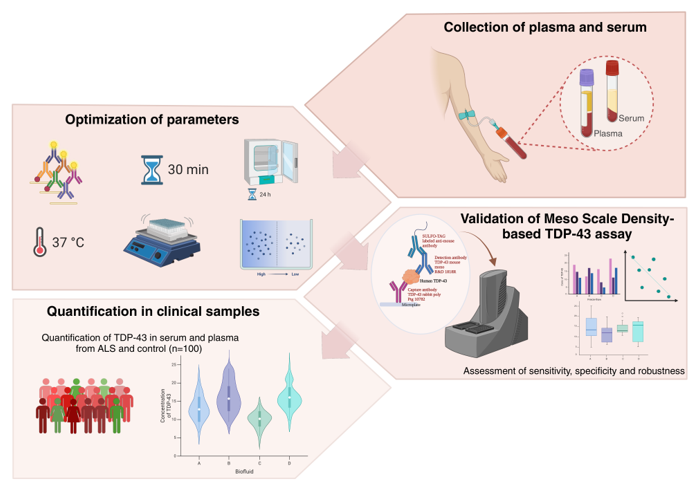
1. Introduction
Neurodegenerative disorders pose a significant and escalating challenge to global healthcare, with a growing impact on the quality of life for millions worldwide. Among these disorders, amyotrophic lateral sclerosis (ALS) is a fatal motor neuron disease characterized by progressive muscle weakness, paralysis, and ultimately, respiratory failure. Despite extensive research efforts, the precise etiology of ALS remains elusive, necessitating a deeper understanding of the molecular mechanisms driving the neurodegenerative processes underlying this debilitating condition.
In recent years, attention has turned towards the role of TAR DNA-binding protein 43 (TDP-43), a nuclear ribonucleoprotein, in the pathogenesis of ALS. Originally identified for its involvement in RNA processing, TDP-43 has emerged as a key player in neurodegeneration, with its aggregation and mis-localization being recognized as prominent pathological hallmarks in ALS and other related disorders such as Frontotemporal lobar degeneration (FTLD), limbic-predominant age-related TDP-43 encephalopathy (LATE) [
1], and Alzheimer’s disease [
2,
3,
4]. TDP-43 likely functions as a key mechanistic contributor in both sporadic and most familial forms of ALS and is a neuropathologic feature in over 95% of all ALS [
5].
TDP-43 is ubiquitously expressed in the central nervous system and plays a crucial role in RNA metabolism, including transcription, splicing, and transport [
6]. Under normal physiological conditions, TDP-43 is predominantly localized in the nucleus, contributing to the maintenance of cellular homeostasis [
7]. However, in ALS and a spectrum of related neurodegenerative disorders collectively known as TDP-43 proteinopathies, this protein undergoes aberrant modifications, leading to its mislocalization from the nucleus and generation of cytoplasmic inclusions, leading to neuronal dysfunction and degeneration [
8]. The cytoplasmic aggregation of TDP-43 has been observed in the affected regions of the central nervous system in ALS patients, including the spinal cord and motor cortex. TDP-43 inclusions in ALS and FTLD contain an accumulation of native protein, but also hyperphosphorylated and truncated forms of TDP-43 protein [
8].
The correlation between the presence of these TDP-43 aggregates and the severity of clinical parameters of disease underscores the significance of understanding the mechanisms governing TDP-43 pathology. Currently, the detection of these pathologic aggregates is limited to post-mortem neuronal tissues. Moreover, emerging evidence suggests that TDP-43 mislocalization may precede the onset of clinical symptoms, pointing towards its potential role as an early contributor of disease [
9]. The cytoplasmic accumulation of TDP-43 suggests that TDP-43 may ultimately be released into the extracellular space via cell degeneration, exosome or autophagosome release from cells [
10,
11]. Reliable detection of TDP-43 in biofluids could serve as a biomarker for TDP-43 proteinopathies, as well as for the development of therapeutic strategies that target TDP-43 function. While several immunoassays have been developed for TDP-43 detection, including enzyme-linked immunosorbent assay (ELISA) and Western blot, the current assays have limitations in terms of sensitivity and reproducibility [
12,
13]. Here, we describe a Meso Scale Discovery (MSD)-based immunoassay for the detection of TDP-43 in blood using an antibody pair that detects full length TDP-43 protein and potential fragments containing the N-terminal region of the protein. The MSD platform is a sensitive and specific immunoassay platform that uses electrochemiluminescence to detect low levels of analytes in complex matrices. Quantification of full-length TDP-43 in biofluids will be a valuable tool to measure levels in many neurodegenerative diseases and evaluate alterations due to pathophysiologic changes in TDP-43 and responses to treatments that target TDP-43 in ALS and other TDP-43 proteinopathies.
2. Materials and Methods
2.1. Materials
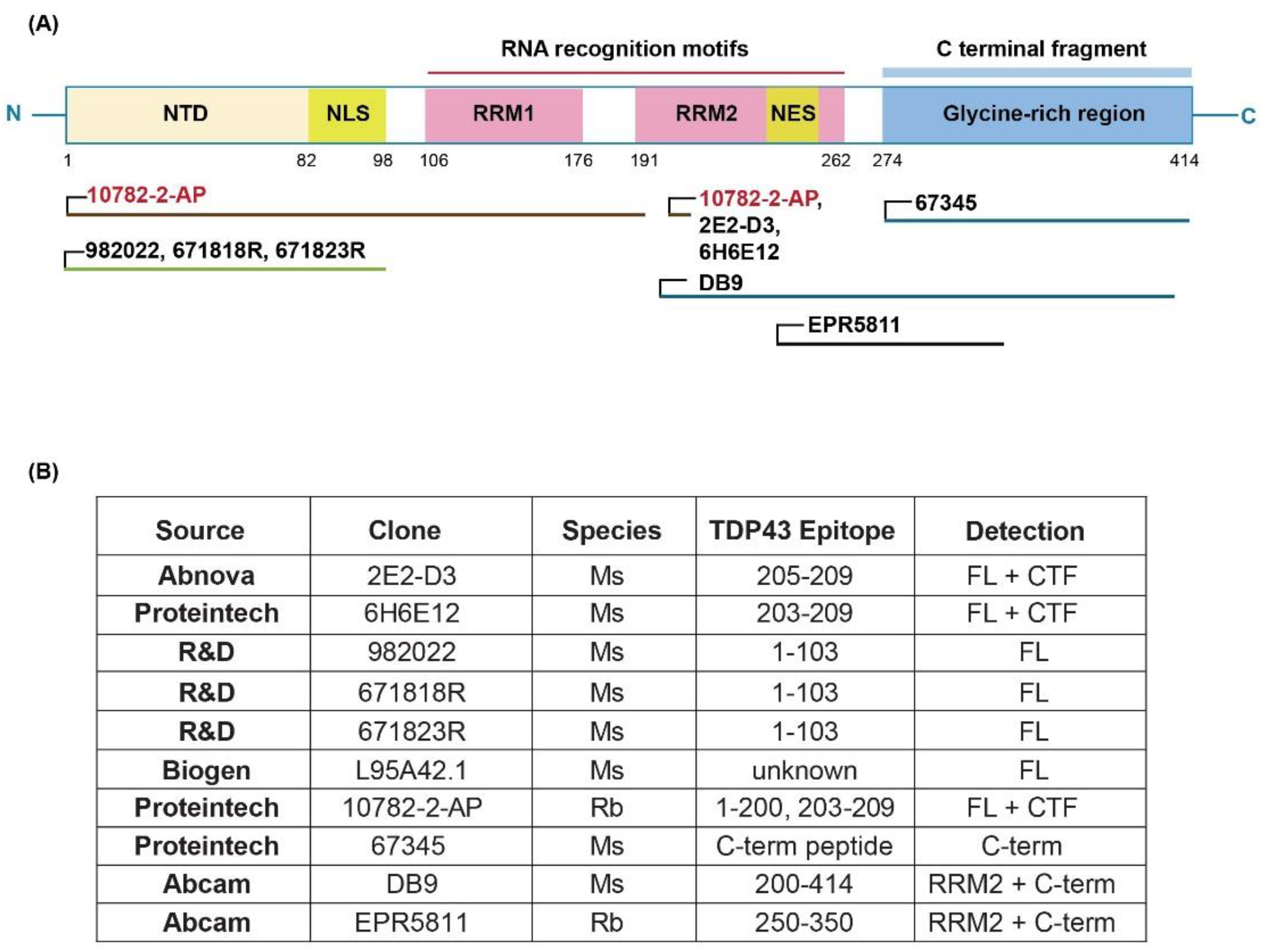
Purified recombinant transactive response DNA-binding protein of 43 kDa (TDP-43) was purchased from OriGene (NM_007375, Cat#TP710010) and used as the assay calibrant. Antibodies and their source are shown in
Figure 1. TDP-43 Rabbit Polyclonal antibody (Proteintech, Catalog #10782-2-AP) and Human TDP-43/TARDBP Mouse monoclonal antibody, clone 671818R (R&D Systems, Catalog #MAB77782-100) were found to be the optimal capture and detection antibodies, respectively. For immunodepletion experiments, we used 7 different TDP-43 antibodies including TARDBP monoclonal antibody (M01), clone 2E2-D3 (Catalog # H00023435-M01), TDP-43 (human specific) Monoclonal antibody, clone 6H6E12 (Proteintech Catalog # 60019-2-Ig), Human/Mouse/Rat TDP-43/TARDBP Antibody, clone: 982022 (R&D Catalog #: MAB77781), Human TDP-43/TARDBP Mouse monoclonal antibody, clone 671818R (R&D Systems, Catalog #MAB77782-100), Mouse monoclonal anti-TDP43 Antibody (Biogen Catalog # L95A42.1), TDP-43 (C-terminal) Monoclonal antibody (Proteintech Catalog # 67345-1-Ig) and Anti-TDP43 antibody DB9 (Abcam Catalog# ab254166).
The calibrant and sample diluent, Iron Horse Assay Diluent (IHAD), was purchased from nVector (Catalog #AD01-10). SULFO-TAG labeled anti-mouse antibody (Catalog #R32AC-1), Read buffer A (Catalog #R92TC) and MSD SECTOR Plates (Catalog #L15XA) were obtained from Meso Scale Discovery (Rockville, MD).
2.2. Sample Collection and Preparation
Plasma and serum samples from ALS and healthy control participants after IRB approved consent were obtained from the NEALS Biorepository and St. Joseph’s Hospital and Medical Center Biobank using standardized protocols. Briefly, blood was collected in K2EDTA tubes, inverted multiple times, and centrifuged at 1500xg for 10 minutes. The plasma was carefully collected, aliquoted, and stored at -80°C until analysis. The serum was isolated by collecting blood in red top tubes lacking anticoagulant, allowed to clot at room temperature for one hour, and centrifuged at 1,100 xg for 15 minutes. The serum was collected, aliquoted, and stored at -80°C for subsequent analysis.
2.3. Meso Scale Discovery (MSD) Assay Conditions
Recombinant full-length TDP-43 calibrator purchased from OriGene was diluted with IHAD to final concentration of 1ug/ml as Standard Stock and aliquot 20 µL per tube and stored in -80˚C. For each standard curve, 980 µL of 20% IHAD was added directly to the standard stock tube and mixed well. A series of 4-fold dilutions with 20% IHAD were performed to generate a standard range from 20,000 pg/ml to 4.88 pg/ml. Standard 8 was 20% IHAD only as a blank. MSD standard sector plates were coated with 40 µL of 1.5 µg/mL Rb poly anti human TDP-43 (ProteinTech, Cat#10782-2-AP) in PBS overnight in 4˚C. Plates were washed with 250 µL of wash buffer (PBST-0.1% Tween 20 in 1X PBS) 4 times then blocked with 1%BSA in 1% Casein/TBS (Bio-Rad, Cat#1610782) overnight at 4˚C. The plasma or serum samples were diluted 4-fold in 20% IHAD. After plates were washed 4 times in PBST, 40 µL of Standards or diluted samples were added to plate wells. Plates were incubated at 4˚C overnight with shaking at 750 rpm using Heidolph Titramax 1000. After 4 washes with 250 µL of PBST 4 times, 40 µL of 1.0 µg/mL mouse mono anti human TDP43(R&D Systems, Cat#MAB77782-100) in Casein/TBS were added to each well and incubated with shaking at 37˚C for 2 hours. After plate washes (250 µL of PBST 4 times), 40 µL of Sulfo-tagged anti-mouse antibody (1.0 µg/mL MSD, Cat#R32AC-1) in Casein/TBS was added to each well and incubated with shaking at 37˚C for 2 hours. After final plate washes (250 µL of PBST 4 times), 150 µL of Read Buffer A (MSD, Cat#R92TG) was added to each well and immediately read on an MSD MESO QuickPlex SQ120. The detailed assay protocol is provided in Appendix 1.
2.4. Assay Development
Several parameters were optimized during the assay development process. The first test was a dot blot analysis using both purified recombinant TDP-43 protein and human frontal cortex sarkosyl tissue extracts to confirm antibodies listed in
Figure 1B detected the recombinant TDP-43 protein and protein in a complex tissue extract. We next performed all possible pairwise combinations of antibodies to determine capture and detection antibody pairs with maximal signal-to-noise ratio using purified recombinant human TDP-43 protein spiked into tris buffered saline (TBS) containing 1% casein or human CSF. Several antibody pairs detected recombinant TDP-43 protein when spiked into buffer or CSF, and these pairs were then tested for their ability to detect TDP-43 protein in a human frontal cortex sarkosyl tissue extract. The best antibody combination was 10782-2-AP as capture and 671818R as detection antibody. We then used this antibody pair to further optimize assay parameters as described below and shown in
Table 1.
2.5. MSD Assay Performance and Reproducibility
Studies were performed to evaluate the reproducibility, precision, dilution linearity and specificity of the final assay.
2.5.1. Reproducibility and Precision
To determine the reproducibility and precision of the assay, we evaluated the precision (intra- and inter-assay variability) of the assay. Intra-assay variability was assessed by repeating samples on the same plate to test within run variations of the assay. Inter-assay variability was determined by measuring the mean precision (coefficient of variability) between assays performed using the same conditions on different days.
2.5.2. Dilution Linearity
The linearity of the assay across dilution ranges was assessed to ensure accurate quantification of TDP-43 levels. The plasma and the serum samples were serially diluted 2-fold 6 times yielding dilutions ranging from 2-, 4-, 8-, 16-, 32- and 64-fold. The measured final concentrations were determined and then compared with the calculated concentrations, considering the relevant dilution factor.
2.5.3. Spike-In Recovery
Experiments were conducted to evaluate the assay accuracy and matrix effects under controlled conditions. A defined concentration (5 ng/mL) of human recombinant TDP-43 protein was added to plasma and serum samples in different dilutions ranging from 2-fold to 64-fold. Samples with no protein spiked in served to define the endogenous amount of TDP-43 protein in the sample. The TDP-43 protein concentration was determined in all samples to evaluate any matrix effect on total TDP-43 protein measured in all samples.
2.5.4. Specificity Test
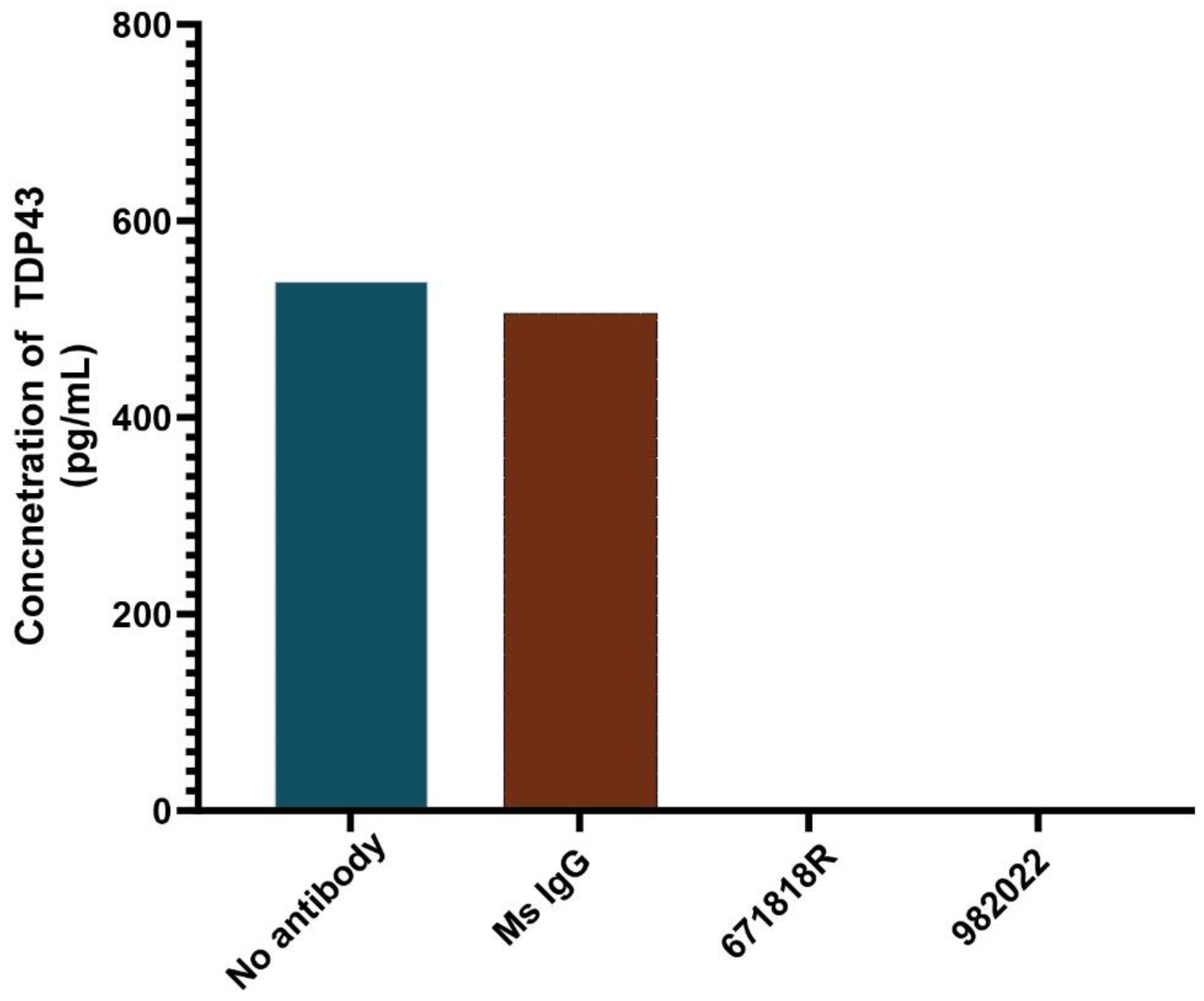
To demonstrate specificity of the assay for TDP-43 in a human biofluid, we removed TDP-43 from human biofluid samples by immunoprecipitation pull-down of endogenous TDP-43 using the mouse monoclonal antibodies 671818R or 982022 (Materials and Methods). Human biofluid samples were pooled from healthy controls and immunoprecipitation was performed using Dynabeads with 4µg of each of the TDP-43 antibodies. The samples were incubated with the antibody overnight at 4°C with high agitation (workflow depicted in Supplementary Figure S1). Following incubation, the antibody-protein complexes were pulled down using protein G magnetic beads. The supernatant, containing the immunodepleted samples, was collected for further analysis. The immunodepleted samples were then analyzed using the MSD assay to quantify full-length TDP-43 and the results were compared to those obtained from non-depleted control samples. As controls, we used a non-specific IgG antibody, and the MSD assay was performed on both IgG/TDP-43 antibody depleted as well as the pooled sample that was not depleted.
2.6. TDP-43 Measures in Clinical Samples
The final assay performance was tested using a set of samples from ALS subjects and healthy controls, obtained from NEALS Biorepository and St. Joseph’s Hospital and Medical Center Biorepository. Full-length TDP-43 was measured in 100 plasma and 100 serum samples (unmatched) from ALS and healthy control subjects constituting a total of 400 biofluid samples. A quality control sample was included in all the plates to evaluate the inter-plate variations and consistency in the results.
2.7. Figures and Tables
The graphs were generated using GraphPad Prism version 10.1.0 GraphPad Software, Boston, Massachusetts USA,
www.graphpad.com. The figures were generated using Adobe Illustrator (27.3.1) and BioRender.com.
3. Results
We used the Meso Scale Discovery (MSD) platform to develop the current full-length TDP-43 immunoassay, which utilizes electrochemiluminescence to detect protein biomarkers with high sensitivity and specificity. We optimized the MSD assay conditions and used the final assay to measure TDP-43 in plasma and serum samples from healthy control and ALS subjects.
3.1. Optimization of Assay Parameters
Figure 1 (A and B) specifies the source and location of the antibody epitopes used to develop the current assay. An optimal antibody pair, consisting of a capture antibody (10782-2-AP, rabbit polyclonal) and a detection antibody (671818R, mouse monoclonal), was selected from a panel of 10 antibodies that detected recombinant TDP-43 protein when spiked into either buffer or human CSF with highest signal to noise ratio. This antibody pair should detect full-length TDP-43 protein and any N-terminal fragments of TDP-43.
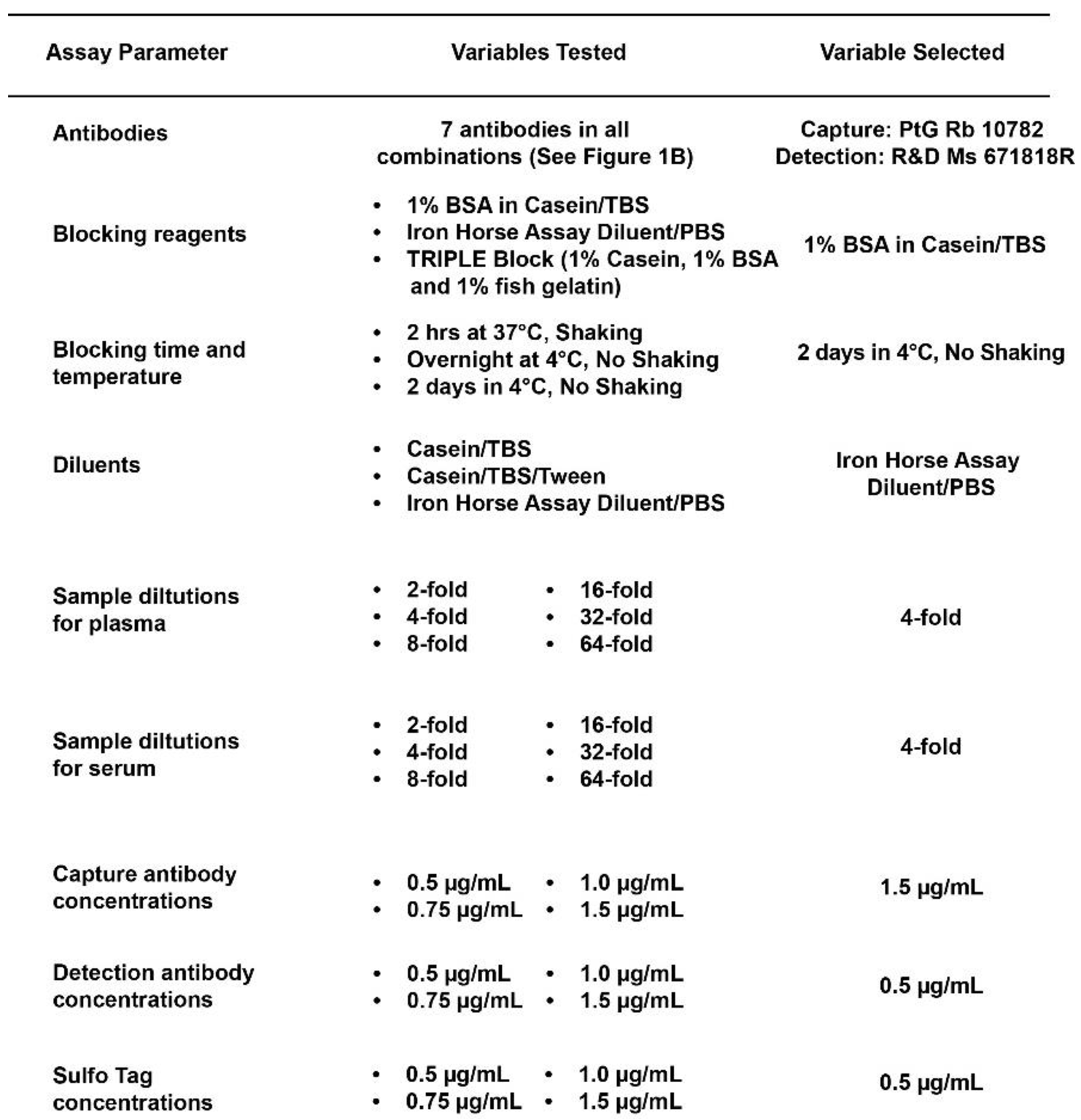
Table 1 highlights MSD assay parameters optimized with this antibody pair. Various blocking reagents were evaluated to identify the most effective for minimizing background signal and enhancing the signal-to-noise ratio. Simultaneously, we evaluated the effects of temperature and shaking of the plate for each blocking reagent. As a result, we selected Casein/TBS as the blocking reagent, with overnight incubation at 4˚C with no shaking. Alternatively, blocking in Casein/TBS for 2 hours at 37˚C with shaking also yielded similar assay results.
We next determined antibody concentrations optimal for the assay. We varied capture antibody concentrations used to coat plates from 0.5 µg/mL to 1.5 µg/mL to determine the levels that provided the best balance between capture efficiency and signal intensity. We found that 1.5 µg/mL capture antibody yielded the maximum detection sensitivity of and dynamic range for measuring TDP-43 protein concentration in human biofluids.
The concentration of detection antibody was also varied between 0.5 µg/mL to 1.5 µg/mL to optimize assay detection and to achieve the ideal balance between sensitivity and specificity. We found that the optimal detection antibody concentration was 0.5 µg/mL providing the highest signal-to-noise ratio and dynamic range, assuring the accurate quantification of TDP-43 levels in human biofluids.
The concentration of the Sulfo-tag anti-mouse antibody was optimized to ensure efficient electrochemical signal generation. A concentration of 1.0 µg/mL Sulfo-tag labeled anti-mouse in 1X TBS with 1% Casein provided optimal sensitivity and accuracy of the immunoassay.
We tested several candidate sample diluents to identify one that maintained the stability of antibodies and TDP-43 protein in biofluids to enable optimal assay performance. The selected diluent, Iron Horse Assay Diluent (IHAD), was integral in preserving the functionality and specificity of the antibodies throughout the assay. To minimize potential matrix effects, we tested multiple biofluid sample dilutions in IHAD. Various sample dilutions were tested to ensure that the chosen dilution factor provided an assay environment in which the TDP-43 levels could be accurately quantified without interference from the complex biofluid matrix. The choice of a 4-fold sample dilution in IHAD maintained the assay's reliability across diverse sample types.
3.2. Precision, Sensitivity, and Specificity
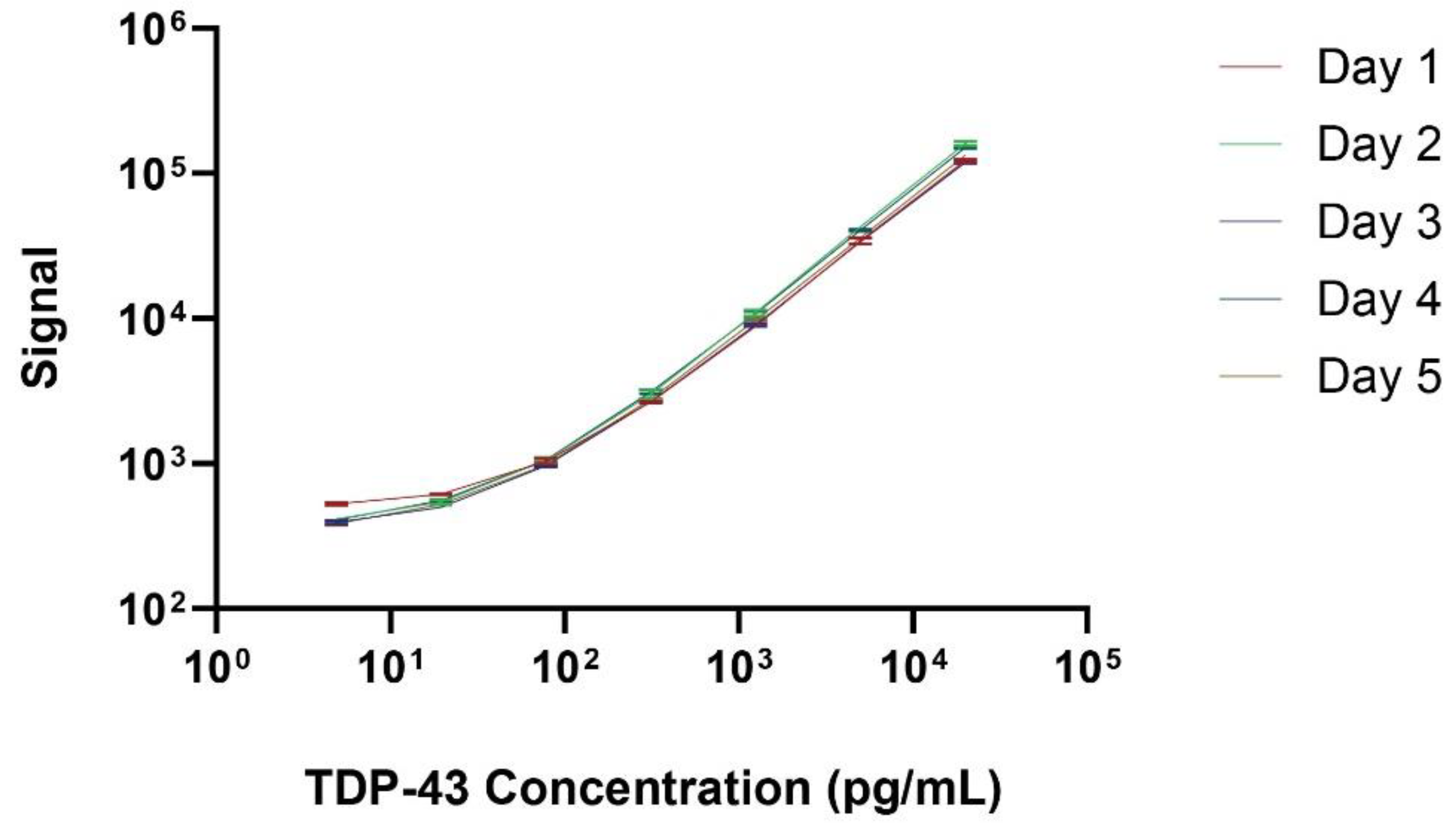
Reproducibility and repeatability were assessed via ten replicates on a single plate for intra-assay variability and repeating the same assay on five consecutive days to evaluate inter-assay variability. The coefficient of variation (CV) was calculated for both intra-assay (within the same day) and inter-assay (across different days) analyses.
Figure 2 illustrates the standard curve over 5 separate days, with a lower limit of quantification of 4.0 pg/mL and upper limit of quantification of 20,000 pg/mL.
Figure 3A displays the intra-assay TDP-43 concentration values for 10 replicates of 3 separate plasma (Left) or serum (Right) samples. The intra-assay CV ranged from 2.7% to 6.3% in plasma and from 4.5% to 5% in serum, indicating minimal variability within the same day. The inter-assay results are shown in
Figure 3B represent a dilution series of TDP-43 measured on 5 consecutive days. The CV ranged from 3.6% to 4.8% in plasma and from 5.7% to 8.8% in serum, reflecting consistent performance across different days. These results underscore the precision and reliability of the assay for quantifying TDP-43 levels in biofluids.
To assess the specificity of the assay, we depleted endogenous TDP-43 from a pooled plasma sample from healthy controls by immunoprecipitation using two different TDP-43 antibodies or an IgG control antibody that does not recognize TDP-43. The starting sample and three immunodepleted samples were subsequently used to measure TDP-43 levels with our immunoassay. The starting sample exhibited TDP-43 protein concentration of approximately 550 pg/ml (
Figure 4). The non-specific IgG control failed to remove TDP-43 and exhibited a TDP-43 concentration similar to the starting sample, whereas those samples incubated with either of the TDP-43 specific antibodies for immunodepletion exhibited a complete loss of TDP-43 signal (
Figure 4). These results confirm that the signal detected with our immunoassay is dependent upon the presence of TDP-43, and not cross-reactive with other proteins within the biofluid.
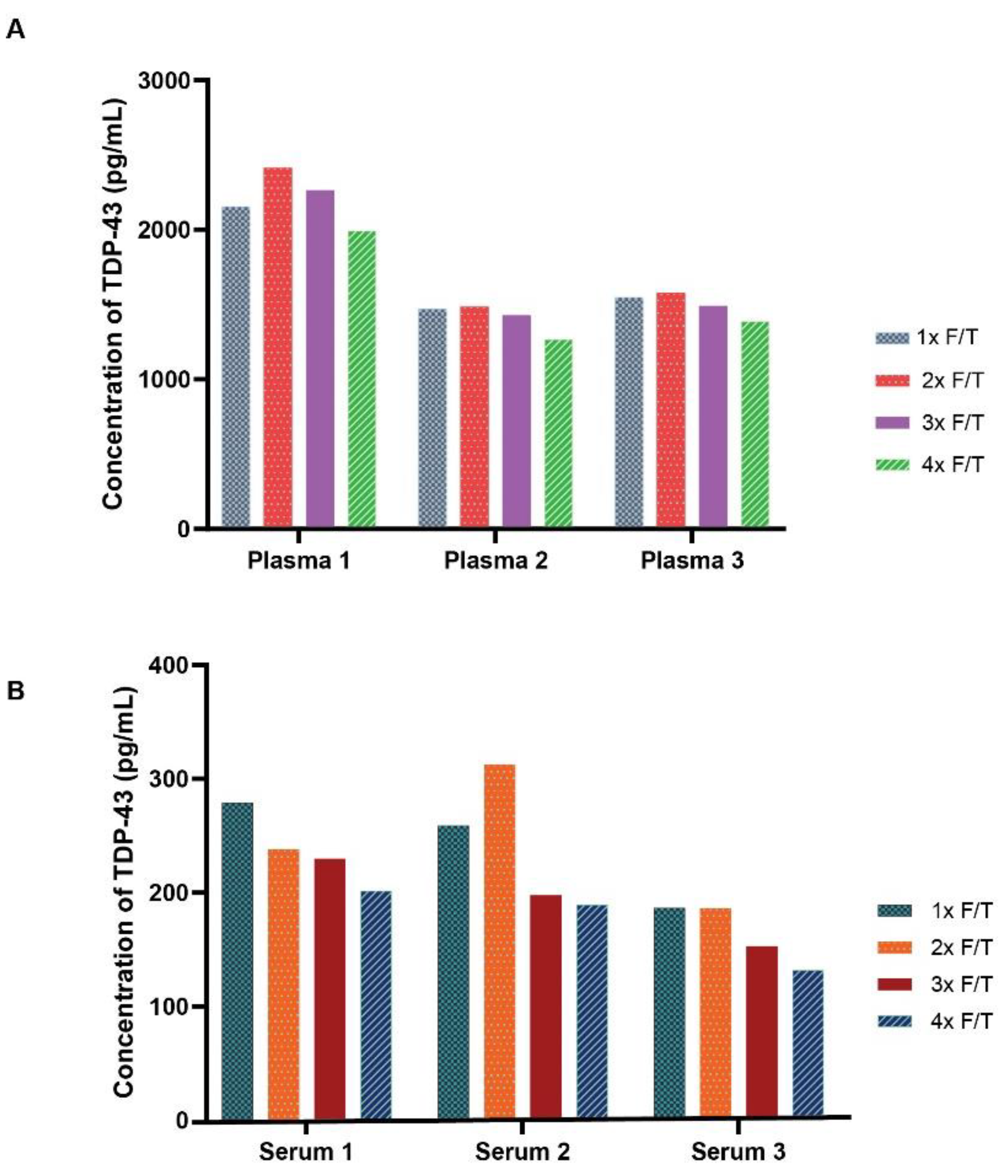
3.3. Freeze-Thaw Stability
The stability of endogenous TDP-43 to numerous freeze-thaw events was next evaluated. To evaluate the impact of freeze-thaw cycles on TDP-43 stability and assay performance, multiple freeze-thaw cycles were performed on plasma and serum from three subjects stored at -80°C. The freeze-thaw results demonstrated a negligible impact on assay performance and observed TDP-43 protein concentration after the first few freeze-thaw cycles, though a modest concentration reduction was observed in the 4th freeze-thaw cycle for plasma (
Figure 5A) and either the 3rd or 4th freeze-thaw cycle for serum (
Figure 5B).
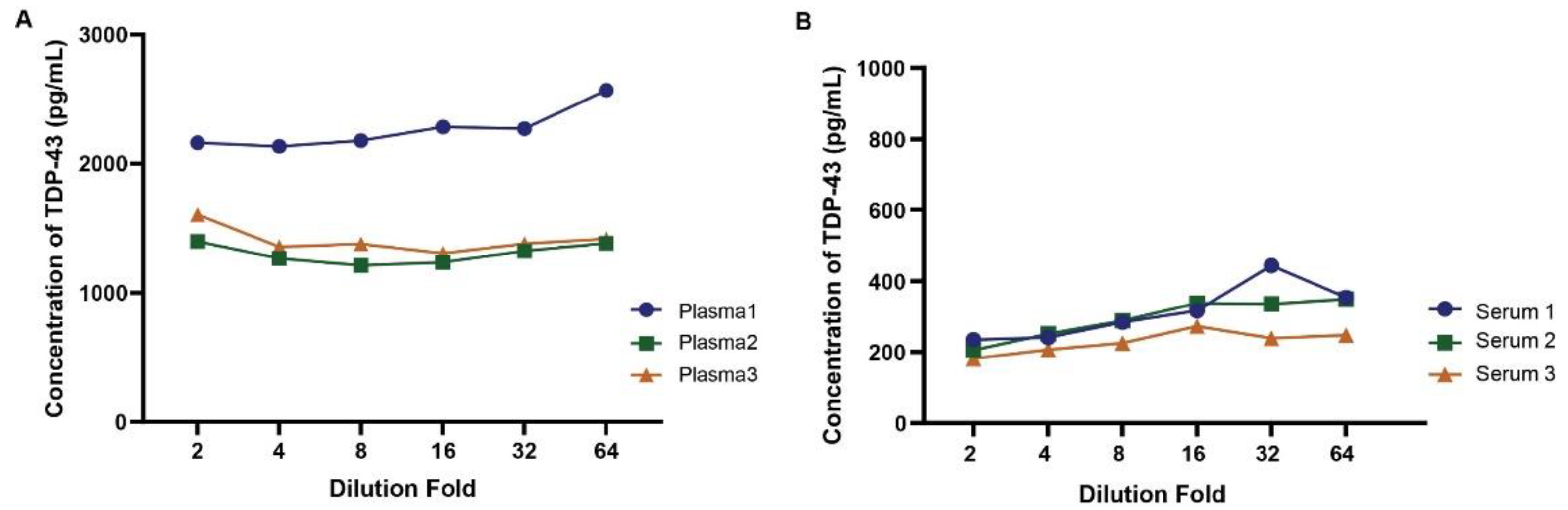
3.4. Parallelism and Spike-In Recovery
Parallelism was assessed to ensure the assay's accuracy across a range of sample concentrations and minimal impact of the matrix at the specified dilutions. Parallelism addresses the relative accuracy of the assay by assessing the effects of dilution on the quantitation of TDP-43 in a biologic matrix. As shown in
Figure 6, the dilution linearity or parallelism assessment revealed a linear relationship up to 64-fold dilution across multiple plasma (
A) or serum (
B) samples. This indicates the assay's ability to accurately quantify TDP-43 levels over a broad range of plasma or serum dilutions with limited impact on overall concentration values.
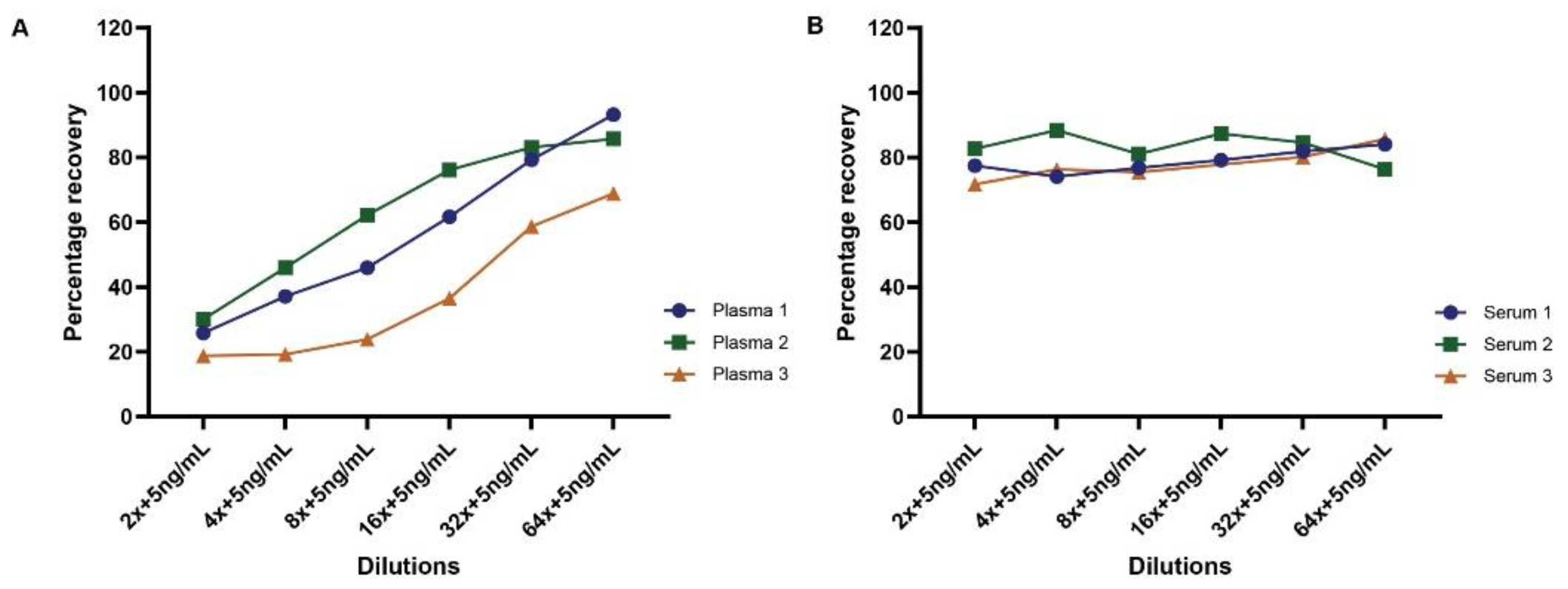
To further evaluate matrix effects on measuring TDP-43 protein concentrations in human biofluids, we performed spike-in recovery tests in both plasma and serum samples. A defined concentration (5.0 ng/mL) of purified human recombinant TDP-43 protein was added to plasma and serum samples at different dilutions ranging from 2-fold to 64-fold. Samples with no spiked in protein served to define the endogenous amount of TDP-43 protein in the sample. As shown in
Figure 7, the results revealed a variable matrix effect trend across all plasma samples, with recovery rates increasing with plasma dilution (
Figure 7A). However, serum samples exhibited recovery rates of approximately 80-90% at all dilutions (
Figure 7B).
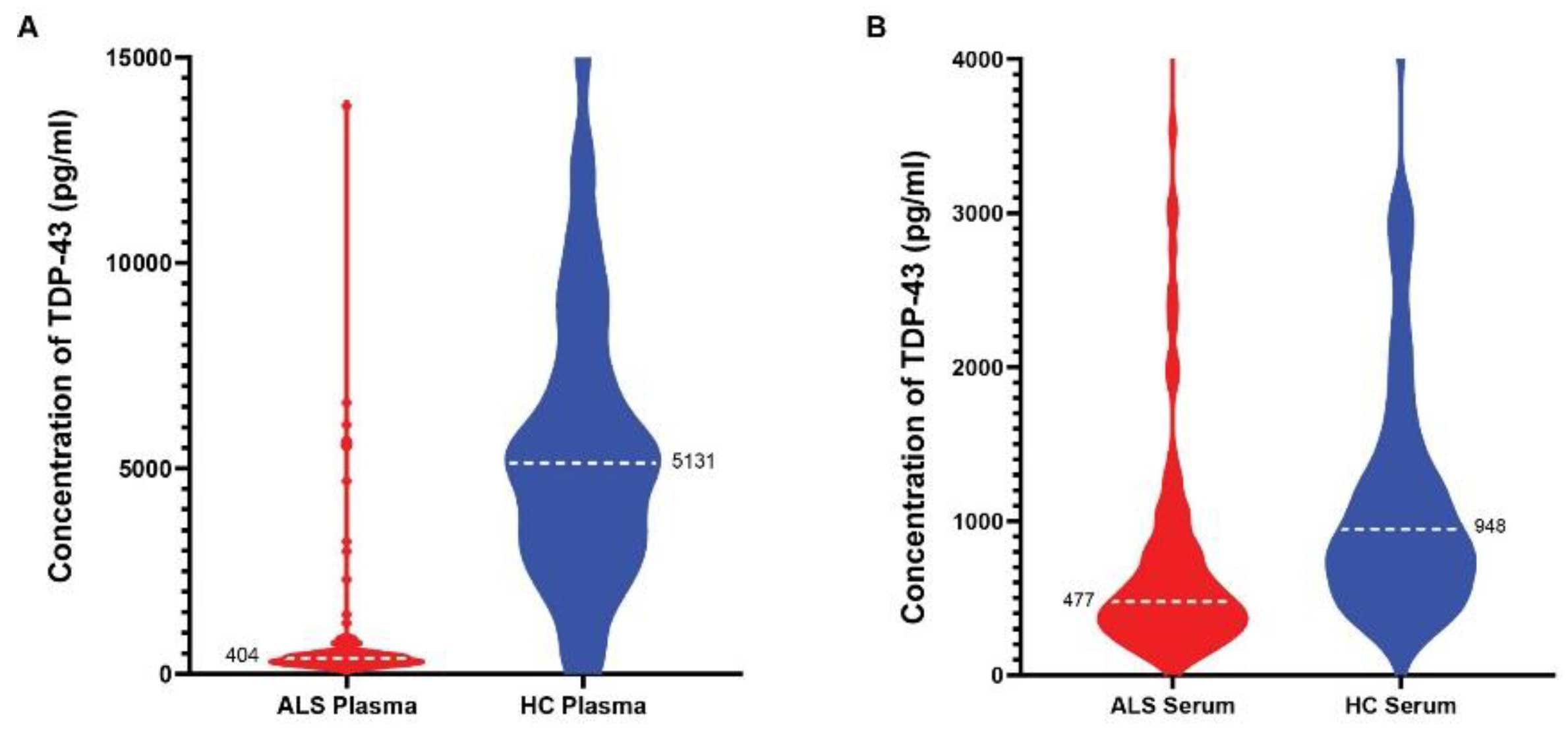
3.5. Quantification of TDP-43 in ALS and Healthy Control Blood Samples
We used our MSD assay to quantitate full-length TDP-43 in plasma and serum (unmatched) from 101 ALS and 115 age-matched healthy control subjects. The limit of quantitation (LLOQ) was 4.0 pg/mL in serum and plasma with a dynamic range of 4-20,000 pg/mL. All plasma and serum samples fell into the working range of this assay. We observed a decrease in full-length TDP-43 in the plasma of ALS patients when compared to healthy control samples (
Figure 8A). The ALS plasma group displayed a mean of 404 pg/mL (95% CI of the mean between 330 – 425 pg/mL) versus the healthy control mean of 5131 pg/mL (95% CI of the mean between 4379 – 5512 pg/mL), with
p = 0.001 using unpaired t-test. We also detected significantly lower levels of TDP-43 in the serum of ALS patients when compared to age-matched healthy controls (
Figure 8B). The mean TDP-43 concentration in the ALS serum group was 477 pg/mL (95% CI of the mean between 430 – 573 pg/mL) versus that of 948 pg/ml mean in the healthy controls (95% CI of the mean between 765 – 1108 pg/mL), with
p = 0.001 using unpaired t-test.
4. Discussion
The present study addresses the growing body of evidence supporting the need for improved immunoassays to measure TDP-43 in ALS and related disorders. Our study defines steps and conditions to create a reliable and reproducible immunoassay for quantifying TDP43 in human biofluids. The final capture and detection antibodies as well as all the reagents are commercially available. The assay protocol as well as the capture 10782-AP polyclonal antibody are freely available to the research community via Target ALS (
https://www.targetals.org/resource/antibody-core/)
Additionally, the potential application of our novel assay in the diagnostic work-up for dementia disorders, including the detection of brain TDP-43 deposition, holds promise for improving clinical diagnosis and patient management. Further validation studies in larger patient cohorts are warranted to confirm the robustness and clinical utility of our novel TDP-43 assay in neurodegenerative disorder diagnosis and research. However, it is crucial to acknowledge that while our results indicate a correlation between reduced full-length TDP-43 levels and ALS, further investigations are necessary to unravel the underlying biology of this reduction.
Antibody Screening
We screened multiple commercially available TDP-43 antibodies during immunoassay development, as antibody specificity and affinity greatly influence assay performance. In our study, we rigorously evaluated a panel of antibodies targeting different epitopes of TDP-43 (
Figure 1B) to identify antibodies that detected TDP-43 in tissue extracts, dot blots of human biofluids, and pure TDP-43 protein spiked into human biofluids, to define an optimal antibody pair for sandwich immunoassay configuration. Despite extensive screening efforts, challenges such as antibody cross-reactivity or poor specificity were noted for some antibodies. To mitigate these issues, we employed stringent validation criteria and confirmed antibody specificity, ensuring the reliability of antibodies used in our final immunoassay. We tested multiple wash conditions, assay diluents, and incubation times and temperatures to maximize assay signal and minimize background (
Table 1). This screening process enabled us to develop an assay with high sensitivity in human plasma and serum samples, as evidenced by the lower limits of detection (LOD) and quantification (LLOQ) achieved.
The nature of TDP-43 protein presents additional challenges in its detection and quantification. TDP-43 is known to undergo fragmentation, aggregation, and formation of insoluble protein aggregates observed in neurodegenerative disorders, including ALS and FTLD [
14,
15]. The detection of soluble and insoluble forms of TDP-43 poses challenges for immunoassay development, though detection of pathologic species of TDP-43 is crucial for generating biomarkers of disease and monitoring the impact of TDP-43 based therapies in clinical trials [
5,
16,
17]. Based on the epitopes detected by each antibody selected in our screening procedure, our immunoassay will detect full-length or N-terminal fragments of TDP-43. Since N-terminal fragments of TDP-43 have not been observed in human tissue extracts or biofluids, we propose that our assay detects full-length TDP-43.
Experiments examining matrix effects of plasma and serum demonstrated that dilutions of either serum or plasma samples provided a linear relationship of measured TDP-43 concentration, though higher dilutions of some samples generated increased TDP-43 protein concentrations (
Figure 6). This result suggests a minimal matrix effect may occur in some human plasma or serum samples. However, our spike-in recovery experiments suggest that plasma has a more significant matrix effect and that spike-in recovery in serum was comparable across a series of sample dilutions (
Figure 7). A reduced recovery was observed in the lower dilutions of plasma when compared to the higher dilutions. Overall, we observed that we could specifically and accurately detect TDP-43 in either plasma or serum, but plasma samples may contain either other proteins, cell types or lipids that impede detection of exogenously added TDP-43.
Decrease in TDP-43 in ALS Plasma and Serum
Contrary to previous reports suggesting elevated TDP-43 levels in ALS blood samples compared to healthy controls [
18], our results revealed a significant decrease in full-length TDP-43 in ALS patients compared to healthy controls (
Figure 8). Similarly, another study also suggested lower levels of TDP-43 in the plasma of ALS patients from an Indian population compared to controls using immunodetection [
19]. A recent study that validated a TDP-43 immunoassay for detecting C-terminal fragments of TDP-43 reported increased TDP-43 C-terminal fragments in human plasma from ALS patients [
18]. These authors observed between 50-100 pg/mL of C-terminal TDP-43 in plasma samples, while we typically detect low ng/ml levels of full-length TDP-43 in plasma. Overall, these results suggest a reduction of full-length TDP-43 in the blood of ALS patients with an increase in C-terminal fragments in the plasma of ALS patients.
The observed reduction in full-length TDP-43 levels in ALS versus healthy control blood samples raises intriguing questions regarding the underlying mechanisms. The decrease in full-length TDP-43 levels may reflect dysregulation in the processing or turnover of this essential RNA-binding protein, which is known to play a crucial role in RNA metabolism and maintaining cellular homeostasis. TDP-43 abnormalities have been extensively linked to ALS, and our findings further strengthen the association between ALS and TDP-43 concentrations in serum and plasma. It is well-established that TDP-43 undergoes various post-translational modifications, including proteolytic cleavage, which can lead to the generation of C-terminal fragments and degradation of the N-terminus [
7,
20]. While our results suggest a correlation between reduced full-length TDP-43 levels in the blood and ALS, additional research is necessary to define the mechanisms leading to this reduction. It is possible that TDP-43 levels in the blood may differ at different disease stages or vary within different blood cell types or biofluid matrices. A prior study suggested higher levels of TDP-43 occur in platelets of ALS patients [
21]. Further studies are required to determine if TDP-43 within the plasma or serum is contained within exosomes, extracellular RNPs, or is localized within a particular cellular component of the plasma or serum such as platelets [
21,
22].
Future studies should focus on generating additional immunoassays that specifically quantitate post-translational modifications of TDP-43. Additionally, exploring the correlations between full-length, C-terminal fragments and post-translational modifications of TDP-43 to clinical parameters of disease may provide insights into disease pathogenesis and provide important biomarkers for use in drug development and clinical trials for therapies targeting TDP-43 function.
5. Conclusions
We describe the development and validation of an MSD-based immunoassay for the detection of full-length TDP-43 in human biofluids, which shows high sensitivity and specificity for TDP-43 in blood samples. The assay has potential clinical utility for the diagnosis and monitoring of TDP-43 associated disorders, as well as for the development of therapeutic strategies targeting TDP-43. Our study highlights a significant decrease in full-length TDP-43 levels in ALS plasma and serum, offering a promising avenue for further research into the molecular mechanisms of ALS and FTD. Further studies are necessary to validate the clinical application of the assay in larger cohorts and to investigate its performance in longitudinal studies. Understanding the specific molecular events that lead to decreased full-length TDP-43 in ALS blood samples could provide valuable insights into disease mechanisms and potentially identify novel therapeutic targets.
Author Contributions
Conceptualization, R.B.; methodology, J.A, L.G. and R.B.; software, J.A., L.G. and V.O.; validation, J.A.; formal analysis, J.A., L.G. and R.B.; investigation, J.A, L.G. V.O. and R.B.; resources, J.A, L.G., V.O., R.K., J.S., and R.B.; data curation, J.A, L.G., V.O., J.S., R.K. and R.B.; writing—original draft preparation, L.G.; writing—review and editing, L.G., J.A. and R.B.; visualization, L.G.; supervision, R.B.; project administration, R.B.; funding acquisition, R.B. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research was funded by a Target ALS grant entitled “Development of TDP-43 Immunoassays” to RB.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of St. Joseph’s Hospital and Medical Center (IRB PHX-21-500-101-70-09 and date of continued approval on January 10, 2023).
Informed Consent Statement
Informed consent for the collection of blood samples was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available via a Data use agreement from the corresponding author upon reasonable request.
Acknowledgments
The authors thank the ALS patients, caregivers, and other healthy control participants for providing blood samples for this study. We thank Target ALS for funding support for this project and detailed assay information is available from Target ALS. We also thank the NEALS Biorepository for providing serum and plasma samples from ALS and healthy controls for use in this study.
Conflicts of Interest
R.B. is a co-founder and shareholder of nVector, Inc., a company developing biomarkers and therapeutics for neurologic disorders. Jiyan An holds shares of nVector, Inc. No conflicts of interest were reported by the other authors.
References
- Nelson, P.T.; Dickson, D.W.; Trojanowski, J.Q.; Jack, C.R.; Boyle, P.A.; Arfanakis, K.; et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019, 142, 1503–1527. [Google Scholar] [CrossRef] [PubMed]
- Matej, R.; Tesar, A.; Rusina, R. Alzheimer's disease and other neurodegenerative dementias in comorbidity: A clinical and neuropathological overview. Clin Biochem. 2019, 73, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Ayala, Y.M.; Zago, P.; D'Ambrogio, A.; Xu, Y.F.; Petrucelli, L.; Buratti, E.; et al. Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci. 2008, 121, 3778–3785. [Google Scholar] [CrossRef] [PubMed]
- Winton, M.J.; Igaz, L.M.; Wong, M.M.; Kwong, L.K.; Trojanowski, J.Q.; Lee, V.M. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J Biol Chem. 2008, 283, 13302–13309. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.R.; Bigio, E.H.; Ince, P.G.; Geser, F.; Neumann, M.; Cairns, N.J.; et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007, 61, 427–434. [Google Scholar] [CrossRef]
- Buratti, E.; Baralle, F.E. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem. 2001, 276, 36337–36343. [Google Scholar] [CrossRef]
- Prasad, A.; Bharathi, V.; Sivalingam, V.; Girdhar, A.; Patel, B.K. Molecular Mechanisms of TDP-43 Misfolding and Pathology in Amyotrophic Lateral Sclerosis. Front Mol Neurosci. 2019, 12, 25. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Huntley, M.L.; Perry, G.; Wang, X. Pathomechanisms of TDP-43 in neurodegeneration. J Neurochem. 2018. [CrossRef]
- Dyer, M.S.; Woodhouse, A.; Blizzard, C.A. Cytoplasmic Human TDP-43 Mislocalization Induces Widespread Dendritic Spine Loss in Mouse Upper Motor Neurons. Brain Sci. 2021, 11. [Google Scholar] [CrossRef]
- Iguchi, Y.; Eid, L.; Parent, M.; Soucy, G.; Bareil, C.; Riku, Y.; et al. Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain. 2016, 139, 3187–3201. [Google Scholar] [CrossRef]
- Zhang, N.; Gu, D.; Meng, M.; Gordon, M.L. TDP-43 Is Elevated in Plasma Neuronal-Derived Exosomes of Patients With Alzheimer's Disease. Front Aging Neurosci. 2020, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Tokuda, T.; Ishigami, N.; Sasayama, H.; Foulds, P.; Mitchell, D.J.; et al. Increased TDP-43 protein in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Acta Neuropathol. 2009, 117, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Steinacker, P.; Hendrich, C.; Sperfeld, A.D.; Jesse, S.; von Arnim, C.A.; Lehnert, S.; et al. TDP-43 in cerebrospinal fluid of patients with frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Arch Neurol. 2008, 65, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.J.; Lee, V.M.; Trojanowski, J.Q. TDP-43 functions and pathogenic mechanisms implicated in TDP-43 proteinopathies. Trends Mol Med. 2011, 17, 659–667. [Google Scholar] [CrossRef]
- Dubowsky, M.; Theunissen, F.; Carr, J.M.; Rogers, M.L. The Molecular Link Between TDP-43, Endogenous Retroviruses and Inflammatory Neurodegeneration in Amyotrophic Lateral Sclerosis: a Potential Target for Triumeq, an Antiretroviral Therapy. Mol Neurobiol. 2023, 60, 6330–6345. [Google Scholar] [CrossRef]
- Shiina, Y.; Arima, K.; Tabunoki, H.; Satoh, J. TDP-43 dimerizes in human cells in culture. Cell Mol Neurobiol. 2010, 30, 641–652. [Google Scholar] [CrossRef]
- Schwab, C.; Arai, T.; Hasegawa, M.; Yu, S.; McGeer, P.L. Colocalization of transactivation-responsive DNA-binding protein 43 and huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol. 2008, 67, 1159–1165. [Google Scholar] [CrossRef]
- Matsuura, S.; Tatebe, H.; Higuchi, M.; Tokuda, T. Validation of a newly developed immunoassay for TDP-43 in human plasma. Heliyon. 2024, 10, e24672. [Google Scholar] [CrossRef]
- Modgil, S.; Khosla, R.; Tiwari, A.; Sharma, K.; Anand, A. Association of Plasma Biomarkers for Angiogenesis and Proteinopathy in Indian Amyotrophic Lateral Sclerosis Patients. J Neurosci Rural Pract. 2020, 11, 573–580. [Google Scholar] [CrossRef]
- Berning, B.A.; Walker, A.K. The Pathobiology of TDP-43 C-Terminal Fragments in ALS and FTLD. Front Neurosci. 2019, 13, 335. [Google Scholar] [CrossRef]
- Hishizawa, M.; Yamashita, H.; Akizuki, M.; Urushitani, M.; Takahashi, R. TDP-43 levels are higher in platelets from patients with sporadic amyotrophic lateral sclerosis than in healthy controls. Neurochem Int. 2019, 124, 41–45. [Google Scholar] [CrossRef]
- Luthi-Carter, R.; Cappelli, S.; Le Roux-Bourdieu, M.; Tentillier, N.; Quinn, J.P.; Petrozziello, T.; et al. Location and function of TDP-43 in platelets: Alterations in neurodegenerative diseases and arising considerations for current plasma biobank protocols. Scientific Reports. In press. 2024. [Google Scholar] [CrossRef]
Figure 1.
Schematic representation of the MSD-based TDP-43 immunoassay. (A): The structure of TDP-43 protein and localization of epitopes against different antibodies screened in this study. All the monoclonal antibodies are marked in black and the polyclonal antibodies in red. (B) Source and description of each commercial antibody used in this study.
Figure 1.
Schematic representation of the MSD-based TDP-43 immunoassay. (A): The structure of TDP-43 protein and localization of epitopes against different antibodies screened in this study. All the monoclonal antibodies are marked in black and the polyclonal antibodies in red. (B) Source and description of each commercial antibody used in this study.
Figure 2.
Representative TDP-43 standard curves generated on five consecutive days. The MSD signal is shown in the Y axis and the TDP-43 concentration in the X axis.
Figure 2.
Representative TDP-43 standard curves generated on five consecutive days. The MSD signal is shown in the Y axis and the TDP-43 concentration in the X axis.
Figure 3.
Assessment of precision and sensitivity of the TDP-43 assay. A: Intra-assay TDP-43 concentration values for 10 replicates of 3 separate plasma (left) and serum (right) samples. The intra-assay coefficient of variation (CV) ranged from 2.7% to 6.3% in plasma and from 4.9% to 5.0% in serum, indicating minimal variability within the same day. B: Inter-assay variability observed in the assay across multiple replicates of the assay conducted on different days for 3 separate plasma and serum samples. Each bar represents the average of replicate wells for each sample.
Figure 3.
Assessment of precision and sensitivity of the TDP-43 assay. A: Intra-assay TDP-43 concentration values for 10 replicates of 3 separate plasma (left) and serum (right) samples. The intra-assay coefficient of variation (CV) ranged from 2.7% to 6.3% in plasma and from 4.9% to 5.0% in serum, indicating minimal variability within the same day. B: Inter-assay variability observed in the assay across multiple replicates of the assay conducted on different days for 3 separate plasma and serum samples. Each bar represents the average of replicate wells for each sample.
Figure 4.
Immunodepletion of TDP-43 demonstrates immunoassay specificity. Plasma was incubated with no antibody (blue bar), control IgG (red bar), anti-TDP43 antibody 671818R, or anti-TDP43 antibody 982022 and immunodepletion performed as described in Methods. Immunodepleted samples were analyzed for TDP-43 protein levels and the average protein concentration from replicate samples displayed. Samples depleted with either anti-TDP43 antibody were devoid of TDP-43 signal.
Figure 4.
Immunodepletion of TDP-43 demonstrates immunoassay specificity. Plasma was incubated with no antibody (blue bar), control IgG (red bar), anti-TDP43 antibody 671818R, or anti-TDP43 antibody 982022 and immunodepletion performed as described in Methods. Immunodepleted samples were analyzed for TDP-43 protein levels and the average protein concentration from replicate samples displayed. Samples depleted with either anti-TDP43 antibody were devoid of TDP-43 signal.
Figure 5.
Stability of Endogenous TDP-43 in Plasma and Serum across Freeze-Thaw Cycles. The bar plots represent the freeze-thaw stability of TDP43 across four freeze-thaw cycles of plasma A and serum B. The results show minimal impact on assay performance and TDP-43 protein concentrations across the first two or three cycles. A modest reduction in TDP-43 concentration was noted in the fourth freeze-thaw cycle for plasma and the third or fourth freeze-thaw cycle for serum. Bars represent average of replicate samples for each sample.
Figure 5.
Stability of Endogenous TDP-43 in Plasma and Serum across Freeze-Thaw Cycles. The bar plots represent the freeze-thaw stability of TDP43 across four freeze-thaw cycles of plasma A and serum B. The results show minimal impact on assay performance and TDP-43 protein concentrations across the first two or three cycles. A modest reduction in TDP-43 concentration was noted in the fourth freeze-thaw cycle for plasma and the third or fourth freeze-thaw cycle for serum. Bars represent average of replicate samples for each sample.
Figure 6.
Dilution linearity of the plasma and serum samples using the TDP-43 assay. Panel A and B illustrate the dilution linearity assessment in plasma and serum, respectively. A linear relationship between dilution factors and TDP-43 concentration was observed both in plasma and serum, with the assay maintaining a stable measurement of TDP-43 even at dilutions up to 64-fold.
Figure 6.
Dilution linearity of the plasma and serum samples using the TDP-43 assay. Panel A and B illustrate the dilution linearity assessment in plasma and serum, respectively. A linear relationship between dilution factors and TDP-43 concentration was observed both in plasma and serum, with the assay maintaining a stable measurement of TDP-43 even at dilutions up to 64-fold.
Figure 7.
Spike in recovery of TDP-43 in human plasma and serum. A fixed concentration (5ng/mL) of human recombinant TDP-43 was spiked into plasma and serum samples at various dilutions (2-fold to 64-fold). The graph illustrates the recovery rates of TDP-43 both in plasma (A) and serum (B).
Figure 7.
Spike in recovery of TDP-43 in human plasma and serum. A fixed concentration (5ng/mL) of human recombinant TDP-43 was spiked into plasma and serum samples at various dilutions (2-fold to 64-fold). The graph illustrates the recovery rates of TDP-43 both in plasma (A) and serum (B).
Figure 8.
The quantification of TDP43 human plasma and serum samples. The violin plots represent the TDP-43 protein levels quantified in ALS (n=101) and Control (n=115) plasma (A) and serum samples (B).
Figure 8.
The quantification of TDP43 human plasma and serum samples. The violin plots represent the TDP-43 protein levels quantified in ALS (n=101) and Control (n=115) plasma (A) and serum samples (B).
Table 1.
The overview of the conditions optimized for the current TDP43 assay. This table presents the optimized parameters for the Meso Scale Discovery (MSD) assay using the specified antibody pair. Parameters include antibody concentrations, incubation times, buffer compositions, and detection settings. Each parameter has been meticulously fine-tuned to ensure maximum assay sensitivity and specificity for the target analyte.
Table 1.
The overview of the conditions optimized for the current TDP43 assay. This table presents the optimized parameters for the Meso Scale Discovery (MSD) assay using the specified antibody pair. Parameters include antibody concentrations, incubation times, buffer compositions, and detection settings. Each parameter has been meticulously fine-tuned to ensure maximum assay sensitivity and specificity for the target analyte.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).