Submitted:
12 September 2024
Posted:
13 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethical Considerations
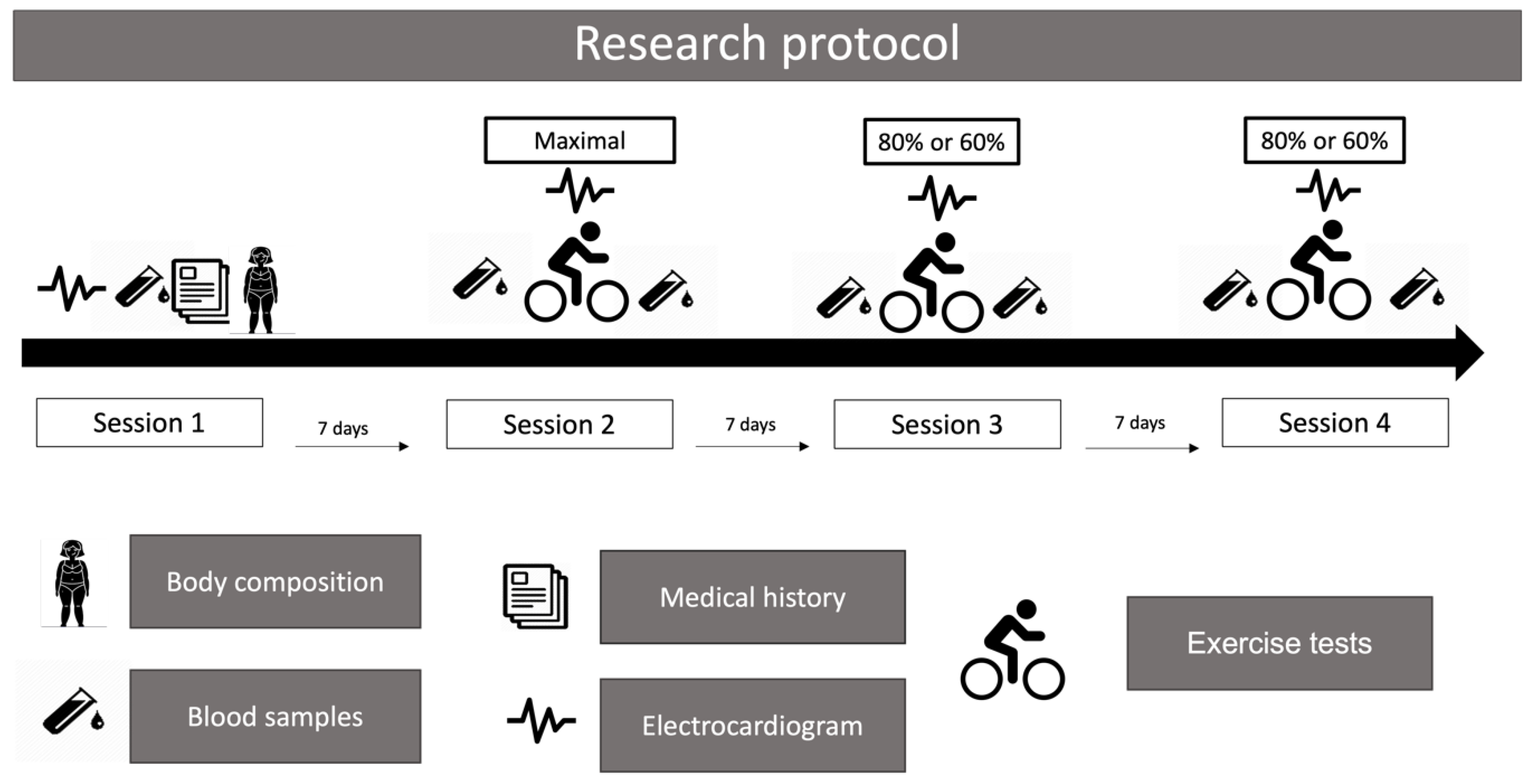
2.3. Research Protocol
2.3.1. First Session
2.3.2. Second Session
2.3.3. Third and Fourth Sessions
2.4. Measurements of Concentrations of Cr and CysC
2.5. Statistical Analysis
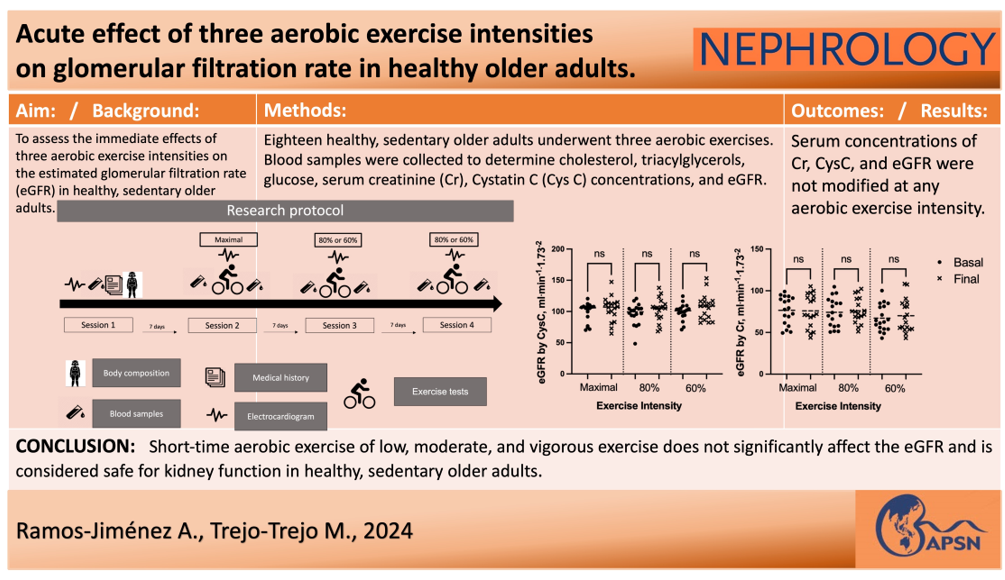
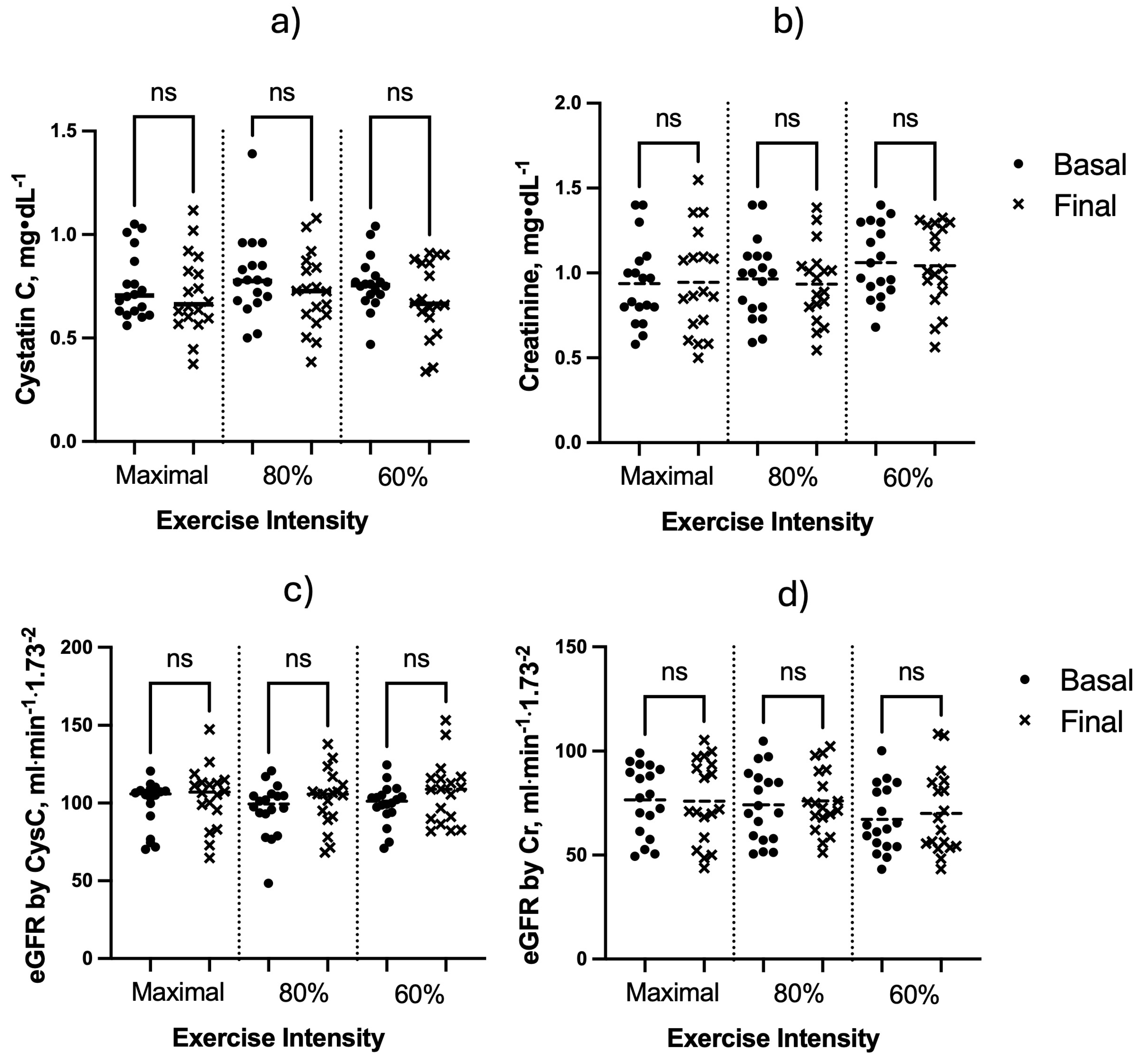
3. Results
4. Discussion
5. Conclusions
6. Future Research Directions
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Amaral, T.L.M.; Amaral, C. de A.; Vasconcellos, M.T.L. de; Monteiro, G.T.R. Prevalence and factors associated to chronic kidney disease in older adults. Rev Saude Publica 2019, 53, 44. [Google Scholar] [CrossRef] [PubMed]
- Villanego, F.; Naranjo, J.; Vigara, L.A.; Cazorla, J.M.; Montero, M.E.; García, T.; Torrado, J.; Mazuecos, A. Impact of physical exercise in patients with chronic kidney disease: Sistematic review and meta-analysis. Nefrologia (Engl Ed) 2020, 40, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Spada, T.C.; Silva, J.M.R.D.; Francisco, L.S.; Marçal, L.J.; Antonangelo, L.; Zanetta, D.M.T.; Yu, L.; Burdmann, E.A. High Intensity Resistance Training Causes Muscle Damage and Increases Biomarkers of Acute Kidney Injury in Healthy Individuals. PLoS One 2018, 13, e0205791. [Google Scholar] [CrossRef] [PubMed]
- Poortmans, J.R.; Ouchinsky, M. Glomerular Filtration Rate and Albumin Excretion after Maximal Exercise in Aging Sedentary and Active Men. J Gerontol A Biol Sci Med Sci 2006, 61, 1181–1185. [Google Scholar] [CrossRef]
- Bongers, C.C.W.G.; Alsady, M.; Nijenhuis, T.; Tulp, A.D.M.; Eijsvogels, T.M.H.; Deen, P.M.T.; Hopman, M.T.E. Impact of Acute versus Prolonged Exercise and Dehydration on Kidney Function and Injury. Physiol Rep 2018, 6, e13734. [Google Scholar] [CrossRef] [PubMed]
- Pryor, R.R.; Pryor, J.L.; Vandermark, L.W.; Adams, E.L.; Brodeur, R.M.; Schlader, Z.J.; Armstrong, L.E.; Lee, E.C.; Maresh, C.M.; Casa, D.J. Acute Kidney Injury Biomarker Responses to Short-Term Heat Acclimation. Int J Environ Res Public Health 2020, 17. [Google Scholar] [CrossRef]
- Ariev, A.L.; Kayukov, I.G.; Beresneva, O.N.; Parastaeva, M.M.; Essaian, A.M.; Kucher, A.G. Aging and kidneys: problems in evaluating of the glomerular filtration rate in elderly. Adv Gerontol 2019, 32, 614–626. [Google Scholar]
- Bibbo, G.; Munn, C.; Kirkwood, I. Comparison of Glomerular Filtration Rates Determined Using Two- and Single-Blood Sample Methods with a Three-Blood Sample Technique for 2922 Paediatric Studies. Nucl Med Commun 2019, 40, 1204–1210. [Google Scholar] [CrossRef]
- Gaspari, F.; Perico, N.; Remuzzi, G. Application of Newer Clearance Techniques for the Determination of Glomerular Filtration Rate. Curr Opin Nephrol Hypertens 1998, 7, 675–680. [Google Scholar] [CrossRef]
- Inoue, Y.; Ohtake, T.; Homma, Y.; Yoshikawa, K.; Nishikawa, J.; Sasaki, Y. Evaluation of Glomerular Filtration Rate by Camera-Based Method in Both Children and Adults. J Nucl Med 1998, 39, 1784–1788. [Google Scholar] [PubMed]
- Agarwal, R. Chromatographic Estimation of Iothalamate and P-Aminohippuric Acid to Measure Glomerular Filtration Rate and Effective Renal Plasma Flow in Humans. J Chromatogr B Biomed Sci Appl 1998, 705, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Laterza, O.F.; Price, C.P.; Scott, M.G. Cystatin C: An Improved Estimator of Glomerular Filtration Rate? Clin Chem 2002, 48, 699–707. [Google Scholar] [CrossRef]
- Poussel, M.; Touzé, C.; Allado, E.; Frimat, L.; Hily, O.; Thilly, N.; Rousseau, H.; Vauthier, J.-C.; Chenuel, B. Ultramarathon and Renal Function: Does Exercise-Induced Acute Kidney Injury Really Exist in Common Conditions? Front Sports Act Living 2019, 1, 71. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, F.; Verdi, M.; Schlemper, B.R.J.; Caponi, S. 50th Anniversary of the Declaration of Helsinki: The Double Standard Was Introduced. Arch Med Res 2014, 45, 600–601. [Google Scholar] [CrossRef] [PubMed]
- Aström, H.; Jonsson, B. Design of Exercise Test, with Special Reference to Heart Patients. Heart 1976, 38, 289–296. [Google Scholar] [CrossRef]
- Miura, K.; Nakagawa, H.; Ohashi, Y.; Harada, A.; Taguri, M.; Kushiro, T.; Takahashi, A.; Nishinaga, M.; Soejima, H.; Ueshima, H. Four Blood Pressure Indexes and the Risk of Stroke and Myocardial Infarction in Japanese Men and Women: A Meta-Analysis of 16 Cohort Studies. Circulation 2009, 119, 1892–1898. [Google Scholar] [CrossRef]
- Mexican Ministry of Health Mexican Official Standard (NOM-167-SSA1-1997), for the provision of social assistance services for minors and older adults. Diario Oficial de la Federación 1999.
- Ramos-Jiménez, A.; Wall-Medrano, A.; Hernández-Lepe, M.A.; Chávez-Treviño, G.; Guereca-Arvizuo, J.; Hernández-Torres, R.P. Borg’s category ratio-scale (CR-10) is useful to predict the onset of blood lactate accumulation (OBLA) in young Mexicans adults, regardless their body mass. CIENCIA ergo-sum 2019, 26, 7. [Google Scholar] [CrossRef]
- Gellish, R.L.; Goslin, B.R.; Olson, R.E.; McDonald, A.; Russi, G.D.; Moudgil, V.K. Longitudinal Modeling of the Relationship between Age and Maximal Heart Rate. Med Sci Sports Exerc 2007, 39, 822–829. [Google Scholar] [CrossRef]
- Storer, T.W.; Davis, J.A.; Caiozzo, V.J. Accurate Prediction of VO2max in Cycle Ergometry. Med Sci Sports Exerc 1990, 22, 704–712. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Farney, T.M. Acute Plasma Volume Change with High-Intensity Sprint Exercise. J Strength Cond Res 2013, 27, 2874–2878. [Google Scholar] [CrossRef] [PubMed]
- Van Beaumont, W. Evaluation of Hemoconcentration from Hematocrit Measurements. J Appl Physiol 1972, 32, 712–713. [Google Scholar] [CrossRef] [PubMed]
- Fadel, P.J. Reflex Control of the Circulation during Exercise. Scand J Med Sci Sports 2015, 25 Suppl 4, 74–82. [Google Scholar] [CrossRef]
- Crecelius, A.R.; Kirby, B.S.; Luckasen, G.J.; Larson, D.G.; Dinenno, F.A. Mechanisms of Rapid Vasodilation after a Brief Contraction in Human Skeletal Muscle. Am J Physiol Heart Circ Physiol 2013, 305, H29–40. [Google Scholar] [CrossRef] [PubMed]
- Tschakovsky, M.E.; Rogers, A.M.; Pyke, K.E.; Saunders, N.R.; Glenn, N.; Lee, S.J.; Weissgerber, T.; Dwyer, E.M. Immediate Exercise Hyperemia in Humans Is Contraction Intensity Dependent: Evidence for Rapid Vasodilation. J Appl Physiol (1985) 2004, 96, 639–644. [Google Scholar] [CrossRef]
- Momen, A.; Handly, B.; Kunselman, A.; Leuenberger, U.A.; Sinoway, L.I. Influence of Sex and Active Muscle Mass on Renal Vascular Responses during Static Exercise. Am J Physiol Heart Circ Physiol 2006, 291, H121–126. [Google Scholar] [CrossRef]
- García-Trabanino, R.; Jarquín, E.; Wesseling, C.; Johnson, R.J.; González-Quiroz, M.; Weiss, I.; Glaser, J.; José Vindell, J.; Stockfelt, L.; Roncal, C.; et al. Heat Stress, Dehydration, and Kidney Function in Sugarcane Cutters in El Salvador--A Cross-Shift Study of Workers at Risk of Mesoamerican Nephropathy. Environ Res 2015, 142, 746–755. [Google Scholar] [CrossRef]
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes With the Aging Kidney. Adv Chronic Kidney Dis 2016, 23, 19–28. [Google Scholar] [CrossRef]
- Hommos, M.S.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes in Human Kidneys with Healthy Aging. J Am Soc Nephrol 2017, 28, 2838–2844. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013, 3, 1–150. [Google Scholar] [CrossRef]
- Gu, X.; Fang, X.; Ji, X.; Tang, Z.; Wang, C.; Guan, S.; Wu, X.; Liu, H.; Zhang, Z. Kidney Dysfunction Is Associated with Risk of Cardiovascular Events in Middle-Aged and Elderly Population with Hypertension: A 5-Year Community-Based Cohort Study in China. Clin Nephrol 2020, 93, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Verbeeck, R.K.; Musuamba, F.T. Pharmacokinetics and Dosage Adjustment in Patients with Renal Dysfunction. Eur J Clin Pharmacol 2009, 65, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Abdulkader, R.C.R.M.; Burdmann, E.A.; Lebrão, M.L.; Duarte, Y.A.O.; Zanetta, D.M.T. Aging and Decreased Glomerular Filtration Rate: An Elderly Population-Based Study. PLoS One 2017, 12, e0189935. [Google Scholar] [CrossRef]
- Mohan, S.G.; Holmgren, N.J.; Jacobson, L.E.; Saxe, J.M.; West-Sell, S.A.; Williams, J.; Harper, P.; Jensen, C.D. Climate Factors May Influence Glomerular Filtration Rate in Nephrology Patients. The FASEB Journal 2022, 36. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N Engl J Med 2012, 367, 20–29. [Google Scholar] [CrossRef]
- Ferguson, T.W.; Komenda, P.; Tangri, N. Cystatin C as a Biomarker for Estimating Glomerular Filtration Rate. Curr Opin Nephrol Hypertens 2015, 24, 295–300. [Google Scholar] [CrossRef]
| Age (years) | 70.1 ± 5.1 |
| Weight (kg) | 71.0 ± 10.9 |
| Height (cm) | 163.3 ± 7.6 |
| BMI (kg·m-2) | 26.8 ± 3.3 |
| Fat mass (%) | 24.1 ± 5.5 |
| Systolic blood pressure (mmHg) | 124.7 ± 13.1 |
| Diastolic blood pressure (mmHg) | 78.9 ± 10.2 |
| Mean blood pressure (mmHg) | 94.2 ± 10.3 |
| Glucose (mg·dl-1) | 103 ± 13 |
| Cholesterol (mg·dl-1) | 185 ± 34 |
| Triacylglycerol (mg·dl-1) | 127 ± 41 |
| Creatinine (mg·dl-1) | 0.937 ± 0.246 |
| Cystatin C (mg·dl-1) | 0.753 ± 0.161 |
| eGFR by Cr (mL·min·1.73 m2) | 76.5 ± 16.7 |
| eGFR by CysC (mL·min·1.73 m2) | 99.3± 15.4 |
| VO2 max (ml·kg-1·min-1) | 28.6 ± 7.1 |
| HRmax (beats·min-1) | 148 ± 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





