Submitted:
15 September 2024
Posted:
16 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- using the same concentration of BB solution (5mg/L);
- adding different doses of ozone (100 - 250 mg O3/ L gaseous mixture) at the BB solution;
- in different contact time (0 - 10 min at pH = 7, 0 - 1.5 min at pH = 4 and 0 - 1 min at pH = 10);
- with continuously stirring (200 rpm) / without stirring;
3. Results and Discussions
3.1. Preliminary Results and Discussion
- -
- the version with stirring is more efficient than the one without stirring;
- -
- at pH = 4.0 the complete discoloration occurs after 1.25 min (for O3 concentration of 100 and 150 mg/L) and after 0.50 min respectively (for O3 concentration of 200 and 250 mg/L);
- -
- at pH = 7.0 the complete discoloration occurs after 10 min (for O3 concentration of 100 and 150 mg/L) and after 1 min respectively (for O3 concentration of 200 and 250 mg/L);
- -
- at pH = 10.0 the final concentration of BB is much lower in a shorter time (0.2 min) compared to previous studied pH values due to OH radical oxidation efficiency is increased.
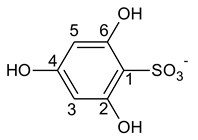
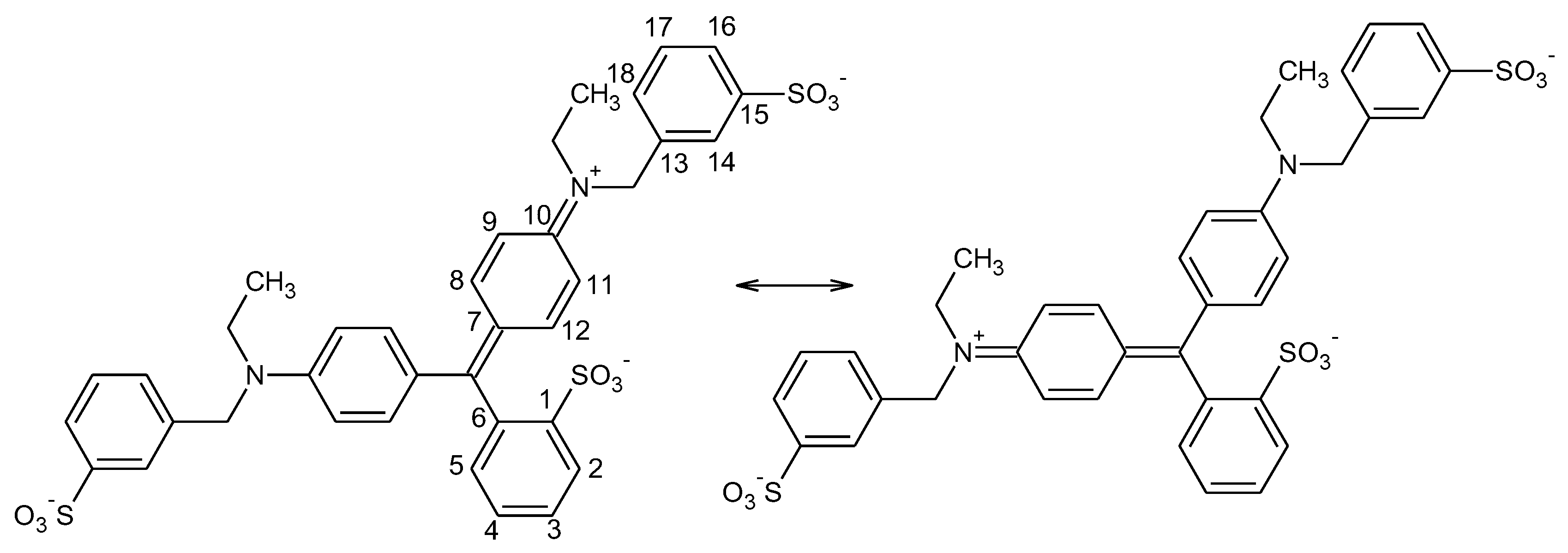
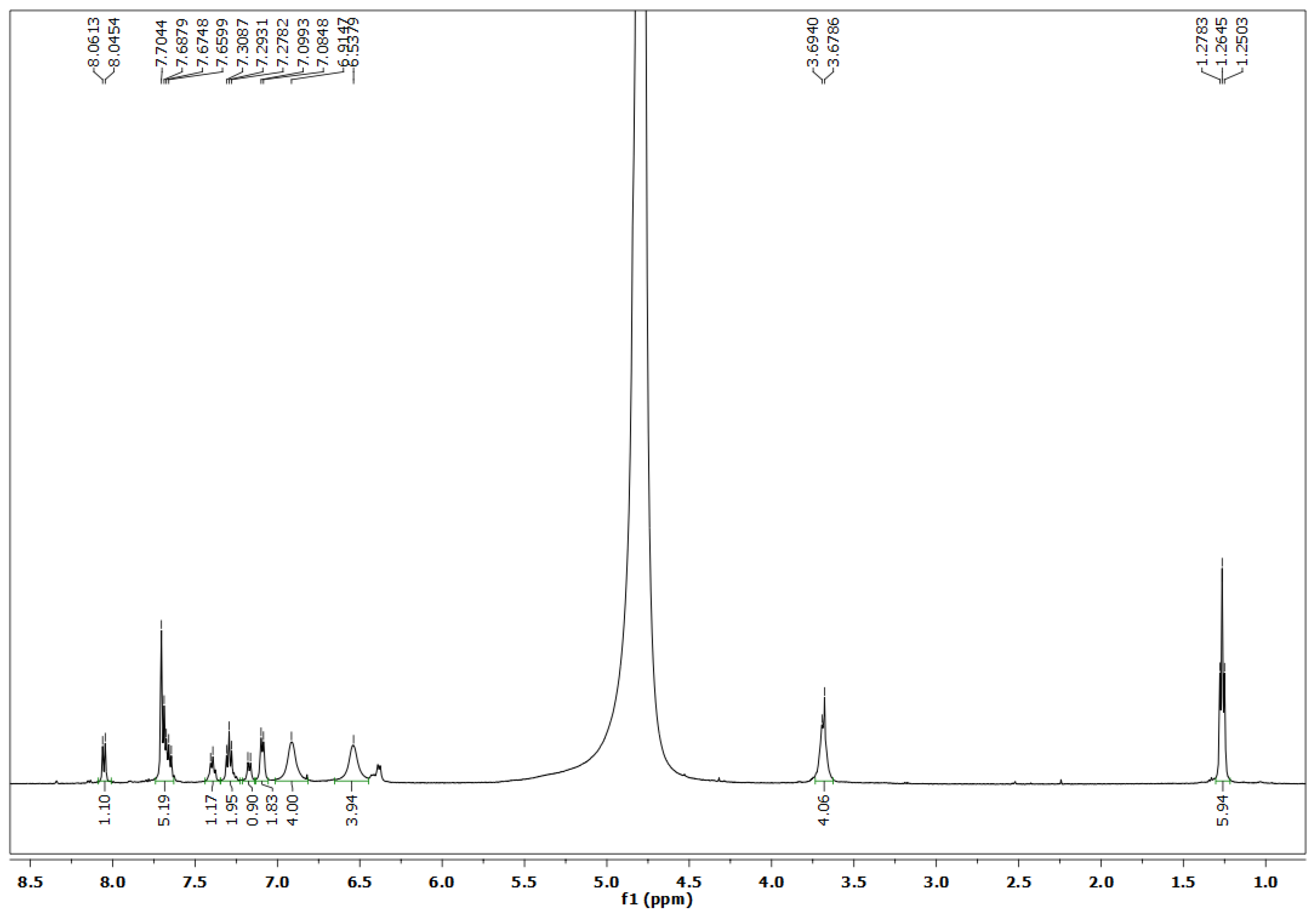
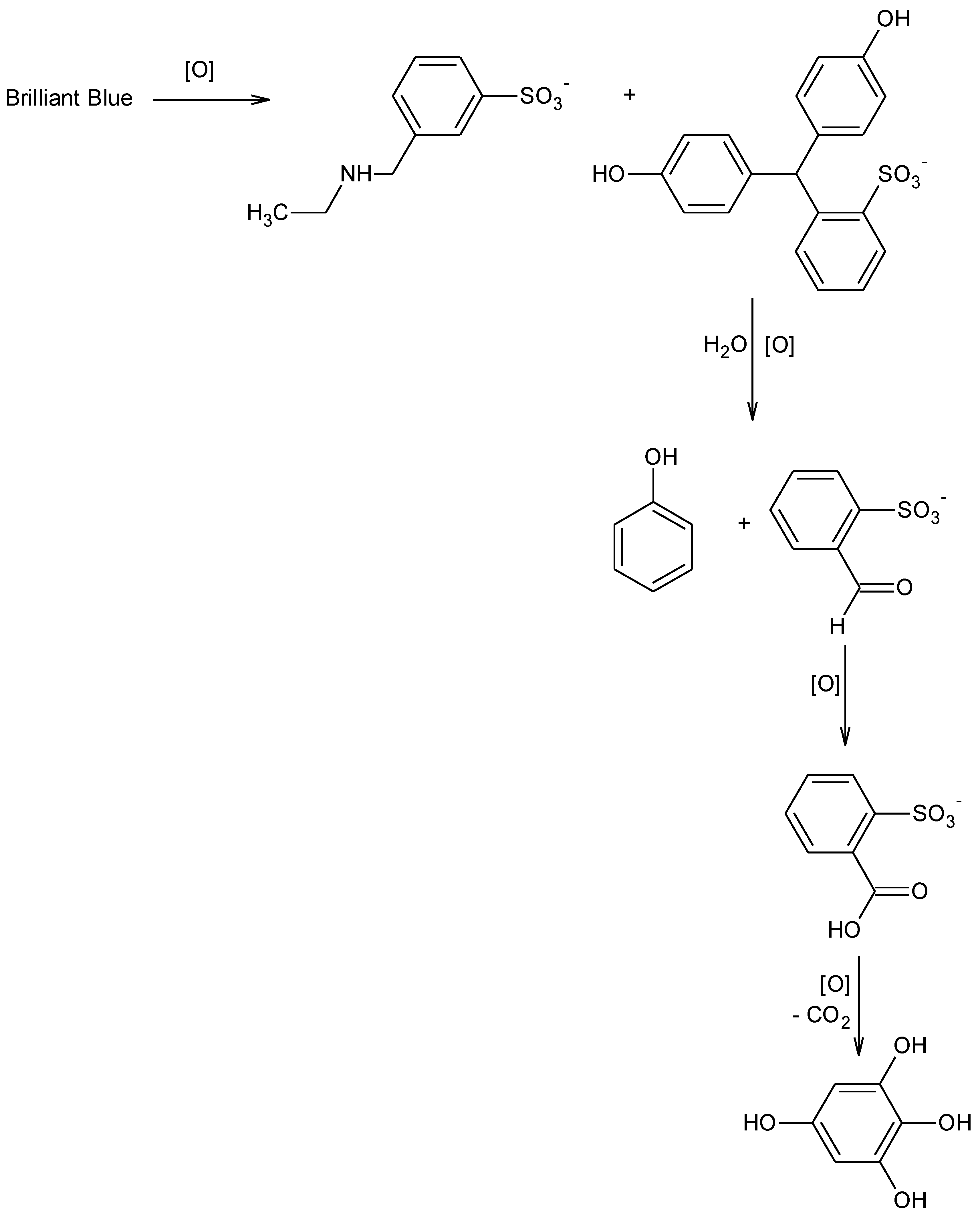
3.2. 1H-NMR Spectroscopy
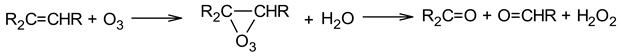
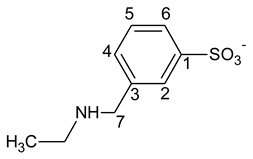
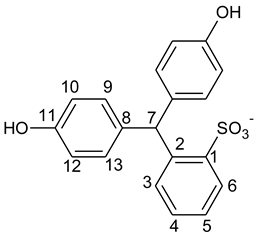
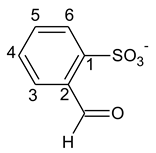
3.3. Kinetic Modelling
3.3.1. Specifying the Kinetic Model
- There are a finite number of reaction sites on the BB molecule;
- The reactivities of these sites are sufficiently different sot that they are attacked in succession (that is, no two sites can be attacked at the same time). This means that the ozone molecule reacts with the initial chromophore at its most reactive site, then with the first ozonation by-product at its most reactive site, etc.;
- The first attack destroys the chromophore, so the rate of discoloration is proportional to the rate of BB decay;
- Each ozonation step follows an apparent second-order kinetics (first order with respect to both ozone and substrate).
3.3.2. Mass Transfer Modelling
- Bulk gas is considered to be homogeneous, so that the partial pressure of the gaseous reagent is a function on time alone. As notated air is continuously fed in the reactor, is taken to be constant;
- In the gas diffusion layer mass transfer is considered to be stationary. Consequently, the total flow of ozone across this region is given by the integrated form of Fick’s equation:where is the mass transfer coefficient of ozone in the gas phase, and and are the partial pressures of ozone in the bulk gas and at gas - liquid interface, respectively.
- The liquid diffusion layer is treated analogously, so the ozone flow across this layer is given by:where is the mass transfer coefficient of ozone in the liquid phase, and and are the partial pressures of ozone in the bulk liquid and at gas - liquid interface, respectively.
- Finally, bulk liquid is considered to be homogeneous, so , the bulk liquid ozone concentration is taken to be a function of time alone. Its value is controlled by both reaction rate an ozone intake from the gas phase, through the stationary condition.
4. Conclusions
References
- National Center for Biotechnology Information, “www.pubchem.ncbi.nlm.nih.gov,” National Center for Biotechnology Information. PubChem Database. Brilliant Blue FCF, CID=19700, [Online]. Available: https://pubchem.ncbi.nlm.nih.gov/compound/BrilliantBlue-FCF. [Accessed 19 10 2019].
- Cardoso da Silva, J. C., Bispo G. L., Pavanelli S. P., de Casia Franco Afonso R. J., and Augusti R., Ozonation of the food dye Brilliant Blue in aqueous medium: monitoring and characterization of products by direct infusion electrospray ionization coupled to high - resolution mass spectrometry, Rapid Commun. Mass Spectrom 2012; 26, pp 1305 - 1310. [CrossRef]
- Lewis R. J., Sr., and I. N. Sax, Sax’s dangerous properties of industrial materials, Wiley & Sons, Inc., Hoboken, N. J., 2004, pp. 1749.
- Chu W., and Ma, C. W., Quantitative Prediction of Direct and Indirect Dye Ozonation Kinetics, Water Research, 2000; 34(12), pp 3153 - 3160. [CrossRef]
- Sabnis R. W., Handbook of biological dyes and stains. Synthesis and industrial applications, New Jersey: John Wiley & Sons, Inc., Hoboken; 2010.
- Zeinab A. A., Ogarite A. Y., and Mouhiaddine M. E. J., Kinetic study of acid blue 1 discoloration with persulfate, Journal of Chemical Technology and Metallurgy, 2017, 52(5), pp. 812 - 824.
- Forgacs E., Cserhati T., and Oros G., Removal of synthetic dyes from wastewaters: a review, Environment international 2004, 30(7), pp 953 - 971. [CrossRef]
- Gupta V. K., Khamparia S., Tyagi I., Jaspal D., and Malviya A., Decoloration of mixture of dyes: A critical review, Global J. Environ. Sci. Manage, 2015; 1(1), pp 71 - 91.
- Shindhal T.,. Rakholiya P,. Varjani S, Pandey A.,. Ngo H. H, Guo W.,. Ng H. Y, and Taherzadeh M. J., A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater, Bioengineering, 2021, 12(1), pp 70 - 87. [CrossRef]
- Ardila - Leal L. D.,. Potou - Pinales R. A, Pedroza Rodriquez A. M., and. Quevedo – Hidalgo B. E, A brief history of colour the environmental impact of synthetic dyes and removal by using Laccases, Molecules, 2021, 26, pp. 3813. [CrossRef]
- Al - Tohamy R., Ali S. S., Li F.,. Okasha K. M, Mahmoud Y. A. G.,. Elsamahy T,. Jiao H, Fu Y., and Sun J., A critical review on the treatment of dye - containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety, Ecotoxicology and Environmental Safety, 2022, 231, pp. 113160. [CrossRef]
- Michelsen D. L., Powell W. W., Woodby R. M., Fulk L. L., and Boardman G. D., Pretreatment of Textile Dye Concentrates using Fenton’s Reagent and Ozonation Prior to Biodegradation, Chemical Oxidation: Technology for the Nineti, AATCC; 1992, p. 135-150.
- 13Warnatz. J., Maas U., and Dibble R. W., Combustion. Physical and Chemical Fundamentals, Modeling and Simulation, Experiments, Pollutant Formation, 4th ed., Germany: SpringerVerlag Berlin Heidelberg; 1996, 1999, 2001, and 2006.
- 14Ikehata. K., . Naghashkarand N. J. J., and Gamal El-Din M., Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: A Review, Journal of the International Ozone Association, 2006; 28(6) pp 353 - 414. [CrossRef]
- Tiwari B. K., Muthukumarappan K., Donnel C. P. O., and Cullen P. J., Modelling colour degradation of orange juice by ozone treatment using response surface methodology, Journal of Food Engineering, 2008, 88(4), pp. 553 - 560. [CrossRef]
- Marcvart M., Constantin C., and Stoica L., Discoloration of food dyes from aqueous media by ozonization. Case study: Brilliant Blue, U.P.B. Sci. Bull., Series B, 2019; 81(3), pp 119-130.
- Hung - Yee S., and Ching-Rong H., Degradation of commercial azo dyes in water using ozonation and UV enhanced ozonation process, Chemosphere 1995, 31(8) pp 3813 - 3825. [CrossRef]
- Shu H. Y., and Chang M. C., Decolorization of six azo dyes by O3, UV/O3 and UV/H2O2 processes, Dyes Pigm. 2005; 65, pp 25 - 31. [CrossRef]
- Urs von Gunten, Ozonation of drinking water: Part I. Oxidation kinetics and product formation, Water Research, 2003; 37, pp. 1443 - 1467. [CrossRef]
- Marcvart Tiron M.,. Lucaciu I. E, Nita - Lazar M., and Gheorghe S., Considerations on the Toxicity of Brilliant Blue FCF aqueous solutions before and after ozonation, Revista de Chimie, 2020; 71(4), pp 356 - 365. [CrossRef]
- Sakamoto - Lopes M., Braga - Moruzzi R., Tomazini - Conceição F., Gabriel - Silva M. S., and Pereira - Antunes M. L., Factors and mass ratio analyses for Reactive Blue 19 dye decolorization using ozone: an experimental and analytical modelling approach. Engenharia Sanitaria e Ambiental, 2019; 24, pp 431 - 438.
- Missen R.W., Mims C.A., and Saville B.A.. Introduction to Chemical Reaction Engineering and Kinetics. John Wiley & Sons.; 1999. [CrossRef]
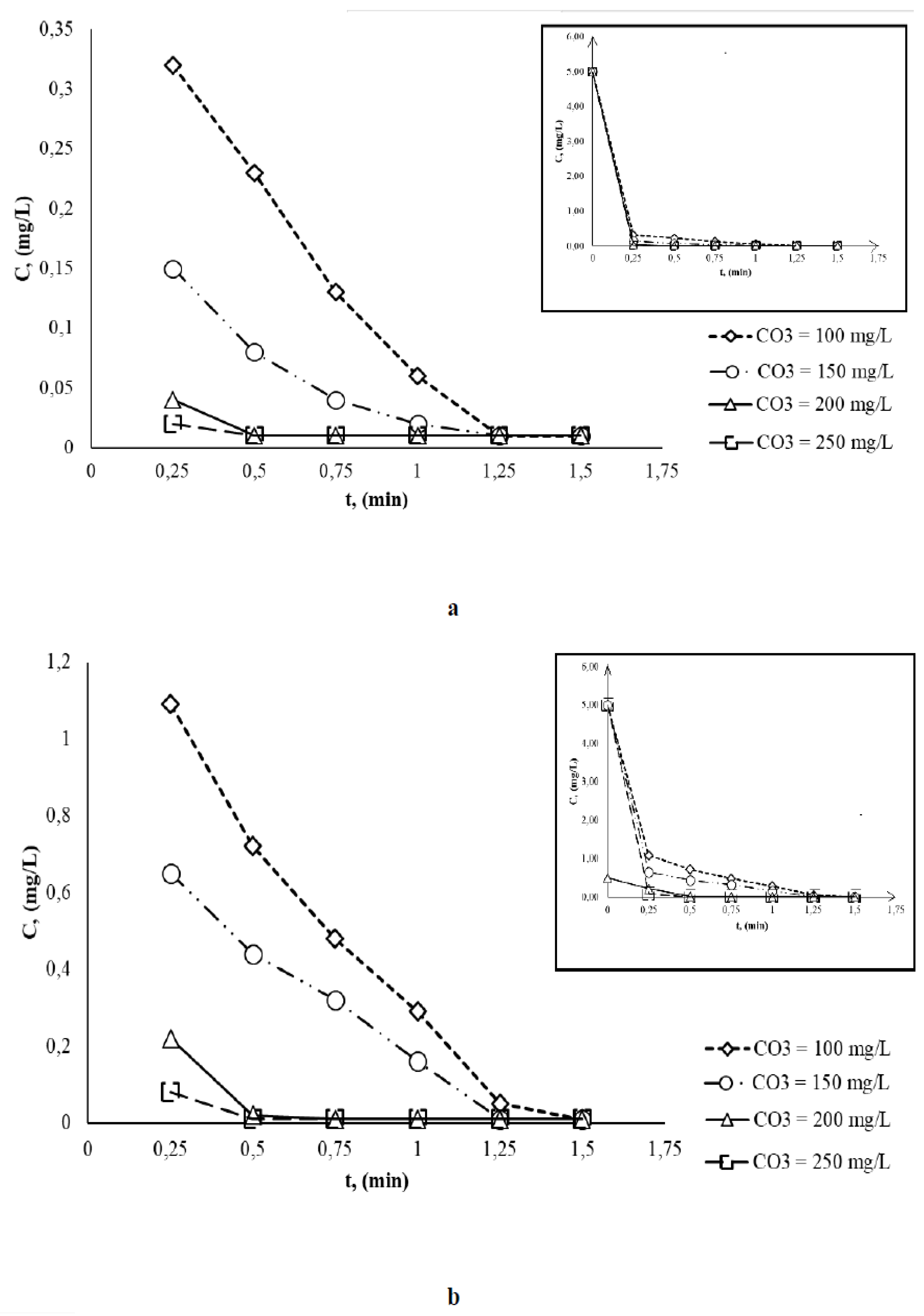
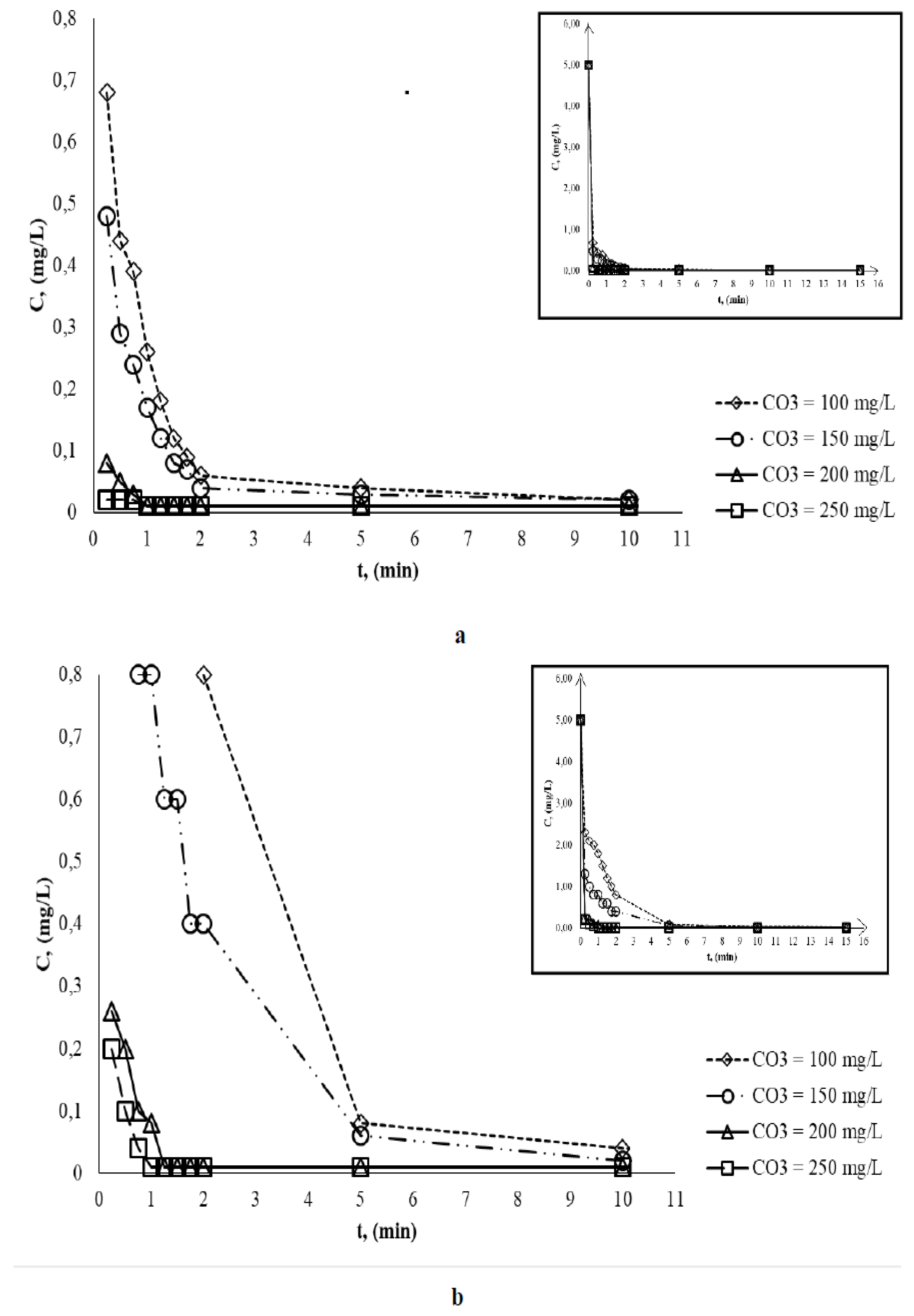

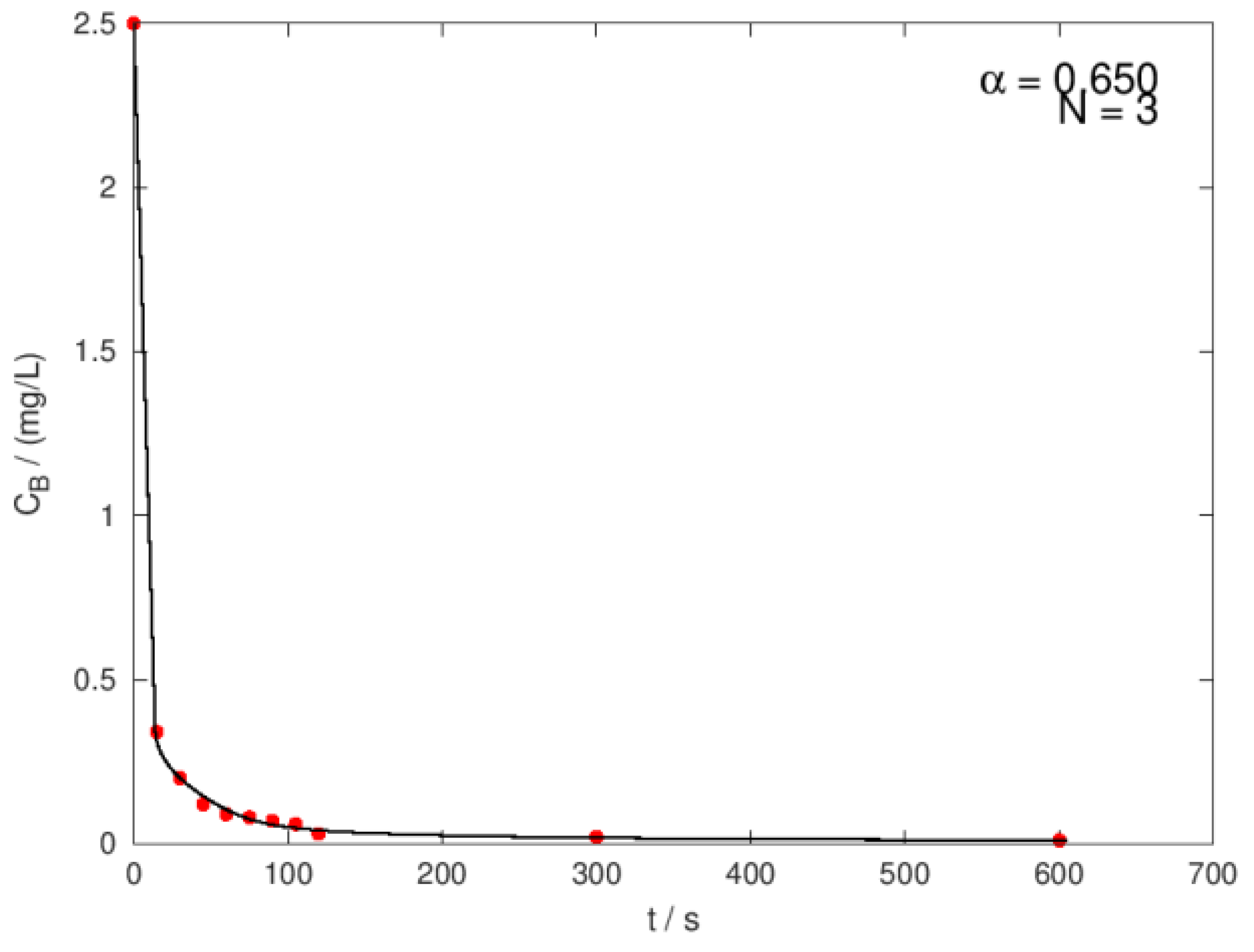
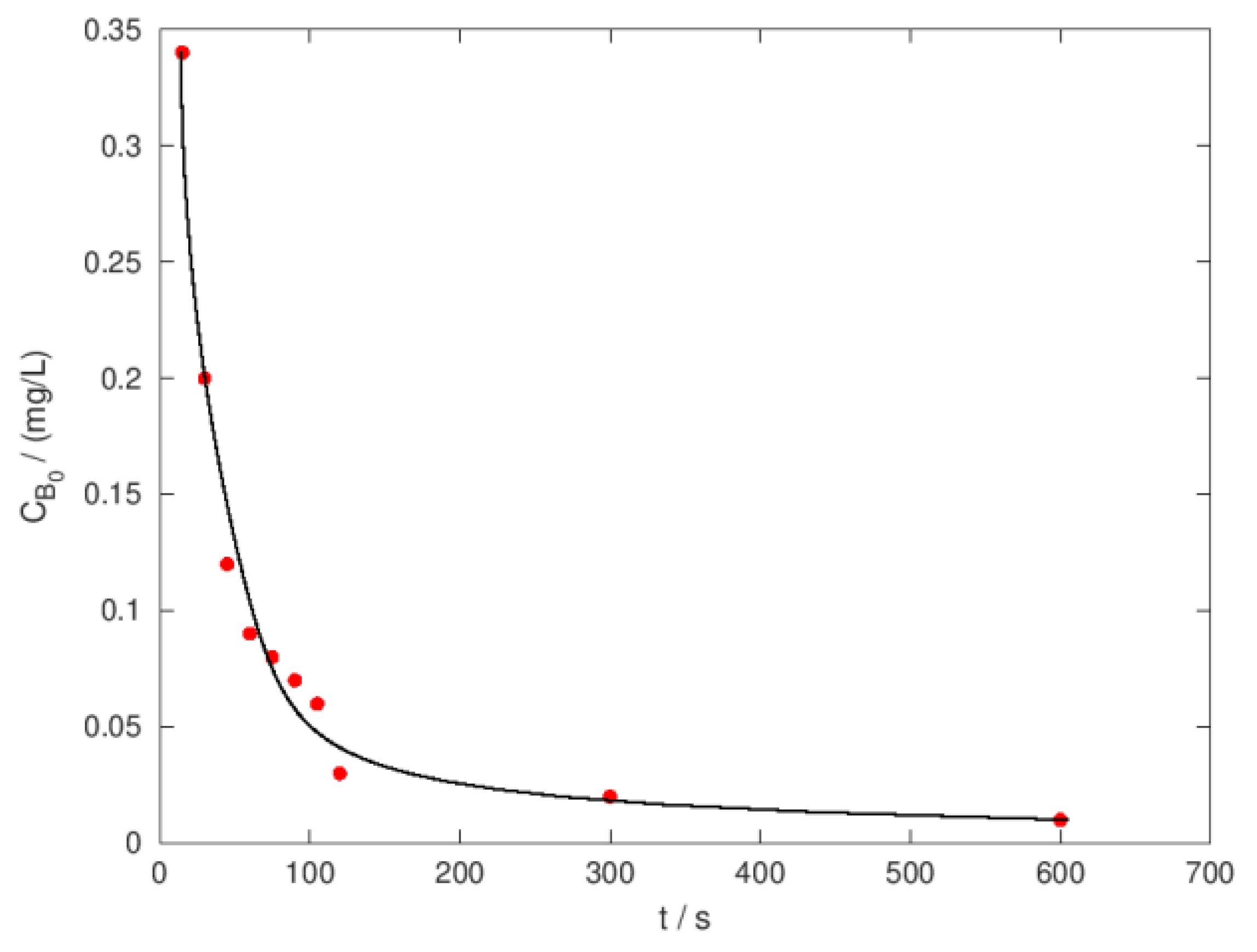
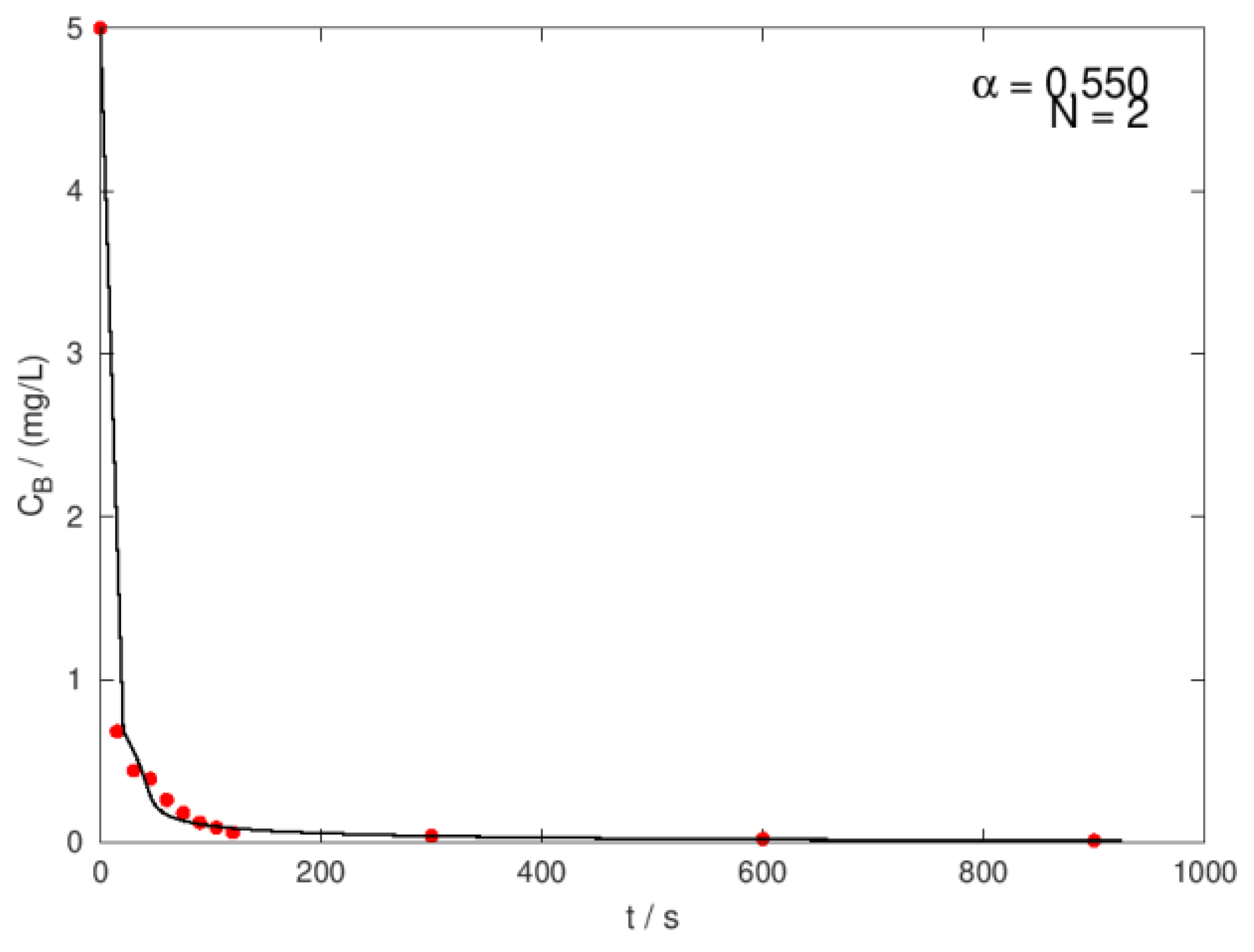
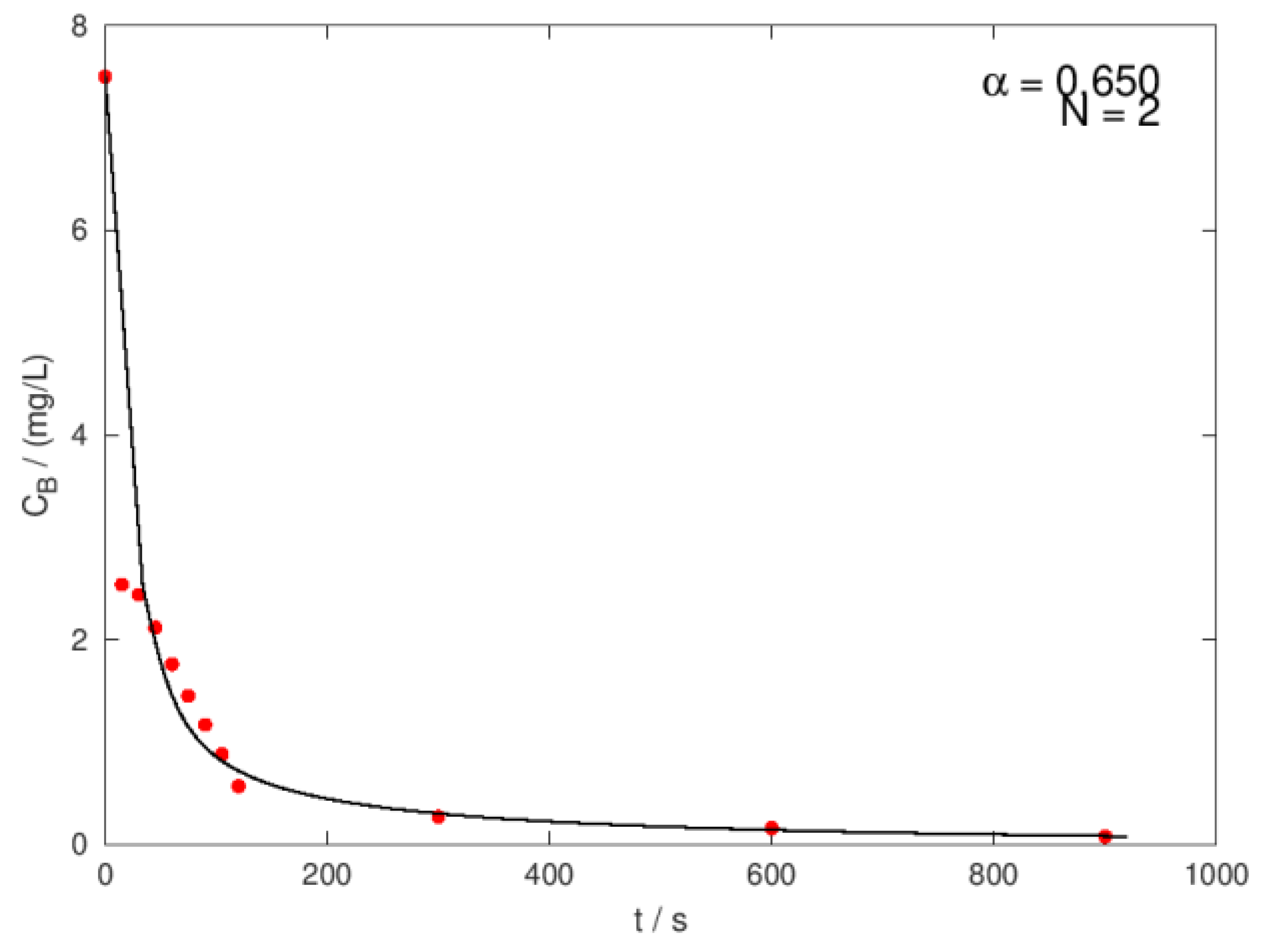
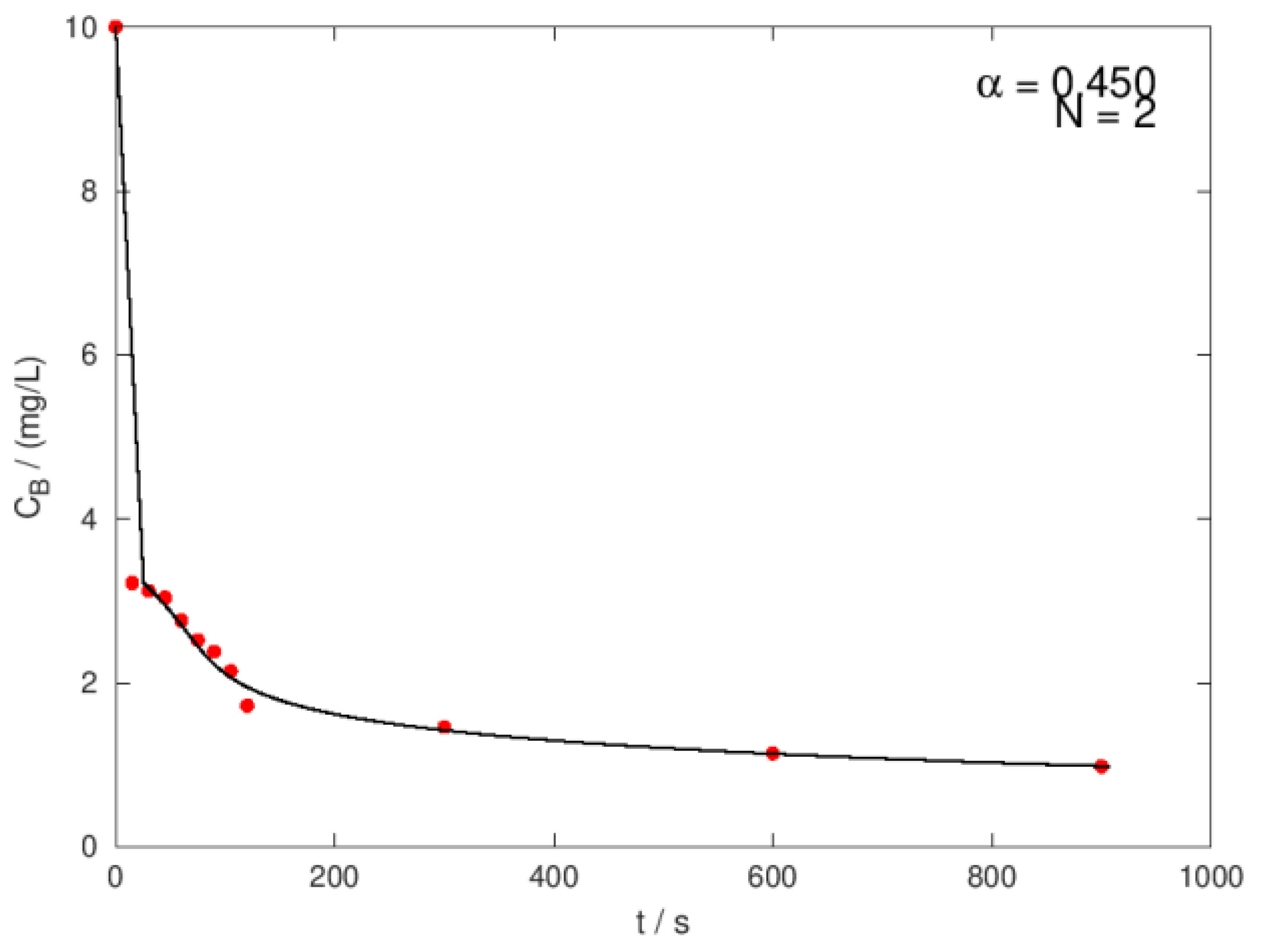
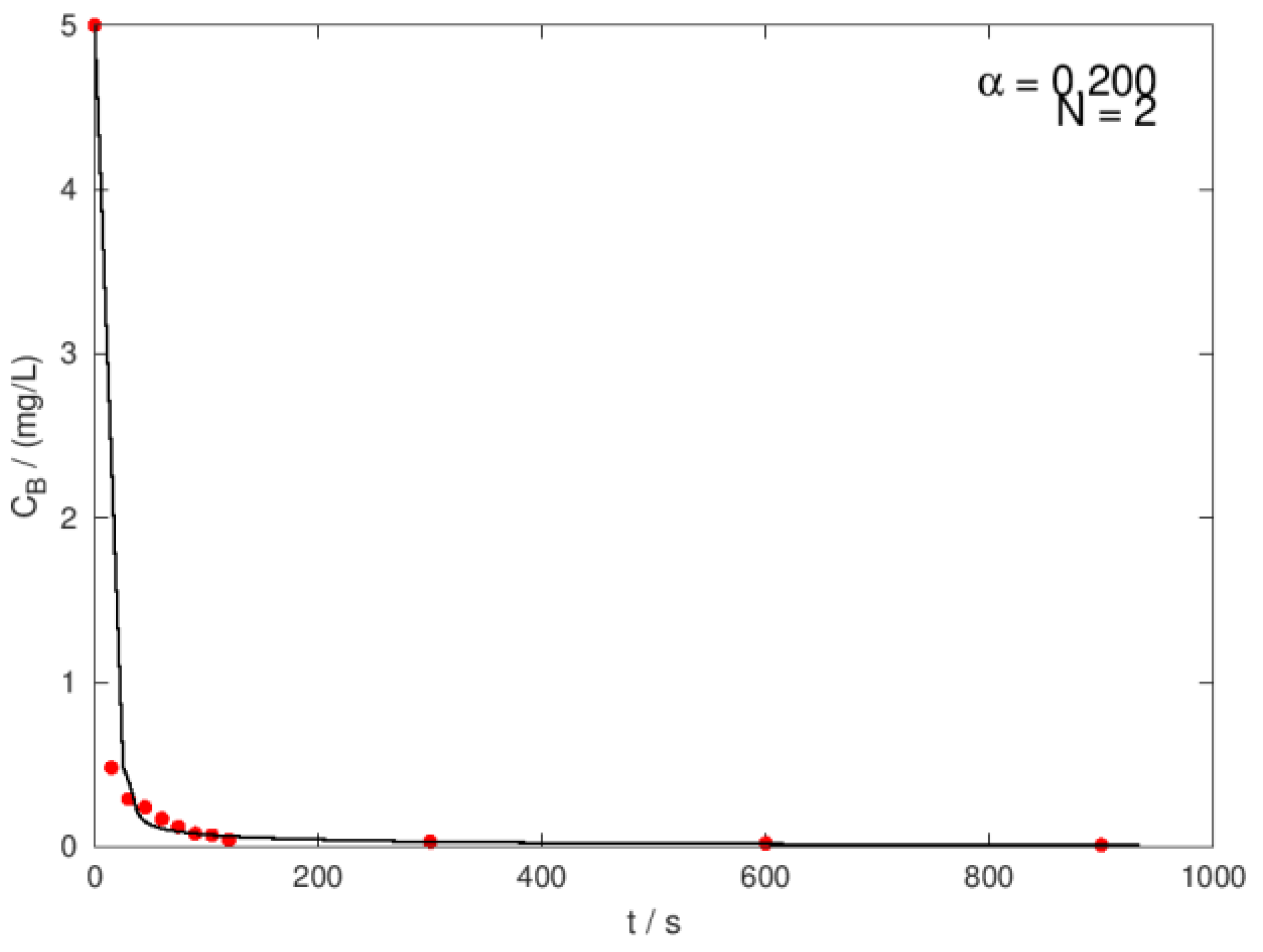
| Ci,O3 mg/L |
N | pH = 4 | pH = 7 | pH = 10 | |||||||||
| k, min-1 | R2 | k, min-1 | R2 | k, min-1 | R2 | ||||||||
| * | ** | * | ** | * | ** | * | ** | * | ** | * | ** | ||
| 100 | 1 | -3,14 | -3,65 | 0,93 | 0,89 | -0,25 | 0,35 | 0,78 | 0,90 | -2,77 | -5,70 | 1 | 0,86 |
| 2 | 89,18 | 63,16 | 0,76 | 0,57 | 6,24 | 3,29 | 0,97 | 0,98 | 115 | 119,98 | 0,92 | 0,64 | |
| 150 | 1 | -2,34 | -3,76 | 0,97 | 0,87 | -0,22 | 0,31 | 0,73 | 0,88 | -1,94 | -4,31 | 0,89 | 0,91 |
| 2 | 86,19 | 90,13 | 0,91 | 0,71 | 6,08 | 3,97 | 0,96 | 0,94 | 110 | 4,32 | 0,90 | 0,91 | |
| 200 | 1 | -0,79 | -2,00 | 0,43 | 0,57 | 0,07 | 0,14 | 0,16 | 0,24 | -6 x 10-15 | -6 x 10-15 | N/A | N/A |
| 2 | 0,79 | 2,00 | 0,43 | 0,57 | 3,20 | 8,21 | 0,17 | 0,27 | 0 | -6 x 10-15 | N/A | N/A | |
| 250 | 1 | -0,40 | -1,19 | 0,43 | 0,43 | -0,03 | 0,09 | 0,17 | 0,16 | -6 x 10-15 | -6 x 10-15 | N/A | N/A |
| 2 | 28,57 | 50,00 | 0,43 | 0,43 | 2,04 | 3,55 | 0,17 | 0,17 | 0 | -6 x 10-15 | N/A | N/A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





