Introduction
Primary Immune thrombocytopenia (ITP) is an acquired autoimmune disease characterized by low platelet count (<100 × 10
9/L) and increased bleeding risk [[
1,
2]] First line therapy for ITP includes steroids and intravenous immunoglobulin (IVIg). Some patients experience a single episode of severe thrombocytopenia and receive effective treatment that induces remission, while 40-70% of adult patients develop chronic ITP (cITP) and require long-term treatment. [
3]
For patients that do not respond to steroids or become corticosteroid-dependent, there are several options of therapy with strong supporting evidence, including thrombopoietin receptor agonists (TPO-RAs) and rituximab. [
1,
2,
4] Second-line treatments with strong supporting evidence include fostamatinib, a spleen tyrosine kinase (SYK) inhibitor. The effects of fostamatinib are mediated through inhibition of signal transduction through B cell receptors and Fc-activating receptors on macrophages. [
5] which reduces antibody-mediated platelet destruction. [
6]
Fostamatinib has been approved (by the US Food and Drug Administration in 2018 and the European Medicines Agency in 2020) for the treatment of cITP in adult patients who are refractory to other treatments. [
7,
8] The FIT1 and FIT2 trials and the open-label extension, FIT3, showed that fostamatinib may achieve a platelet response in around 40% of patients [
9,
10] Post hoc analysis of FIT program described a global response rate of 86-94% in case of early second line use of fostamatinib. [
11] Real world evidence confirms these data. [
12,
13,
14,
15,
16]
Sustained response off therapy (SROT) is defined as the possibility to discontinue cITP treatment and maintain a safe platelet count for a prolonged period of time. [
1,
17] Obtaining a SROT has become a key goal of ITP therapy. Previous studies have demonstrated that SROT may be achieved after splenectomy (60-70%), rituximab (20-30%), and TPO-RAs (10-30%). [
2,
4,
17,
18,
19]
TPO-RAs may produce SROT by modifying the immunologic responses in the bone marrow, making the immune system tolerant to platelets. [
20,
21] The induction of this immune tolerance may allow the safe discontinuation of TPO-RAs. There is little information on SROT with fostamatinib. At present, there is one published case report describing a SROT of 24 months with fostamatinib in a multi-agent refractory cITP patient [
15] and the description of five patients who got SROT in a real-world study of fostamatinib safety and efficacy, without any description of patients characteristics, tapering methodology, quality response or duration. [
12]
The Andalusian Group of Congenital Coagulopathies (GACC), recently collected and summarized our experience with fostamatinib in clinical practice in the Fostasur Study (NCT06071520). [
22] Now, reflecting that in our clinical practice, TPO-RA tapering is recommended in subjects with platelets counts 50-100x10
9/L stable during 4-6 months without rescue treatment. [
4] We have applied a similar strategy to fostamatinib. In this paper, we present an update of the Fostasur Study that includes data on tapering, discontinuation and SROT in our patients.
Methods
Study Design
In this retrospective, multicenter study adult patients diagnosed with primary ITP (newly diagnosed, persistent or chronic) who were on fostamatinib treatment were evaluated. Fostamatinib was prescribed according to the Summary of Product Characteristics [
7] or based on the healthcare provider’s clinical judgement in regard to dosage, monitoring, and avoiding contraindicated medications.
Given that SROT is a goal for ITP therapy and SROT has been reported with fostamatinib, a tapering/discontinuation protocol was developed for patients showing complete responses to fostamatinib. The criterion for selection of patients for tapering/discontinuation (T/D) was platelet count (PLT) greater than 100×109/L for at least 6 months. In patients with platelets counts higher than 250x109/, dose was reduce to despite of less than 6 months of CR, and this was considered the start of tapering. No other criteria were used for patient selection to enter the T/D protocol. Clinical parameters for patients eligible for T/D were compared with parameters of patients not eligible for T/D. The patient characteristics included, dosages, PLT responses, previous treatments and adverse events (AEs).
All patients treated with fostamatinib at the participating clinics and that provided informed consent were evaluated consecutively over the period October 2021 and May 2023. Patients that were eligible for T/D followed this dose reduction protocol. Scheme of tapering was from the dose of 150 mg every 12 hours, the dose was reduced to 150 mg alternating with 100 mg every 12 hours, then to 100 mg every 12 hours, then 100 mg every 24 hours, then 100 mg every 48 hours, then 100 mg twice a week and then discontinued. PLTs were monitored every two weeks and if the PLT remained above 50×109/L during four weeks after a dose reduction, an additional dose decrease was initiated.
The primary objective was to describe the efficacy and safe of fostamatinib in real world use and to describe the characteristics of the patients eligible for T/D, compare them with patients ineligible for T/D and to describe the rate of SROT and characteristics of this group of patients. These characteristics included type of ITP and response to fostamatinib.
Phases of ITP and responses to fostamatinib treatment were classified according to international guidelines. [
1,
17] ITP phases were: newly diagnosed ≤ three months from diagnosis; persistent ITP > three months < 12 months from diagnosis; chronic ITP > 12 months. Responses to fostamatinib were defined as: complete response (CR) PLT ≥100×10
9/L, response (R) PLT ≥30×10
9/L < 100×10
9/L and non-response (NR) PLT <30×10
9/L. CR and R were at least a doubling of PLT from baseline and the absence of bleeding events. CR and R were combined to give the overall response (OR).
Study Population
The study population were patients treated with fostamatinib for ITP between October 2021 (marketing date in Spain) and May 2023 at participating clinics in the Andalusia region of Spain. Inclusion criteria included patients that were 18 years or older, had a diagnosis of ITP according to clinical practice guidelines [
2,
17,
23] and were being treated with fostamatinib. Patients meeting the selection criterion while on fostamatinib treatement were initiated on the T/D protocol.
Patients were excluded from the study if they had any medical or psychological condition that prevented them from following normal clinical practice procedures as determined by the investigator. In addition, patients with concomitant defects in hemostasis , documented history of non-ITP medical conditions that were the underlying cause of their thrombocytopenia, secondary ITP, or a known allergy to fostamatinib or its formulation were excluded.
Variables
The following data were collected: demographics, medical history including the type of ITP, ITP treatment history and fostamatinib dosage, periodic PLT, and need for rescue medication.
AEs were also recorded for all patients. AEs included fostamatinib discontinuation due to lack of effectiveness (PLT level insufficient to prevent significant bleeding). Other AEs included high blood pressure, hepatotoxicity, diarrhea, neutropenia, infections, and any other AEs.
Sample Size
In this study, all patients who met the criteria in the defined study period and who signed the informed consent were included. All patients that met the criterion for T/D followed the T/D protocol. Patients that did not meet the T/D criterion were analyzed for comparison with the T/D group. Assessment of the treatment of ITP in Andalusia indicated that 50-70 patients could be enrolled in the study based on ITP prevalence and the Andalusian population (~8,600,224 persons). [
24] .
Analysis Plan
Study data was recorded and stored in a specifically designed database. Discrete variables were summarized as numbers and percentages. Continuous variables were described by median (interquartile range [IQR]). The Mann-Whitney U-test was used to compare non-parametrically distributed continuous variables. Spearman’s Rho coefficient was calculated to analyze the correlation between non-parametrically distributed variables. The Fisher exact test was used to compare qualitative variables.
Ethical Considerations
Patients, or their legal representative provided written informed consent to participate in this study. Any product-related AE was reported to the Spanish Agency of Medicines and Medical Devices (AEMPS). The study protocol was approved by the institutional ethics committee (Comité Ético de Investigación Provincial de Sevilla) and was conducted in accordance with the 2013 revision of the Declaration of Helsinki. Ethics committee code 0169-N-23, approved 22nd may 2023. The trial was registered at clinicaltrials.gov (NCT06071520).
Results
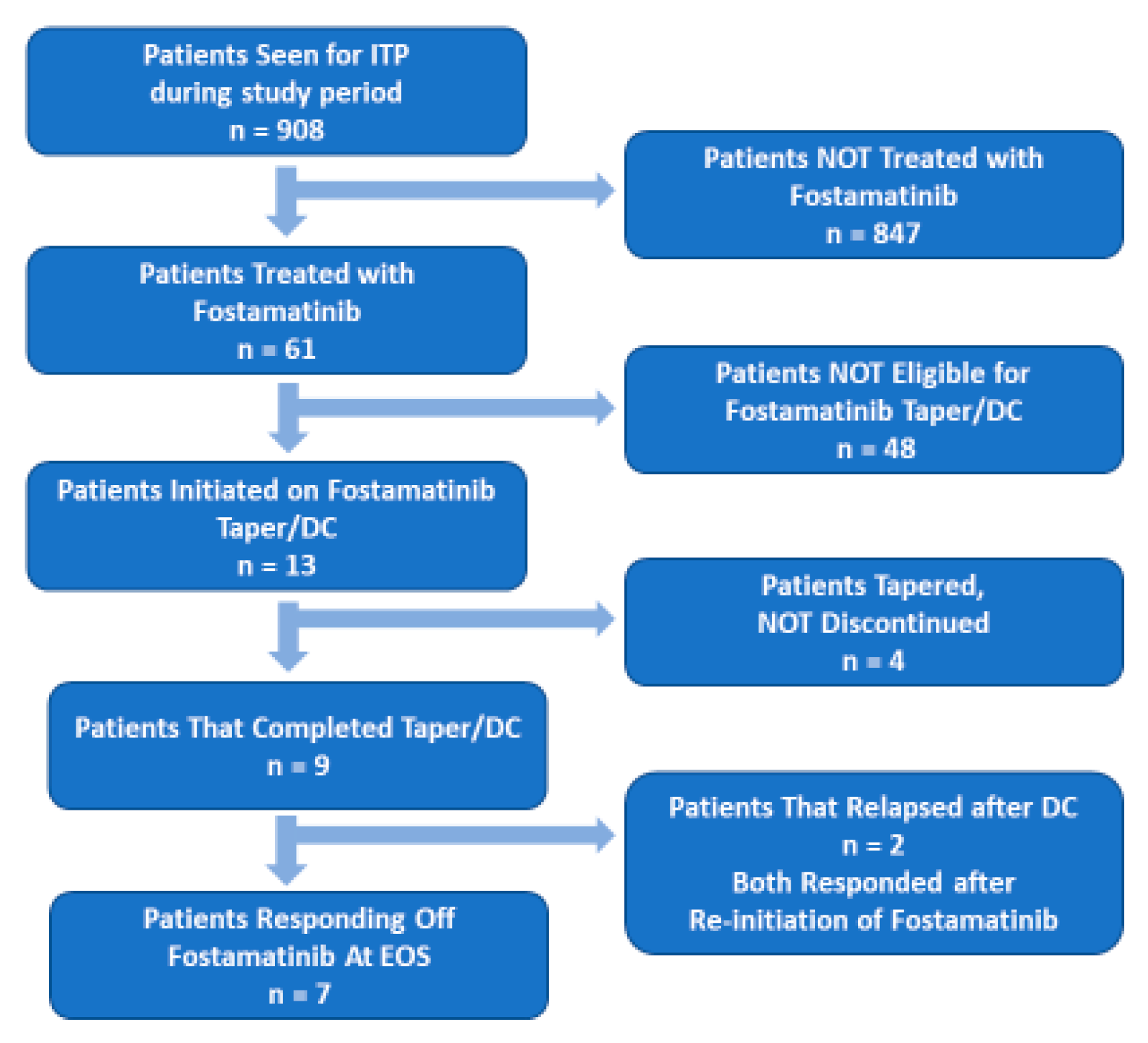
The overall population of fostamatinib-treated ITP patients this study was 61 patients. The population had a slight preponderance of male patients (54.1%) over female patients (45.9%) and a median age of 59 years (
Table 1). The most common co-morbidities in this cohort were hypertension, dyslipidemia, heart disease, obesity, diabetes and arterial thrombosis (
Table 2). The WHO hemorrhagic classification was 0-1 for most of the patients (85.2%) and most had cITP (75.4%). Most of the patient population was heavily pre-treated for ITP with a median of four prior treatments before they were treated with fostamatinib (range 1-9). The most common treatments prior to fostamatinib in these patients were corticosteroids, intravenous immunoglobulins and TPO-RAs.
The OR rate to fostamatinib was 72.1% (n = 44;
Table 1). Of the responders, 15 (24.6% of fostamatinib-treated patients) had a CR (PLT ≥100×10
9/L). Based on the selection criterion described above (PLTs > 100×10
9/L (CR) for at least six months), 13 patients were entered into the T/D protocol. Median PLT in the selected patients at the initiation of the T/D protocol were 232×10
9/L (IQR 152-345×10
9/L). For the nine patients that completed the protocol (fostamatinib was discontinued), median PLT at the end of T/D was 190×10
9/L (IQR 142.5-316.5×10
9/L). As shown in
Figure 1, two of the patients that completed the T/D protocol relapsed. PLT count at relapse were 9.5 and 11 ×10
9/L. Both patients had complete responses with reinstatement of fostamatinib at 100 mg every 12 hours. No rescue treatment was used between relapse and the second complete response.
The time from the onset of fostamatinib therapy to the initiation of the T/D protocol was a median of 116 days (IQR 38.5-157). Tapering took a median of 112.5 days (IQR 94.5-191) indicating that most patients completed the T/D protocol without delays due to PLT below the threshold set in the protocol (≤ 50x109/L). For the two patients that relapsed after the T/D protocol, time from discontinuation to relapse 63-73 days. The time between the end of the T/D protocol and the last reported follow-up was over six months (median 263 days (IQR: 247-313 days). Median platelet counts at last visit was 156x109/L (IQR 135-312x109/L) in the T/D and SROT group (7/61 patients) and 112x109/L (IQR 47-143.5x109/L) in the non-T/D group (31/61 patients).
Comparison between Eligible and Ineligible Patients for the T/D Protocol
When the patients eligible for the T/D protocol were compared to ineligible patients, the T/D group had a greater proportion of female patients and were slightly younger, but these differences were not statistically significant. Both groups had similar WHO Hemorrhagic Classifications (
Table 1). More patients in the non-T/D group required a dose increase (62.5%) than in the T/D group (30.8%). The T/D eligible group had a higher percentage of patients previously treated with rituximab (T/D 38.5% versus non-T/D 18.8%) and splenectomy (T/D 30.8% versus non-T/D 6.3%) than the non-eligible group. A higher proportion of the non-T/D group was refractory or had a suboptimal response to TPO-RAs (60.4% non-T/D versus 38.5% T/D) while the T/D group was more likely to be intolerant to TPO-RAs (T/D 38.5% versus 16.7% non-T/D). These differences between the T/D and the non-T/D groups were not statistically significant.
Comparisons between the T/D and non-T/D groups regarding previous treatments, time since diagnosis, baseline PLT, starting, responsive and final doses, associated therapies and need for rescue therapy showed no significant differences between the groups.
Some statistically significant differences were observed between the two groups when temporal PLT were examined.
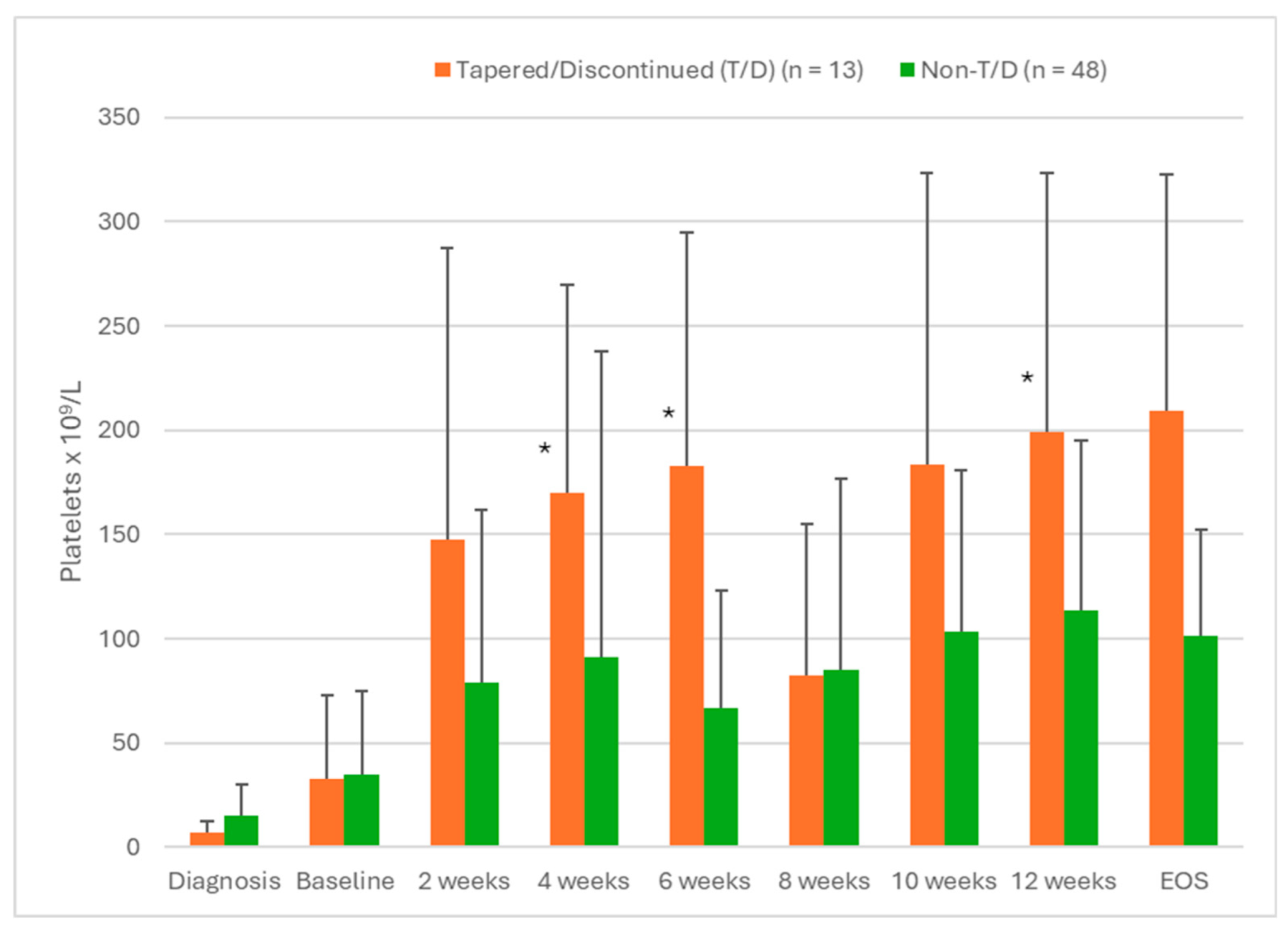
Figure 2A shows the PLT for both groups (tapered versus non-tapered) over the first 12 weeks of fostamatinib therapy. These data showed that patients eligible for T/D had significantly higher PLT at weeks 4 (p = 0.003), 6 (p = 0.012) and 12 (p = 0.007) than non-eligible patients. In addition, patients that were eligible for T/D were more likely to have PLT > 100 × 10
9/L at 12 weeks than patients that were not eligible for T/D (p = 0.011).
Comparison between Patients That Achieved SROT and the Rest of Patients
Comparisons were also made between the group that completed the T/D protocol and remained responsive after fostamatinib therapy was discontinued (sustained response off therapy (SROT)) and the other patients treated with fostamatinib in this study.
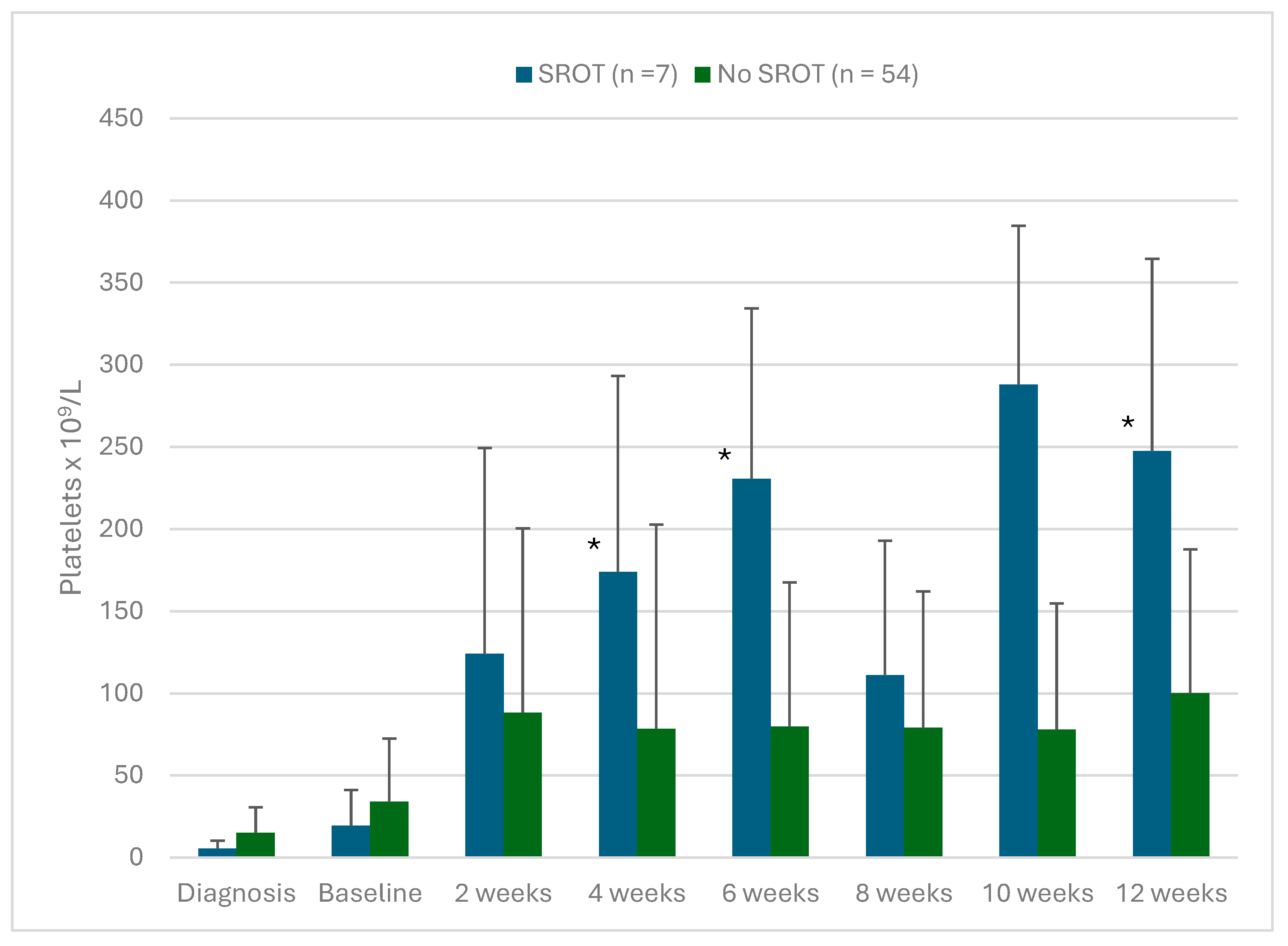
Figure 2B shows that platelet counts were higher during the first 12 weeks of therapy in patients that developed SROT. These differences were statistically significant at 4, 6 and 12 weeks of fostamatinib treatment. As noted in
Figure 1, of the 13 patients that entered into the T/D protocol, four patients had their doses tapered but not discontinued, nine completed the T/D protocol and two of those nine relapsed. The two patients that relapsed were responsive to re-introduction of fostamatinib therapy.
Figure 2A.
Platelet levels in patients with ITP treated with fostamatinib comparing patients eligible for the tapering/discontinuation (T/D) protocol with those not eligible for T/D. The selection criterion was PLT > 100 × 109/L for at least six months. Values are mean ± SD. * Significant differences between T/D eligible patients and non-eligible patients (p < 0.05).
Figure 2A.
Platelet levels in patients with ITP treated with fostamatinib comparing patients eligible for the tapering/discontinuation (T/D) protocol with those not eligible for T/D. The selection criterion was PLT > 100 × 109/L for at least six months. Values are mean ± SD. * Significant differences between T/D eligible patients and non-eligible patients (p < 0.05).
Figure 2B.
Platelet levels in patients with ITP treated with fostamatinib comparing patients that achieved a sustained response off treatment (SROT) after the tapering/discontinuation protocol (T/D) with those patients that did not. Values are mean ± SD. * Significant differences between SROT patients all other patients (p < 0.05).
Figure 2B.
Platelet levels in patients with ITP treated with fostamatinib comparing patients that achieved a sustained response off treatment (SROT) after the tapering/discontinuation protocol (T/D) with those patients that did not. Values are mean ± SD. * Significant differences between SROT patients all other patients (p < 0.05).
Discussion
In ITP patients, SROT has been documented with treatments including corticosteroids [
25], corticosteroids in combination with IVIG [
26], eltrombopag [
27,
28,
29,
30], and romiplostim. [
27,
31,
32]. For SROT with fostamatinib, there is little information. One case report 24-month SROT in a multirefractary chronic ITP patient who achieved a complete response with fostamatinib. [
15] González-López et al. in their series of 138 patients with ITP treated with fostamatinib had five patients with apparent SROT. However, no additional information was provided regarding patient characteristcs and follow up. [
12] Finally, the phase 3 randomized, double blind placebo-controlled study of another SYK inhibitor, sovleplenib, in ITP patients did not describe discontinuation or SROT. [
33]
Fostasur study was designed to retrospectively to describe real world practice management, efficacy and safety of fostamatinib in ITP patients in Andalusia. Since a common goal of ITP therapy is to minimize or discontinue therapy while maintaining a clinical response, i.e. SROT, [
1,
2,
4,
23], a secondary objective of the study was to identify clinical characteristics of patients that achieved SROT after fostamatinib treatment. This could allow better prediction of which fostamatinib-treated patients would be the best candidates for T/D in routine clinical practice. In the current study, we determined that early responses to fostamatinib may help to predict which patients may amenable to T/D while maintaining a therapeutic response.
When patients eligible for the T/D protocol were compared with those not eligible, most of the demographic, clinical and treatment parameters analyzed in this study did not show significant differences between these groups. The only clinical factors that showed a significant difference between the T/D eligible patients versus non-eligible and patients with SROT versus all other patients were platelet counts at 4, 6 and 12 weeks of fostamatinib treatment (
Figure 2A,B). Patients eligible for T/D and with SROT also had more frequently PLT > 100 × 10
9/L at 12 weeks of fostamatinib treatment (p = 0.007 and p = 0.011, respectively). These data suggest that early efficacy of fostamatinib may be a prognostic factor indicating which patients will eventually be able to discontinue therapy but continue to have PLT in the therapeutic range.
It is also interesting to note that the two patients that relapsed after completing the T/D protocol were fully responsive to fostamatinib when therapy was reinstated. This suggests that efficacy of fostamatinib is maintained or reinstated after discontinuation and subsequent loss of response.
SROT, since it occurs while patients are off therapy it may not be able to be completely separated from spontaneous remission. The rate of spontaneous remissions in subjects with ITP is estimated at 25-74% in the pediatric population decreasing with age. [
34,
35] The reported spontaneous remission rates are lower in adults. [
36,
37,
38,
39] For some ITP treatments, the rates of SROT were higher than the rates of spontaneous remissions.
Additionally, the prognostic factors for SROT and the mechanisms by which it occurs are not clear. In subjects with ITP receiving steroids as first-line treatment, SROT rates after 6 months were close to 40%. [
40] For the second-line treatment, rituximab, 20% SROT has been reported. [
41] For TPO-RAs, SROT rates at six months were between 3-48%. [
42] To date, no clear predictive factors for SROT have been found. In the case of TPO-RAs, achieving a response before 14 days of treatment [
3] and starting treatment with romiplostim without conversion to another TPO-RA [
43] are good prognostic factors for achieving SROT. Other factors such as the type of ITP, chronicity or previous lines of treatment have not been validated as prognostic factors for SROT.
There are several limitations and possible sources of bias in this study. The Fostasur study was a retrospective real-world evidence study, so there was a lack of control of treatment and outcome assessments, dependence on accurate recordkeeping by others, and the potential for selection bias.
Although the number of patients with SROT was relatively small, these findings suggest that early responses to fostamatinib may help predict which patients may be amenable to T/D while maintaining a therapeutic response. Additional studies will be required to confirm the advisability of T/D after fostamatinib treatment and determine if predictive factors exist and possible mechanisms for SROT.
Data Availability Statement
Data supporting this article are available from the corresponding author on request due to privacy/ethical restrictions.
Funding Statement
The writing of this study was supported by Grifols. Grifols had no role in the study design, data collection, data interpretation or the decision to submit this study for publication.
Conflicts of Interest disclosure
MEMC received grants from Amgen and Novartis and served as An advisor or speaker for Amgen, Sanofi, Grifols, Novartis, Novo Nordisk, and Takeda. RJB received speaker honoraria from Amgen, CSL Behring, Grifols, Novo Nordisk, Pfyzer, Roche, Sobi and participated in advisory boards with Sobi. LEU served as an advisor or speaker for Amgen, Novartis, Novo Nordisk, Roche, CSL Behring and Sobi. RJNV received speaker honoraria from Novo Nordisk, Takeda, Grifols, Roche, Pfizer, Octapharma, CSL-Behring, Sobi, Alexion. and served as an advisor for Novo Nordisk, Takeda, Roche, Grifols, Pfizer, Octapharma, CSL-Behring, Sobi. Rest of the authors Declare no competing financial interests.
Ethics Approval statement
The appropriate ethics committee (Comité de Ético de la Investigación con medicamentos provincial de Sevilla) approved this study. It was conducted in accordance with the 2013 revision of the Declaration of Helsinki. Ethics committee code 0169-N-23, approved 22nd may 2023.
Patient Consent Statement
Patients, or their legal representatives, provided written informed consent form to participate in this study. Informed consent included permission for their anonymized data to appear in any publications resulting from the study.
Permission to Reproduce Material from Other Sources
No material from other sources is reproduced in this article.
Clinical Trial Registration
The trial was registered at clinicaltrials.gov (NCT06071520).
Acknowledgments
Michael K. James, PhD, CMPP (Grifols) and Eugenio Rosado, PhD, CMPP (Grifols) are acknowledged for medical writing and editorial support.
Author Contributions
MEMC and RJB contributed to the design of the study, data collection, statistical analysis, and writing of the final manuscript; RJB and MEMC coordinated the team; and all authors contributed to the data collection, participated in the clinical management of these patients, contributed to the literature review on the topic, and critically reviewed the manuscript.
References
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393. [CrossRef]
- Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780-3817. [CrossRef]
- Iino M, Sakamoto Y, Sato T. Treatment-free remission after thrombopoietin receptor agonist discontinuation in patients with newly diagnosed immune thrombocytopenia: an observational retrospective analysis in real-world clinical practice. Int J Hematol. 2020;112(2):159-168. [CrossRef]
- Mingot-Castellano ME, Canaro Hirnyk M, Sanchez-Gonzalez B, et al. Recommendations for the Clinical Approach to Immune Thrombocytopenia: Spanish ITP Working Group (GEPTI). J Clin Med. 2023;12(20). [CrossRef]
- Braselmann S, Taylor V, Zhao H, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319(3):998-1008. [CrossRef]
- Podolanczuk A, Lazarus AH, Crow AR, Grossbard E, Bussel JB. Of mice and men: an open-label pilot study for treatment of immune thrombocytopenic purpura by an inhibitor of Syk. Blood. 2009;113(14):3154-3160. [CrossRef]
- Tavlesse (fostamatinib) Summary of Product Characteristics. 2020; https://www.ema.europa.eu/en/documents/product-information/tavlesse-epar-product-information_en.pdf. Accessed 24 March 2023.
- Tavalisse (fostamatinib disodium hexahydrate) tablets - Package Insert. 2020; https://www.tavalissehcp.com/downloads/pdf/TAVALISSE-Full-Prescribing-Information.pdf. Accessed 19 May 2023.
- Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: Results of two phase 3, randomized, placebo-controlled trials. American journal of hematology. 2018;93(7):921-930. [CrossRef]
- Bussel JB, Arnold DM, Boxer MA, et al. Long-term fostamatinib treatment of adults with immune thrombocytopenia during the phase 3 clinical trial program. American journal of hematology. 2019;94(5):546-553. [CrossRef]
- Boccia R, Cooper N, Ghanima W, et al. Fostamatinib is an effective second-line therapy in patients with immune thrombocytopenia. British Journal of Haematology. 2020;190(6):933-938. [CrossRef]
- Gonzalez-Lopez TJ, Bermejo N, Cardesa-Cabrera R, et al. Fostamatinib effectiveness and safety for immune thrombocytopenia in clinical practice. Blood. 2024. [CrossRef]
- Hughes DM, Toste C, Nelson C, Escalon J, Blevins F, Shah B. Transitioning From Thrombopoietin Agonists to the Novel SYK Inhibitor Fostamatinib: A Multicenter, Real-World Case Series. J Adv Pract Oncol. 2021;12(5):508-517.
- Liu J, Hsia CC. The Efficacy and Safety of Fostamatinib in Elderly Patients with Immune Thrombocytopenia: A Single-Center, Real-World Case Series. Adv Hematol. 2022;2022:8119270. [CrossRef]
- Auteri G, Biondo M, Mazzoni C, et al. Sustained response off therapy after fostamatinib: A chronic refractory ITP case report. Heliyon. 2023;9(2):e13462. [CrossRef]
- Dranitsaris G, Peevyhouse A, Wood T, Kreychman Y, Neuhalfen H, Moezi M. Fostamatinib or Thrombopoietic Receptor Agonists for the Treatment of Chronic Immune Thrombocytopenia in Adult Patients: A Real-World Assessment of Safety, Effectiveness and Cost. Acta Haematol. 2023.
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829-3866. [CrossRef]
- Kashiwagi H, Kuwana M, Hato T, et al. Reference guide for management of adult immune thrombocytopenia in Japan: 2019 Revision. Int J Hematol. 2020;111(3):329-351. [CrossRef]
- Choi PY, Merriman E, Bennett A, et al. Consensus guidelines for the management of adult immune thrombocytopenia in Australia and New Zealand. Med J Aust. 2022;216(1):43-52. [CrossRef]
- Bao W, Bussel JB, Heck S, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116(22):4639-4645. [CrossRef]
- Schifferli A, Kuhne T. Thrombopoietin receptor agonists: a new immune modulatory strategy in immune thrombocytopenia? Semin Hematol. 2016;53 Suppl 1:S31-34.
- Jimenez-Barcenas R, Garcia-Donas-Gabaldon G, Campos-Alvarez RM, et al. Treatment with fostamatinib in patients with immune thrombocytopenia: Experience from the Andalusian region in Spain-The Fostasur Study. Br J Haematol. 2024. [CrossRef]
- Lozano ML, Sanz MA, Vicente V. Guidelines of the Spanish ITP Group for the diagnosis, treatment and follow-up of patients with immune thrombocytopenia. Medicina Clínica (English Edition). 2021;157(4):191-198. [CrossRef]
- Andalusia - Population: The population of Andalusia grows by 25,533 people. https://datosmacro.expansion.com/demografia/poblacion/espana-comunidades-autonomas/andalucia. Accessed 8 January 2025.
- Pizzuto J, Ambriz R. Therapeutic experience on 934 adults with idiopathic thrombocytopenic purpura: Multicentric Trial of the Cooperative Latin American group on Hemostasis and Thrombosis. Blood. 1984;64(6):1179-1183.
- Godeau B, Chevret S, Varet B, et al. Intravenous immunoglobulin or high-dose methylprednisolone, with or without oral prednisone, for adults with untreated severe autoimmune thrombocytopenic purpura: a randomised, multicentre trial. Lancet. 2002;359(9300):23-29. [CrossRef]
- Mahevas M, Fain O, Ebbo M, et al. The temporary use of thrombopoietin-receptor agonists may induce a prolonged remission in adult chronic immune thrombocytopenia. Results of a French observational study. Br J Haematol. 2014;165(6):865-869.
- Gonzalez-Lopez TJ, Pascual C, Alvarez-Roman MT, et al. Successful discontinuation of eltrombopag after complete remission in patients with primary immune thrombocytopenia. Am J Hematol. 2015;90(3):E40-43. [CrossRef]
- Lucchini E, Palandri F, Volpetti S, et al. Eltrombopag second-line therapy in adult patients with primary immune thrombocytopenia in an attempt to achieve sustained remission off-treatment: results of a phase II, multicentre, prospective study. Br J Haematol. 2021;193(2):386-396.
- Cooper N, Ghanima W, Vianelli N, et al. Sustained response off-treatment in eltrombopag-treated adult patients with ITP who are refractory or relapsed after first-line steroids: Primary, final, and ad-hoc analyses of the Phase II TAPER trial. Am J Hematol. 2024;99(1):57-67.
- Newland A, Godeau B, Priego V, et al. Remission and platelet responses with romiplostim in primary immune thrombocytopenia: final results from a phase 2 study. Br J Haematol. 2016;172(2):262-273. [CrossRef]
- Mingot-Castellano ME, Grande-Garcia C, Valcarcel-Ferreiras D, Conill-Cortes C, de Olivar-Oliver L. Sustained Remission in Patients with Primary Immune Thrombocytopenia after Romiplostim Tapering and Discontinuation: A Case Series in Real Life Management in Spain. Case Rep Hematol. 2017;2017:4109605. [CrossRef]
- Hu Y, Liu X, Zhou H, et al. Efficacy and safety of sovleplenib (HMPL-523) in adult patients with chronic primary immune thrombocytopenia in China (ESLIM-01): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Haematol. 2024. [CrossRef]
- Bennett CM, Neunert C, Grace RF, et al. Predictors of remission in children with newly diagnosed immune thrombocytopenia: Data from the Intercontinental Cooperative ITP Study Group Registry II participants. Pediatr Blood Cancer. 2018;65(1). [CrossRef]
- Imbach P, Kühne T, Müller D, et al. Childhood ITP: 12 months follow-up data from the prospective registry I of the Intercontinental Childhood ITP Study Group (ICIS). Pediatr Blood Cancer. 2006;46(3):351-356. [CrossRef]
- Sailer T, Lechner K, Panzer S, Kyrle PA, Pabinger I. The course of severe autoimmune thrombocytopenia in patients not undergoing splenectomy. Haematologica. 2006;91(8):1041-1045.
- George JN, Woolf SH, Raskob GE, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88(1):3-40.
- Stasi R, Stipa E, Masi M, et al. Long-term observation of 208 adults with chronic idiopathic thrombocytopenic purpura. Am J Med. 1995;98(5):436-442. [CrossRef]
- Schifferli A, Holbro A, Chitlur M, et al. A comparative prospective observational study of children and adults with immune thrombocytopenia: 2-year follow-up. Am J Hematol. 2018;93(6):751-759. [CrossRef]
- Wei Y, Ji XB, Wang YW, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood. 2016;127(3):296-302; quiz 370. [CrossRef]
- Marangon M, Vianelli N, Palandri F, et al. Rituximab in immune thrombocytopenia: gender, age, and response as predictors of long-term response. Eur J Haematol. 2017;98(4):371-377.
- Zaja F, Carpenedo M, Barate C, et al. Tapering and discontinuation of thrombopoietin receptor agonists in immune thrombocytopenia: Real-world recommendations. Blood Rev. 2020;41:100647. [CrossRef]
- Lozano ML, Mingot-Castellano ME, Perera MM, et al. Deciphering predictive factors for choice of thrombopoietin receptor agonist, treatment free responses, and thrombotic events in immune thrombocytopenia. Sci Rep. 2019;9(1):16680. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).