Submitted:
12 September 2024
Posted:
13 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Analysis and Data Collection
2.3. Statistical Analysis
3. Results
3.1. Vitamin D Status in Mothers and Newborns
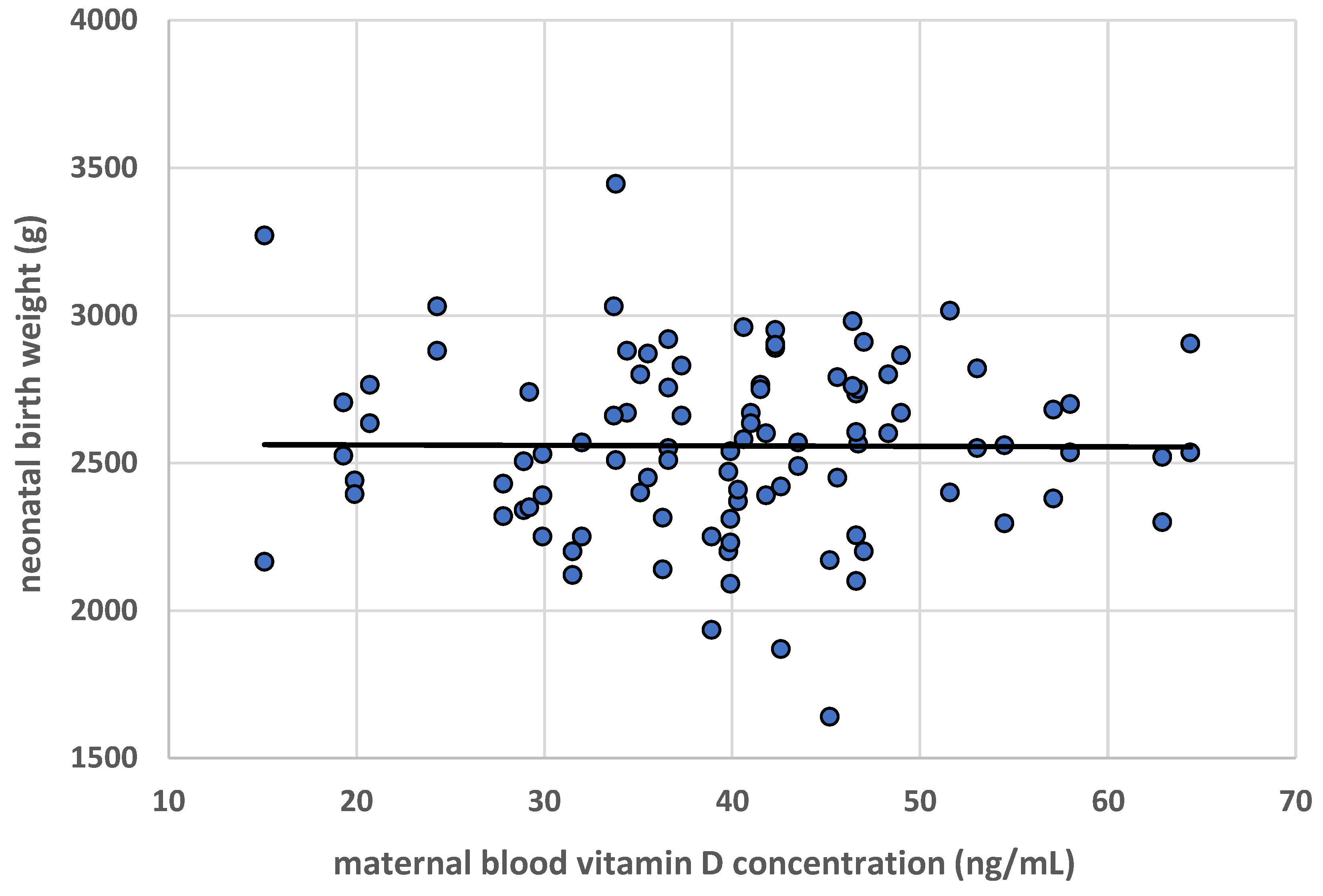
3.2. Neonatal Weight
3.3. Neonatal Length
3.4. Head Circumference
3.5. Neonatal Chest Circumference
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- van der Pligt, P.F.; Ellery, S.J.; de Guingand, D.L.; Abbott, G.; Della Gatta, P.A.; Daly, R.M. Maternal plasma vitamin D levels across pregnancy are not associated with neonatal birthweight: Findings from an Australian cohort study of low-risk pregnant women. BMC Pregnancy Childbirth 2023, 23(1), 67. [Google Scholar] [CrossRef]
- Gallo, S.; McDermid, J.M.; Al-Nimr, R.I.; Hakeem, R.; Moreschi, J.M.; Pari-Keener, M.; Stahnke, B.; Papoutsakis, C.; Handu, D.; Cheng, F.W. Vitamin D supplementation during pregnancy: An evidence analysis center systematic review and meta-analysis. J. Acad. Nutr. Diet. 2020, 120(5), 898–924.e4. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, A.; Shinde, V.; Ravindran, R. Pooled estimate of vitamin D deficiency among pregnant women in India: A systematic review and meta-analysis. J. Health Popul. Nutr. 2021, 40(1), 28. [Google Scholar] [CrossRef]
- Blarduni, E.; Arrospide, A.; Galar, M.; Castaño, L.; Mar, J.; Grupo GOIVIDE. Factors associated with the prevalence of hypovitaminosis D in pregnant women and their newborns. An. Pediatr. (Engl Ed). 2019, 91(2), 96–104. [Google Scholar] [CrossRef] [PubMed]
- Zgliczyńska, M.; Kosińska-Kaczyńska, K. Micronutrients in multiple pregnancies-the knowns and unknowns: A systematic review. Nutrients 2021, 13(2), 386. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.L.; Oliveri, B.; Giacoia, E.; Fusaro, D.; Costanzo, P.R. Vitamin D: Before, during and after pregnancy: Effect on neonates and children. Nutrients 2022, 14(9), 1900. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.; Tung, K.T.S.; Mak, R.T.W.; Leung, W.C.; Yam, J.C.; Chua, G.T. Fung, G.P.G.; Ho, M.H.K.; Wong, I.C.K.; Ip, P. Vitamin D concentrations during pregnancy and in cord blood: A systematic review and meta-analysis. Nutr. Rev. 2022, 80(12), 2225–2236. [Google Scholar] [CrossRef]
- Curtis, E.M.; Moon, R.J.; Harvey, N.C.; Cooper, C. Maternal vitamin D supplementation during pregnancy. Br. Med. Bull. 2018, 126(1), 57–77. [Google Scholar] [CrossRef]
- Dovnik, A.; Mujezinović, F.; Treiber, M.; Pečovnik Balon, B.; Gorenjak, M.; Maver, U.; Takač, I. Seasonal variations of vitamin D concentrations in pregnant women and neonates in Slovenia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 6–9. [Google Scholar] [CrossRef]
- Cadario, F.; Savastio, S.; Pozzi, E.; Capelli, A.; Dondi, E.; Gatto, M.; Zaffaroni, M.; Bona, G. Vitamin D status in cord blood and newborns: Ethnic differences. Ital. J. Pediatr. 2013, 39, 35. [Google Scholar] [CrossRef]
- Goswami, D.; Rani, R.; Saxena, A.; Arora, M.S.; Batra, S.; Sreenivas, V. Maternal and neonatal vitamin-D status in twin versus singleton pregnancies. J. Obstet. Gynaecol. Res. 2016, 42(10), 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.B.; Mărginean, C. The impact of vitamin D deficiency on infants’ health. Nutrients 2023, 15(20), 4379. [Google Scholar] [CrossRef]
- Ma, K.; Wei, S.Q.; Bi, W.G.; Weiler, H.A.; Wen, S.W. Effect of vitamin D supplementation in early life on children’s growth and body composition: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2021, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.L.; Lu, F.G.; Yang, S.H.; Xu, H.L.; Luo, B.A. Does maternal vitamin D deficiency increase the risk of preterm birth: A meta-analysis of observational studies. Nutrients 2016, 8(5), 301. [Google Scholar] [CrossRef] [PubMed]
- Tous, M.; Villalobos, M.; Iglesias, L.; Fernández-Barrés, S.; Arija, V. Vitamin D status during pregnancy and offspring outcomes: A systematic review and meta-analysis of observational studies. Eur. J. Clin. Nutr. 2020, 74(1), 36–53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhou, L.; Wang, S.; Yin, H.; Yang, X.; Hao, L. Effect of maternal vitamin D status on risk of adverse birth outcomes: A systematic review and dose-response meta-analysis of observational studies. Eur. J. Nutr. 2022, 61(6), 2881–2907. [Google Scholar] [CrossRef]
- Bi, W.G.; Nuyt, A.M.; Weiler, H.; Leduc, L.; Santamaria, C.; Wei, S.Q. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: A systematic review and meta-analysis. JAMA Pediatr. 2018, 172(7), 635–645. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7(7), CD008873. [Google Scholar] [CrossRef]
- Bialy, L.; Fenton, T.; Shulhan-Kilroy, J.; Johnson, D.W.; McNeil, D.A.; Hartling, L. Vitamin D supplementation to improve pregnancy and perinatal outcomes: An overview of 42 systematic reviews. BMJ Open 2020, 10(1), e032626. [Google Scholar] [CrossRef]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for preventing and treating vitamin D deficiency: A 2023 update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef]
- Palmrich, P.; Thajer, A.; Schirwani, N.; Haberl, C.; Zeisler, H.; Ristl, R.; Binder, J. Longitudinal assessment of serum 25-hydroxyvitamin D levels during pregnancy and postpartum—Are the current recommendations for supplementation sufficient? Nutrients 2023, 15(2), 339. [Google Scholar] [CrossRef]
- Sato, Y.; Kamei, A.; Endo, F.; Matsuyama, S.; Toda, H.; Kasai, T. Vitamin D supplementation at a dose of 10 µg/day in institutionalized children with severe motor and intellectual disabilities. Nutrients 2024, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Vierucci, F.; Fusani, L.; Saba, A.; Minucciani, T.; Belluomini, M.P.; Domenici, R.; Bracco, G.L.; Vaccaro, A.; Federico, G. Gestational vitamin D3 supplementation and sun exposure significantly influence cord blood vitamin D status and 3-epi-25-hydroxyvitamin D3 levels in term newborns. Clin. Chim. Acta 2022, 524, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Wagner, C.L.; Castracane, V.D. Understanding vitamin D metabolism in pregnancy: From physiology to pathophysiology and clinical outcomes. Metabolism 2018, 86, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Streym, S.; Moller, U.K.; Rejnmark, L.; Heickendorff, L.; Mosekilde, L.; Vestergaard, P. Maternal and infant vitamin D status during the first 9 months of infant life—A cohort study. Eur. J. Clin. Nutr. 2013, 67, 1022–1028. [Google Scholar] [CrossRef]
- Kiely, M.E.; Wagner, C.L.; Roth, D.E. Vitamin D in pregnancy: Where we are and where we should go. J. Steroid. Biochem. Mol. Biol. 2020, 201, 105669. [Google Scholar] [CrossRef]
- Ku, C.W.; Lee, A.J.W.; Oh, B.; Lim, C.H.F.; Chang, T.Y.; Yap, F.; Chan, J.K.Y.; Loy, S.L. The effect of vitamin D supplementation in pregnant women with overweight and obesity: A randomised controlled trial. Nutrients 2024, 16, 146. [Google Scholar] [CrossRef]
- Le, J.; Lv, Z.H.; Peng, R.; Li, Y.; Wang, S.T. Evaluation of vitamin D status and the analysis of risk factors of vitamin D deficiency in twin pregnancies. Lab. Med. 2023, 54(5), 534–542. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, A.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press (US): Washington, DC, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK56070/ (accessed on 5 January 2024).
- Kiely, M.; O’Donovan, S.M.; Kenny, L.C.; O´B Hourihane, J.; Irvine, A.D.; Murray, D.M. Vitamin D metabolite concentrations in umbilical cord blood serum and associations with clinical characteristics in large prospective mother-infant cohort in Ireland. J. Steroid. Biochem. Mol. Biol. 2017, 167, 162–168. [Google Scholar] [CrossRef]
- Mao, D.; Yuen, L.Y.; Ho, C.S.; Wang, C.C.; Tam, C.H.; Chan, M.H.; Lowe, W.L., Jr.; Ma, R.C.; Tam, W.H. Maternal and neonatal 3-epi-25-hydroxyvitamin D concentration and factors influencing their concentrations. J. Endocr. Soc. 2021, 6(1), vab170. [Google Scholar] [CrossRef]
- Bomba-Opoń, D.; Drews, K.; Huras, H.; Laudański, P.; Paszkowski, T.; Wielgoś, M. Rekomendacje Polskiego Towarzystwa Ginekologów i Położników dotyczące indukcji porodu. Aktualizacja 2021. Ginekol. Perinatol. Prakt. 2020, 5, 86–99. [Google Scholar]
- Rizzini, N.; Fratelli, N.; Negri, B.; Odicino, F.E.; Sartori, E.; Risso, F.M.; Prefumo, F.; Fichera, A. Chorionicity, birth weight discordance and neonatal morbidity in uncomplicated twin pregnancies delivered from 36 weeks. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 271, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine and National Research Council. Weight gain during Pregnancy: Reexamining the Guidelines; National Academies Press (US): Washington, DC, USA, 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK32813/ (accessed on 8 May 2024).
- Obata, S.; Shimura, M.; Misumi, T.; Nakanishi, S.; Shindo, R.; Miyagi, E.; Aoki, S. Weight gain during twin pregnancy with favorable pregnancy outcomes in Japan: A retrospective investigation for new criteria based on perinatal registry data. PLoS ONE 2021, 16, e0253596. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Wen, L.; Li, Q.; Yan, J.; Tian, J.; Tong, C.; Tong, Q.; Qi, H.; Saffery, R. Vitamin D status in women with dichorionic twin pregnancies and their neonates: A pilot study in China. BMC Pregnancy Childbirth 2021, 21, 279. [Google Scholar] [CrossRef]
- Roero, S.; Ingala, A.; Arduino, S.; Folino Gallo, M.; Arese, A.; Ferrando, I.; Bossotti, C.; Revelli, A. Maternal circulating vitamin D level, targeted supplementation, and perinatal outcomes in twin pregnancy. Nutrients 2024, 16(14), 2239. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Robinson, S.M.; Harvey, N.C.; Javaid, M.K.; Jiang, B.; Martyn, C.N.; Godfrey, K.M.; Cooper, C. Princess Anne Hospital Study Group. Maternal vitamin D status during pregnancy and child outcomes. Eur. J. Clin. Nutr. 2008, 62(1), 68–77. [Google Scholar] [CrossRef]
- Wierzejska, R.; Jarosz, M.; Klemińska-Nowak, M.; Tomaszewska, M.; Sawicki, W.; Bachanek, M.; Siuba-Strzelińska, M. Maternal and cord blood vitamin D status and anthropometric measurements in term newborns at birth. Front. Endocrinol. (Lausanne) 2018, 9, 9. [Google Scholar] [CrossRef]
- Wierzejska, R.; Jarosz, M.; Bachanek, M.; Sawicki, W. Gestational vitamin D concentration and other risk factors versus fetal femur length. J. Matern. Fetal Neonatal. Med. 2020, 33(12), 2012–2016. [Google Scholar] [CrossRef]
- Luo, L.M.; Wu, N.; Zhang, J.; Yang, D. Maternal vitamin D levels correlate with fetal weight and bone metabolism during pregnancy: A materno-neonatal analysis of bone metabolism parameters. J. Perinat. Med. 2022, 51(4), 538–545. [Google Scholar] [CrossRef]
- Kılıcaslan, A.Ö.; Kutlu, R.; Kilinc, I.; Ozberk, D.I. The effects of vitamin D supplementation during pregnancy and maternal vitamin D levels on neonatal vitamin D levels and birth parameters. J. Matern. Fetal Neonatal. Med. 2018, 31(13), 1727–1734. [Google Scholar] [CrossRef]
- Hauta-Alus, H.H.; Viljakainen, H.T.; Holmlund-Suila, E.M.; Enlund-Cerullo, M.; Rosendahl, J.; Valkama, S.M.; Helve, O.M.; Hytinantti, T.K.; Mäkitie, O.M.; Andersson, S. Maternal vitamin D status, gestational diabetes and infant birth size. BMC Pregnancy Childbirth 2017, 17(1), 420. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, F.; Dabbaghmanesh, M.H.; Samsami, A.; Nasiri, S.; Shirazi, P.T. Vitamin D supplementation during pregnancy on infant anthropometric measurements and bone mass of mother-infant pairs: A randomized placebo clinical trial. Early Hum. Dev. 2016, 103, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Alizadeh-Charandabi, S.; Mirghafourvand, M.; Mansouri, A.; Najafi, M.; Khodabande, F. The effect of vitamin d and calcium plus vitamin D during pregnancy on pregnancy and birth outcomes: A randomized controlled trial. J. Caring. Sci. 2015, 4(1), 35–44. [Google Scholar] [CrossRef]
- Naghshineh, E.; Sheikhaliyan, S. Effect of vitamin D supplementation in the reduce risk of preeclampsia in nulliparous women. Adv. Biomed. Res. 2016, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Sablok, A.; Batra, A.; Thariani, K.; Batra, A.; Bharti, R.; Aggarwal, A.R.; Kabi, B.C.; Chellani, H. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin. Endocrinol. (Oxf.) 2015, 83(4), 536–541. [Google Scholar] [CrossRef]
- Roth, D.E.; Morris, S.K.; Zlotkin, S.; Gernand, A.D.; Ahmed, T.; Shanta, S.S.; Papp, E.; Korsiak, J.; Shi, J.; Islam, M.M.; et al. Vitamin D supplementation in pregnancy and lactation to promote infant growth. N. Engl. J. Med. 2018, 379, 535–546. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Blanco, I.; Agodi, A. Effects of vitamin D supplementation during pregnancy on birth size: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2019, 11(2), 442. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, C.; Xu, R.; Wang, K.; Zhang, D.; Pang, W.; Tu, W.; Chen, Y. Effects of vitamin D supplementation during pregnancy on offspring health at birth: A meta-analysis of randomized controlled trails. Clin. Nutr. 2022, 41(7), 1532–1540. [Google Scholar] [CrossRef]
- Luo, T.; Lin, Y.; Lu, J.; Lian, X.; Guo, Y.; Han, L.; Guo, Y. Effects of vitamin D supplementation during pregnancy on bone health and offspring growth: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2022, 17(10), e0276016. [Google Scholar] [CrossRef]
- Irwinda, R.; Hiksas, R.; Lokeswara, A.W.; Wibowo, N. Vitamin D supplementation higher than 2000 IU/day compared to lower dose on maternal-fetal outcome: Systematic review and meta-analysis. Womens Health (Lond) 2022, 18, 17455057221111066. [Google Scholar] [CrossRef]
- Hack, K.; Derks, J.B.; Elias, S.G.; Franx, A.; Roos, E.J.; Voerman, S.K.; Bode, C.L.; Koopman-Esseboom, C.; Visser, G.H. Increased perinatal mortality and morbidity in monochorionic versus dichorionic twin pregnancies: Clinical implications of a large Dutch cohort study. BJOG 2008, 115, 58–67. [Google Scholar] [CrossRef]
- Rissanen, A.S.; Gissler, M.; Nupponen, I.K.; Nuutila, M.E.; Jernman, R.M. Perinatal outcome of dichorionic and monochorionic-diamniotic Finnish twins: A historical cohort study. Acta Obstet. Gynecol. Scand. 2022, 101(1), 153–162. [Google Scholar] [CrossRef]
- Seetho, S.; Kongwattanakul, K.; Saksiriwuttho, P.; Thepsuthammarat, K. Epidemiology and factors associated with preterm births in multiple pregnancy: A retrospective cohort study. BMC Pregnancy Childbirth 2023, 23(1), 872. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Pugh, S.J.; Abrams, B.; Himes, K.P.; Hutcheon, J.A. Gestational weight gain in twin pregnancies and maternal and child health: A systematic review. J. Perinatol. 2014, 34, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.L.; Boyle, J.A.; Harrison, C.L.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; et al. Gestational weight gain across continents and ethnicity: Systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Pölzlberger, E.; Hartmann, B.; Hafner, E.; Stümpflein, I.; Kirchengast, S. Maternal height and pre-pregnancy weight status are associated with fetal growth patterns and newborn size. J. Biosoc. Sci. 2017, 49(3), 392–407. [Google Scholar] [CrossRef] [PubMed]
- Masalin, S.; Laine, M.K.; Kautiainen, H.; Gissler, M.; Raina, M.; Pennanen, P.; Eriksson, J.G. Impact of maternal height and gestational diabetes mellitus on offspring birthweight. Diabetes Res. Clin. Pract. 2019, 148, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Lutsiv, O.; Mulla, S.; McDonald, S.D. Maternal height and the risk of preterm birth and low birth weight: A systematic review and meta-analyses. J. Obstet. Gynaecol. Can. 2012, 34(8), 721–746. [Google Scholar] [CrossRef]
- Pickett, K.E.; Abrams, B.; Selvin, S. Maternal height, pregnancy weight gain, and birthweight. Am. J. Hum. Biol. 2000, 12(5), 682–687. [Google Scholar] [CrossRef]

| Number of women, n including: monochorionic pregnancies, n (%) dichorionic pregnancies, n (%) |
50 23 (46%) 27 (54%) |
| Age (in years), mean ± SD | 31.7 ± 4.6 |
| Education, n (%) higher other |
36 (72) 14 (28) |
| Place of residence, n (%) city/town rural/village |
46 (92) 4 (8) |
| Number of pregnancies, n (%) first subsequent |
26 (52) 24 (48) |
| Gestational age (in weeks), median (min-max) | 36 (36–38) |
| Gestational age of monochorionic pregnancy (in weeks), median (min-max) | 36 (36–37) |
| Gestational age of dichorionic pregnancy (in weeks), median (min-max) | 37 (36–38) |
| Due date season, n (%) spring-summer autumn-winter |
25 (50) 25 (50) |
| Maternal Body Mass Index (BMI) prior to conception, median (min-max) | 22.5 (16.6–38.9) |
| Gestational weight gain, n (%) low normal excessive |
24 (48) 20 (40) 6 (12) |
| Gestational diabetes, n (%) | 10 (20) |
| Hypertension, n (%) | 3 (6) |
| Anaemia, n (%) | 17 (34) |
| Smoking during pregnancy, n (%) | 0 (0) |
| Vitamin D supplementation, n (%) | 49 (98) |
| Daily vitamin D intake with food (µg), median (min-max) with food and dietary supplements (µg), median (min-max) |
2.4 (0.4–9.1) 52.1 (1.2–154.6) |
| Supplementation with vitamin-mineral preparations (multicomponent), n (%) | 49 (98) |
| Calcium intake from milk and dairy products (mg), median (min-max) | 641.1 (0.0–2900.4) |
| Caffeine intake from coffee and tea (mg), mean ± SD | 91.7 ± 73.2 |
| Fish consumption (at least once a week), n (%) | 20 (40) |
| Sex of the newborn, n (%) male female |
45 (45) 55 (55) |
| Neonatal weight (g), mean ± SD | 2557.8 ± 295.6 |
| Number of newborns with low birth weight (<2500 g), n (%) | 40 (40) |
| Number of twin pairs with low birth weight (<2500 g), n (%) | 12 (24) |
| Birth weight discordance, n (%) | 6 (12%) |
| Neonatal length (cm), median (min-max) | 51 (46–56) |
| Neonatal head circumference (cm), median (min-max) | 33 (30–35) |
| Neonatal chest circumference (cm), median (min-max) | 31 (27–35) |
| Apgar score at 5 minutes (points), median (min-max) | 10 (8–10) |
| Maternal 25(OH)D concentration (ng/mL), mean ± SD | 39.7 ± 10.7 |
| Maternal 25(OH)D concentration in women in monochorionic pregnancies (ng/mL), mean ± SD | 39.0 ± 9.4 |
| Maternal 25(OH)D concentration in women in dichorionic pregnancies (ng/mL), mean ± SD | 40.3 ± 11.6 |
| Cord blood 25(OH)D concentration (ng/mL), mean ± SD | 24.4 ± 5.9 |
| Cord blood 25(OH)D concentration in neonates born from monochorionic pregnancies (ng/mL), mean ± SD | 24.5 ± 5.2 |
| Cord blood 25(OH)D concentration in neonates born from dichorionic pregnancies (ng/mL), mean ± SD | 24.4 ± 6.4 |
| Cord blood 25(OH)D concentration in first-born twins, (ng/mL), mean ± SD | 24.8 ± 6.0 |
| Cord blood 25(OH)D concentration in second-born twins, (ng/mL), mean ± SD | 24.1 ± 5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





