Submitted:
11 September 2024
Posted:
12 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Chemical Analyses of Propolis Samples
2.1.1. Total Polyphenols and Total Flavonoids Quantification
| Sample code | TP (% w/w) |
TF (% w/w) |
Sample code | TP (% w/w) |
TF (% w/w) |
|---|---|---|---|---|---|
| N1 | 5.34 ± 0.64 | 3.38 ± 0.17 | C10 | 23.84 ± 1.08 | 21.78 ± 1.78 |
| N2 | 21.86 ± 3.07 | 18.13 ± 0.06 | C11 | 22.70 ± 2.06 | 19.63 ± 2.36 |
| N3 | 10.45 ± 1.88 | 9.26 ± 0.18 | C12 | 27.36 ± 3.35 | 21.42± 0.98 |
| N4 | 18.20 ± 2.09 | 11.04 ± 0.37 | C13 | 16.58 ± 1.61 | 11.60 ± 0.14 |
| N5 | 14.78 ± 3.28 | 12.28 ± 0.63 | S1 | 27.88 ± 4.24 | 24.28 ± 3.27 |
| C1 | 28.31 ± 4.61 | 26.64 ± 2.01 | S2 | 21.41 ± 2.58 | 16.65 ± 0.05 |
| C2 | 23.24 ± 4.74 | 21.29 ± 0.02 | S3 | 22.53 ± 2.06 | 19.90 ± 0.65 |
| C3 | 22.67 ± 4.28 | 18.15 ± 0.60 | S4 | 8.26 ± 2.19 | 5.33 ± 0.01 |
| C4 | 27.85 ± 6.44 | 20.70 ± 2.16 | S5 | 12.10 ± 1.04 | 11.39 ± 0.01 |
| C5 | 36.41 ± 0.30 | 18.33 ± 2.29 | S6 | 20.14 ± 2.30 | 16.56 ± 0.90 |
| C6 | 31.45 ± 4.82 | 25.65 ± 3.74 | S7 | 19.64 ± 0.22 | 17.67 ± 0,04 |
| C7 | 31.90 ± 5.78 | 20.50 ± 1.45 | I1 | 3.68 ± 0.37 | 3.57 ± 0.12 |
| C8 | 21.67 ± 2.30 | 18.94 ± 0.24 | I2 | 3.04 ± 0.15 | 0.90 ± 0.10 |
| C9 | 20.82 ± 2.01 | 20.43 ± 0.06 |
2.1.2. Quantification of Pinocembrin, Chrysin, Galangin and CAPE through HPLC-DAD
| Sample code | PIN (% w/w) | CHR and GAL (% w/w) | CAPE (% w/w) | Samples code | PIN (% w/w) | CHR and GAL (% w/w) | CAPE (% w/w) |
|---|---|---|---|---|---|---|---|
| N1 | 1.04 ± 0.14 | 0.60 ± 0.01 | 0.86 ± 0.41 | C10 | 10.16 ± 0.05 | 8.67 ± 0.14 | 1.65 ± 0.05 |
| N2 | 8.20 ± 0.16 | 2.79 ± 0.17 | 1.30 ± 0.04 | C11 | 9.30 ± 0.08 | 10.03 ± 0.34 | 1.70 ±0.04 |
| N3 | 2.28 ± 0.10 | 2.52 ± 0.07 | 0.96 ± 0.03 | C12 | 11.27 ± 0.05 | 7.13 ± 0.13 | 1.62 ± 0.07 |
| N4 | *coeluition | <0.05 | 0.81 ± 0.03 | C13 | 4.91 ± 0.05 | 3.15 ± 0.07 | 1.01 ± 0.05 |
| N5 | 5.53± 0.03 | 4.87 ± 0.11 | 1.37 ± 0.01 | S1 | 9.87 ± 0.32 | 12.22 ± 0.13 | 1.93 ± 0.05 |
| C1 | 10.07 ± 0.12 | 9.38 ± 0.15 | 1.52 ± 0.02 | S2 | 8.14 ± 0.15 | 4.55 ± 0.01 | 1.96 ± 0.05 |
| C2 | 8.16 ± 0.15 | 5.68 ± 0.10 | 1.80 ± 0.02 | S3 | 8.33 ± 0.03 | 6.24 ± 0.10 | 1.43 ± 0.01 |
| C3 | 6.88 ± 0.09 | 5.38 ± 0.01 | 1.16 ± 0.01 | S4 | 3.08 ± 0.01 | 1.20 ± 0.03 | 1.59 ± 0.03 |
| C4 | 8.05 ± 0.09 | 6.11 ± 0.18 | 1.53 ± 0.01 | S5 | 5.13 ± 0.22 | 2.63 ± 0.12 | 1.54 ± 0.05 |
| C5 | 8.01 ± 0.24 | 6.58 ± 0.42 | 1.37 ± 0.01 | S6 | 8.01 ± 0.84 | 7.53 ± 0.42 | 1.74 ± 0.21 |
| C6 | 11.53 ± 0.12 | 10.02 ± 0.40 | 1.57 ± 0.01 | S7 | 7.13 ± 0.13 | 7.70 ± 0.14 | 1.17 ± 0.07 |
| C7 | 5.14 ± 0.17 | 4.31 ± 0.18 | 1.19 ± 0.01 | I1 | 1.46 ± 0.10 | 0.28 ± 0.01 | 0.14 ± 0.03 |
| C8 | 9.97 ± 0.03 | 4.57 ± 0.04 | 1.54 ± 0.43 | I2 | 0.24 ± 0.11 | 0.07 ± 0.01 | 0.10 ± 0.01 |
| C9 | 10.95 ± 0.13 | 6.47 ± 0.27 | 1.96 ± 0.41 |
2.2. Antiradicalic Activity of Propolis Samples
3. Conclusions
4. Materials and Methods
4.1. Samples Collection
4.2. Phytochemical Analyses
4.2.1. Quantification of Total Polyphenols and Total Flavonoids
4.2.2. HPLC-DAD Analysis
4.3. Antiradical Activity of Propolis
4.4. Multivariate Modelling
4.5. Statistical Analysis
Author Contributions
Funding
Data Availability
Acknowledgments
Conflicts of Interest
References
- Burlando, B.; Cornara, L. Honey in Dermatology and Skin Care: A Review. J Cosmet Dermatol 2013, 12, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M.; Spivak, M. Propolis and Bee Health: The Natural History and Significance of Resin Use by Honey Bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Bankova, V. Chemical Diversity of Propolis and the Problem of Standardization. J Ethnopharmacol 2005, 100, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Tazawa, S.; Ohta, S.; Rhyu, M.-R.; Misaka, T.; Ichihara, K. Artepillin C, a Major Ingredient of Brazilian Propolis, Induces a Pungent Taste by Activating TRPA1 Channels. PLoS One 2012, 7, e48072. [Google Scholar] [CrossRef] [PubMed]
- Valipour, M. Therapeutic Prospects of Naturally Occurring P38 MAPK Inhibitors Tanshinone IIA and Pinocembrin for the Treatment of SARS-CoV -2-induced CNS Complications. Phytotherapy Research 2023, 37, 3724–3743. [Google Scholar] [CrossRef] [PubMed]
- Governa, P.; Cusi, M.G.; Borgonetti, V.; Sforcin, J.M.; Terrosi, C.; Baini, G.; Miraldi, E.; Biagi, M. Beyond the Biological Effect of a Chemically Characterized Poplar Propolis: Antibacterial and Antiviral Activity and Comparison with Flurbiprofen in Cytokines Release by LPS-Stimulated Human Mononuclear Cells. Biomedicines 2019, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, 2010.
- European Parliament; European Council; European Commission Regulation Regulation (EU) 2015/2283.
- Biagi, M.; Pecorari, R.; Appendino, G.; Miraldi, E.; Magnano, A.; Governa, P.; Cettolin, G.; Giachetti, D. Herbal Products in Italy: The Thin Line between Phytotherapy, Nutrition and Parapharmaceuticals; A Normative Overview of the Fastest Growing Market in Europe. Pharmaceuticals 2016, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Gardini, S.; Bertelli, D.; Marchetti, L.; Graziosi, R.; Pinetti, D.; Plessi, M.; Marcazzan, G.L. Chemical Composition of Italian Propolis of Different Ecoregional Origin. J Apic Res 2018, 57, 639–647. [Google Scholar] [CrossRef]
- Papotti, G.; Bertelli, D.; Bortolotti, L.; Plessi, M. Chemical and Functional Characterization of Italian Propolis Obtained by Different Harvesting Methods. J Agric Food Chem 2012, 60, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Aliboni, A.; D’Andrea, A.; Massanisso, P. Propolis Specimens from Different Locations of Central Italy: Chemical Profiling and Gas Chromatography−Mass Spectrometry (GC−MS) Quantitative Analysis of the Allergenic Esters Benzyl Cinnamate and Benzyl Salicylate. J Agric Food Chem 2011, 59, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.P.; Bankova, V.S.; Bogdanov, S.; Tsvetkova, I.; Naydenski, C.; Marcazzan, G.L.; Sabatini, A.-G. Chemical Characteristics of Poplar Type Propolis of Different Geographic Origin. Apidologie 2007, 38, 306–306. [Google Scholar] [CrossRef]
- Parco Nazionale foreste casentinesi Popolus Spp. Available online: https://www.parcoforestecasentinesi.it/sites/default/files/Quattordicesima.pdf (accessed on 2 September 2024).
- Cui-ping, Z.; Shuai, H.; Wen-ting, W.; Shun, P.; Xiao-ge, S.; Ya-jing, L.; Fu-liang, H. Development of High-Performance Liquid Chromatographic for Quality and Authenticity Control of Chinese Propolis. J Food Sci 2014, 79. [Google Scholar] [CrossRef] [PubMed]
- Tolba, M.F.; Azab, S.S.; Khalifa, A.E.; Abdel-Rahman, S.Z.; Abdel-Naim, A.B. Caffeic Acid Phenethyl Ester, a Promising Component of Propolis with a Plethora of Biological Activities: A Review on Its Anti-inflammatory, Neuroprotective, Hepatoprotective, and Cardioprotective Effects. IUBMB Life 2013, 65, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, A.; Faraloni, C.; Venturini, S.; Baini, G.; Miraldi, E.; Biagi, M. Characterization of Phenolic Profile and Antioxidant Activity of the Leaves of the Forgotten Medicinal Plant Balsamita Major Grown in Tuscany, Italy, during the Growth Cycle. Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology 2021, 155, 908–913. [Google Scholar] [CrossRef]
- Pressi, G.; Bertaiola, O.; Guarnerio, C.; Barbieri, E.; Rigillo, G.; Governa, P.; Biagi, M.; Guzzo, F.; Semenzato, A. In Vitro Cell Culture of Rhus Coriaria L.: A Standardized Phytocomplex Rich of Gallic Acid Derivatives with Antioxidant and Skin Repair Activity. Cosmetics 2022, 9, 12. [Google Scholar] [CrossRef]
- Biagi, M.; Collodel, G.; Corsini, M.; Pascarelli, N.A.; Moretti, E. Protective Effect of Propolfenol ® on Induced Oxidative Stress in Human Spermatozoa. Andrologia 2018, 50, e12807. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Biagi, M.; Ercoli, J.; Macrì, G.; Miraldi, E.; Trabalzini, L. Phaseolus Vulgaris L. Var. Venanzio Grown in Tuscany: Chemical Composition and In Vitro Investigation of Potential Effects on Colorectal Cancer. Antioxidants 2020, 9, 1181. [Google Scholar] [CrossRef] [PubMed]
- Governa, P.; Biagi, M. Copaifera Langsdorffii Desf.: In Vitro Investigation on Anti- Helicobacter Pylori and Anti-Inflammatory Activities of Oleoresin and Fruit Methanolic Extract. Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology 2020, 154, 117–124. [Google Scholar] [CrossRef]
- Sberna, G.; Biagi, M.; Marafini, G.; Nardacci, R.; Biava, M.; Colavita, F.; Piselli, P.; Miraldi, E.; D’Offizi, G.; Capobianchi, M.R.; et al. In Vitro Evaluation of Antiviral Efficacy of a Standardized Hydroalcoholic Extract of Poplar Type Propolis Against SARS-CoV-2. Front Microbiol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Scikit Learn. Available online: https://scikit-learn.org/stable/modules/generated/sklearn.decomposition.PCA.html (accessed on 2 September 2024).
- Matplotlib. Available online: https://matplotlib.org/ (accessed on 2 September 2024).
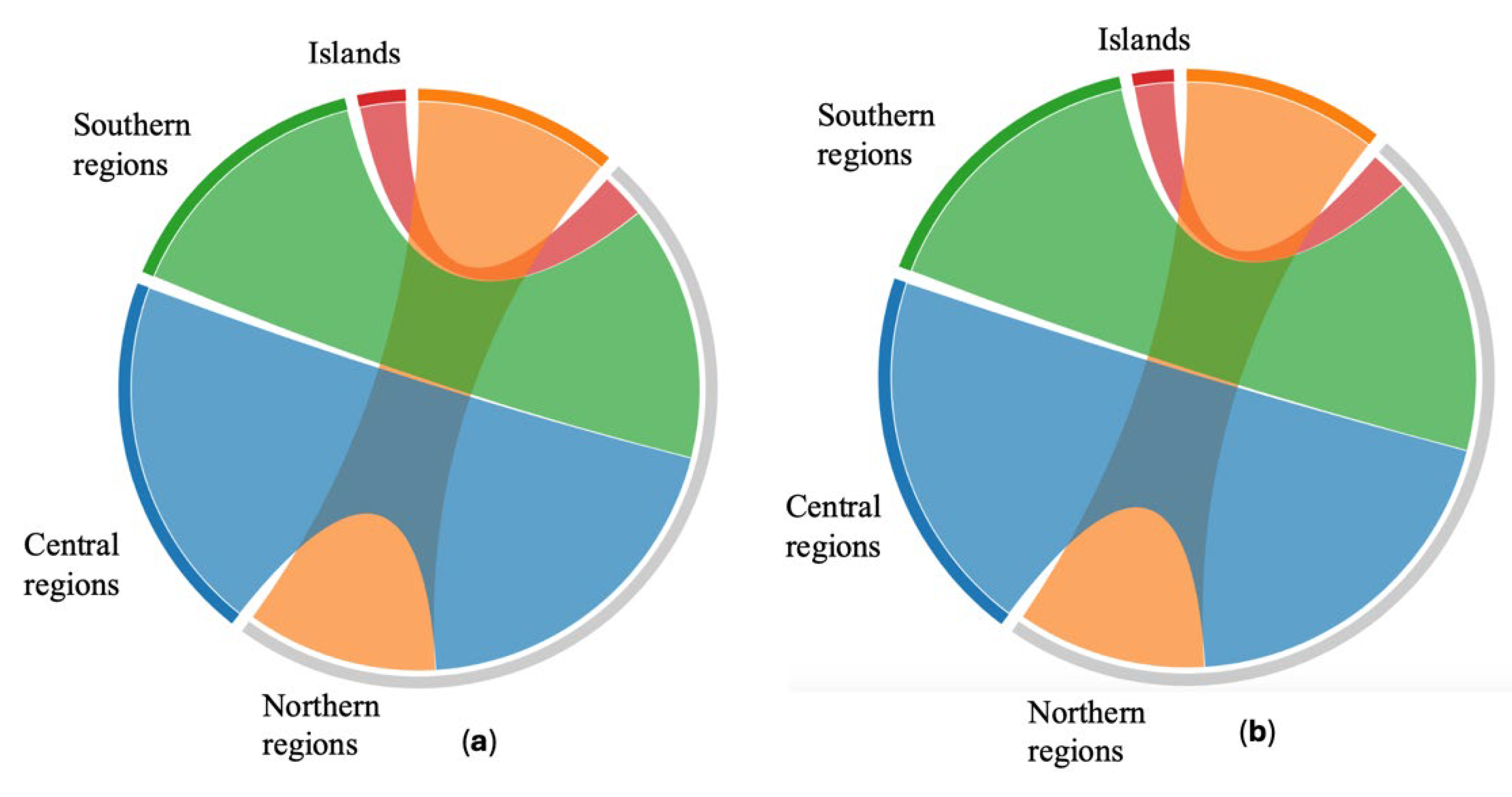
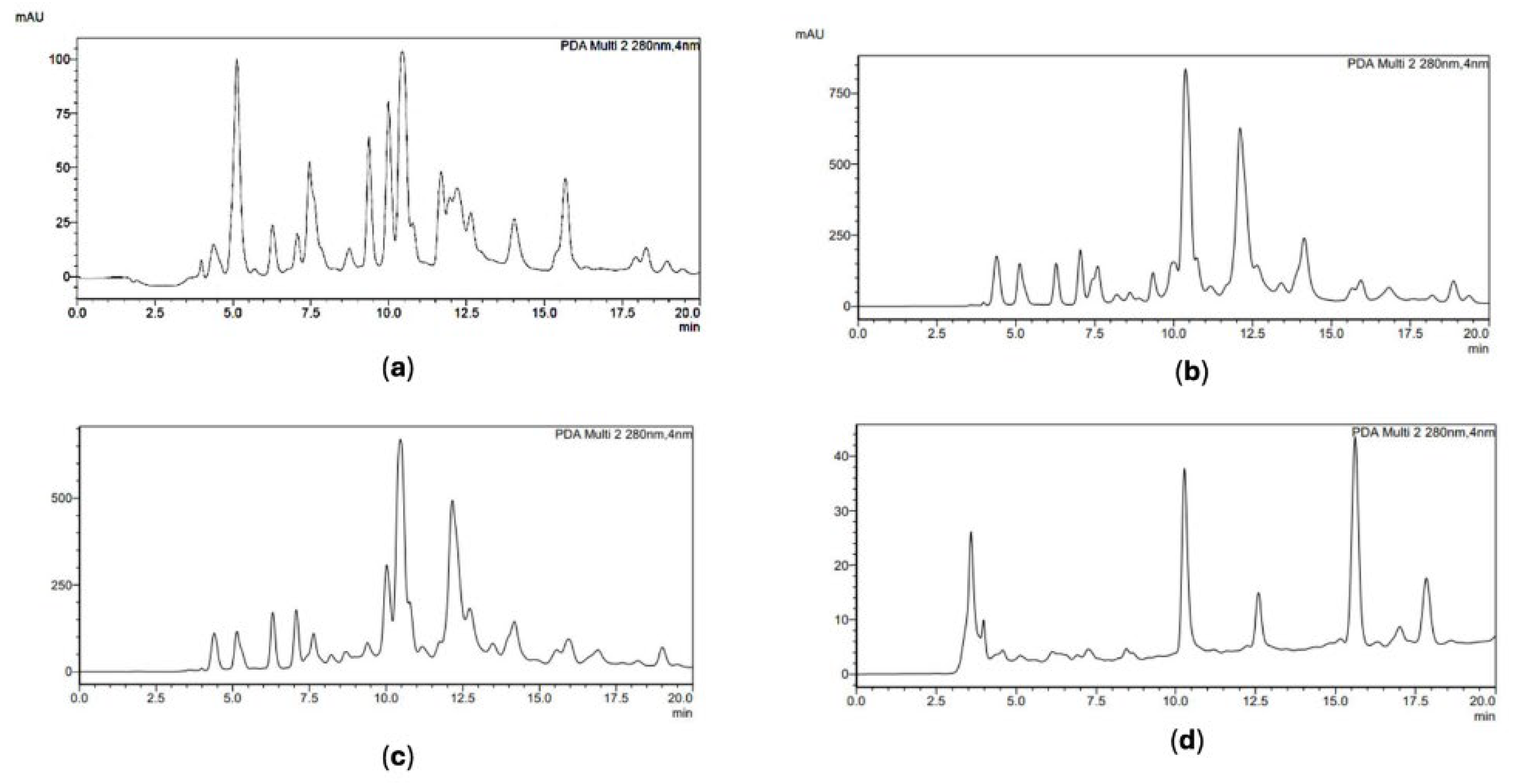
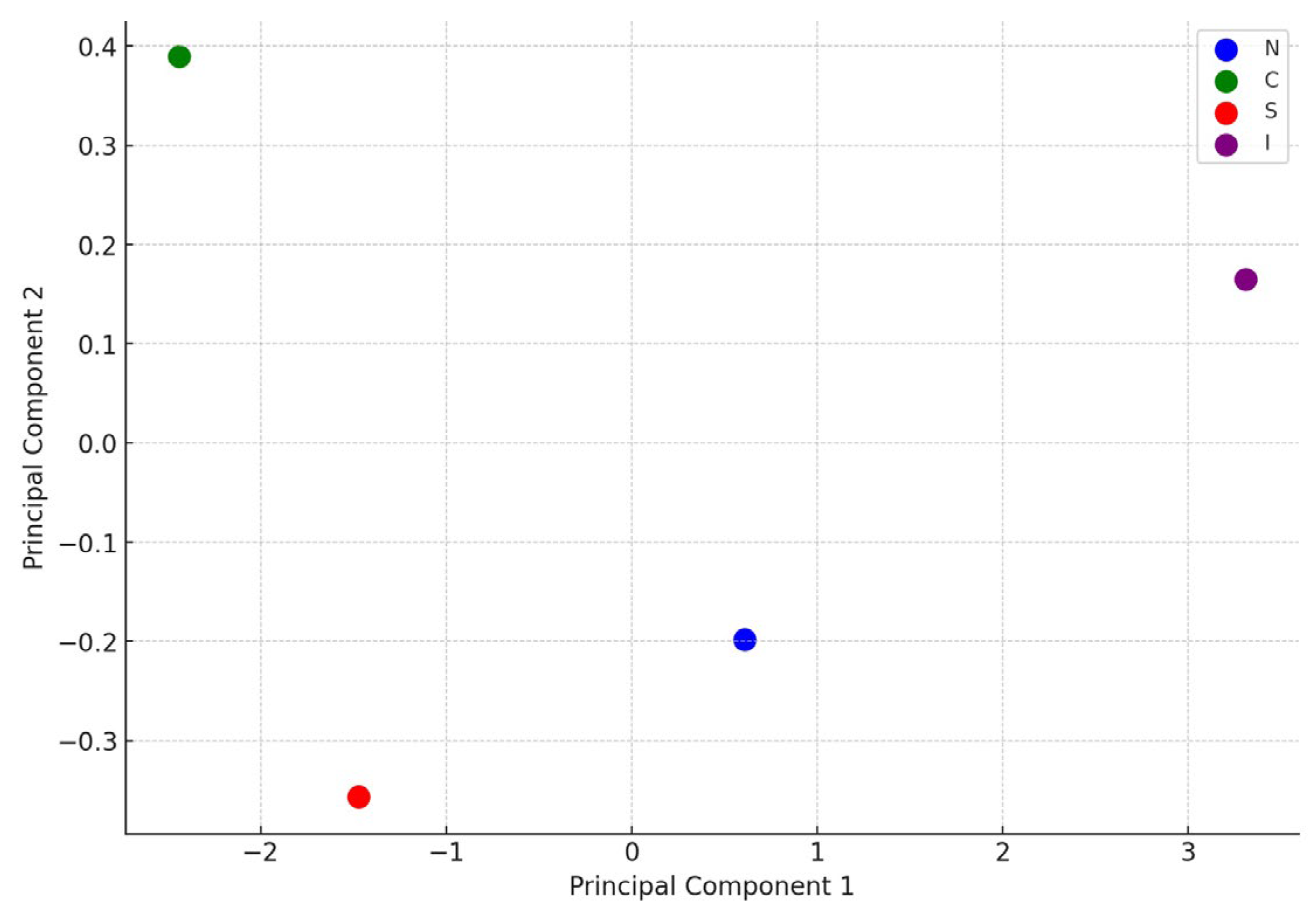
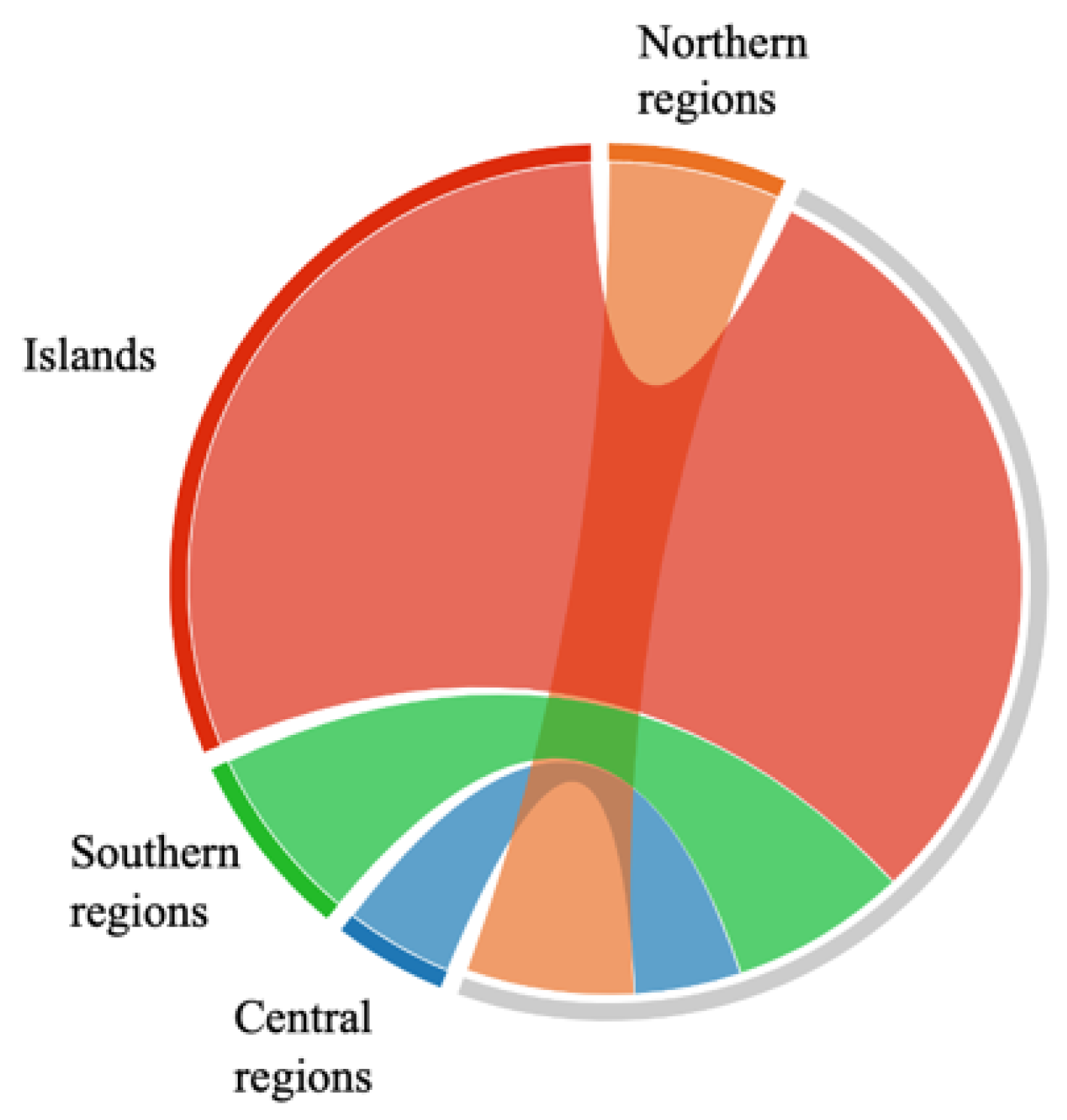
| Geographical region | TP (% w/w) |
TF (% w/w) |
Flavonoids/Polyphenols ratio |
|---|---|---|---|
| N | 14.13 ± 6.47a | 10.82 ± 5.32a | 0.76 ± 0.13a |
| C | 25.75 ± 5.41b | 20.39 ± 3.65b | 0.81 ± 0.13a |
| S | 18.85 ± 6.60ab | 15.97 ± 6.09ab | 0.83 ± 0.10a |
| I | 3.36 ± 0.45c | 2.24 ± 1.89c | 0.63 ± 0.48a |
| Geographical region | PIN (%w/w) |
PIN/TF ratio | CHR and GAL (%w/w) | CHR and GAL /TF ratio | CAPE (%w/w) | CAPE/TP ratio |
|---|---|---|---|---|---|---|
| N | 4.26 ± 3.24a | 0.29 ± 0.19a | 2.16 ± 1.93a | 0.20 ± 0.15a | 1.06 ± 0.26a | 0.09 ± 0.04a |
| C | 8.80 ± 2.18b | 0.43 ± 0.08a | 6.73 ± 2.22bc | 0.33 ± 0.08ab | 1.51 ± 0.27b | 0.06 ± 0.02ab |
| S | 7.10 ± 2.28ab | 0.46 ± 0.06a | 6.01 ± 3.66c | 0.35 ± 0.11b | 1.62 ± 0.28b | 0.10 ± 0.05a |
| I | 0.85 ± 0.86c | 0.34 ± 0.10a | 0.18 ± 0.15a | 0.08 ± 0.01ca | 0.12 ± 0.03c | ± 0.01ab |
| Samples | IC50 (µg/mL) | Samples | IC50 (µg/mL) |
|---|---|---|---|
| N1 | 67.27 | C10 | 23.67 |
| N2 | 27.34 | C11 | 26.78 |
| N3 | 40.76 | C12 | 18.92 |
| N4 | 37.78 | C13 | 32.42 |
| N5 | 33.48 | S1 | 21.93 |
| C1 | 25.82 | S2 | 32.71 |
| C2 | 30.04 | S3 | 29.48 |
| C3 | 26.80 | S4 | 100.16 |
| C4 | 27.09 | S5 | 64.75 |
| C5 | 24.12 | S6 | 26.24 |
| C6 | 27.70 | S7 | 30.46 |
| C7 | 26.18 | I1 | 162.67 |
| C8 | 36.13 | I2 | 220.59 |
| C9 | 24.25 |
| Geographical region | IC50 |
|---|---|
| N | 41.33 ± 15.36° |
| C | 26.46 ± 4.09° |
| S | 43.68 ± 28.60° |
| I | 191.63 ± 40.96b |
| Samples code | Area of origin | Gps coordinates | Region | Samples code | Area of origin | Gps coordinates | Region |
|---|---|---|---|---|---|---|---|
| N1 | Valdilana (BI) | 45°39′25.66″N 8°09′01.85″E | Piedmont | C10 | Arcidosso (GR) | 42°52′20″N 11°32′15″E | Tuscany |
| N2 | Arcisate (VA) | 45°51′18.98″N 8°52′03.3″E | Lombardia | C11 | Castello delle Forme, Marsciano (PG) | 42°58′47.06″N 12°21′22.21″E | Umbria |
| N3 | Castellanza (VA) | 45°37′N 8°54′E | Lombardia | C12 | Deruta (PG) | 42°59′N 12°25′E | Umbria |
| N4 | Pergine Valsugana (TN) | 46°04′N 11°14′E | Trentino-Alto Adige | C13 | Norma, Monti Lepini (LT) | 41°35′N 12°58′E | Lazio |
| N5 | Castel San Pietro Terme (BO) | 44°23′52″N 11°35′22″E | Emilia-Romagna | S1 | Bellante (TE) | 42°45′N 13°48′E | Abruzzo |
| C1 | Quarrata (PT) | 43°50′51″N 10°59′00″E | Tuscany | S2 | Massiccio Del Matese | 41°26′59.87″N 14°22′19.21″E | Molise/Campania |
| C2 | Firenze Valdarno (FI) | 43°39′24″N 11°26′58″E | Tuscany | S3 | Campobasso (CB) | 41°33′39.6″N 14°40′06.24″E | Molise |
| C3 | Figline Valdarno (FI) | 43°37′N 11°28′E | Tuscany | S4 | Rodi Garganico (FG) | 41°55′19.9″N 15°52′37.86″E | Puglia |
| C4 | Grassina Ponte a Ema (FI) | 43°44′22.42″N 11°17′51.73″E | Tuscany | S5 | San Severo (FG) | 41°41′42.4″N 15°22′45.4″E | Puglia |
| C5 | Greve in Chianti (FI) | 43°35′N 11°19′E | Tuscany | S6 | San Basile (CS) | 39°48′34.56″N 16°09′47.81″E | Calabria |
| C6 | Reggello (FI) | 43°41′N 11°32′E | Tuscany | S7 | Cicala (CZ) | 39°01′19.88″N 16°29′09.96″E | Calabria |
| C7 | San Polo in Chianti (FI) | 43°40′18.13″N 11°21′46.13″E | Tuscany | I1 | Isola di Capraia (LI) | 43°02′55.32″N 9°50′25.08″E | Tuscany |
| C8 | Batignano (GR) | 42°52′02.22″N 11°09′57.84″E | Tuscany | I2 | Pianoconte (ME) | 38°28'38.2"N 14°55'43.8"E | Sicily |
| C9 | Montorsaio (GR) | 42°53′26″N 11°12′13″E | Tuscany |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





