Submitted:
13 September 2024
Posted:
16 September 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Case Report
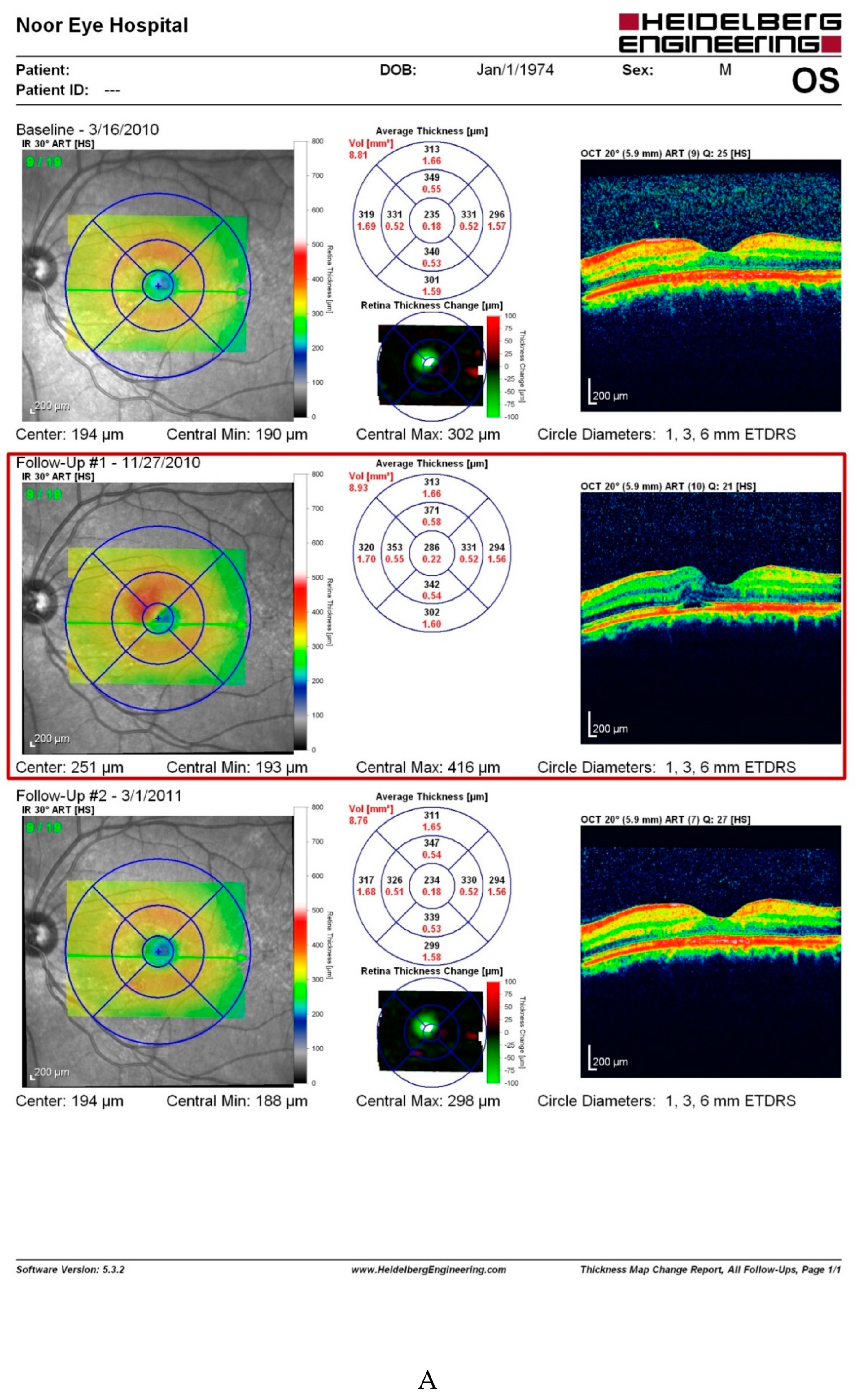
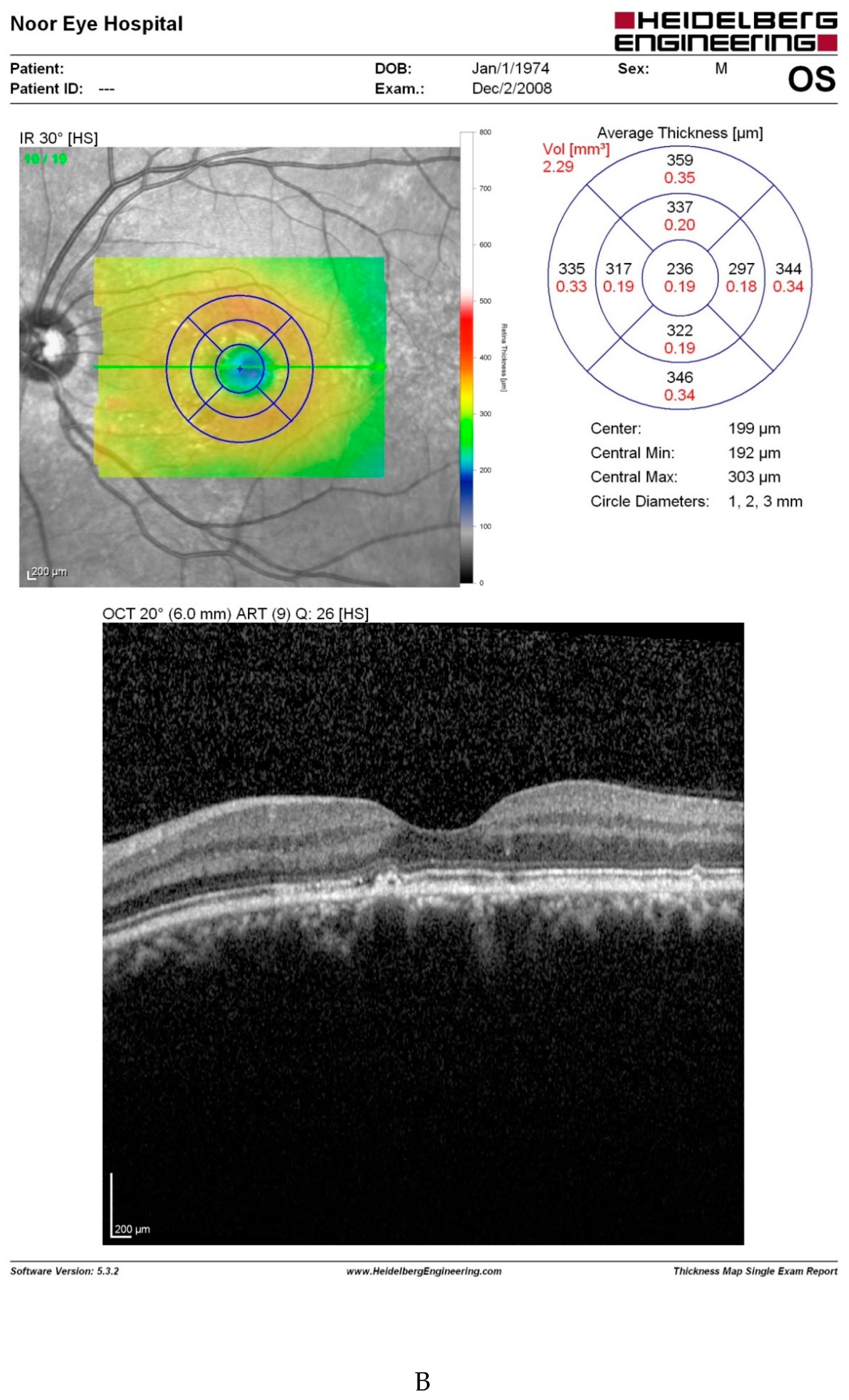
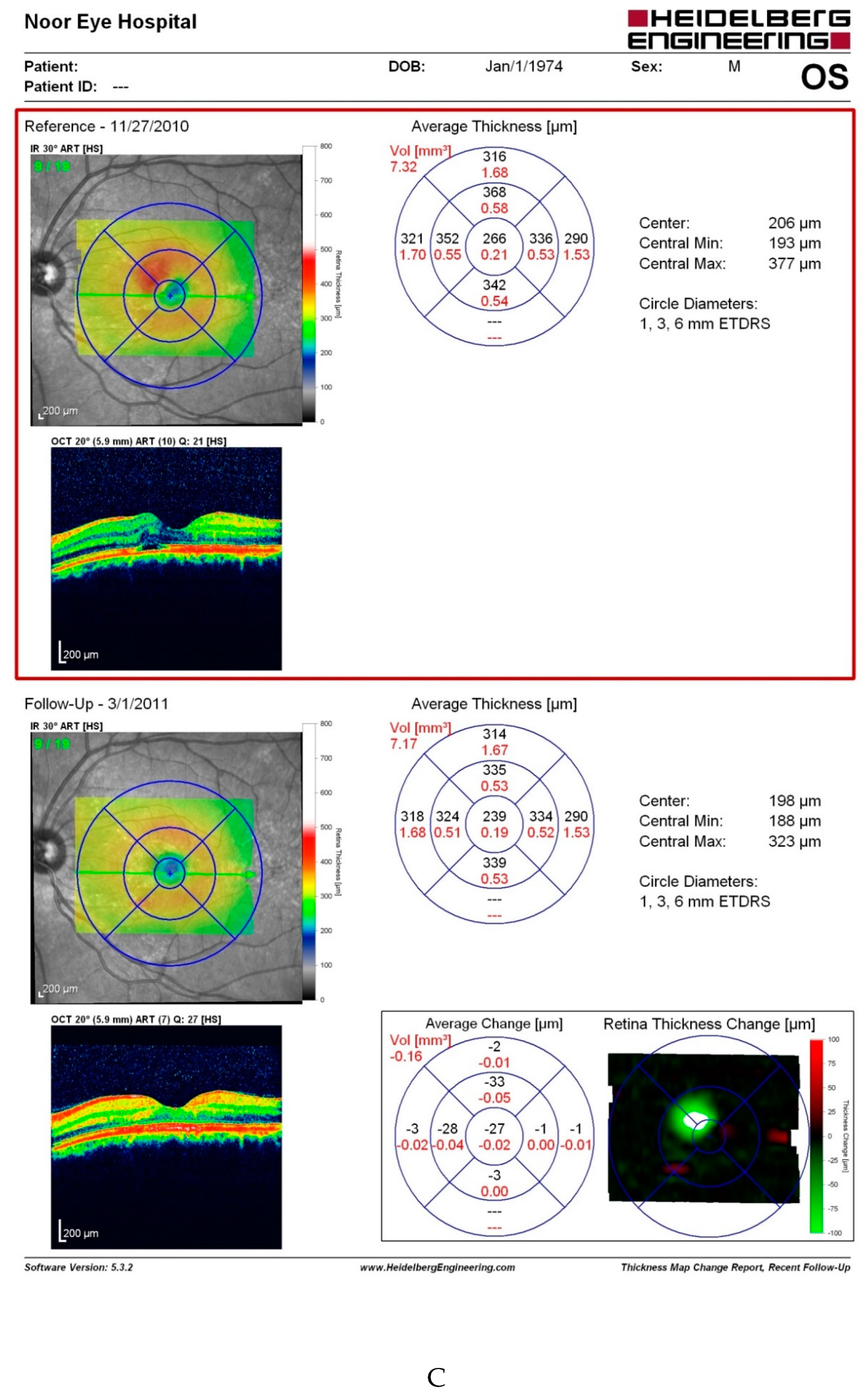
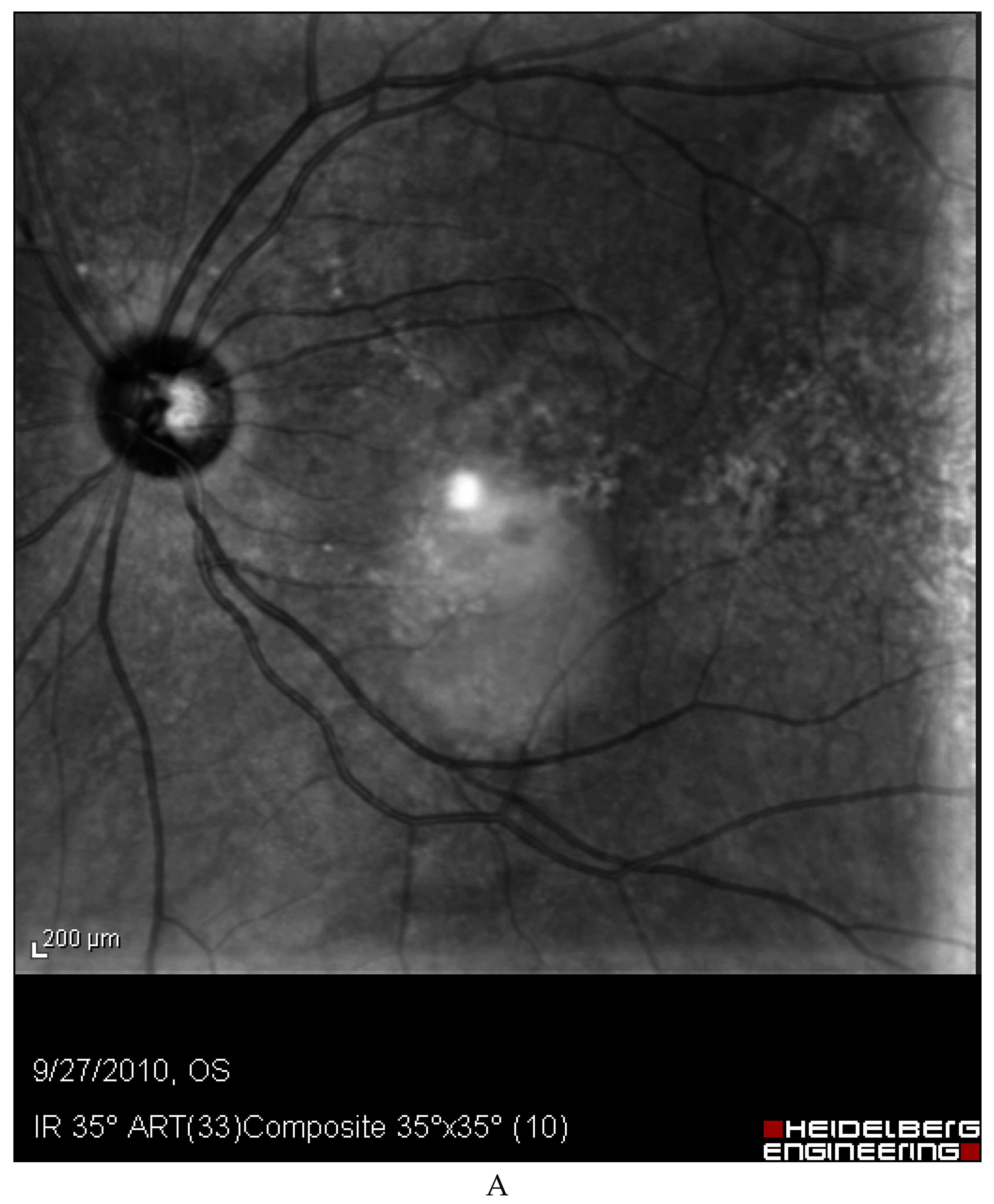
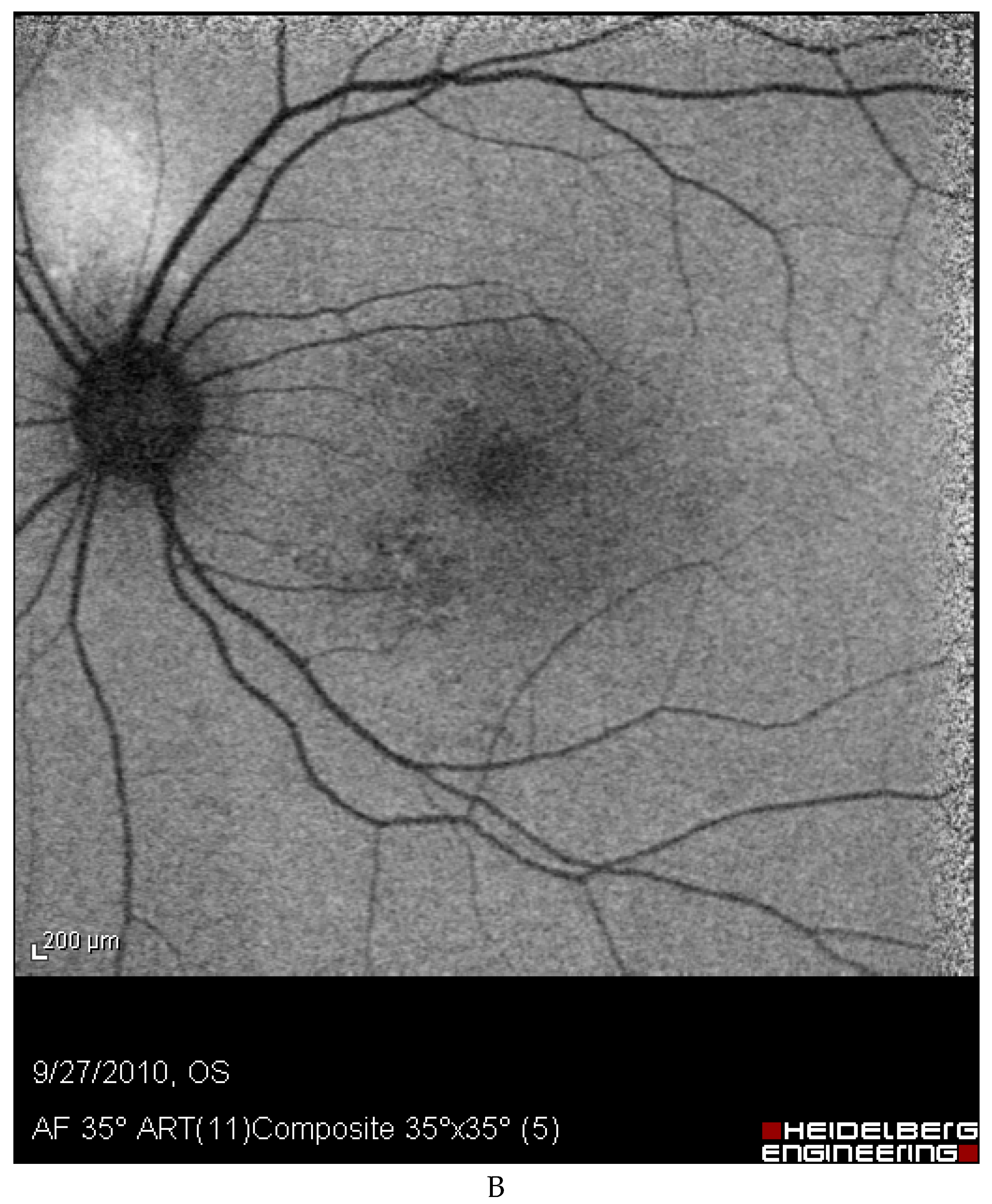
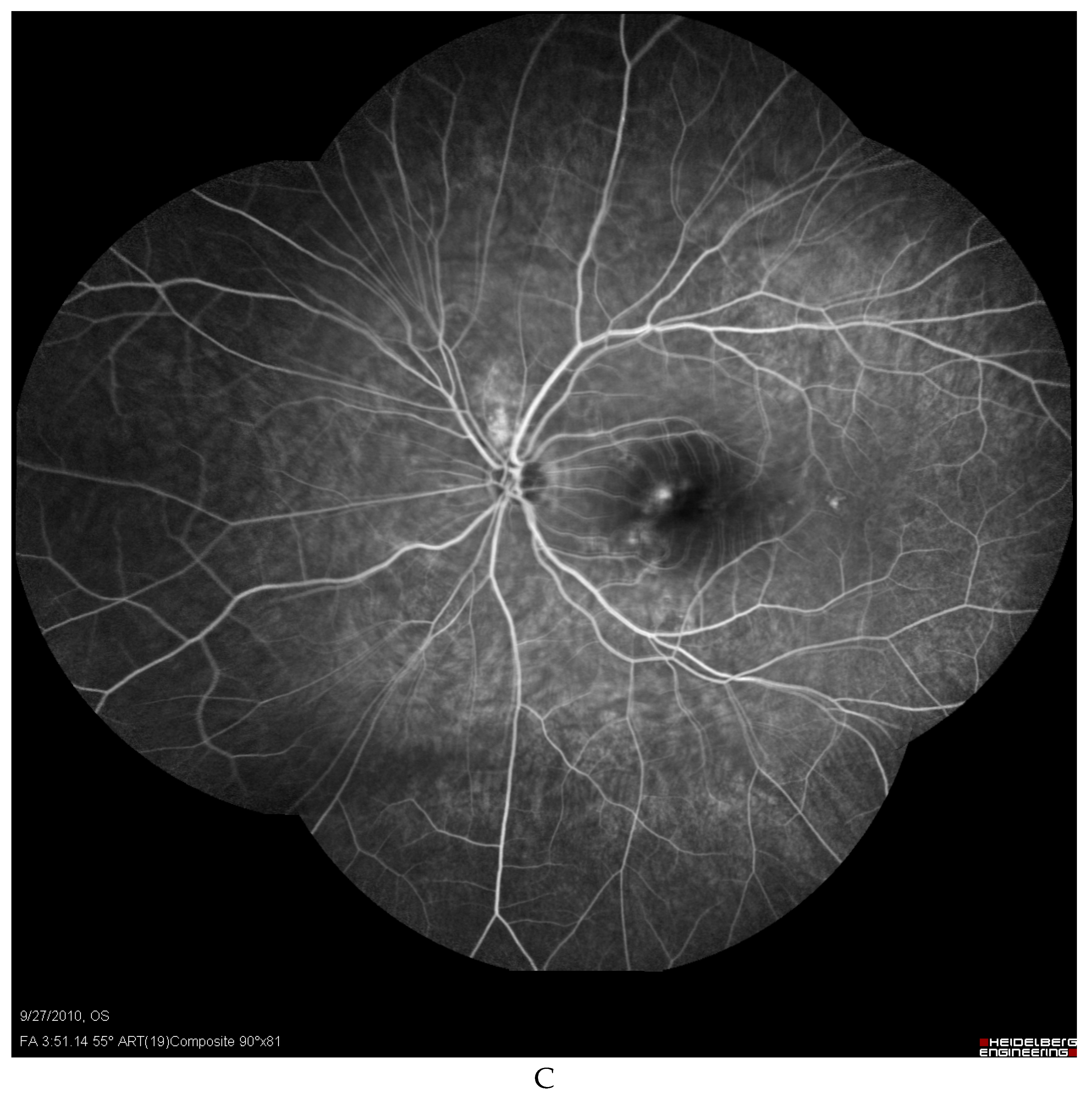
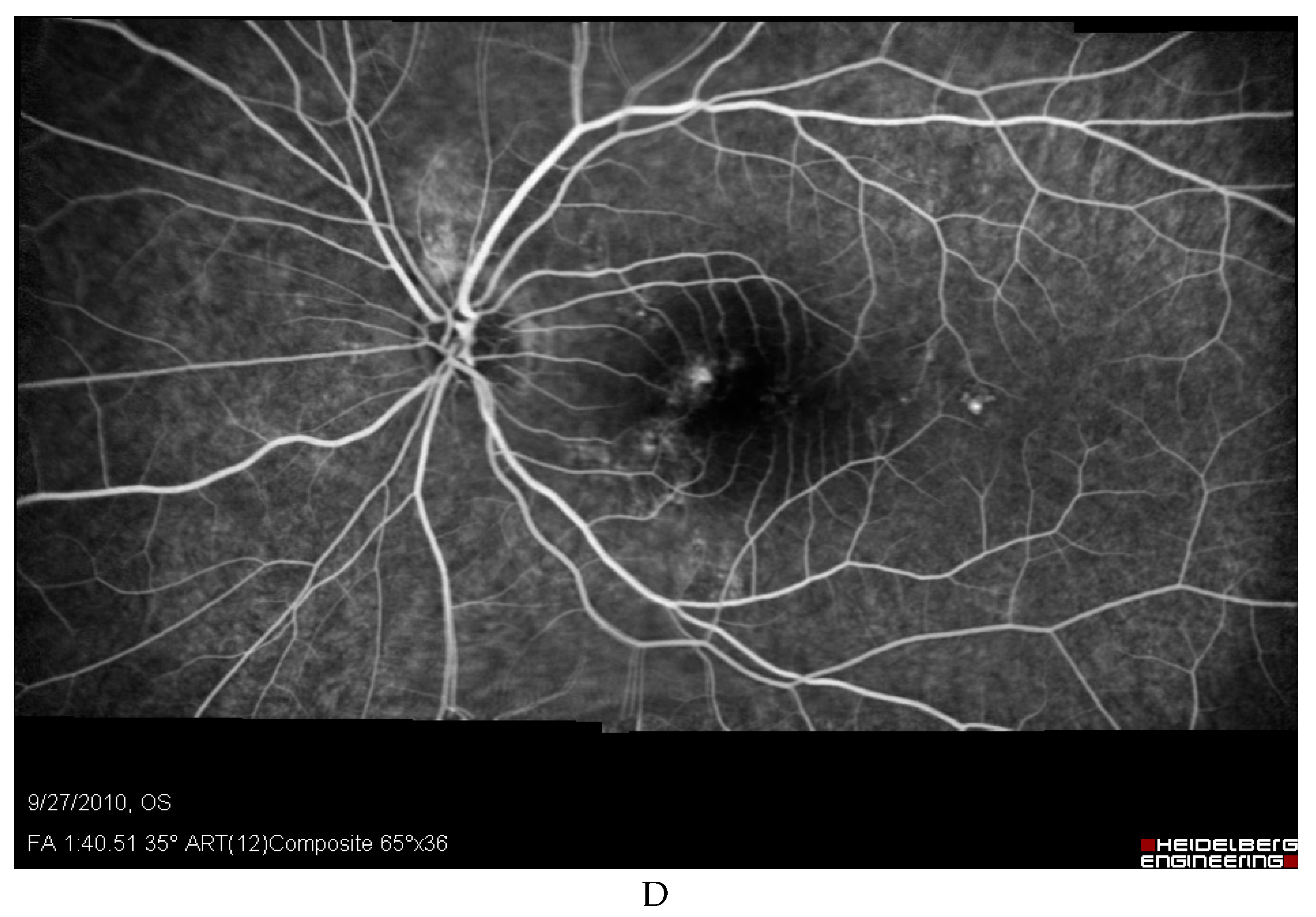
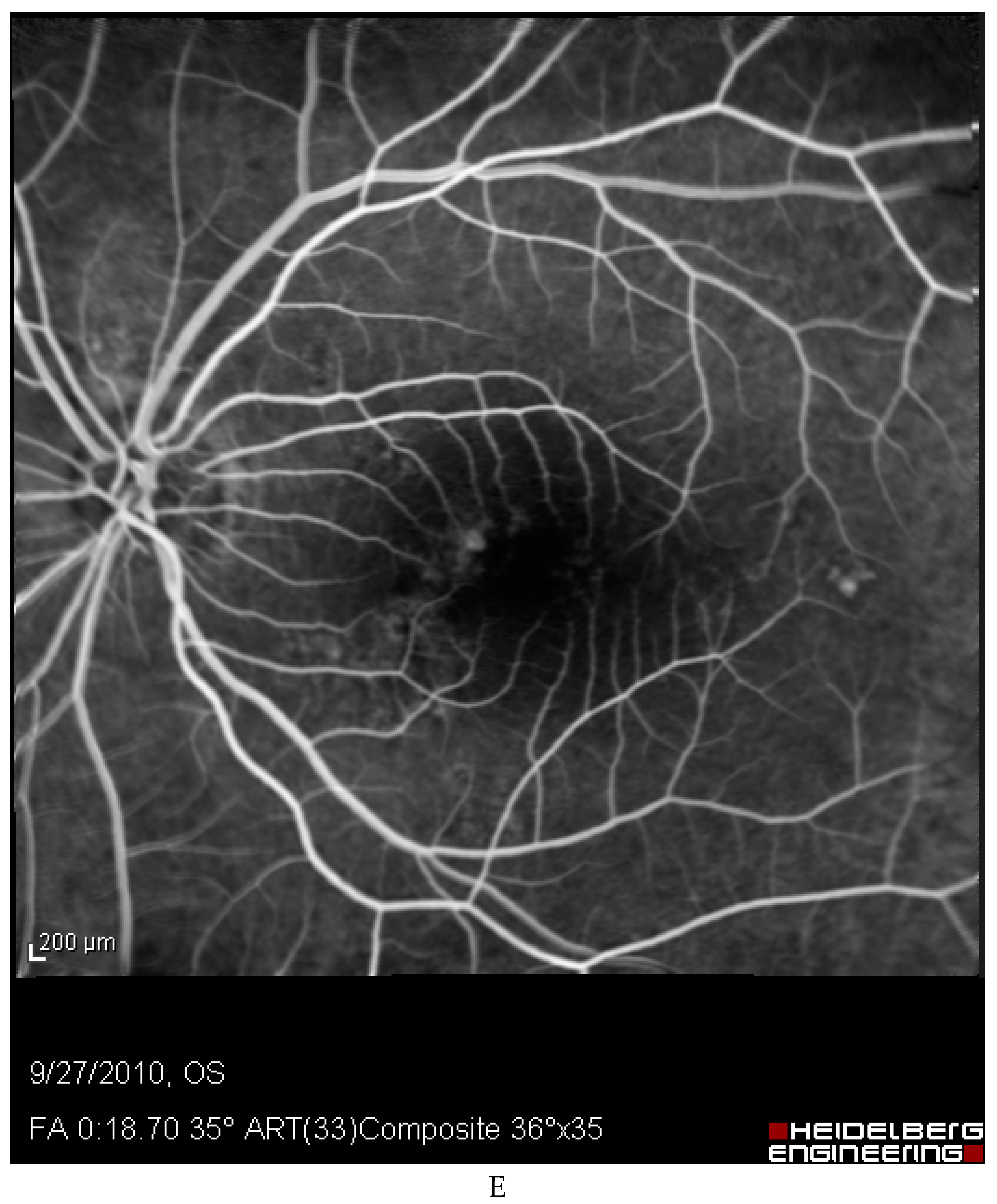
Discussion
- (i)
- The most straightforward explanation for this observation is that retinal issues following capsaicin consumption are not consistent. However, the fact that our patient experienced the same retinal problems three times following considerable amounts of red pepper, merits investigations.
- (ii)
- The second possibility-albeit with low likelihood- is that capsaicin may have been capable of crossing the blood-brain-barrier (BBB). Indeed, there is evidence to support this possibility [6,7,16,17]. After being transported into the portal vein and then into the whole body in both human and rodents, about 5% of unmodified capsaicin crosses the BBB and goes into the brain tissue [16,17]. This suggests that acute consumption of capsaicin could be another rationale explanation. This, in turn, may create a further possibility, i.e., there might be individuals who are extremely sensitive or hyper-reactive to high and acute intake of capsaicin. There is also evidence showing that capsaicin increases the permeability of the blood-spinal cord barrier and this effect is mediated by the activation of TRPV1-expressing primary sensory neurons [18]. If it is conceivable that capsaicin can cross in sufficient amounts in hyper-reactive individuals, then the common belief that red pepper is safe requires reconsideration. This also may affect the design of future studies investigating related ocular conditions, because there is a robust possibility that high intake of red pepper may create an uncertainty or a bias, particularly in non-randomized clinical trials. This case report may also have implications for the design of capsaicin-related food supplements and drugs, particularly considering high inter-individual differences in capsaicin bioavailability [19] and metabolism, detoxification, and bioactivation of capsaicin as well as alkyl dehydrogenation and oxygenation of capsaicinoids by P450 enzymes [20]. In this regard, it of interest to mention the hypertensive crisis in a 19-year-old man with a a prior abundant ingestion of peppers likely associated to a decrease in protecting calcitonin gene-related peptide [21] as well of myocardial infarction due to cayenne pepper pills in a young man [22] .
- (iii)
- Another hypothetical explanation for this case report is the involvement of aquaporins (AQPs). Experimental animals have a set of homeostatic mechanisms that continuously work together to maintain body-fluid osmolality within a narrow range around a set point (∼300 mOsm/kg) [23]. AQPs have been implicated in retinal neovascularization both in animal models and humans [24,25,26]. Namely, AQP1 is required for hypoxia-inducible angiogenesis in human retinal vascular endothelial cells [24]. Relatedly, activating stimuli such as AQPs, protons and temperature synergistically enhance osmosensitivity of TRPV1.
Author Contributions
Data Availability Statement
References
- Venkata, M. Kanukollu and Syed Shoeb Ahmad. Retinal Hemorrhage. in StatPearls [Internet], StatPearls Publishing, 2022).
- Christopher, A. Reilly. Cytochrome P450-Dependent Modification of Capsaicinoids: Pharmacological Inactivation and Bioactivation Mechanisms. Role of Capsaicin in Oxidative Stress and Cancer 2013, 107–129. [Google Scholar]
- Kou Liu, Xiang Gao, Chengyang Hu, Yanchao Gui, Siyu Gui, Qinyu Ni, Liming Tao, and Zhengxuan Jiang. Capsaicin ameliorates diabetic retinopathy by inhibiting poldip2-induced oxidative stress. Redox biology 2022, 56, 102460. [Google Scholar]
- Jun Wang, Wenke Tian, Shuaiwei Wang, Wenqiang Wei, Dongdong Wu, Honggang Wang, Li Wang, Ruisheng Yang, Ailing Ji, and Yanzhang Li. AntiGÇÉinflammatory and retinal protective effects of capsaicin on ischaemiaGÇÉinduced injuries through the release of endogenous somatostatin. Clinical and Experimental Pharmacology and Physiology 2017, 44, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Hércules Rezende Freitas, Alinny Rosendo Isaac, Thayane Martins Silva, Geyzzara Oliveira Ferreira Diniz, Yara dos Santos Dabdab, Eduardo Cosendey Bockmann, Mar+ lia Zaluar Passos Guimar+úes, Karin da Costa Calaza, Fernando Garcia de Mello, and Ana Lucia Marques Ventura. Cannabinoids induce cell death and promote P2X7 receptor signaling in retinal glial progenitors in culture. Molecular neurobiology 2019, 56, 6472–6486. [Google Scholar] [CrossRef] [PubMed]
- Jessica O’Neill, Christina Brock, Anne Estrup Olesen, Trine Andresen, Matias Nilsson, and Anthony H. Dickenson. Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacological reviews 2012, 64, 939–971. [Google Scholar] [CrossRef]
- A. Saria, G. Skofitsch, and F. Lembeck. Distribution of capsaicin in rat tissues after systemic administration. Journal of Pharmacy and Pharmacology 1982, 34, 273–275. [Google Scholar]
- Sanjay Chanda, Mohammad Bashir, Sunita Babbar, Aruna Koganti, and Keith Bley. In vitro hepatic and skin metabolism of capsaicin. Drug Metabolism and Disposition 2008, 36, 670–675. [Google Scholar] [CrossRef]
- Maria Wang, Inger Christine Munch, Pascal W. Hasler, Christian Pr++nte, and Michael Larsen. Central serous chorioretinopathy. Acta ophthalmologica 2008, 86, 126–145. [Google Scholar] [CrossRef]
- Mark, S. Blumenkranz, Stephen R. Russell, Marcia G. Robey, Recia Kott-Blumenkranz, and Neal Penneys. Risk factors in age-related maculopathy complicated by choroidal neovascularization. Ophthalmology 1986, 93, 552–558. [Google Scholar]
- Junwon Lee, Seonghee Choi, Christopher Seungkyu Lee, Min Kim, Sung Soo Kim, Hyoung Jun Koh, Sung Chul Lee, and Suk Ho Byeon. Neovascularization in fellow eye of unilateral neovascular age-related macular degeneration according to different drusen types. American Journal of Ophthalmology 2019, 208, 103–110. [Google Scholar] [CrossRef]
- Junwon Lee, Min Kim, Christopher Seungkyu Lee, Sung Soo Kim, Hyoung Jun Koh, Sung Chul Lee, and Suk Ho Byeon. Drusen subtypes and choroidal characteristics in Asian eyes with typical neovascular age-related macular degeneration. Retina 2020, 40, 490–498. [Google Scholar] [CrossRef]
- Richard, F. Spaide, Donald Armstrong, and Richard Browne. Choroidal neovascularization in age-related macular degenerationGÇöwhat is the cause? Retina 2003, 23, 595–614. [Google Scholar]
- W. C. Huang, W. L. Chen, Y. Y. Tsai, C. C. Chiang, and J. M. Lin. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eye 2009, 23, 488–489. [Google Scholar] [CrossRef] [PubMed]
- Karen, B. Schaal, Alexandra E. Hoeh, Alexander Scheuerle, Florian Schuett, and Stefan Dithmar. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. European journal of ophthalmology 2009, 19, 613–617. [Google Scholar]
- V. S. Govindarajan and M. N. Sathyanarayana. CapsicumGÇöproduction, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences. Critical Reviews in Food Science & Nutrition 1991, 29, 435–474. [Google Scholar]
- Peijian Wang, Zhencheng Yan, Jian Zhong, Jing Chen, Yinxing Ni, Li Li, Liqun Ma, Zhigang Zhao, Daoyan Liu, and Zhiming Zhu. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes 2012, 61, 2155–2165. [Google Scholar] [CrossRef]
- Simon Beggs, Xue Jun Liu, Chun Kwan, and Michael W. Salter. Peripheral nerve injury and TRPV1-expressing primary afferent C-fibers cause opening of the blood-brain barrier. Molecular pain 2010, 6, 1744–8069. [Google Scholar]
- William, D. Rollyson, Cody A. Stover, Kathleen C. Brown, Haley E. Perry, Cathryn D. Stevenson, Christopher A. McNees, John G. Ball, Monica A. Valentovic, and Piyali Dasgupta. Bioavailability of capsaicin and its implications for drug delivery. Journal of Controlled Release 2014, 196, 96–105. [Google Scholar]
- Christopher, A. Reilly and Garold S. Yost. Metabolism of capsaicinoids by P450 enzymes: a review of recent findings on reaction mechanisms, bio-activation, and detoxification processes. Drug metabolism reviews 2006, 38, 685–706. [Google Scholar]
- Patanè S, Marte F, La Rosa FC, La Rocca R. Capsaicin and arterial hypertensive crisis. Int J Cardiol. 2010, 144, e26-7. [Google Scholar] [CrossRef]
- Akçay M, Gedikli Ö, Yüksel S. An unusual side effect of weight loss pills in a young man; acute myocardial infarction due to cayenne pepper pills. Anatol J Cardiol. 2017, 18, 310–311. [Google Scholar] [CrossRef]
- B. Anderson. Regulation of body fluids. Annual review of physiology 1977, 39, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Kentaro Kaneko, Kazuo Yagui, Asami Tanaka, Kei Yoshihara, Kou Ishikawa, Kazuo Takahashi, Hideaki Bujo, Kenichi Sakurai, and Yasushi Saito. Aquaporin 1 is required for hypoxia-inducible angiogenesis in human retinal vascular endothelial cells. Microvascular research 2008, 75, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Javier Ruiz-Ederra and A., S. Verkman. Aquaporin-1 Independent Microvessel Proliferation in a Neonatal Mouse Model of Oxygen-Induced Retinopathy. Investigative ophthalmology & visual science 2007, 48, 4802–4810. [Google Scholar]
- Wang Xun, Yanbo Liu, Gu Qing, Xu Xun, Zhu Dongqing, and Wu Haixiang. Aquaporin 1 expression in retinal neovascularization in a mouse model of retinopathy of prematurity. Preparative Biochemistry & Biotechnology 2009, 39, 208–217. [Google Scholar]
- Holly, C. Cappelli, Brianna D. Guarino, Anantha K. Kanugula, Ravi K. Adapala, Vidushani Perera, Matthew A. Smith, Sailaja Paruchuri, and Charles K. Thodeti. Transient receptor potential vanilloid 4 channel deletion regulates pathological but not developmental retinal angiogenesis. Journal of cellular physiology 2021, 236, 3770–3779. [Google Scholar]
- Caitriona O’Leary, Mary K. McGahon, Sadaf Ashraf, Jennifer McNaughten, Thomas Friedel, Patrizia Cincola, Peter Barabas, Jose A. Fernandez, Alan W. Stitt, and J. Graham McGeown. Involvement of TRPV1 and TRPV4 channels in retinal angiogenesis. Investigative ophthalmology & visual science 2019, 60, 3297–3309. [Google Scholar]
- Catarina Micaelo-Fernandes, Joseph Bouskila, Roberta M. Palmour, Jean Fran+ºois Bouchard, and Maurice Ptito. Age and Sex-Related Changes in Retinal Function in the Vervet Monkey. Cells 2022, 11, 2751. [Google Scholar] [CrossRef]
- Eric, J. Vallender, Charlotte E. Hotchkiss, Anne D. Lewis, Jeffrey Rogers, Joshua A. Stern, Samuel M. Peterson, Betsy Ferguson, and Ken Sayers. Nonhuman primate genetic models for the study of rare diseases. Orphanet journal of rare diseases 2023, 18, 20. [Google Scholar]
- Anri Nishinaka, Miruto Tanaka, Kentaro Ohara, Eiji Sugaru, Yuji Shishido, Akemi Sugiura, Yukiko Moriguchi, Amane Toui, Shinsuke Nakamura, and Kaoru Shimada. TRPV4 channels promote vascular permeability in retinal vascular disease. Experimental Eye Research 2023, 228, 109405. [Google Scholar] [CrossRef]
- Lei Wen, Yue Chun Wen, Gen Jie Ke, Si Qin Sun, Kai Dong, Lin Wang, and Rong Feng Liao. TRPV4 regulates migration and tube formation of human retinal capillary endothelial cells. BMC ophthalmology 2018, 18, 1–6. [Google Scholar]
- Lee D, Ahn H, Lee KG. Analysis of 207 residual pesticides in hot pepper powder using LC-MS/MS. Food Sci Biotechnol. 2023, 33, 1337–1350. [CrossRef]
- Yaduvanshi SK, Srivastava N, Marotta F, Jain S, Yadav H. Evaluation of micronuclei induction capacity and mutagenicity of organochlorine and organophosphate pesticides. Drug Metab Lett. 2012, 6, 187–197. [Google Scholar] [CrossRef]
- Tyagi S, Shekhar N, Thakur AK. Protective Role of Capsaicin in Neurological Disorders: An Overview. Neurochem Res. 2022, 47, 1513–1531. [Google Scholar] [CrossRef] [PubMed]
- Marotta F, Mao GS, Liu T, Chui DH, Lorenzetti A, Xiao Y, Marandola P. Anti-inflammatory and neuroprotective effect of a phytoestrogen compound on rat microglia. Ann N Y Acad Sci. 2006, 1089, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo M, Marotta F, Dominguez LJ. Oxidative stress in patients with Alzheimer’s disease: effect of extracts of fermented papaya powder. Mediators Inflamm. 2015, 2015, 624801. [Google Scholar] [CrossRef]
- Sedriep S, Xia X, Marotta F, Zhou L, Yadav H, Yang H, Soresi V, Catanzaro R, Zhong K, Polimeni A, Chui DH. Beneficial nutraceutical modulation of cerebral erythropoietin expression and oxidative stress: an experimental study. J Biol Regul Homeost Agents 2011, 25, 187–194. [Google Scholar]
- Marotta F, Chui DH, Yadav H, Lorenzetti A, Celep G, Jain S, Bomba A, Polimeni A, Zhong K, Allegri F. Effective properties of a sturgeon-based bioactive compound on stress-induced hippocampal degeneration and on in vitro neurogenesis. J Biol Regul Homeost Agents 2012, 26, 327–35. [Google Scholar]
- Cervi J, Rastmanesh R, Moghadam Ahmad A, Ayala A, Aperio C, et al. Effect of a fermented functional food versus vitamin e on nrf2 and are-signalling in untrained middle-age/elderly after exercise: a rct, double-blind clinical study. Functional Food Science (.
- Elkhedir A, Iqbal A, Albahi A, Tao M, Rong L, Xu X. Capsaicinoid-Glucosides of Fresh Hot Pepper Promotes Stress Resistance and Longevity in Caenorhabditis elegans. Plant Foods Hum Nutr. 2022, 77, 30. [Google Scholar] [CrossRef]
- Kumar P, Chand S, Chandra P, Maurya PK. Influence of Dietary Capsaicin on Redox Status in Red Blood Cells During Human Aging. Adv Pharm Bull. 2015, 5, 583–586. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




