Submitted:
13 September 2024
Posted:
17 September 2024
You are already at the latest version
Abstract
Keywords:
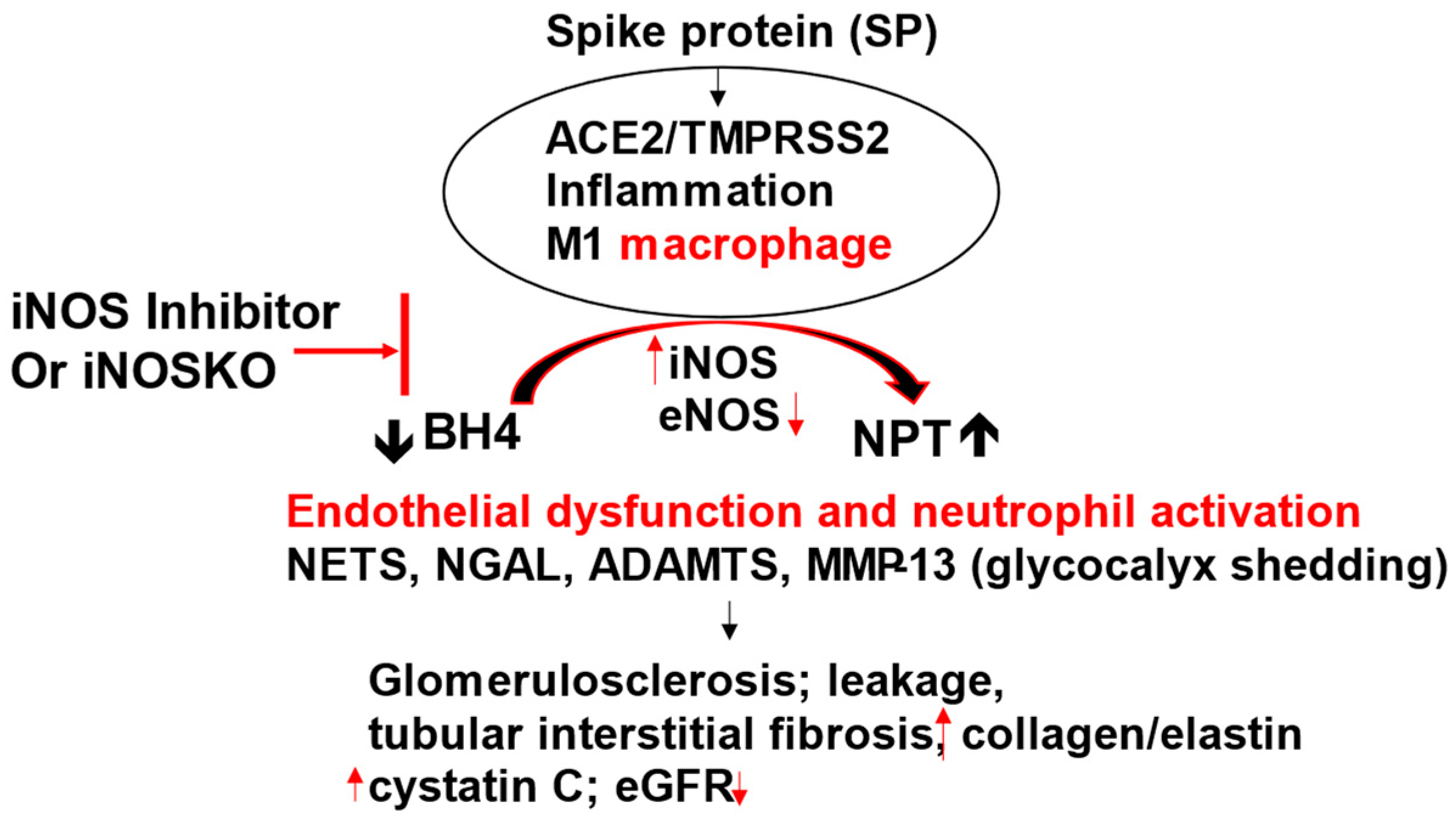
- Key Contributions: The acute kidney injury post Covid-19 can lead to CKD by depletion of renal microvascular nitric oxide, leading to kidney stones
- Running Title: Covid-19 AKI to CKD
- *A part of this study was supported by NIH grants AR-71789; HL139047; and DK116591
- DISCLOSURES: No conflicts of interest; financial or otherwise; are declared by the author
Introduction
Discussion
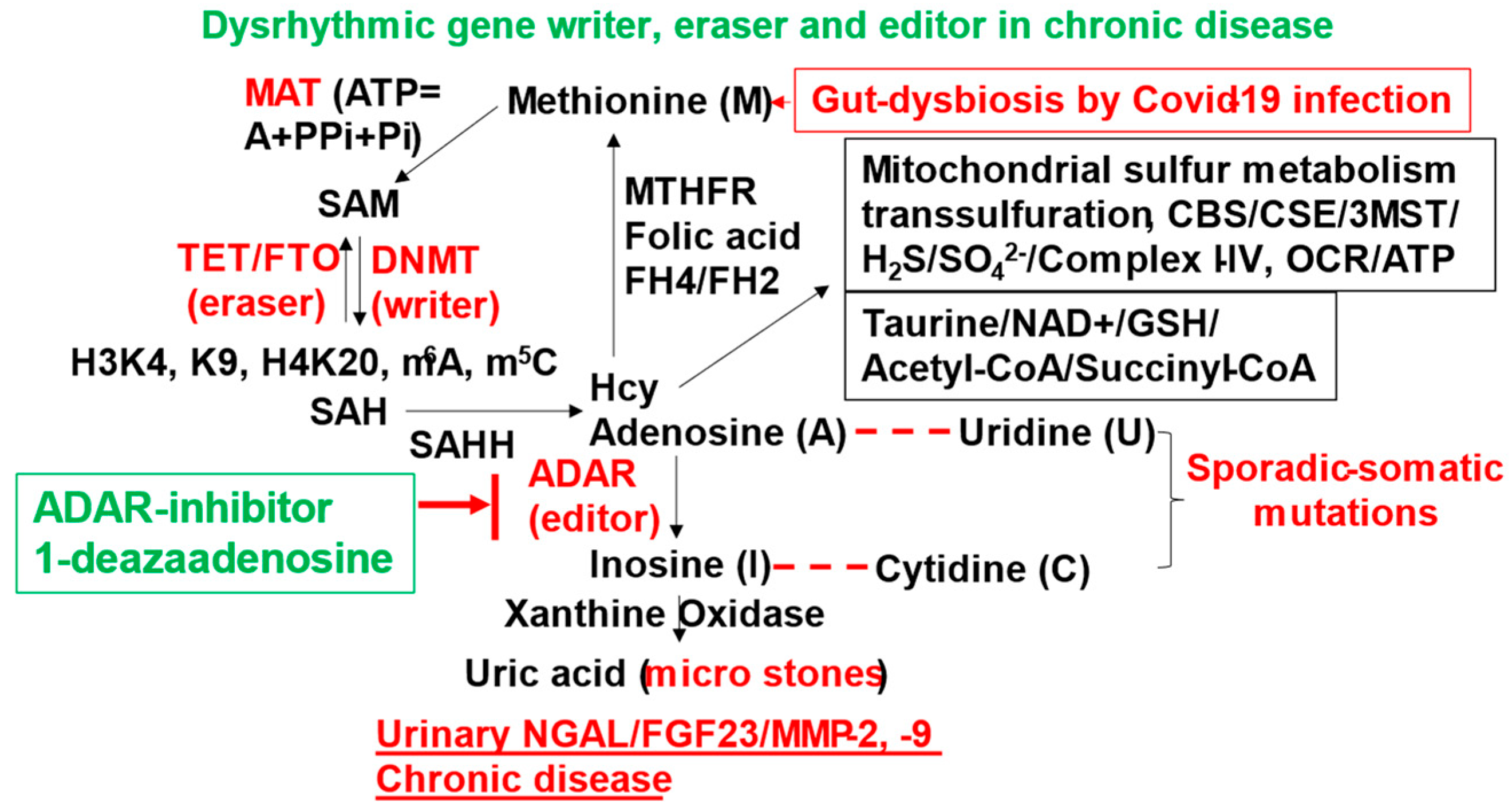
Kidney Stones
What are the underlying mechanisms for COVID-19 infection to kidney stone formation:
Conclusions and Future Direction:
References
- Martin de Francisco, A. & Fernandez Fresnedo, G. Long COVID-19 renal disease: A present medical need for nephrology. Nefrologia (Engl Ed) 43, 1-5 (2023). [CrossRef]
- Schiffl, H. & Lang, S. M. Long-term interplay between COVID-19 and chronic kidney disease. Int Urol Nephrol 55, 1977-1984 (2023). [CrossRef]
- Teng, L. et al. The pattern of cytokines expression and dynamic changes of renal function at 6 months in patients with Omicron COVID-19. J Med Virol 95, e28477 (2023). [CrossRef]
- Atiquzzaman, M. et al. Long-term effect of COVID-19 infection on kidney function among COVID-19 patients followed in post-COVID recovery clinic in British Columbia, Canada. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association (2023). [CrossRef]
- Chancharoenthana, W. et al. Gastrointestinal manifestations of long-term effects after COVID-19 infection in patients with dialysis or kidney transplantation: An observational cohort study. World J Gastroenterol 29, 3013-3026 (2023). [CrossRef]
- Wang CS, Glenn DA, Helmuth M, Smith AR, Bomback AS, Canetta PA, Coppock GM, Khalid M, Tuttle KR, Bou-Matar R, Greenbaum LA, Robinson BM, Holzman LB, Smoyer WE, Rheault MN, Gipson D, Mariani LH; Cure Glomerulonephropathy (CureGN) Study Consortium. Association of COVID-19 Versus COVID-19 Vaccination With Kidney Function and Disease Activity in Primary Glomerular Disease: A Report of the Cure Glomerulonephropathy Study. Am J Kidney Dis. 2024 Jan;83(1):37-46. Epub 2023 Aug 31. [CrossRef]
- Bai S, Zhan Y, Pan C, Liu G, Li J, Shan L. Prospective comparison of extracorporeal shock wave lithotripsy versus flexible ureterorenoscopy in patients with non-lower pole kidney stones under the COVID-19 pandemic. Urolithiasis. 2023 Feb 16;51(1):38. [CrossRef] [PubMed]
- Spooner J, Masoumi-Ravandi K, MacNevin W, Ilie G, Skinner T, Powers AL. Septic and febrile kidney stone presentations during the COVID-19 pandemic What is the effect of reduced access to care during pandemic restrictions? Can Urol Assoc J. 2024 Jan;18(1):E19-E25. [CrossRef] [PubMed]
- Rajabian N, Ikhapoh I, Shahini S., et al, has shown that the Methionine adenosyltransferase2A (MAT) inhibition restores metabolism to improve regenerative capacity and strength of aged skeletal muscle, Nature Communications, 14, article number: 886; Feb 16, 2023.
- Kottur J, White KM, Rodriguez ML, Rechkoblit O, Quintana-Feliciano R, Nayar A, García-Sastre A, Aggarwal AK. Structures of SARS-CoV-2 N7-methyltransferase with DOT1L and PRMT7 inhibitors provide a platform for new antivirals. PLoS Pathog. 2023 Jul 31;19(7):e1011546. eCollection 2023 Jul. [CrossRef] [PubMed]
- Martins MC, Meyers AA, Whalley NA, Rodgers AL. Cystine: a promoter of the growth and aggregation of calcium oxalate crystals in normal undiluted human urine. J Urol, 2002, Jan;167(1):317-321. [CrossRef]
- Wallace B, Chmiel JA, Al KF, Bjazevic J, Burton JP, Goldberg HA, Razvi H. The Role of Urinary Modulators in the Development of Infectious Kidney Stones. J Endourol. 2023 Mar;37(3):358-366. Epub 2023 Jan 20. [CrossRef] [PubMed]
- Kalan Sarı I, Keskin O, Seremet Keskin A, Elli Dağ HY, Harmandar O. Is Homocysteine Associated with the Prognosis of Covid-19 Pneumonia. Int J Clin Pract. 2023 Mar 2;2023:9697871. eCollection 2023. Free PMC article. [CrossRef] [PubMed]
- Govender, N., Khaliq, O., Moodley, J. & Naicker, T. Unravelling the Mechanistic Role of ACE2 and TMPRSS2 in Hypertension: A Risk Factor for COVID-19. Curr Hypertens Rev 18, 130-137 (2022). [CrossRef]
- South, A. M., Diz, D. I. & Chappell, M. C. COVID-19, ACE2, and the cardiovascular consequences. American journal of physiology. Heart and circulatory physiology 318, H1084-H1090 (2020). [CrossRef]
- South, A. M. et al. Fetal programming and the angiotensin-(1-7) axis: a review of the experimental and clinical data. Clin Sci (Lond) 133, 55-74 (2019). [CrossRef]
- Singh, M. et al. Simulation of COVID-19 symptoms in a genetically engineered mouse model: implications for the long haulers. Mol Cell Biochem 478, 103-119 (2023). [CrossRef]
- Srivastava, S. P., Srivastava, R., Chand, S. & Goodwin, J. E. Coronavirus Disease (COVID)-19 and Diabetic Kidney Disease. Pharmaceuticals (Basel) 14 (2021). [CrossRef]
- Borczuk, A. C. & Yantiss, R. K. The pathogenesis of coronavirus-19 disease. J Biomed Sci 29, 87 (2022). [CrossRef]
- Sachetto, A. T. A. & Mackman, N. Tissue Factor and COVID-19: An Update. Curr Drug Targets 23, 1573-1577 (2022). [CrossRef]
- Altillero, M., Jr., Danguilan, R. & Arakama, M. H. Incidence of, and Risk Factors and Outcomes Associated with, Acute Kidney Injury in COVID-19 at the National Kidney and Transplant Institute, Philippines. Trop Med Infect Dis 8 (2023). [CrossRef]
- Kim, I. S. et al. Role of increased neutrophil extracellular trap formation on acute kidney injury in COVID-19 patients. Front Immunol 14, 1122510 (2023). [CrossRef]
- Zhang, W. et al. Identification of common molecular signatures of SARS-CoV-2 infection and its influence on acute kidney injury and chronic kidney disease. Front Immunol 14, 961642 (2023). [CrossRef]
- Narayanan, A., Cunningham, P., Mehta, M., Lang, T. & Hammes, M. Acute Kidney Injury in Coronavirus Disease and Association with Thrombosis. American journal of nephrology 54, 156-164 (2023). [CrossRef]
- Pickkers, P. et al. Study protocol of a randomised, double-blind, placebo-controlled, two-arm parallel-group, multi-centre phase 3 pivotal trial to investigate the efficacy and safety of recombinant human alkaline phosphatase for treatment of patients with sepsis-associated acute kidney injury. BMJ Open 13, e065613 (2023). [CrossRef]
- Singh, M. et al. Novel mechanism of the COVID-19 associated coagulopathy (CAC) and vascular thromboembolism. npj Viruses 1, 3 (2023). [CrossRef]
- AbdelHamid, S. G. et al. Deciphering epigenetic(s) role in modulating susceptibility to and severity of COVID-19 infection and/or outcome: A systematic rapid review. Environ. Sci. Pollut. Res. Int. 28, 54209–54221 (2021). [CrossRef]
- Chlamydas, S., Papavassiliou, A. G. & Piperi, C. Epigenetic mechanisms regulating COVID-19 infection. Epigenetics 16, 263–270 (2021). [CrossRef]
- Jadali Z. Double-edged sword effect of platelets in COVID-19. J Vasc Bras. 2023 Mar 6;22:e20220101. eCollection 2023. Free PMC article. No abstract available. [CrossRef] [PubMed]
- Vuorio A, Raal F, Kovanen PT. Familial hypercholesterolemia: The nexus of endothelial dysfunction and lipoprotein metabolism in COVID-19. Curr Opin Lipidol. 2023 Mar 10. Online ahead of print. [CrossRef] [PubMed]
- Zhou S, Yu Z, Chen Z, Ning F, Hu X, Wu T, Li M, Xin H, Reilly S, Zhang X. Olmesartan alleviates SARS-CoV-2 envelope protein induced renal fibrosis by regulating HMGB1 release and autophagic degradation of TGF-beta1. Front Pharmacol. 2023 May 15;14:1187818. eCollection 2023. [CrossRef] [PubMed]
- Tudoran, C. et al. Correspondence between Aortic and Arterial Stiffness, and Diastolic Dysfunction in Apparently Healthy Female Patients with Post-Acute COVID-19 Syndrome. Biomedicines 11 (2023). [CrossRef]
- Wierzbicki, T. & Bai, Y. Finite element modeling of alpha-helices and tropocollagen molecules referring to spike of SARS-CoV-2. Biophys J 121, 2353-2370 (2022). [CrossRef]
- Brennan, G. T. COVID-19-Induced Collagenous Colitis. Gastro Hep Adv 1, 976 (2022). [CrossRef]
- Gutman, H. et al. Matrix Metalloproteinases Expression Is Associated with SARS-CoV-2-Induced Lung Pathology and Extracellular-Matrix Remodeling in K18-hACE2 Mice. Viruses 14 (2022). [CrossRef]
- Brusa, S. et al. Circulating tissue inhibitor of metalloproteinases 1 (TIMP-1) at COVID-19 onset predicts severity status. Front Med (Lausanne) 9, 1034288 (2022). [CrossRef]
- Belen Apak, F. B. et al. Coagulopathy is Initiated with Endothelial Dysfunction and Disrupted Fibrinolysis in Patients with COVID-19 Disease. Indian J Clin Biochem, 1-11 (2023). [CrossRef]
- Chauvin, M. et al. Elevated Neopterin Levels Predict Fatal Outcome in SARS-CoV-2-Infected Patients. Front Cell Infect Microbiol 11, 709893 (2021). [CrossRef]
- Al-Kuraishy, H. M., Al-Gareeb, A. I., Alzahrani, K. J., Cruz-Martins, N. & Batiha, G. E. The potential role of neopterin in Covid-19: a new perspective. Mol Cell Biochem 476, 4161-4166 (2021). [CrossRef]
- Zhang, Q. et al. Effects of convalescent plasma infusion on the ADAMTS13-von Willebrand factor axis and endothelial integrity in patients with severe and critical COVID-19. Res Pract Thromb Haemost 7, 100010 (2023). [CrossRef]
- Hoffmann, M. et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181, 271-280 e278 (2020). [CrossRef]
- Matthew D. Cheung, Elise N. Erman, Shanrun Liu, Nathaniel B. Erdmann, Gelare Ghajar-Rahimi, Kyle H. Moore, Jeffrey C. Edberg, James F. George, and Anupam Agarwal. Single-Cell RNA Sequencing of Urinary Cells Reveals Distinct Cellular Diversity in COVID-19–Associated AKI. American Society of Nephrology www.kidney360.org Vol 3 January, 2022.
- Jadali Z. Double-edged sword effect of platelets in COVID-19. J Vasc Bras. 2023 Mar 6;22:e20220101. eCollection 2023. Free PMC article. No abstract available. [CrossRef] [PubMed]
- Vuorio A, Raal F, Kovanen PT. Familial hypercholesterolemia: The nexus of endothelial dysfunction and lipoprotein metabolism in COVID-19. Curr Opin Lipidol. 2023 Mar 10. Online ahead of print. [CrossRef] [PubMed]
- Zhou, S. et al. ADAMTS13 protects mice against renal ischemia-reperfusion injury by reducing inflammation and improving endothelial function. American journal of physiology. Renal physiology 316, F134-F145 (2019). [CrossRef]
- Henry, B. M. et al. Cell-Free DNA, Neutrophil extracellular traps (NETs), and Endothelial Injury in Coronavirus Disease 2019- (COVID-19-) Associated Acute Kidney Injury. Mediators Inflamm 2022, 9339411 (2022). [CrossRef]
- Li, H., Yu, Z., Gan, L., Peng, L. & Zhou, Q. Serum NGAL and FGF23 may have certain value in early diagnosis of CIN. Ren Fail 40, 547-553 (2018). [CrossRef]
- Faul, C. et al. FGF23 induces left ventricular hypertrophy. The Journal of clinical investigation 121, 4393-4408 (2011). [CrossRef]
- Zheng, X. L. ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med 66, 211-225 (2015). [CrossRef]
- Chen, C., Wang, J., Liu, Y. M. & Hu, J. Single-cell analysis of adult human heart across healthy and cardiovascular disease patients reveals the cellular landscape underlying SARS-CoV-2 invasion of myocardial tissue through ACE2. J Transl Med 21, 358 (2023). [CrossRef]
- Bolignano, D. et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 4, 337-344 (2009). [CrossRef]
- Zhang, Q. et al. Clinical Significance of Urinary Biomarkers in Patients With Primary Focal Segmental Glomerulosclerosis. Am J Med Sci 355, 314-321 (2018). [CrossRef]
- Viveiros, A. et al. Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones. American journal of physiology. Heart and circulatory physiology 320, H296-H304 (2021). [CrossRef]
- Viveiros A, Gheblawi M, Aujla PK, Sosnowski DK, Seubert JM, Kassiri Z, Oudit GY. Sex- and age-specific regulation of ACE2: Insights into severe COVID-19 susceptibility. J Mol Cell Cardiol. 2022 Mar;164:13-16. Epub 2021 Nov 11. Free PMC article. [CrossRef] [PubMed]
- Schroder, S. K., Gasterich, N., Weiskirchen, S. & Weiskirchen, R. Lipocalin 2 receptors: facts, fictions, and myths. Front Immunol 14, 1229885 (2023). [CrossRef]
- Thevenod, F. et al. Role of the SLC22A17/lipocalin-2 receptor in renal endocytosis of proteins/metalloproteins: a focus on iron- and cadmium-binding proteins. American journal of physiology. Renal physiology 325, F564-F577 (2023). [CrossRef]
- Cabedo Martinez, A. I. et al. Biochemical and Structural Characterization of the Interaction between the Siderocalin NGAL/LCN2 (Neutrophil Gelatinase-associated Lipocalin/Lipocalin 2) and the N-terminal Domain of Its Endocytic Receptor SLC22A17. The Journal of biological chemistry 291, 2917-2930 (2016). [CrossRef]
- Langelueddecke, C., Roussa, E., Fenton, R. A. & Thevenod, F. Expression and function of the lipocalin-2 (24p3/NGAL) receptor in rodent and human intestinal epithelia. PloS one 8, e71586 (2013). [CrossRef]
- Langelueddecke, C. et al. Lipocalin-2 (24p3/neutrophil gelatinase-associated lipocalin (NGAL)) receptor is expressed in distal nephron and mediates protein endocytosis. The Journal of biological chemistry 287, 159-169 (2012). [CrossRef]
- Kim, J. K. et al. Prognostic role of circulating neutrophil extracellular traps levels for long-term mortality in new end-stage renal disease patients. Clin Immunol 210, 108263 (2020). [CrossRef]
- Veras, F. P. et al. Targeting neutrophils extracellular traps (NETs) reduces multiple organ injury in a COVID-19 mouse model. Respiratory research 24, 66 (2023). [CrossRef]
- AbdelHamid, S. G. et al. Deciphering epigenetic(s) role in modulating susceptibility to and severity of COVID-19 infection and/or outcome: A systematic rapid review. Environ. Sci. Pollut. Res. Int. 28, 54209–54221 (2021). [CrossRef]
- Chlamydas, S., Papavassiliou, A. G. & Piperi, C. Epigenetic mechanisms regulating COVID-19 infection. Epigenetics 16, 263–270 (2021). [CrossRef]
- Michael S Xydakis, Mark W Albers, Eric H Holbrook, Dina M Lyon, Robert Y Shih, Johannes A Frasnelli, Axel Pagenstecher, Alexandra Kupke, Lynn W Enquist, Stanley Perlman. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021 Sep;20(9):753-761. Epub 2021 Jul 30. [CrossRef] [PubMed] [PubMed Central]
- Eileen P Scully, Jenna Haverfield , Rebecca L Ursin, Cara Tannenbaum, Sabra L Klein. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020 Jul;20(7):442-447. Epub 2020 Jun 11. [CrossRef] [PubMed] [PubMed Central]
- Camp, T. M., Smiley, L. M., Hayden, M. R. & Tyagi, S. C. Mechanism of matrix accumulation and glomerulosclerosis in spontaneously hypertensive rats. Journal of hypertension 21, 1719-1727 (2003). [CrossRef]
- Rucklidge, G. J., Milne, G., McGaw, B. A., Milne, E. & Robins, S. P. Turnover rates of different collagen types measured by isotope ratio mass spectrometry. Biochimica et biophysica acta 1156, 57-61 (1992). [CrossRef]
- Ramnath RD, Butler MJ, Newman G, Desideri S, Russell A, Lay AC, Neal CR, Qiu Y, Fawaz S, Onions KL, Gamez M, Crompton M, Michie C, Finch N, Coward RJ, Welsh GI, Foster RR, Satchell SC. Blocking matrix metalloproteinase-mediated syndecan-4 shedding restores the endothelial glycocalyx and glomerular filtration barrier function in early diabetic kidney disease. Kidney Int. 2020 May;97(5):951-965. Epub 2019 Nov 2. Free PMC article. [CrossRef] [PubMed]
- Sarah Fawaz, Aldara Martin Alonso, Yan Qiu, Raina Ramnath, Holly Stowell-Connolly, Monica Gamez, Carl May, Colin Down, Richard J Coward, Matthew J Butler, Gavin I Welsh, Simon C Satchell, Rebecca R Foster. Adiponectin reduces glomerular endothelial glycocalyx disruption and restores glomerular barrier function in a mouse model of type 2 diabetes, Diabetes, 2024 Mar 26:db230455. [CrossRef]
- McDonald, J. T. et al. Role of miR-2392 in driving SARS-CoV-2 infection. Cell Rep 37, 109839 (2021). [CrossRef]
- Jiang, Q. et al. Ursolic acid induced anti-proliferation effects in rat primary vascular smooth muscle cells is associated with inhibition of microRNA-21 and subsequent PTEN/PI3K. Eur J Pharmacol 781, 69-75 (2016). [CrossRef]
- Del Nogal Avila M, Das R, Kharlyngdoh J, Molina-Jijon E, Donoro Blazquez H, Gambut S, Crowley M, Crossman DK, Gbadegesin RA, Chugh SS, Chugh SS, Avila-Casado C, Macé C, Clement LC, Chugh SS. Cytokine storm-based mechanisms for extrapulmonary manifestations of SARS-CoV-2 infection. JCI Insight. 2023 May 22;8(10):e166012. [CrossRef] [PubMed]
- Carreño JM, Alshammary H, Singh G, Raskin A, Amanat F, Amoako A, Gonzalez-Reiche AS, van de Guchte A, Study Group P, Srivastava K, Sordillo EM, Sather DN, van Bakel H, Krammer F, Simon V. Evidence for retained spike-binding and neutralizing activity against emerging SARS-CoV-2 variants in serum of COVID-19 mRNA vaccine recipients. EBioMedicine. 2021 Nov;73:103626. Epub 2021 Oct 20. [CrossRef] [PubMed]
- Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, Nakao C, Rayaprolu V, Rawlings SA, Peters B, Krammer F, Simon V, Saphire EO, Smith DM, Weiskopf D, Sette A, Crotty S. Immunological memory to SARS-CoV-2 assessed for up to eight months after infection. bioRxiv [Preprint]. 2020 Dec 18:2020.11.15.383323. [CrossRef] [PubMed]
- Thomas S Metkus, Lori J Sokoll, Andreas S Barth, Matthew J Czarny, Allison G Hays, Charles 419 J Lowenstein, Erin D Michos, Eric P Nolley, Wendy S Post, Jon R Resar, David R 420 BioTech 2024, 5, x FOR PEER REVIEW 12 of 13; Thiemann, Jeffrey C Trost, Rani K Hasan. Myocardial Injury in Severe COVID-19 421 Compared with Non-COVID-19 Acute Respiratory Distress Syndrome, Circulation. 2021 422 Feb 9;143(6):553-565. Epub 2020 Nov 13. 423. [CrossRef] [PubMed] [PubMed Central]
- Zhou S, Yu Z, Chen Z, Ning F, Hu X, Wu T, Li M, Xin H, Reilly S, Zhang X. 425 Olmesartan alleviates SARS-CoV-2 envelope protein induced renal fibrosis by regulating 426 HMGB1 release and autophagic degradation of TGF-beta1. Front Pharmacol. 2023 May 427 15;14:1187818. eCollection 2023. [CrossRef] [PubMed]
- Greene C, Connolly R, Brennan D, Laffan A, O'Keeffe E, Zaporojan L, O'Callaghan J, 429 Thomson B, Connolly E, Argue R, Martin-Loeches I, Long A, Cheallaigh CN, Conlon N, 430 Doherty CP, Campbell M. Blood-brain barrier disruption and sustained systemic 431 inflammation in individuals with long COVID-associated cognitive impairment. Nat 432 Neurosci. 2024 Feb 22. Online ahead of 433 print. [CrossRef] [PubMed]
- Cathomas F, Lin HY, Chan KL, Li L, Parise LF, Alvarez J, Durand-de Cuttoli R, Aubry AV, 435 Muhareb S, Desland F, Shimo Y, Ramakrishnan A, Estill M, Ferrer-Pérez C, Parise EM, 436 Wilk CM, Kaster MP, Wang J, Sowa A, Janssen WG, Costi S, Rahman A, Fernandez 437 N, Campbell M, Swirski FK, Nestler EJ, Shen L, Merad M, Murrough JW, Russo SJ. 438 Circulating myeloid-derived MMP8 in stress susceptibility and depression. Nature. 2024 439 Feb 7. Online ahead of print. [CrossRef] [PubMed]
- Valle A, Soto Z, Muhamadali H, Hollywood KA, Xu Y, Lloyd JR, Goodacre R, Cantero D, 441 Cabrera G, Bolivar J. Metabolomics for the design of new metabolic engineering strategies 442 for improving aerobic succinic acid production in Escherichia coli. Metabolomics. 2022 Jul 443 20;18(8):56. Free PMC article. 444 RESULTS: Most of the 65 identified metabolites showed lower relative levels in the M4-445 deltaiclR and M4-deltagnd mutants than those of the M4. However, fructose 1,6-446 biphosphate, trehalose, isovaleric acid and mannitol relative concentrations were increased 447 in M4-deltaiclR a … 448. [CrossRef] [PubMed]
- Tong W, Hannou SA, Wang Y, Astapova I, Sargsyan A, Monn R, Thiriveedi V, Li D, McCann 449 JR, Rawls JF, Roper J, Zhang GF, Herman MA. The intestine is a major contributor to 450 circulating succinate in mice. FASEB J. 2022 Oct;36(10):e22546. The tricarboxylic acid (TCA) cycle is the 452 epicenter of cellular aerobic metabolism. ...Despite the importance of circulating TCA cycle 453 metabolites as signaling molecules, the source of circulating TCA cycle intermediates 454 remains uncertain. ... 455. [CrossRef] [PubMed]
- Kalan Sarı I, Keskin O, Seremet Keskin A, Elli Dağ HY, Harmandar O. Is Homocysteine 456 Associated with the Prognosis of Covid-19 Pneumonia. Int J Clin Pract. 2023 Mar 457 2;2023:9697871. eCollection 2023. Free 458 PMC article. 459. [CrossRef] [PubMed]
- Giovanni Carpenè, Davide Negrini, Brandon M Henry, Martina Montagnana, Giuseppe Lippi 460 Homocysteine in coronavirus disease (COVID-19): a systematic literature review, 461 BioTech 2024, 5, x FOR PEER REVIEW 13 of 13.
- Diagnosis (Berl) 2022 Jun 16;9(3):306-310. doi: 10.1515/dx-2022-0042. eCollection 2022 462 Aug 1. [CrossRef] [PubMed]
- Sang R. Lee, Jeong Yeon Roh, Jihoon Ryu, Hyun-Jin Shin & Eui-Ju Hong, Activation of TCA 464 cycle restrains virus-metabolic hijacking and viral replication in mouse hepatitis 465 virus-infected cells, Cell & Bioscience volume 12, Jan 18, 2022; Article number: 7 (2022). 466.
- Liu Q, Wang H, Zhang H, Sui L, Li L, Xu W, Du S, Hao P, Jiang Y, Chen J, Qu X, Tian M, 467 Zhao Y, Guo X, Wang X, Song W, Song G, Wei Z, Hou Z, Wang G, Sun M, Li X, Lu H, 468 Zhuang X, Jin N, Zhao Y, Li C, Liao M. The global succinylation of SARS-CoV-2-infected 469 host cells reveals drug targets. Proc Natl Acad Sci U S A. 2022 Jul 470 26;119(30):e2123065119. Epub 2022 Jul 471 12. Free PMC article. SARS-CoV-2 infection promotes succinylation of 472 several key enzymes in the TCA, leading to inhibition of cellular metabolic pathways. We 473 demonstrated that host protein succinylation is regulated by viral nonstructural protein 474 (NSP14) through interaction with sirtuin 5 (SIRT … 475. [CrossRef] [PubMed]
- Ruyu Tan, Santao Ou, Ting Kang, Weihua Wu, Lin Xiong, Tingting Zhu and Liling Zhang. 476 Altered serum metabolome associated with vascular calcification developed from CKD and 477 the critical pathways. Frontiers in Cardiovascular Medicine, TYPE Original Research 478 PUBLISHED 11 April 2023 | DOI 10.3389/fcvm.2023.1114528. 479. [CrossRef]
- Cui J, Hong P, Li Z, Lin J, Wu X, Nie K, Zhang X, Wan J. Chloroquine inhibits NLRP3 481 inflammasomes activation and alleviates renal fibrosis in mouse model of 482 hyperuricemic nephropathy with aggravation by a high-fat-diet. Int Immunopharmacol. 483 2023 Jun 3;120:110353. Online ahead of 484 print. Numerous epidemiological studies have demonstrated that 485 hyperuricemia (HUA) is a risk factor for renal diseases and renal fibrosis. Dietary patterns 486 can influence serum urate levels and hyperuricemic nephropathy (HN). ...Additionally, the 487 HN + HFD group displ …. [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



