Submitted:
12 September 2024
Posted:
13 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Overview of Chemerin as a Multifunctional Cytokine and Adipokine
1.2. Rationale for Exploring Chemerin in the Context of Upper GI Cancers
2. Chemerin: Biochemistry and General Functions
2.1. Biochemical Properties and Synthesis of Chemerin
2.2. Main Receptors (CMKLR1, CCRL2, GPR1) and Signaling Pathways
2.3. Main Receptors (CMKLR1, CCRL2, GPR1) and Signaling Pathways
2.3.1. Inflammatory Response
2.3.2. Immune Regulation
2.3.3. Metabolic Processes
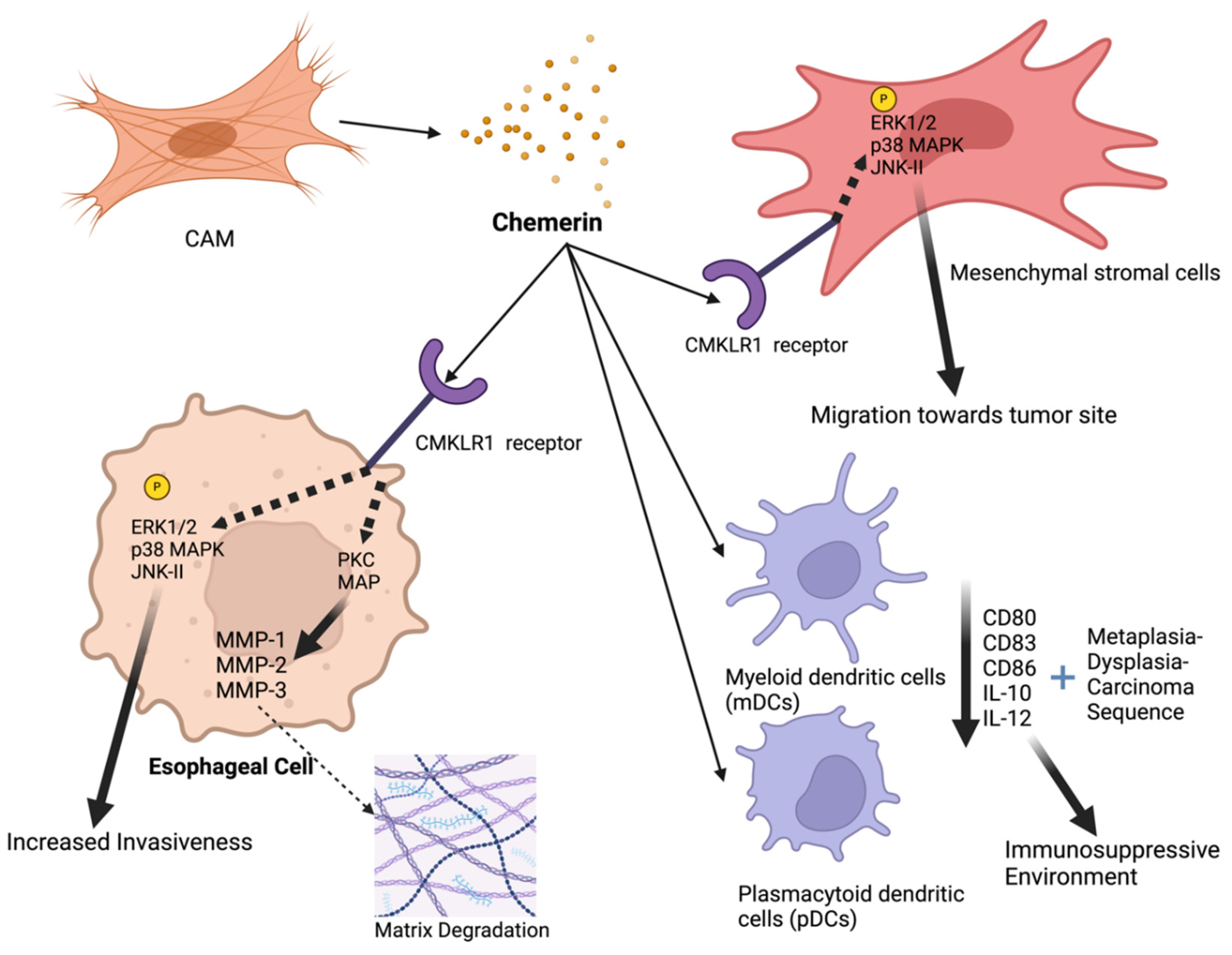
3. Chemerin in Esophageal Cancer
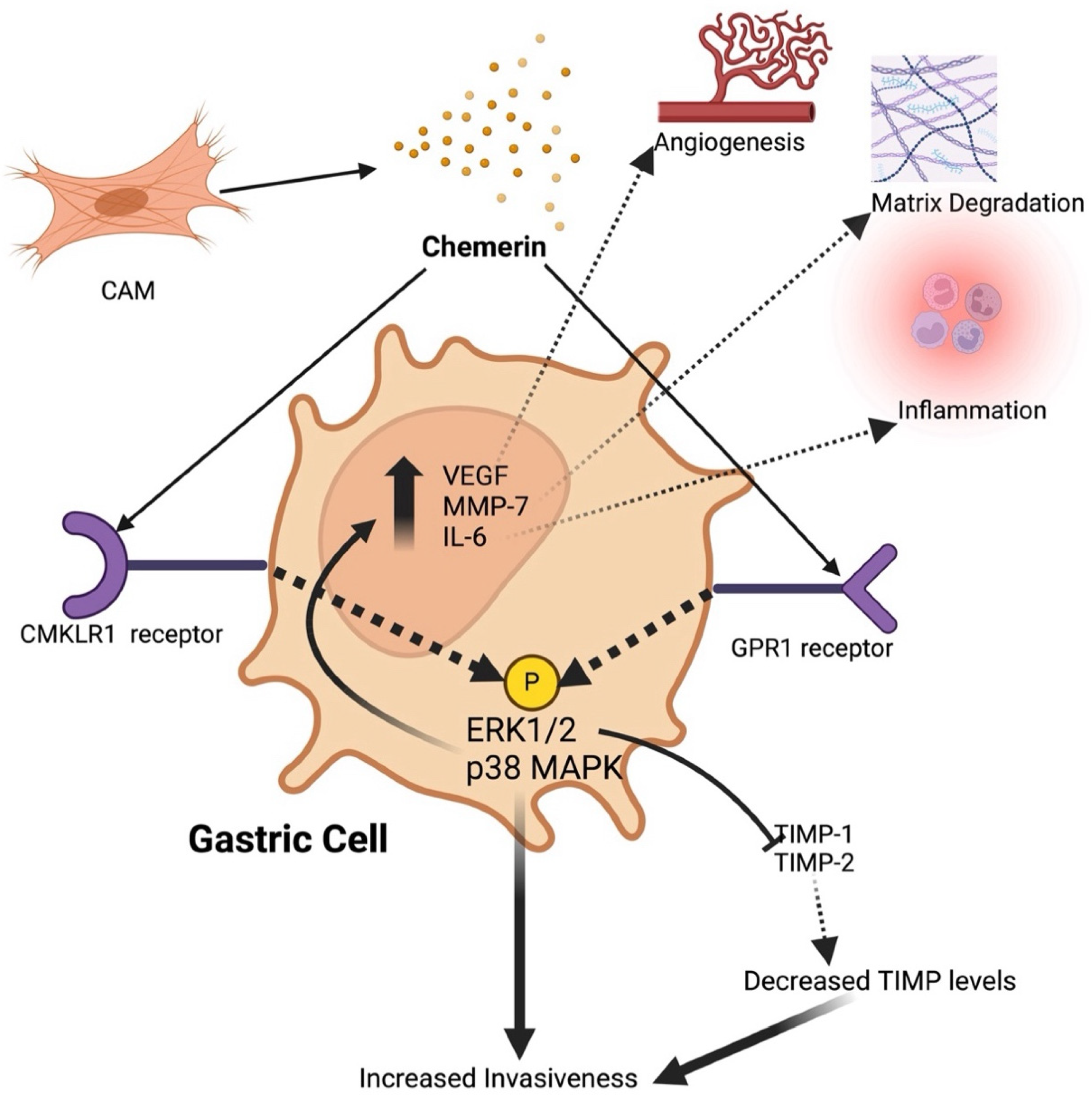
4. Chemerin in Gastric Cancer
5. Clinical Implications of Chemerin
5.1. Prognostic Value of Chemerin Levels
5.2. Therapeutic Targeting of Chemerin and its Receptors
5.3. Integration into Current Clinical Practice
6. Future Directions and Research Needs
6.1. What Is Missing About Chemerin’s Role in Upper GI Cancers?
6.2. State-of-the-Art Assessment of Chemerin
6.3. Proposals for Future Research Focusing on Translational and Clinical Studies
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jacenik, D.; Fichna, J. Chemerin in Immune Response and Gastrointestinal Pathophysiology. Clin Chim Acta 2020, 504, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Towards an Integrative Approach to Understanding the Role of Chemerin in Human Health and Disease—Rourke—2013—Obesity Reviews—Wiley Online Library Available online:. Available online: https://onlinelibrary.wiley.com/doi/10.1111/obr.12009 (accessed on 6 May 2024).
- Ernst, M.C.; Sinal, C.J. Chemerin: At the Crossroads of Inflammation and Obesity. Trends Endocrinol Metab 2010, 21, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Allen, S.J.; Kulig, P.; Allen, J.A.; Cichy, J.; Handel, T.M.; Butcher, E.C. Chemerin Activation by Serine Proteases of the Coagulation, Fibrinolytic, and Inflammatory Cascades. J Biol Chem 2005, 280, 34661–34666. [Google Scholar] [CrossRef]
- Du, X.-Y.; Zabel, B.A.; Myles, T.; Allen, S.J.; Handel, T.M.; Lee, P.P.; Butcher, E.C.; Leung, L.L. Regulation of Chemerin Bioactivity by Plasma Carboxypeptidase N, Carboxypeptidase B (Activated Thrombin-Activable Fibrinolysis Inhibitor), and Platelets. J Biol Chem 2009, 284, 751–758. [Google Scholar] [CrossRef]
- Wittamer, V.; Franssen, J.-D.; Vulcano, M.; Mirjolet, J.-F.; Le Poul, E.; Migeotte, I.; Brézillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M.; et al. Specific Recruitment of Antigen-Presenting Cells by Chemerin, a Novel Processed Ligand from Human Inflammatory Fluids. Journal of Experimental Medicine 2003, 198, 977–985. [Google Scholar] [CrossRef]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a Novel Adipokine That Regulates Adipogenesis and Adipocyte Metabolism. J Biol Chem 2007, 282, 28175–28188. [Google Scholar] [CrossRef]
- Parolini, S.; Santoro, A.; Marcenaro, E.; Luini, W.; Massardi, L.; Facchetti, F.; Communi, D.; Parmentier, M.; Majorana, A.; Sironi, M.; et al. The Role of Chemerin in the Colocalization of NK and Dendritic Cell Subsets into Inflamed Tissues. Blood 2007, 109, 3625–3632. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Nakae, S.; Zúñiga, L.; Kim, J.-Y.; Ohyama, T.; Alt, C.; Pan, J.; Suto, H.; Soler, D.; Allen, S.J.; et al. Mast Cell-Expressed Orphan Receptor CCRL2 Binds Chemerin and Is Required for Optimal Induction of IgE-Mediated Passive Cutaneous Anaphylaxis. J Exp Med 2008, 205, 2207–2220. [Google Scholar] [CrossRef]
- Monnier, J.; Lewén, S.; O’Hara, E.; Huang, K.; Tu, H.; Butcher, E.C.; Zabel, B.A. Expression, Regulation, and Function of Atypical Chemerin Receptor CCRL2 on Endothelial Cells. J Immunol 2012, 189, 956–967. [Google Scholar] [CrossRef]
- Barnea, G.; Strapps, W.; Herrada, G.; Berman, Y.; Ong, J.; Kloss, B.; Axel, R.; Lee, K.J. The Genetic Design of Signaling Cascades to Record Receptor Activation. Proceedings of the National Academy of Sciences 2008, 105, 64–69. [Google Scholar] [CrossRef]
- Gurevich, V.V.; Gurevich, E.V. GPCR Signaling Regulation: The Role of GRKs and Arrestins. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Docherty, J.M.; Nguyen, T.; Heiber, M.; Cheng, R.; Heng, H.H.; Tsui, L.C.; Shi, X.; George, S.R.; O’Dowd, B.F. Cloning of Human Genes Encoding Novel G Protein-Coupled Receptors. Genomics 1994, 23, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Soda, Y.; Kanbe, K.; Liu, H.Y.; Jinno, A.; Kitamura, T.; Hoshino, H. An Orphan G Protein-Coupled Receptor, GPR1, Acts as a Coreceptor to Allow Replication of Human Immunodeficiency Virus Types 1 and 2 in Brain-Derived Cells. J Virol 1999, 73, 5231–5239. [Google Scholar] [CrossRef] [PubMed]
- Wittamer, V.; Bondue, B.; Guillabert, A.; Vassart, G.; Parmentier, M.; Communi, D. Neutrophil-Mediated Maturation of Chemerin: A Link between Innate and Adaptive Immunity. J Immunol 2005, 175, 487–493. [Google Scholar] [CrossRef]
- Rowicka, G.; Dyląg, H.; Chełchowska, M.; Weker, H.; Ambroszkiewicz, J. Serum Calprotectin and Chemerin Concentrations as Markers of Low-Grade Inflammation in Prepubertal Children with Obesity. Int J Environ Res Public Health 2020, 17, 7575. [Google Scholar] [CrossRef]
- Gonzalez-Ponce, F.; Gamez-Nava, J.I.; Perez-Guerrero, E.E.; Saldaña-Cruz, A.M.; Vazquez-Villegas, M.L.; Ponce-Guarneros, J.M.; Huerta, M.; Trujillo, X.; Contreras-Haro, B.; Rocha-Muñoz, A.D.; et al. Serum Chemerin Levels: A Potential Biomarker of Joint Inflammation in Women with Rheumatoid Arthritis. PLoS One 2021, 16, e0255854. [Google Scholar] [CrossRef]
- Gisondi, P.; Lora, V.; Bonauguri, C.; Russo, A.; Lippi, G.; Girolomoni, G. Serum Chemerin Is Increased in Patients with Chronic Plaque Psoriasis and Normalizes Following Treatment with Infliximab. Br J Dermatol 2013, 168, 749–755. [Google Scholar] [CrossRef]
- Horn, P.; Metzing, U.B.; Steidl, R.; Romeike, B.; Rauchfuß, F.; Sponholz, C.; Thomas-Rüddel, D.; Ludewig, K.; Birkenfeld, A.L.; Settmacher, U.; et al. Chemerin in Peritoneal Sepsis and Its Associations with Glucose Metabolism and Prognosis: A Translational Cross-Sectional Study. Crit Care 2016, 20, 39. [Google Scholar] [CrossRef]
- Ye, Y.; Fang, L.; Li, J.; Wu, H.; Tan, X.; Luo, H.; Li, X.; Huang, L. Chemerin/ChemR23 Regulates Cementoblast Function and Tooth Resorption in Mice via Inflammatory Factors. J Periodontol 2021, 92, 1470–1482. [Google Scholar] [CrossRef]
- Shang, J.; Wang, L.; Zhang, Y.; Zhang, S.; Ning, L.; Zhao, J.; Cheng, G.; Liu, D.; Xiao, J.; Zhao, Z. Chemerin/ChemR23 Axis Promotes Inflammation of Glomerular Endothelial Cells in Diabetic Nephropathy. J Cell Mol Med 2019, 23, 3417–3428. [Google Scholar] [CrossRef]
- Hu, S.; Shao, Z.; Zhang, C.; Chen, L.; Mamun, A.A.; Zhao, N.; Cai, J.; Lou, Z.; Wang, X.; Chen, J. Chemerin Facilitates Intervertebral Disc Degeneration via TLR4 and CMKLR1 and Activation of NF-kB Signaling Pathway. Aging (Albany NY) 2020, 12, 11732–11753. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.L.; Louis, N.A.; Tomassetti, S.E.; Canny, G.O.; Arita, M.; Serhan, C.N.; Colgan, S.P. Resolvin E1 Promotes Mucosal Surface Clearance of Neutrophils: A New Paradigm for Inflammatory Resolution. FASEB J 2007, 21, 3162–3170. [Google Scholar] [CrossRef] [PubMed]
- Cash, J.L.; Hart, R.; Russ, A.; Dixon, J.P.C.; Colledge, W.H.; Doran, J.; Hendrick, A.G.; Carlton, M.B.L.; Greaves, D.R. Synthetic Chemerin-Derived Peptides Suppress Inflammation through ChemR23. J Exp Med 2008, 205, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Bondue, B.; Wittamer, V.; Parmentier, M. Chemerin and Its Receptors in Leukocyte Trafficking, Inflammation and Metabolism. Cytokine & Growth Factor Reviews 2011, 22, 331–338. [Google Scholar] [CrossRef]
- Su, X.; Cheng, Y.; Zhang, G.; Wang, B. Chemerin in Inflammatory Diseases. Clinica Chimica Acta 2021, 517, 41–47. [Google Scholar] [CrossRef]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin Is a Novel Adipokine Associated with Obesity and Metabolic Syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.; Wu, Q.-F. Chemerin: A Multifaceted Adipokine Involved in Metabolic Disorders. The Journal of Endocrinology 2018, 238, 79–94. [Google Scholar] [CrossRef]
- Lehrke, M.; Becker, A.; Greif, M.; Stark, R.; Laubender, R.P.; von Ziegler, F.; Lebherz, C.; Tittus, J.; Reiser, M.; Becker, C.; et al. Chemerin Is Associated with Markers of Inflammation and Components of the Metabolic Syndrome but Does Not Predict Coronary Atherosclerosis. European Journal of Endocrinology 2009, 161, 339–344. [Google Scholar] [CrossRef]
- Stejskal, D.; Karpisek, M.; Hanulova, Z.; Svestak, M. CHEMERIN IS AN INDEPENDENT MARKER OF THE METABOLIC SYNDROME IN A CAUCASIAN POPULATION—A PILOT STUDY. Biomedical Papers 2008, 152, 217–221. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, J.; Wang, Y.; Yang, Z.; Chen, X. Chemerin Causes Lipid Metabolic Imbalance and Induces Passive Lipid Accumulation in Human Hepatoma Cell Line via the Receptor GPR1. Life Sciences 2021, 278, 119530. [Google Scholar] [CrossRef]
- Zhang, Y. Epidemiology of Esophageal Cancer. World Journal of Gastroenterology 2013, 19, 5598–5606. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of Esophageal Cancer: Update in Global Trends, Etiology and Risk Factors. Clin J Gastroenterol 2020, 13, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Arnal, M.J.D.; Arenas, Á.F.; Arbeloa, Á.L. Esophageal Cancer: Risk Factors, Screening and Endoscopic Treatment in Western and Eastern Countries. World Journal of Gastroenterology 2015, 21, 7933–7943. [Google Scholar] [CrossRef]
- Somja, J.; Demoulin, S.; Roncarati, P.; Herfs, M.; Bletard, N.; Delvenne, P.; Hubert, P. Dendritic Cells in Barrett’s Esophagus Carcinogenesis: An Inadequate Microenvironment for Antitumor Immunity? Am J Pathol 2013, 182, 2168–2179. [Google Scholar] [CrossRef]
- Kumar, J.D.; Holmberg, C.; Kandola, S.; Steele, I.; Hegyi, P.; Tiszlavicz, L.; Jenkins, R.; Beynon, R.J.; Peeney, D.; Giger, O.T.; et al. Increased Expression of Chemerin in Squamous Esophageal Cancer Myofibroblasts and Role in Recruitment of Mesenchymal Stromal Cells. PLoS One 2014, 9, e104877. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.D.; Kandola, S.; Tiszlavicz, L.; Reisz, Z.; Dockray, G.J.; Varro, A. The Role of Chemerin and ChemR23 in Stimulating the Invasion of Squamous Oesophageal Cancer Cells. Br J Cancer 2016, 114, 1152–1159. [Google Scholar] [CrossRef]
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clinical Gastroenterology and Hepatology 2020, 18, 534–542. [Google Scholar] [CrossRef]
- Rawla, P.; Barsouk, A. Epidemiology of Gastric Cancer: Global Trends, Risk Factors and Prevention. Gastroenterology Rev 2018, 14, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ying, X.; Liu, S.; Lyu, G.; Xu, Z.; Zhang, X.; Li, H.; Li, Q.; Wang, N.; Ji, J. Gastric Cancer: Epidemiology, Risk Factors and Prevention Strategies. CJCR 2020, 32, 695–704. [Google Scholar] [CrossRef]
- Venerito, M.; Vasapolli, R.; Rokkas, T.; Malfertheiner, P. Gastric Cancer: Epidemiology, Prevention, and Therapy. Helicobacter 2018, 23, e12518. [Google Scholar] [CrossRef]
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, G.J.A.; Maciejewski, R.; Polkowski, W.P. Gastric Cancer: Epidemiology, Prevention, Classification, and Treatment. CMAR 2018, 10, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. International Journal of Molecular Sciences 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; Grieken, N.C. van; Lordick, F. Gastric Cancer. The Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, H.-C.; Zhu, A.-K.; Ying, R.-C.; Wei, W.; Zhang, F.-J. Prognostic Significance of Plasma Chemerin Levels in Patients with Gastric Cancer. Peptides 2014, 61, 7–11. [Google Scholar] [CrossRef]
- Wang, C.; Wu, W.K.K.; Liu, X.; To, K.-F.; Chen, G.G.; Yu, J.; Ng, E.K.W. Increased Serum Chemerin Level Promotes Cellular Invasiveness in Gastric Cancer: A Clinical and Experimental Study. Peptides 2014, 51, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.D.; Aolymat, I.; Tiszlavicz, L.; Reisz, Z.; Garalla, H.M.; Beynon, R.; Simpson, D.; Dockray, G.J.; Varro, A. Chemerin Acts via CMKLR1 and GPR1 to Stimulate Migration and Invasion of Gastric Cancer Cells: Putative Role of Decreased TIMP-1 and TIMP-2. Oncotarget 2019, 10, 98–112. [Google Scholar] [CrossRef]
- Alkady, M.M.; Abdel-Messeih, P.L.; Nosseir, N.M. Assessment of Serum Levels of the Adipocytokine Chemerin in Colorectal Cancer Patients. J Med Biochem 2018, 37, 313–319. [Google Scholar] [CrossRef]
- Yagi, M.; Sasaki, Y.; Abe, Y.; Yaoita, T.; Sakuta, K.; Mizumoto, N.; Shoji, M.; Onozato, Y.; Kon, T.; Nishise, S.; et al. Association between High Levels of Circulating Chemerin and Colorectal Adenoma in Men. Digestion 2019, 101, 571–578. [Google Scholar] [CrossRef]
- Erdogan, S.; Yilmaz, F.M.; Yazici, O.; Yozgat, A.; Sezer, S.; Ozdemir, N.; Uysal, S.; Purnak, T.; Sendur, M.A.; Ozaslan, E. Inflammation and Chemerin in Colorectal Cancer. Tumor Biol. 2016, 37, 6337–6342. [Google Scholar] [CrossRef]
- Rennier, K.; Shin, W.J.; Krug, E.; Virdi, G.; Pachynski, R.K. Chemerin Reactivates PTEN and Suppresses PD-L1 in Tumor Cells via Modulation of a Novel CMKLR1-Mediated Signaling Cascade. Clin Cancer Res 2020, 26, 5019–5035. [Google Scholar] [CrossRef]
- Pachynski, R.K.; Wang, P.; Salazar, N.; Zheng, Y.; Nease, L.; Rosalez, J.; Leong, W.-I.; Virdi, G.; Rennier, K.; Shin, W.J.; et al. Chemerin Suppresses Breast Cancer Growth by Recruiting Immune Effector Cells Into the Tumor Microenvironment. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.F.; Czerniak, A.S.; Weiß, T.; Zellmann, T.; Zielke, L.; Els-Heindl, S.; Beck-Sickinger, A.G. Cyclic Derivatives of the Chemerin C-Terminus as Metabolically Stable Agonists at the Chemokine-like Receptor 1 for Cancer Treatment. Cancers 2021, 13, 3788. [Google Scholar] [CrossRef] [PubMed]
- Klose, R.; Krzywinska, E.; Castells, M.; Gotthardt, D.; Putz, E.M.; Kantari-Mimoun, C.; Chikdene, N.; Meinecke, A.-K.; Schrödter, K.; Helfrich, I.; et al. Targeting VEGF-A in Myeloid Cells Enhances Natural Killer Cell Responses to Chemotherapy and Ameliorates Cachexia. Nat Commun 2016, 7, 12528. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.-H.; Lee, S.K.; Song, N.-Y.; Son, S.H.; Kim, K.R.; Chung, W.-Y. Chemerin Treatment Inhibits the Growth and Bone Invasion of Breast Cancer Cells. International Journal of Molecular Sciences 2020, 21, 2871. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor Microenvironment as a Therapeutic Target in Cancer. Pharmacol Ther 2021, 221, 107753. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M.; Chishti, M.A.; Moustafa, A.S.; Fatma, S.; Alomaim, W.S.; Al-Naami, M.Y.; Bassas, A.F.; Chrousos, G.P.; Jo, H. Differential Patterns of Serum Concentration and Adipose Tissue Expression of Chemerin in Obesity: Adipose Depot Specificity and Gender Dimorphism. Molecules and Cells 2012, 33, 591–596. [Google Scholar] [CrossRef]
- Goralski, K.B.; Jackson, A.E.; McKeown, B.T.; Sinal, C.J. More Than an Adipokine: The Complex Roles of Chemerin Signaling in Cancer. Int J Mol Sci 2019, 20, 4778. [Google Scholar] [CrossRef]
- Sell, H.; Divoux, A.; Poitou, C.; Basdevant, A.; Bouillot, J.-L.; Bedossa, P.; Tordjman, J.; Eckel, J.; Clément, K. Chemerin Correlates with Markers for Fatty Liver in Morbidly Obese Patients and Strongly Decreases after Weight Loss Induced by Bariatric Surgery. The Journal of Clinical Endocrinology & Metabolism 2010, 95, 2892–2896. [Google Scholar] [CrossRef]
- Waniczek, D.; Swiętochowska, E.; Lorenc, Z. Serum and Salivary Chemerin Concentrations in Patients with Colorectal Cancer and Obesity. Ann. Acad. Med. Siles. 2021, 75, 11–17. [Google Scholar] [CrossRef]
- Buechler, C.; Feder, S.; Haberl, E.M.; Aslanidis, C. Chemerin Isoforms and Activity in Obesity. Int J Mol Sci 2019, 20, 1128. [Google Scholar] [CrossRef]
- Erdmann, S.; Niederstadt, L.; Koziolek, E.J.; Gómez, J.D.C.; Prasad, S.; Wagener, A.; von Hacht, J.L.; Reinicke, S.; Exner, S.; Bandholtz, S.; et al. CMKLR1-Targeting Peptide Tracers for PET/MR Imaging of Breast Cancer. Theranostics 2019, 9, 6719–6733. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




