Sengayi-Muchengeti, M. Cervical cancer in women living in South Africa: A record linkage study of the National Health Laboratory Service and the National Cancer Registry. ecancermedicalscience 2022, 16. [Google Scholar] [CrossRef] [PubMed]
- Olorunfemi, G.; Libhaber, E.; Ezechi, O.C.; Musenge, E. Population-Based Temporal Trends and Ethnic Disparity in Cervical Cancer Mortality in South Africa (1999–2018): A Join Point and Age–Period–Cohort Regression Analyses. Cancers 2022, 14, 6256. [Google Scholar] [CrossRef] [PubMed]
- Boily, M.-C.; Barnabas, R.V.; Rönn, M.M.; Bayer, C.J.; van Schalkwyk, C.; Soni, N.; Rao, D.W.; Staadegaard, L.; Liu, G.; Silhol, R. Estimating the effect of HIV on cervical cancer elimination in South Africa: Comparative modelling of the impact of vaccination and screening. EClinicalMedicine 2022, 54. [Google Scholar] [CrossRef]
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Ibrahim Khalil, A.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health 2021, 9, e161–e169. [Google Scholar] [CrossRef]
- Williamson, A.-L. The Interaction between Human Immunodeficiency Virus and Human Papillomaviruses in Heterosexuals in Africa. Journal of Clinical Medicine 2015, 4, 579–592. [Google Scholar] [CrossRef]
- Dreyer, G. Clinical implications of the interaction between HPV and HIV infections. Best practice & research. Clinical obstetrics & gynaecology 2018, 47, 95–106. [Google Scholar] [CrossRef]
- Castle, P.E.; Einstein, M.H.; Sahasrabuddhe, V.V. Cervical cancer prevention and control in women living with human immunodeficiency virus. CA: A Cancer Journal for Clinicians 2021, 71, 505–526. [Google Scholar] [CrossRef]
- UNAIDS. UNAIDS data 2023. 2023. [Google Scholar]
- Rahatgaonkar, V.G.; Deshpande, A.A.; Oka, G.A. Screening for cervical cancer in HIV-infected women: A review of literature. Indian Journal of Cancer 2021, 58, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Amponsah-Dacosta, E.; Blose, N.; Nkwinika, V.V.; Chepkurui, V. Human papillomavirus vaccination in South Africa: Programmatic challenges and opportunities for integration with other adolescent health services? Frontiers in Public Health 2022, 10, 799984. [Google Scholar] [CrossRef] [PubMed]
- Delany-Moretlwe, S.; Kelley, K.F.; James, S.; Scorgie, F.; Subedar, H.; Dlamini, N.R.; Pillay, Y.; Naidoo, N.; Chikandiwa, A.; Rees, H. Human papillomavirus vaccine introduction in South Africa: Implementation lessons from an evaluation of the national school-based vaccination campaign. Global Health: Science and Practice 2018, 6, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Botha, M.; Mabenge, M.; Makua, M.; Mbodi, M.; Rogers, L.; Sebitloane, M.; Smith, T.; Van der Merwe, F.; Williamson, A.; Whittaker, J. Cervical Cancer Screening Guidelines for South Africa. African Journal of Obstetrics and Gynaecology 2023, 1, 27–31. [Google Scholar]
- Kremer, W.W.; Van Zummeren, M.; Breytenbach, E.; Richter, K.L.; Steenbergen, R.D.; Meijer, C.J.; Dreyer, G. The use of molecular markers for cervical screening of women living with HIV in South Africa. Aids 2019, 33, 2035–2042. [Google Scholar] [CrossRef]
- Mbulawa, Z.Z.; Marais, D.J.; Johnson, L.F.; Coetzee, D.; Williamson, A.L. Impact of human immunodeficiency virus on the natural history of human papillomavirus genital infection in South African men and women. The Journal of Infectious Diseases 2012, 206, 15–27. [Google Scholar] [CrossRef]
- Clarke, M.A.; Risley, C.; Stewart, M.W.; Geisinger, K.R.; Hiser, L.M.; Morgan, J.C.; Owens, K.J.; Ayyalasomayajula, K.; Rives, R.M.; Jannela, A. Age-specific prevalence of human papillomavirus and abnormal cytology at baseline in a diverse statewide prospective cohort of individuals undergoing cervical cancer screening in Mississippi. Cancer Medicine 2021, 10, 8641–8650. [Google Scholar] [CrossRef]
- Mbulawa, Z.Z.; Coetzee, D.; Williamson, A.L. Human papillomavirus prevalence in South African women and men according to age and human immunodeficiency virus status. BMC Infectious Diseases 2015, 15, 459. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, X.; Lin, X.; Guo, B.; Yu, Y. Deciphering age-specific molecular features in cervical cancer and constructing an angio-immune prognostic model. Medicine 2024, 103, e37717. [Google Scholar] [CrossRef]
- Seyoum, A.; Assefa, N.; Gure, T.; Seyoum, B.; Mulu, A.; Mihret, A. Prevalence and genotype distribution of high-risk human papillomavirus infection among Sub-Saharan African women: A systematic review and meta-analysis. Frontiers in Public Health 2022, 10, 890880. [Google Scholar] [CrossRef] [PubMed]
- Mbulawa, Z.Z.A.; van Schalkwyk, C.; Hu, N.C.; Meiring, T.L.; Barnabas, S.; Dabee, S.; Jaspan, H.; Kriek, J.M.; Jaumdally, S.Z.; Muller, E.; et al. High human papillomavirus (HPV) prevalence in South African adolescents and young women encourages expanded HPV vaccination campaigns. 2018, 13, e0190166. PLoS ONE 2018, 13, e0190166. [Google Scholar] [CrossRef] [PubMed]
- Volpini, L.P.B.; Dias, J.A.; de Freitas, L.B.; Silva, M.; Miranda, A.E.; Spano, L.C. Viral load and high prevalence of HR-HPV52 and 58 types in black women from rural communities. BMC infectious diseases 2021, 21, 362. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liang, H.; Yan, Y.; Bian, R.; Huang, W.; Zhang, X.; Nie, J. Distribution of HPV types among women with HPV-related diseases and exploration of lineages and variants of HPV 52 and 58 among HPV-infected patients in China: A systematic literature review. Human Vaccines & Immunotherapeutics 2024, 20, 2343192. [Google Scholar] [CrossRef]
- Mbulawa, Z.Z.; Phohlo, K.; Garcia-Jardon, M.; Williamson, A.-L.; Businge, C.B. High human papillomavirus (HPV)-35 prevalence among South African women with cervical intraepithelial neoplasia warrants attention. PloS ONE 2022, 17, e0264498. [Google Scholar] [CrossRef] [PubMed]
- Okeke, S.U. Fighting cervical cancer in Africa: Taking a closer look at human papillomavirus 35. African Journal of Laboratory Medicine 2024, 13, 1–3. [Google Scholar] [CrossRef]
- Lemba, P.C.T.; Boumba, L.M.A.; Péré, H.; Nganga, P.C.; Veyer, D.; Puech, J.; Bouassa, R.-S.M.; Malanda-Kiminou, P.; Moukassa, D.; Bélec, L. Human papillomavirus genotype distribution by cytological status and associated risk factors in the general population of Congolese women living in urban and rural areas: Implications for cervical cancer prevention. Infectious Diseases Now 2023, 53, 104762. [Google Scholar] [CrossRef]
- Clifford, G.M.; de Vuyst, H.; Tenet, V.; Plummer, M.; Tully, S.; Franceschi, S. Effect of HIV infection on human papillomavirus types causing invasive cervical cancer in Africa. Journal of acquired immune deficiency syndromes (1999) 2016, 73, 332. [Google Scholar] [CrossRef]
- Wang, W.; Kothari, S.; Skufca, J.; Giuliano, A.R.; Sundström, K.; Nygård, M.; Koro, C.; Baay, M.; Verstraeten, T.; Luxembourg, A. Real-world impact and effectiveness of the quadrivalent HPV vaccine: An updated systematic literature review. Expert Review of Vaccines 2022, 21, 1799–1817. [Google Scholar] [CrossRef]
- Zhao, J.; Zhan, Q.; Guo, J.; Liu, M.; Ruan, Y.; Zhu, T.; Han, L.; Li, F. Phylogeny and polymorphism in the E6 and E7 of human papillomavirus: Alpha-9 (HPV16, 31, 33, 52, 58), alpha-5 (HPV51), alpha-6 (HPV53, 66), alpha-7 (HPV18, 39, 59, 68) and alpha-10 (HPV6, 44) in women from Shanghai. Infectious agents and cancer 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Mbulawa, Z.Z.; Mahlangu, L.L.; Makhabane, E.; Mavivane, S.; Nongcula, S.; Phafa, A.; Sihlobo, A.; Zide, M.; Mkiva, A.; Ngobe, T.N. Poor Cervical Cancer Knowledge and Awareness among Women and Men in the Eastern Cape Province Rural Community. Int. J. Environ. Res. Public Health 2023, 20, 6916. [Google Scholar] [CrossRef] [PubMed]
- Ncane, Z.; Faleni, M.; Pulido-Estrada, G.; Apalata, T.R.; Mabunda, S.A.; Chitha, W.; Nomatshila, S.C. Knowledge on Cervical Cancer Services and Associated Risk Factors by Health Workers in the Eastern Cape Province. Healthcare 2023, 11, 325. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Prevalence and distribution of different human papillomavirus types according to single and multiple infection among women of Eastern Cape Province of South Africa.
Figure 1.
Prevalence and distribution of different human papillomavirus types according to single and multiple infection among women of Eastern Cape Province of South Africa.
Figure 2.
Prevalence and distribution of different human papillomavirus types according to single or multiple infection among HIV-positive (A) and HIV-negative (B) women of Eastern Cape Province of South Africa.
Figure 2.
Prevalence and distribution of different human papillomavirus types according to single or multiple infection among HIV-positive (A) and HIV-negative (B) women of Eastern Cape Province of South Africa.
Figure 3.
The effect of age and human immunodeficiency virus (HIV) status on overall, single and multiple human papillomavirus infection among women of Eastern Cape Province.
Figure 3.
The effect of age and human immunodeficiency virus (HIV) status on overall, single and multiple human papillomavirus infection among women of Eastern Cape Province.
Figure 4.
The high-risk and low-risk human papillomavirus (HPV) prevalence by age and HIV status among women who were HPV infected by any of the detected types.
Figure 4.
The high-risk and low-risk human papillomavirus (HPV) prevalence by age and HIV status among women who were HPV infected by any of the detected types.
Figure 5.
The prevalence of alphapapillomavirus species according to cervical cytology among women of Eastern Cape Province. Alpha-1: HPV-42. Alpha-3: HPV-61. Alpha-5: HPV-26, -51, -69, -82. Alpha-6: HPV-53, -56, -66. Alpha-7: HPV-18, -39, -45, -59, -68, -70. Alpha-8: HPV-40, -43. Alpha-9: HPV-16, -31, -33, -35, -52, -58. Alpha-10: HPV-6, -11, -44. Alpha-11: HPV-73. Alpha-13: HPV-54.
Figure 5.
The prevalence of alphapapillomavirus species according to cervical cytology among women of Eastern Cape Province. Alpha-1: HPV-42. Alpha-3: HPV-61. Alpha-5: HPV-26, -51, -69, -82. Alpha-6: HPV-53, -56, -66. Alpha-7: HPV-18, -39, -45, -59, -68, -70. Alpha-8: HPV-40, -43. Alpha-9: HPV-16, -31, -33, -35, -52, -58. Alpha-10: HPV-6, -11, -44. Alpha-11: HPV-73. Alpha-13: HPV-54.
Table 1.
Brief study participants’ characteristics.
Table 1.
Brief study participants’ characteristics.
| Characteristic |
% |
n/N |
| Age groups |
|
|
| 18–25 years |
14.5 |
47/325 |
| 26–35 years |
33.8 |
110/325 |
| 36–45 years |
26.8 |
87/325 |
| 46–60 years |
24.6 |
80/325 |
| Missing |
0.3 |
1/325 |
| HIV status |
|
|
| HIV-positive |
64.0 |
208/325 |
| HIV-negative |
35.4 |
115/325 |
| Missing |
0.6 |
2/325 |
| Cervical cytology |
|
|
| NILM1
|
82.8 |
269/325 |
| ASCUS2
|
2.5 |
8/325 |
| LSIL3
|
4.9 |
16/325 |
| ASC-H4
|
1.2 |
4/325 |
| HSIL5
|
4.3 |
14/325 |
| CDD6
|
4.0 |
13/325 |
| Missing |
0.3 |
1/325 |
Table 2.
Prevalence of single and multiple human papillomavirus (HPV) infections according to human immunodeficiency virus (HIV) status and age among women of Eastern Cape Province of South Africa.
Table 2.
Prevalence of single and multiple human papillomavirus (HPV) infections according to human immunodeficiency virus (HIV) status and age among women of Eastern Cape Province of South Africa.
| |
All Women |
HIV-Positive Women |
|
HIV-Negative Women |
|
| |
% |
95% CI |
n/N |
% |
95% CI |
n/N |
p-Value |
% |
95% CI |
n/N |
p-Value |
| Overall HPV |
65.2 |
59.9–70.2 |
212/325 |
67.8 |
61.2–73.8 |
141/208 |
|
60.0 |
50.9–68.5 |
69/115 |
|
| Single HPV infection |
26.5 |
22.0–31.5 |
86/325 |
26.9 |
21.3–33.3 |
56/208 |
|
24.3 |
17.4–33.0 |
28/115 |
|
| Dual HPV infection |
14.2 |
10.8–18.4 |
46/325 |
16.8 |
12.3–22.5 |
35/208 |
|
9.6 |
5.3–16.5 |
11/115 |
|
| Triple HPV infection |
10.5 |
7.6–14.3 |
34/325 |
10.1 |
6.6–15.0 |
21/208 |
|
11.3 |
6.6–18.5 |
13/115 |
|
| Quadruple+ HPV infection* |
14.2 |
10.8–18.4 |
46/325 |
13.9 |
9.8–19.4 |
29/208 |
|
14.8 |
9.3–22.5 |
17/115 |
|
| Multiple HPV infection |
38.8 |
33.6–44.2 |
126/325 |
40.9 |
34.4–47.7 |
85/208 |
|
35.7 |
27.5–44.8 |
41/115 |
|
| HR-HPV |
53.8 |
48.4–59.2 |
175/325 |
56.7 |
49.9–63.3 |
118/208 |
|
47.8 |
38.9–56.9 |
55/115 |
|
| Probable HR-HPV |
18.2 |
14.3–22.7 |
59/325 |
17.8 |
13.2–23.6 |
37/208 |
|
19.1 |
12.9–27.3 |
22/115 |
|
| LR-HPV |
31.1 |
26.3–36.3 |
101/325 |
32.2 |
26.2–38.8 |
67/208 |
|
29.6 |
22–38.5 |
34/115 |
|
| Overall HPV infection by age groups |
|
|
|
|
|
|
|
|
|
|
|
| 18–25 years |
80.9 |
67.2–89.8 |
38/47 |
91.7 |
62.5–99.9 |
11/12 |
ref |
77.1 |
60.7–88.2 |
27/35 |
ref |
| 26–35 years |
73.6 |
64.7–81.0 |
81/110 |
71.8 |
60.4–81.0 |
51/71 |
0.279 |
76.3 |
60.6–87.2 |
29/38 |
>0.999 |
| 36–45 years |
63.2 |
52.7–72.6 |
55/87 |
72.5 |
60.9–81.7 |
50/69 |
0.277 |
27.8 |
12.2–51.2 |
5/18 |
0.001 |
| 46–60 years |
46.3 |
35.8–57.1 |
37/80 |
51.8 |
39.0–64.3 |
29/56 |
0.011 |
33.3 |
17.8–53.4 |
8/24 |
0.001 |
| p for trend |
|
p < 0.0001 |
|
|
p = 0.004 |
|
|
|
p < 0.001 |
|
|
Table 3.
Prevalence of human papillomavirus (HPV) types targeted by current commercial HPV vaccines according to HIV status and age.
Table 3.
Prevalence of human papillomavirus (HPV) types targeted by current commercial HPV vaccines according to HIV status and age.
| |
Cervarix® |
Gardasil®4 |
Gardasil®9 |
| |
n |
% |
n |
% |
n |
% |
| All women, n = 325 |
42 |
12.9 |
56 |
17.2 |
137 |
42.2 |
| HIV-positive women, n = 208 |
28 |
13.5 |
37 |
17.8 |
92 |
44.2 |
| HIV-negative women, n = 115 |
13 |
11.3 |
18 |
15.7 |
45 |
39.1 |
| 18–25 years, n = 47 |
8 |
17.0 |
14 |
29.8 |
28 |
59.6 |
| 26–35 years, n = 110 |
16 |
14.5 |
19 |
21.8 |
51 |
46.4 |
| 36–45 years, n = 87 |
13 |
14.9 |
14 |
16.1 |
34 |
39.1 |
| 46–60 years, n = 80 |
5 |
6.3 |
8 |
10.0 |
24 |
30.0 |
Table 4.
Alphapapillomavirus prevalence among women of Eastern Cape Province.
Table 4.
Alphapapillomavirus prevalence among women of Eastern Cape Province.
| |
|
|
All women, N = 325 |
HIV-positive women, N = 208 |
HIV-negative women, N = 115 |
| |
All infection |
Single infection |
Multiple infection |
All infection |
Single infection |
Multiple infection |
All infection |
Single infection |
Multiple infection |
| Species |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
| Alpha-1 |
13 |
4.0 |
2 |
0.6 |
11 |
3.4 |
10 |
4.8 |
2 |
1.0 |
8 |
3.8 |
3 |
2.6 |
0 |
0.0 |
3 |
2.6 |
| Alpha-3 |
18 |
5.5 |
3 |
0.9 |
15 |
4.6 |
11 |
5.3 |
2 |
1.0 |
9 |
4.3 |
7 |
6.1 |
1 |
0.9 |
6 |
5.2 |
| Alpha-5 |
48 |
14.8 |
4 |
1.2 |
44 |
13.5 |
35 |
16.8 |
3 |
1.4 |
32 |
15.4 |
13 |
11.3 |
1 |
0.9 |
12 |
10.4 |
| Alpha-6 |
50 |
15.4 |
7 |
2.2 |
43 |
13.2 |
33 |
15.9 |
3 |
1.4 |
30 |
14.4 |
17 |
14.8 |
4 |
3.5 |
13 |
11.3 |
| Alpha-7 |
97 |
29.8 |
19 |
5.8 |
78 |
24.0 |
72 |
34.6 |
13 |
6.3 |
59 |
28.4 |
24 |
20.9 |
5 |
4.3 |
19 |
16.5 |
| Alpha-8 |
34 |
10.5 |
10 |
3.1 |
24 |
7.4 |
22 |
10.6 |
7 |
3.4 |
15 |
7.2 |
12 |
10.4 |
3 |
2.6 |
9 |
7.8 |
| Alpha-9 |
132 |
40.6 |
38 |
11.7 |
94 |
28.9 |
90 |
43.3 |
25 |
12.0 |
65 |
31.3 |
41 |
35.7 |
12 |
10.4 |
29 |
25.2 |
| Alpha-10 |
40 |
12.3 |
5 |
1.5 |
35 |
10.8 |
22 |
10.6 |
3 |
1.4 |
19 |
9.1 |
18 |
15.7 |
2 |
1.7 |
16 |
13.9 |
| Alpha-11 |
6 |
1.8 |
1 |
0.3 |
5 |
1.5 |
4 |
1.9 |
0 |
0.0 |
4 |
1.9 |
2 |
1.7 |
1 |
0.9 |
1 |
0.9 |
| Alpha-13 |
21 |
6.5 |
2 |
0.6 |
19 |
5.8 |
17 |
8.2 |
2 |
1.0 |
15 |
7.2 |
4 |
3.5 |
0 |
0.0 |
4 |
3.5 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).