1. Introduction
The availability of multibranch stent-grafts devices for endovascular repair of thoraco-abdominal aortic aneurysms (TAAA) represented a significant advancement in endovascular techniques. These devices aim to provide a minimally invasive alternative to open surgery, potentially reducing recovery time and associated complications usually linked to more invasive procedures.
However, an endovascular approach is not suitable for every patient or all types of TAAAs. Therefore, patient’s selection and detailed aortic anatomy evaluation by a multidisciplinary team are crucial in determining the best treatment option for each individual case. As well know, the aorto-iliac morphology represents a significant constraint which affects the feasibility of TAAAs treatment with currently available stent-grafts. E-nside thoraco-abdominal multibranch stent-graft system (Artivion, Hechingen, Germany) is the first pre-cannulated, inner branch based, off-the-shelf solution for endovascular TAAA repair accessible on the market. The inner branch technology enables the treatment of a wider range of aortic anatomies with a consistent approach: internal tunnels can be used in narrow and kinked anatomies as well as large aneurysms. Also, pre-cannulation is designed and aims to minimize fluoroscopy and implantation time as well as to limit the usage of contrast media.
The purpose of this manuscript is to report clinical and technical outcomes of our initial experience with the E-nside stent-graft system.
2. Materials and Methods
Patients undergoing endovascular repair of TAAA or pararenal abdominal aortic aneurysm (PAAA) with E-nside stent-graft system between May 2021 and March 2023 were included in this retrospective single-center cohort study.
Clinical, technical and follow-up data were collected and entered in a specifically maintained database and retrospectively analyzed. Demographics characteristics and anatomical features, procedure related complications, reinterventions, Intensive Care Unit (ICU) and in-hospital length of stay, aneurysm- and all-causes related mortality were recorded.
Preoperative computed tomography were used to classify aneurysms according to the Crawford classification. Even though endovascular repairs often require a more extensive proximal seal than the one required in open surgery, we believe that the clinical outcomes (except for SCI [
1]) and patient recovery after surgery are not strictly dependent of the extent of the aortic coverage [
2].
2.1. Device Description
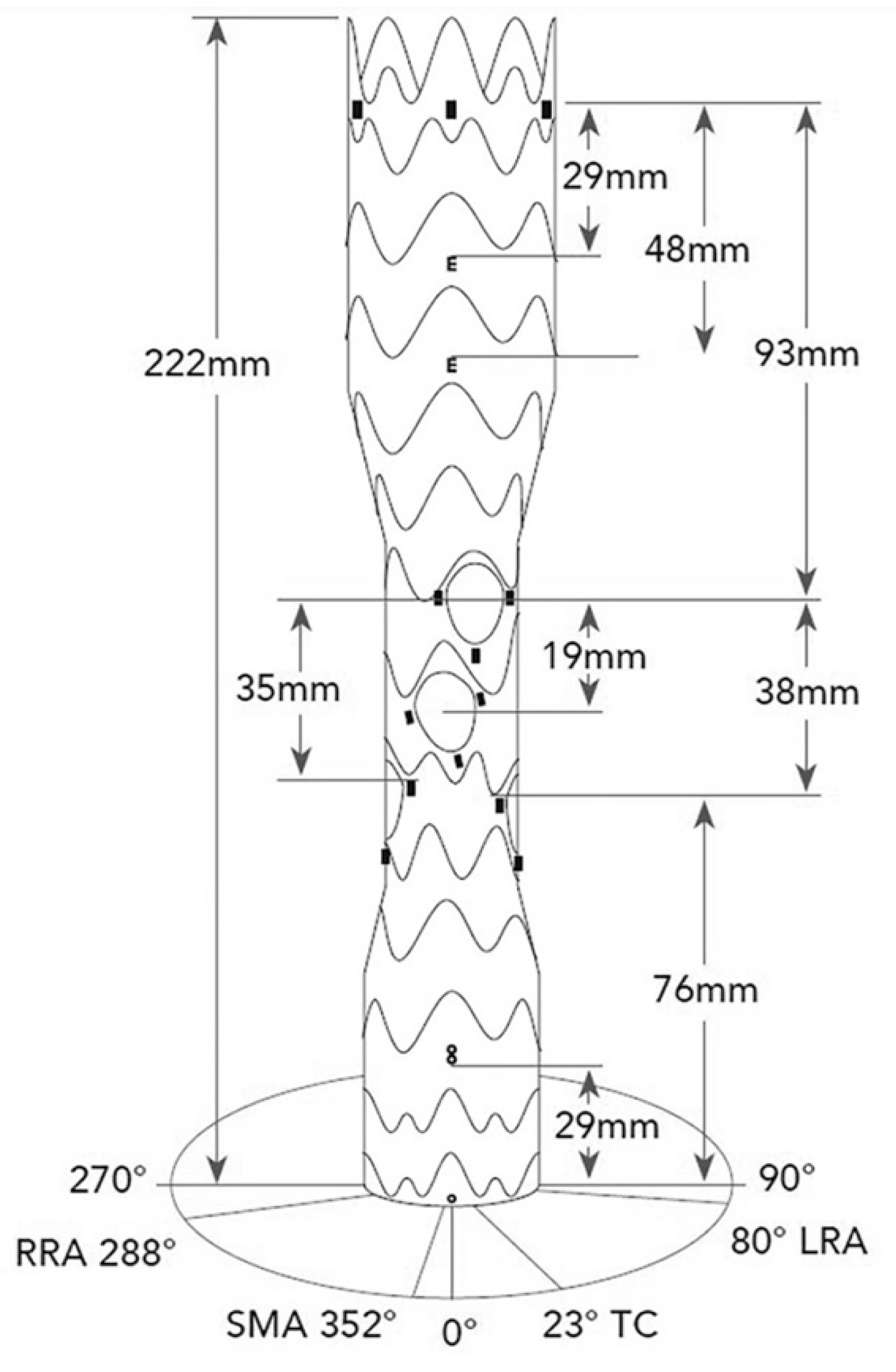
The E-nside device is designed as a tube graft made of a Polytetrafluoroethylene (PTFE) fabric with a nitinol frame. (
Figure 1) A full technical description of the device has been already published [
3,
4,
5]. Nonetheless, a brief description is given here. Proximally the graft is designed to dock in a thoracic stent-graft; thus, it has no ancillary proximal fixation (barbs) and it only relies on the outward radial force of the nitinol frame [
5].
The stent-graft is available in four configurations with proximal diameters of 38 or 33 mm, a total length of 222 mm and distal diameters of 30 or 26 mm. The central section has always a diameter of 24 mm, featuring four internal branches: 20 mm in length and 8 mm in diameter for the celiac trunk (CT) and superior mesenteric artery (SMA), and 20 mm in length and 6 mm in diameter for the renal arteries (
Figure 1). The inner branches are pre-cannulated with polyamid hypotubes to facilitate snaring and direct anterograde catheterization during the procedure. The device is loaded in a large bore sheath of 24 F (outer diameter, OD).
2.2. Procedural Details
All procedures were performed under general anesthesia by a team of vascular surgeons experienced on BEVAR with off-the-shelf (e.g., Zenith t-Branch Thoracoabdominal Endovascular Graft by Cook Medical Inc, Bloomington, Ind, USA) and custom-made devices (CMDs).
In most of the cases, a surgical cutdown to expose the common femoral artery (CFA) was performed, as well as a surgical exposure of the axillary artery. According to our current standard, lumbar drainage for SCI prevention was performed at the surgeon's discretion [
6], depending on the extent of the aortic coverage. For spinal perfusion protection, a mean arterial pressure of >80 mmHg was aimed in accordance with the current European Society for Vascular Surgery (ESVS) guidelines [
7]. In addition, the prevention of SCI was obtained with temporary aneurysm sac perfusion (TASP) [
8]. TASP was performed with bare metal stent (BMS) as bridging stent, leaving an open branch, leaving open iliac limb, or leaving both open iliac limb and BMS as bridging stent. The secondary intervention, which was intended to complete the aneurysm exclusion procedure, was always performed under local anesthesia, with ultrasound-guided percutaneous femoral access and a total femoral approach using a steerable sheath (Aptus HeliFX; Medtronic, Minneapolis, United States).
Ballon-expandable Gore Viabahn VBX stent grafts (W.L. Gore & Associates, Flagstaff, AZ, United States) were used as bridging stents in all cases.
After the intervention, all patients received dual antiplatelet therapy for six months without loading doses. Follow-up protocol consisted of a duplex ultrasound examination (DUS), control computed tomography angiography (CTA) and clinical examination performed after one, six and twelve months from surgery. After one year, an annually DUS and CTA was also performed.
Institutional review board approval was requested and obtained.
Informed written consent of patients was obtained. Patients gave their consent to the publication.
2.3. Endpoints
The primary endpoint was the technical success, defined as the triad of the proper deployment of the stent-graft, target vessels (TVs) cannulation and exclusion of the target pathology without evidence of type 1 or 3 endoleaks in accordance with the reporting standards for endovascular aortic aneurysm repair [
9]. Assisted primary technical success was defined if further unplanned procedures (e.g., due to a type 1A endoleak) were necessary during the primary procedure for the exclusion of the target pathology.
Target vessel success was defined as successful cannulation and bridging stent implantation in the TV without evidence of embolism or dissection and proper branch perfusion [
7].
Secondary endpoint was the clinical success, defined as the absence of perioperative (≤30 days) major adverse events (MAE), such as acute myocardial infarction (AMI), acute renal injury, respiratory failure required invasive or non-invasive ventilation (NIV), acute mesenteric ischemia, stroke, and SCI. Access-site related complications were also reported, as the ICU- and the in-hospital length of stay.
Perioperative (≤30 day) and early mortality rates (≤180 day), freedom from aortic re-intervention, TVs patency and endoleak rate were evaluated during the follow-up, as mid-term mortality.
Technical metrics such as minimum EIA diameter, iliac tortuosity index (X), mean aortic diameter at E-nside proximal sealing zone, mean aortic diameter at TV ostium level, time of TV catheterization, and extent of aortic coverage were retrospectively evaluated.
Iliac artery tortuosity index (
X) was calculated as
X=L/D, where L is the centerline length and D is the Euclidean (straight line) distance between its end points [
10].
Time of TV catheterization was defined as the time from preloaded system cannulation, when used, to deployment of bridging stent for each vessel.
A descriptive analysis of all variables was performed. SPSS (Version 25; SPSS Inc, Chicago, IL, USA) and Excel (Microsoft Corporation, Redmond, WA, USA) were used for statistical analysis.
A p-value < .05 was considered as statistically significant.
3. Results
Between May 2021 and March 2023, 22 patients (16/22 male, 72.7%; 6/22 female, 27.3%) with a mean age of 75.9±5.5 years (range, 69-88), underwent elective iBEVAR for TAAA or PAAA at our University Hospital Center.
Patients’ demographics are listed in
Table 1. Aneurysm extent was Crawford I in 4 patients (18.2%), III in 8 (36.4%), IV in 5 (22.7%), V in 1 (4.5%) and PAAA in 4 (18.2%). All patients presented a degenerative aneurysm. Mean aortic diameter was 63.5±9.9 mm (range, 52-97).
Five patients had history of prior aortic surgery (22.7%; 1 EVAR, 1 TEVAR, 2 open abdominal aortic aneurysm repair, 1 frozen elephant trunk).
The technical success was 95.5%, as there was evidence of one intraoperative type 1A endoleak.
Concomitant proximal thoracic endograft was deemed necessary in seven patients. Six patients required TEVAR to extend the proximal sealing because of type I and III TAAA, whereas one patient required TEVAR due to the evidence of type 1A endoleak at completion angiography. The assisted primary success was 100%.
Eighteen patients (81.8%) received concomitant bifurcated stent-graft, and one of them (4.5%) also an iliac branch device. Internal iliac artery (IIA) was bilaterally patent in nineteen patients. One patient presented the occlusion of both IIAs, whereas two patients presented the occlusion of the left IIA only. Two patients presented IIA’s aneurysm. In these latter cases, IIA’s embolization with Amplatzer Vascular Plugs (Abbott Vascular, CA, USA) was performed without complications.
Seven out of the 88 TVs were preoperatively occluded (7.9%), whereas fourteen presented a severe stenosis (15.9%). Seventy-eight TVs were successfully incorporated through a bridging stent (96.3%). One asymptomatic SMA dissection was observed at control CTA (1.3%); one patient died before CT’s bridging stent implantation, and one patient suffered left renal artery and CT occlusion before the secondary intervention, due to the improper apposition of the fabric of the graft in front of its ostium. TV success was 95.1%.
A total of 86 bridging stents was used for 78 TVs (1.1 stents/TV). The preloaded system was used for 52 TVs (52/78, 66.7%). Nine intentional occlusions of the inner branches were successful.
3.1. Perioperative Outcomes
The clinical success was 86.4%. Three MAEs were reported (n=X, %). One patient suffered from AMI, one patient suffered from cerebellar ischemia caused by the distal embolization in left vertebral artery from the left subclavian access, and one patient suffered a respiratory failure, which required NIV and prolonged ICU-stay. Lumbar drainage was never been deemed necessary. To prevent SCI, TASP was performed with BMS as bridging stent in two cases (9.1%), leaving an open branch in two (9.1%), and leaving open iliac limb in seven (31.8%), and leaving both open iliac limb and BMS as bridging stent in seven patients (31.8%). TASP was not performed in four patients (18.2%), due to the large size of the aneurysm.
The average time between primary and secondary intervention was 54,7±30.5 days (range, 13-113). No case of inter-stage aortic-related mortality was reported, as well as nor permanent or temporary SCI.
One access-site related complication was observed due to the avulsion of the right EIA during removal of the 24 F introducer sheath, requiring urgent conversion.
The mean ICU length of stay was 1.2±0.8 (range, 0.25-4); the mean in-hospital length of stay was 6.6±2.9 (range, 4-14).
Perioperative and early freedom from all-cause mortality rate was 90.9% and 90%, respectively. Two patients died for heart failure within 30 days from the intervention.
Routine CTA one year after surgery revealed a type III endoleak in two patients. One patient underwent elective TEVAR; one patient refused any additional procedure and is still under a stricter follow-up protocol. A type II endoleak were detected in three cases, without sac enlargement. Freedom from aortic related reintervention rate was 95.5%. No bridging stents stenosis or occlusion were observed during the follow-up, as no case of IIA’s occlusion.
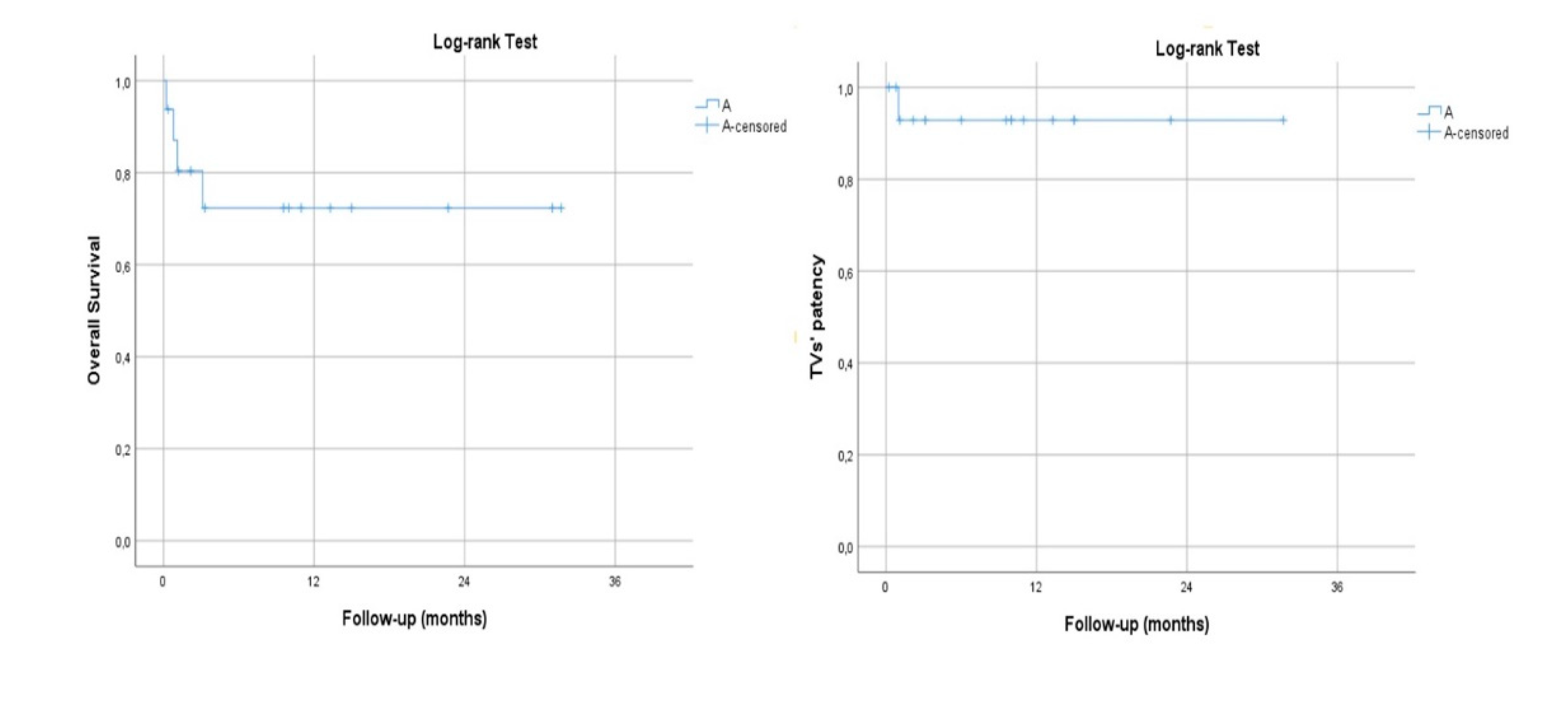
The mean follow-up time was 21.3±12.7 months (range, 9.6-35.3). No case of aneurysm-related mortality was observed during the follow-up. Estimated Kaplan–Meier 1-year survival manifested at 100% and branch patency at 100% (78/78) (
Figure 2).
3.2. Technical Metrics
The mean EIA diameter was 7.5±1.1 mm (range, 5.8-10.1), whereas the mean iliac tortuosity index was 1.34±0,2 (range, 1.1-1.9). Twelve patients (54.5%; six males, 37.5%; six women, 100%) were considered outside the instruction for use (IFU) for E-nside stent-graft due to narrow iliac arteries.
The mean outer-to-outer diameter at the level of E-nside stent-graft proximal sealing zone in function of TAAA class is listed in
Table 2.
The mean aortic diameter at TVs ostium level and the mean time of TV catheterization is listed in
Table 3.
The mean operative time was 292.9.4±61.9 minutes (range, 155-450); the mean fluoroscopy time was 63.4±15.7 minutes (range, 39.1-90); the mean contrast volume was 142±40.7 mL (range, 90-200); the mean radiant dose was 30295.9±7386.3 cGy (range, 16353-43606).
The mean extent of aortic coverage (above the celiac trunk, CT) was 11.7±0.9 cm (range, 10.1-13.2).
4. Discussion
The E-nside multibranch stent-graft system is a novel off-the-shelf solution for the treatment of TAAAs and PAAAs, both in elective and urgent setting (
Figure 1).
Although CMDs, fenestrated or polymer-filled based stent-grafts [
11] still play a leading role in TAAAs and PAAAs treatment, this novel off-the-shelf device might fit a wider range of aortic anatomies thanks to its four configurations. A potential downside of off-the-shelf devices (e.g Cook Zenith T- Branch, E-nside) is that they require a longer sacrifice of healthy aorta and intercostal arteries compared with open repair [
12] and CMDs [
13], which could lead to a higher rate of permanent or transient SCI.
Nonetheless, because of its configurations, E-nside stent-graft can fix large proximal and distal aorta sealing zones resulting in a sparing of proximal thoracic aortic coverage and of intercostal and lumbar arteries’ patency, potentially reducing the risk of SCI. Although the manufacture’s IFU recommend docking in a thoracic stent-graft, we indeed evaluated the feasibility and safety of the E-nside stent-graft without proximal thoracic extension.
In our cohort, prior TEVAR was present in two patients (9.1%), whereas a proximal thoracic endograft was deployed in about one third of patients (7/20, 35%). As reported in the literature [
14], our analysis confirms that proximal thoracic endograft deployment is more frequent in type I-III TAAA than type IV-V TAAA and PAAA (66.7% vs 0%, p= .003).
Notably, a sub-group analysis among patients with type III-IV TAAA and PAAA (n=17) demonstrated that the proximal thoracic stent-graft was deployed in four patients with type III TAAA, whereas it was never deemed as necessary to extend the proximal sealing in patients with type IV TAAA and PAAA (50% vs 0%, p= .029).
In this sub-group of patients, the mean outer-to-outer aortic diameter at the level of E-nside stent-graft’s proximal sealing zone was 32.2±3.1 mm (range, 26-36; anatomical characteristics of the proximal sealing zone in function of aneurysms extent in
Table 2). As emerged, differently from other off-the-shelf concurrent available device (e.g Cook Zenith T-Branch), and despite no ancillary proximal fixation (barbs), E-nside seems capable to fix also large proximal thoracic sealing zone (>31 mm), reducing the use of proximal thoracic stent-graft (4/17, 23.5%) and healthy proximal aorta coverage.
The shorter sacrifice of healthy aorta, as well as TASP and other additional measures (preservation of antegrade perfusion of the left subclavian artery and hypogastric artery, minimization of lower limb ischemia reperfusion injury), may explain the absence of cases of SCI in our study, significantly lower than reported in a recent meta-analysis on the use of other similar devices (13.4%) [
15,
16].
Piazza M et al. reported a rate of SCI of 6,9% with E-nside stent-graft. Also Kapalla M et al. reported a complete permanent SCI in 6,8% of patients, immediately after the procedure. All these patients were treated for TAAA (n= 2 type II, n= 1 type IV), whereas no transient or late SCI was observed4. Differently from these reports, in our series no transient or permanent SCI were noted, while the MAE rate is consistent with the ones reported (24% vs 13.6%).
Aortic and iliac axis anatomy still represent an important limiting factor of E-nside stent-graft wider applicability.
Due to aortic anatomy at paravisceral level, Abisi S
et al. reported a major suitability of their patients with iBEVAR than outer-branched device [
17].
In a retrospective analysis evaluating the anatomical feasibility, Bilman V
et al. reported an overall treatment suitability of 43% with E-nside stent-graft for type I-IV TAAA [
18]. The authors concluded that the main limiting factors for its application are EIA’s diameter (21%), infrarenal aortic diameter (16%) and size of aortic lumen at the level of visceral vessels (14%).
In our series, the mean EIA’s artery diameter was 7.5±1.1 mm (range, 5.8-10.1), consistently smaller than indicated in the manufacture’s IFU (≥8,2 mm). Twelve patients (54.5%; six males, 37.5%; six women, 100%) were considered outside the IFU for E-nside stent-graft due to narrow iliac arteries; of whom, eight of these twelve patients presented an EIA’s diameter ≤7 mm. Fourteen patients (63.6%) presented a moderate to severe tortuosity of the iliac axis (X>1,25). Despite these poor anatomical features, only one unexpected access-site related complication was reported (EIA’s avulsion required urgent conversion). In this latter case, the EIA allowed the insertion and retrieval of a thoracic stent-graft (22 F, 8.5 mm of EIA diameter required), and we were confident about the E-nside safety and feasibility.
Also, Piazza M
et al. reported a fatal intraoperative complication due to EIA rupture. The rupture was related to the unsuccessful E-nside deployment caused by a difficult stent-graft advancement through a severely diseased and narrow EIA (<7 mm) [
14].
Although the IFU recommends performing a surgical conduit in the case of EIA <8.2 mm, our experience shows the feasibility and safety of E-nside even in the case of narrow EIA. Additional measures are suggested to safety manage the 24 F large sheath, such as multiple serial dilations with 18, 20, and 22 F sheaths before the insertion, and the use of the contralateral access to assess the integrity of the vessel during the removal. In addition, we suggest performing surgical conduit or iliac endoconduit in case of an association between EIA diameter <7 mm and iliac tortuosity index >1.4, or in case of serious and circumferential calcifications of the iliac axis which could hamper the safety insertion and removal of the device. A narrow iliac axis could lead to a suboptimal device’s orientation, which could hamper the TVs’ catheterization and lead to longer operative time. The longer operative time could result in a tenacious adhesion of the arterial wall to the sheath, that could hamper its safety retrieval.
Several reports focused on access-site complications as a major weakness of this stent-graft due to the large bore sheath [
19,
20]. The Inbreed Investigators reported an access-site related reintervention rate at 90-days up to 4.3%, whereas Kapalla M
et al. reported an access-site complications in seven patients (15.9%; 4 false aneurysm, 3 surgical site infection) [
4]. In our series, one intraoperative but no late access-site related complications were observed. A careful case planning of the access is therefore crucial to improve the outcomes and to reduce the complications. Performing a surgical cutdown to expose the CFA seems viable to reduce the risk of false aneurysms and blood loss in the early post-operative period.
Aortic anatomy at the paravisceral level is the last limiting factor that affects E-nside applicability. In our series, four patients (18.2%) had a lumen <24 mm. This data is consistent with the recently published reports (15.5% of a narrow aortic diameter <25 mm). No differences in terms of TVs’ success and stability were recorded in comparing these cases to those with a larger lumen (p= ns). Precannulation is useful for facilitating the advancement of the sheath into the inner branch and to reduce the time of TVs’ stenting, but no statistical differences in time of TVs catheterization were observed between cases in which the preloaded system was used and cases in which it was not.
Although Katsargyris A
et al. found that inner branches were often difficult to cannulate [
21], our experience is consistent with most recent reports in which TVs’ success can be easily achieved, from axillary of femoral approach, also without precannulation system.
Our TVs success rate was 95.1%. These data are comparable to previous reports recently published, in which the TVs success rate ranged from 96 to 99%, with an estimated branch patency rate al twelve months of 98%. In our series, no branch related reintervention was observed during the follow-up.
Previous Literature on the outcomes of BEVAR reported good technical success rate and a low incidence of perioperative morbidity and mortality rate [
13,
22,
23].
To date, there are few available reports in the L literature on the early- to mid-term results of the E-nside stent-graft [
4,
14,
17,
24,
25,
26].
Yazar O et al. reported a series of 23 patients treated with E-nside stent-graft over a period of 56 months and a mean follow-up time of 15 months with a technical success of 96%. Our data are consistent with this publication, with a technical success of 95.5%, and a primary assisted technical success of 100%.
Our perioperative mortality rate was 9.1% (2/22), higher than that reported by Yazar O (8.3%) and by Piazza M (5.2%). Nevertheless, our patients were older (75.9 vs 73 years), and seven patients (mean age of 80.3 years) were considered frail, with previous stroke and moderate to severe cognitive impairment. As reported, frail patients have a worse ability to recover after surgery especially if following MAE or procedure-related complications. The frail patients had a significantly longer hospitalization and more frequent non-home discharge than the fit patients. This increase in mortality rate could be not only justified by the frequency of complications following iBEVAR, but also by the greater risks inherent to longer hospitalization (immobilization, loss of muscle mass, functional and cognitive decline) that are strictly related with frailty.
Limitetions
This research has certain limitations. First, bias related to a retrospective data collection, but also by the small number of cases and the retrospective non-randomized single-center study design. Additionally, over the course of the two-years study period, there has been a learning curve and an increase in proficiency with this endovascular technique and the device itself, which could have had an impact on technical and clinical outcomes.
5. Conclusions
Our initial experience confirms that E-nside thoraco-abdominal multibranch stent-graft system seems having promising early technical and clinical success rates, and low reintervention rate.
Drawbacks include the extensive sacrifice of the healthy aorta and the 24 F delivery system which represent a limitation in patients with narrow iliac arteries. Nevertheless, our analysis showed a promising low rate of access-site related complications, also in case of patients treated outside the IFU for iliac axis anatomy.
The preservation of precannulation system, as well as the reduction of the diameter of the delivery system, is crucial to establish the E-nside as a preeminent endograft for the treatment of complex aortic disease, especially among female patients.
Mid- to long-term confirmatory larger studies and prospective registries are mandatory to evaluate long term results as its efficacy and safety, as a comparison between E-nside and other alternative off-the-shelf solution, such as in situ fenestration, chimney techniques or outer-branched stent-grafts.
Author Contributions
Conceptualization, S.C.. and A.M.; methodology, S.C.; software, S.C.; validation, E.S., S.C. and A.M.; formal analysis, S.C.; investigation, S.C., A.M., O.M., J.J. and A.Mol.; resources, V.B. and E.S..; data curation, S.C. and O.M..; writing—original draft preparation, S.C., E.S. and O.M.; writing—review and editing, A.M..; visualization, V.B.,A.Mol and J.J..; supervision, E.S.; project administration, S.C.; funding acquisition, E.S.
All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of "University of Rome, La Sapienza" (protocol code SUR 2021-58, 6 April 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not available due to privacy restriction.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Feezor RJ, Martin TD, Hess PJ Jr, Daniels MJ, Beaver TM, Klodell CT, et al. Extent of aortic coverage and incidence of spinal cord ischemia after thoracic endovascular aneurysm repair. Ann Thorac Surg. 2008, 86, 1809–1814. [Google Scholar] [CrossRef] [PubMed]
- Diamond KR, Simons JP, Crawford AS, Arous EJ, Judelson DR, Aiello F, et al. Effect of thoracoabdominal aortic aneurysm extent on outcomes in patients undergoing fenestrated/branched endovascular aneurysm repair. J Vasc Surg. 2021, 74, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann A, Menges AL, Rancic Z, Meuli L, Dueppers P, Reutersberg B. E-nside Off-the-Shelf Inner BranchStentGraft: Technical Aspects of Planning and Implantation. J Endovasc Ther. 2022, 29, 167–174. [Google Scholar] [CrossRef]
- Kapalla M, Busch A, Lutz B, Nebelung H, Wolk S, Reeps C. Single-center initial experience with inner-branch complex EVAR in 44 patients. Front Cardiovasc Med. 2023, 10, 1188501. [Google Scholar] [CrossRef] [PubMed]
- Toro D, Ohrlander T, Resch T. Experience with inner branch off the shelf device for thoracoabdominal aneurysms. J Cardiovasc Surg (Torino). 2023, 64, 475–480. [Google Scholar]
- Jónsson GG, Mani K, Mosavi F, D'Oria M, Semenas E, Wanhainen A, et al. Spinal drain-related complications after complex endovascular aortic repair using a prophylactic automated volume-directed drainage protocol. J Vasc Surg. 2023, 78, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Riambau V, Böckler D, Brunkwall J, Cao P, Chiesa R, Coppi G. Editor’s choice - Management of descending thoracic aorta diseases: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef]
- Kasprzak PM, Gallis K, Cucuruz B, Pfister K, Janotta M, Kopp R. Editor's choice - Temporary aneurysm sac perfusion as an adjunct for prevention of spinal cord ischemia after branched endovascular repair of thoracoabdominal aneurysms. Eur J Vasc Endovasc Surg. 2014, 48, 258–265. [Google Scholar] [CrossRef]
- Verzini F, Loschi D, De Rango P, Ferrer C, Simonte G, Coscarella C, et al. Current results of total endovascular repair of thoracoabdominal aortic aneurysms. J Cardiovasc Surg (Torino). 2014, 55, 9–19. [Google Scholar]
- Piccinelli M, Veneziani A, Steinman DA, Remuzzi A, Antiga L. A framework for geometric analysis of vascular structures: application to cerebral aneurysms. IEEE Trans Med Imaging. 2009, 28, 1141–1155. [Google Scholar] [CrossRef]
- Cuozzo S, Martinelli O, Brizzi V, Miceli F, Flora F, Sbarigia E, Gattuso R. Early Experience with Ovation Alto Stent-Graft. Ann Vasc Surg. 2023, 88, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio L, Cambiaghi T, Ferrer C, Baccellieri D, Verzini F, Melissano G, et al. Comparison of sacrificed healthy aorta during thoracoabdominal aortic aneurysm repair using off-the-shelf endovascular branched devices and open surgery. J Vasc Surg. 2018, 67, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Spath P, Tsilimparis N, Furlan F, Hamwi T, Prendes CF, Stana J. Additional Aortic Coverage With an Off The Shelf, Multibranched Endograft Compared With Custom Made Devices For Endovascular Repair of Pararenal Abdominal Aortic Aneurysms. Eur J Vasc Endovasc Surg. 2023, 65, 710–718. [Google Scholar] [CrossRef]
- Piazza M, Squizzato F, Pratesi G, Tshomba Y, Gaggiano A, Gatta E, et al. ; INBREED Investigators. Editor's Choice - Early Outcomes of a Novel Off the Shelf Preloaded Inner Branch Endograft for the Treatment of Complex Aortic Pathologies in the ItaliaN Branched Registry of E-nside EnDograft (INBREED). Eur J Vasc Endovasc Surg. 2023, 65, 811–817. [Google Scholar] [CrossRef]
- Chen Y, Liu Z, Wang S, D'Oria M, Zhang X, Bi J, et al. Systematic Review and Meta-analysis of Short-term and Mid-term Outcomes After Use of t-Branch Off-the-shelf Multibranched Endograft for Elective and Urgent Treatment of Thoracoabdominal Aortic Aneurysms. J Endovasc Ther. 5266.
- Konstantinou N, Antonopoulos CN, Jerkku T, Banafsche R, Kölbel T, Fiorucci B, et al. Systematic review and meta-analysis of published studies on endovascular repair of thoracoabdominal aortic aneurysms with the t-Branch off-the-shelf multibranched endograft. J Vasc Surg. 2020, 72, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Abisi S, Zymvragoudakis V, Gkoutzios P, Sallam M, Donati T, Saha P, et al. Early outcomes of Jotec inner-branched endografts in complex endovascular aortic aneurysm repair. J Vasc Surg. 2021, 74, 871–879. [Google Scholar] [CrossRef]
- Bilman V, Cambiaghi T, Grandi A, Carta N, Melissano G, Chiesa R, et al. Anatomical feasibility of a new off-the-shelf inner branch stent graft (E-nside) for endovascular treatment of thoraco-abdominal aneurysms. Eur J Cardiothorac Surg. 2020, 58, 1296–1303. [Google Scholar] [CrossRef]
- Silverberg D, Bar-Dayan A, Hater H, Khaitovich B, Halak M. Short-term outcomes of inner branches for endovascular repair of complex abdominal and thoracoabdominal aortic aneurysms. Vascular. 2021, 29, 644–651. [Google Scholar] [CrossRef]
- Youssef M, Deglise S, Szopinski P, Jost-Philipp S, Jomha A, Vahl CF, et al. A multicenter experience with a new fenestrated-branched device for endovascular repair of thoracoabdominal aortic aneurysms. J Endovasc Ther. 2018, 25, 209–219. [Google Scholar] [CrossRef]
- Katsargyris A, Marques de Marino P, Mufty H, et al. Early experience with the use of inner branches in endovascular repair of complex abdominal and thoraco-abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2018, 55, 640–646. [Google Scholar] [CrossRef]
- Oderich GS, Greenberg RK, Farber M, Lyden S, Sanchez L, Fairman R, et al. Results of the United States multicenter prospective study evaluating the Zenith fenestrated endovascular graft for treatment of juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2014, 60, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Doonan RJ, Bin-Ayeed S, Charbonneau P, Hongku K, Mackenzie K, Steinmetz O, et al. Mortality and major adverse events improve with increased institutional experience for fenestrated and branched endovascular aortic repair. J Endovasc Ther. 2021, 29, 746–754. [Google Scholar]
- Spinella G, Pane B, Finotello A, Bastianon M, Mena Vera JM, Di Gregorio S, et al. Early Experience of Inner Branch Retrograde Cannulation With E-nside Branch Stent Graft for Thoracoabdominal Aortic Aneurysms. J Endovasc Ther. 2023, 15266028231163067.
- Yazar O, Huysmans M, Lacquet M, Salemans PB, Wong CY, Bouwman LH. Single-Center Experience With Inner-Branched Endograft for the Treatment of Pararenal Abdominal Aortic Aneurysms. J Endovasc Ther. 2023, 15266028231204286.
- Silverberg D, Bar Dayan A, Speter C, Fish M, Halak M. The Use of the Off-the-Shelf Inner Branch E-nside Endograft for the Treatment of Elective and Emergent Complex Aortic Aneurysms-A Single-Center Experience. Ann Vasc Surg. 2023, S0890-5096(23)00528-9.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).