1. Introduction
Alzheimer’s disease (AD) is a progressive age-related neurodegeneration, which represents the most common cause of dementia and cognitive decline in the elderly. [
1] According to statistics, the true prevalence is unknown; however, it is the leading cause of death in the UK, surpassing cardiovascular diseases [
2], and the fifth leading cause in individuals aged > 65 in the United States. [
3] Additionally, it is twice as common in women compared to men. [
4] Unfortunately, AD leads to a high level of health loss and mortality worldwide, with limited treatment options. Approximately, 10-15% of AD patients are misdiagnosed by specialists, and the diagnosis can only be confirmed post-mortem. Early symptoms include cognitive deterioration, memory loss, and confusion.
The etiology of Alzheimer’s remains unknown; however, most cases present sporadically with a late onset (≥ 65 years), while 5-10% of cases have a familial component, characterized by an early onset (< 65 years) and commonly associated with specific genetic mutations. The neuropathogenesis of AD is marked by the misfolding and aggregation of two proteins, amyloid precursor protein (APP), and tau protein (responsible for microtubule stabilization). [
5] APP leads to the formation of Aβ monomers, which aggregate to form Aβ fibrils and plaques, particularly toxic and damaging. On the other hand, hyperphosphorylation of tau protein leads to the formation of neurofibrillary tangles (NFT), [
6] associated with cognitive decline and the neurodegeneration typical of AD. Other genetic factors contributing to neurodegeneration are related to apolipoprotein E (apoE), influencing cytoskeletal integrity and neuronal repair efficiency. Studies have shown that other factors such as hypertension, diabetes, dyslipidemia, smoking, hormonal changes, and metal exposure can increase the risk of developing AD. [
6]
Metals are natural components of the Earth’s crust and can be inhaled or ingested through food, water, and air. Some heavy metals such as copper (Cu), zinc (Zn) and selenium (Se) can be detected in the body at low concentrations as trace metals. Metal ion homeostasis is essential in regulating certain brain functions. However, variations in concentration and form can make them toxic to the body. In particular, metals like Cu can promote the maturation of Aβ monomer aggregation and tau protein hyperphosphorylation. Evidence suggests that dysregulation in essential metal homeostasis and exposure to non-essential metals significantly impact AD pathogenesis. [
7]
Copper is a redox-active essential metal normally found in blood [
8]. It is essential for the metabolism of many enzymes, such as Superoxide Dismutase 1 (SOD1), implicated in numerous antioxidant processes. Cu is an important catalyst for iron absorption and heme synthesis. It’s the third-most abundant transition metal in the liver, after Iron and Zinc. [
3] In the brain, Cu concentrations of approximately 60~110 μM can be detected, especially in the frontal lobe, brain, and hippocampus. [
8] Usually, about 85%-95% of the total copper is found bound to ceruloplasmin (CP), a blue-looking copper glycoprotein. CP is mainly synthesized by the liver, brain, kidney, and fat. It plays important roles in Cu transport, iron (Fe) regulation, and antioxidant processes. Its structure is composed of 6 domains that can bind to six Cu atoms; when all six domains are bound to Cu, the CP becomes unstable. [
9]
The remaining 5%-15% of Cu is represented by loosely bound Cu to albumin and other little molecules, also known as “free Copper” or “non-Cp-Cu”. Free Copper, due to its loose binding, is freely available to meet tissue needs in the body.
The moment this 5%-15% pool expands, the Copper becomes toxic; this is in line with what occurs in the case of Wilson’s disease. [
10] Moreover, Cu is considered a pro-oxidant factor [
11]: the increase in its serum levels lead to may feed the brain’s copper reservoir which can enter oxidative stress cycles. [
11,
12] Neuronal damage caused by Cu homeostasis failure could be attributed to its numerous roles in processes essential for normal brain function, including catecholamine synthesis, activation of neuropeptides and hormones, antioxidant defence, connective tissue production, immune function, and synaptic transmission.
Considering the strong evidence of copper’s essential roles in the brain, it is not surprising that several studies have proposed that an imbalance in its homeostasis is associated with neurodegenerative disorders such as AD.
In this review, we will examine the role of copper in the pathophysiology of AD, as well as the mechanisms involved in neurotoxicity and cognitive decline, through a careful synthesis of literature published in the last 10 years, to identify new evidence.
2. Materials and Methods
2.1. Protocol
A draft protocol was written according to the Cochrane Handbook for Systematic Reviews of Interventions [
13] for an updated systematic review on the role of the Cu in AD etiopathogenesis. The protocol is presented in supplementary materials (S1).
2.2. Eligibility Criteria, Search Method, Information Sources and Study Selection
The study adopted inclusion and exclusion criteria. These criteria were designed to ensure the selection of relevant studies while excluding those that did not meet the specified criteria.
The search was conducted in PubMed, Google Scholar and across all databases available to us. We included only the original research articles in full text, published in a peer-reviewed journal, in English and Italian languages and published from January 2013 up to January 2023. All case-control studies that addressed the analysis of Cu and AD were considered, following the combinations of keywords: “Alzheimer disease”, “blood”, “copper”, “risk”. We didn’t use any AND or OR in our research, thanks to the usage of the Boolean operators which read the space between words as an implied AND.
We considered only late-onset Alzheimer’s disease; so, the population consisted in only over 60 years old people. Both sexes were taken into account. The nationality in which the study was conducted and/or where the samples were from was not cause of exclusion.
After the initial screening of the title and abstract, the full texts of potentially eligible studies were examined independently by authors and the discrepancies at all stages of study selection were resolved through discussion and consensus among the authors’ group.
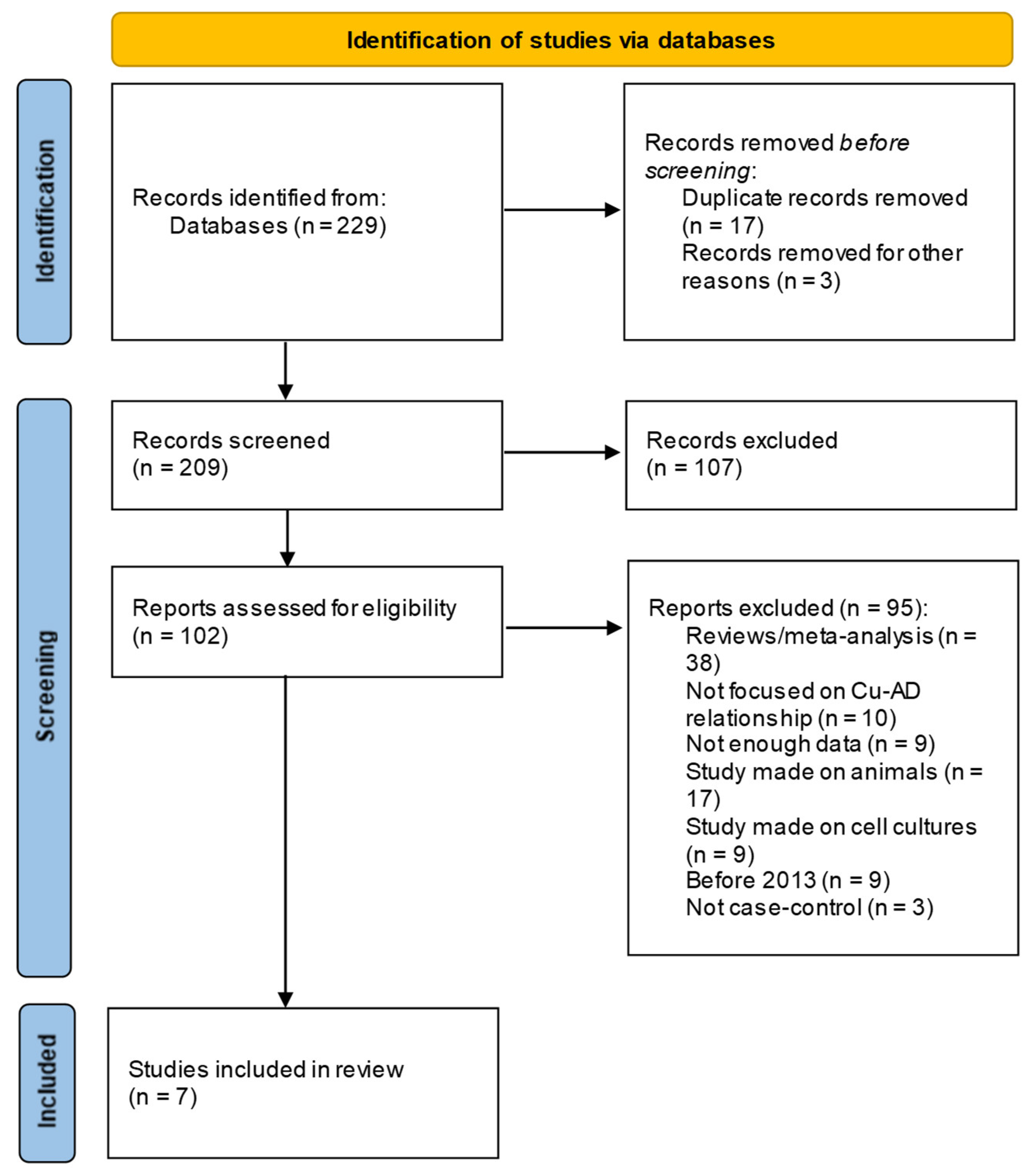
Finally, the studies inclusions in the review, accompanied with reasons for exclusion, are presented in the flow Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA) [
14] diagram. (
Figure 1) . For data extraction, a standardized form is used (
https://www.ncbi.nlm.nih.gov/books/NBK355732/) and screening data is done using Excel spreadsheets.
3. Results
As reported in PRISMA flow diagram (
Figure 1) the search results from the electronic databases are 229 records; titles and abstracts of 209 total records were accessed at the screening phase, from which only 102 were selected for eligibility criteria. Finally, only 7 studies (
Table 1) were considered eligible for inclusion to full text level review.
A summary table of the characteristics of the seven records included in the review with authors, year of publication, area of the study, age, gender number, levels of non-Cp-Cu (when stated) and Cp-Cu, for cases and controls, with the p-value emphasizing the difference between the two Cu type, were reported (
Table 1) Out of the 7 articles that were picked for this review, 3 studies focused on non-Cp-Cu and 4 on Cp-Cu. As follows, a narrative synthesis of the articles included in the review was undertaken for non-Cp-Cu and Cp-Cu, respectively.
3.1. Ceruloplasmin Bound Copper Results
Ceruloplasmin bound copper represent the total pool of blood Cu. In this section we present the 4 studies of choice on this topic. Al-khateeb et al. [
11] studied the association between serum copper, lipids concentration and changes in cognitive function in elder Jordanian subjects (52 dementia patients and 50 controls). Results showed no significant difference between dementia and control groups for age (p = 0.215) and gender (p = 0.290) respectively, while educational level (p = 0.006) and coffee intake (p = 0.000) showed significant association. In this study serum Cu in both groups was within the normal physiological range (114.55 ± 57.6 µg/dL in patients vs 126.12 ± 71.78 µg/dL in controls). Finally, the correlation analysis did not show a correlation between Mental scales (MMSE) scores and serum Total cholesterol (TC), Triglycerides (TG), High density lipoprotein HDL, low density lipoprotein LDL, and Cu in dementia group except the correlation for age (p = 0.005).
In 2021, Yadav J et al. and colleagues [
15] analysed the correlation between different essential and non-essential metals concentration and gene expression in Alzheimer’s patients (n=50) with age-matched control subjects (n=50). Results showed higher levels of Cadmium (Cd), Mercury (Hg) (p-value<0.0001) and Cu compared to Iron (Fe) and Zinc (Zn) which were found in lower concentrations in AD’s patients. On the other hand, although apolipoprotein E (Apo E) have been associated with the amyloid-beta pathology involved in AD and showed a maximum affinity with copper, in this study, the correlation between metals concentration and gene expression was not found significant in AD cases.
Negahdar H et al. [
16] have enrolled 120 elderly patients with cognitive impairment (MCI), and 120 elderly healthy people who were differentiated using MMSE in order to determinate serum levels of oxidant and antioxidant, Cu, Mn, Zn and homocysteine (Hcy) concentrations in both groups. The results showed lower levels of oxidant and antioxidant in patients than healthy controls (p<0.001). Moreover, showed the decrease of gradient from mild to severe cognitive impairment patients (MCI I to MCI II). There was no significant different in trace elements between groups and Hcy levels for age, gender and education. Thus, the results of this study confirmed the association between oxidative status and increase the severity of cognitive impairment.
Paglia et al. [
17] have proposed a study aimed to profile the serum biomarkers during the progression of AD. The study involved 40 healthy subjects, 24 subjects with subjective memory complaint (SMC), 20 MCI subjects and 20 AD patients. They analysed 22 serum elements such as Mn, Fe, Cu, Zn, Se, Hg, thallium (TI), antimony (Sb), vanadium (V), and molybdenum (Mo). All these elements have resulted in significant changes in the 4 groups examined. Several essential elements, such as manganese, selenium, zinc and iron tended to increase in SMC and then progressively to decrease in MCI and AD. Toxic elements show a variable behavior, since some elements tended to increase, while others tend to decrease in AD.
3.2. Non-Ceruloplasmin Bound Copper (non-Cp-Cu) Results
For several years non-Cp-Cu was considered a topic only regarding Wilson’s disease (WD), but recently it’s been studied there might be a correlation with Alzheimer’s disease as well. Interesting studies focused on non-Cp-Cu were conducted by Squitti R. et. al. [
12] The studies showed homogeneity in the selection of their patients: they were in a range between 72-80 years old, with mostly female participants. Where the age between cases and controls was over 9 years, it has been corrected through the usage of statistical means. All studies showed a correlation between the increase of non-Cp-Cu and the risk of AD.
Squitti R. et al. 2013 [
12] studied the serum levels of copper, ceruloplasmin and free copper in 399 AD patients and 303 healthy controls. Their results showed that AD patients had higher levels of copper and free copper compared to controls.
Also, Squitti R. et al. 2016 [
18] in their study, analyzed a total of 287 AD patients, divided in two groups based on the difference in their non-Cp-Cu levels using a cut-off of 1.9µM. These AD patients have been furtherly compared with a control group of 250 participants from an older study. They focused on how AD patients with different genotypes (GG and TT for rs732774 C > T ATP7B) had a higher risk of higher levels of non-Cp-Cu. These two studies focused on how patients who showed the variants of the ATP7B gene had a bigger concentration of non-Cp-Cu, mainly due to an abnormal activation or acceleration of additional pathogenic pathways of the disease cascade. Furthermore, one of these two studies added a second control group with AD patients with normal non-Cp-Cu values but still higher than normal Cu values, showing that there might exist a high copper subtype of AD caused by a diverse frequency of some ATP7B SNPs. Overall, 60% of the patients evaluated in the study had values of non-Cp-Cu higher than normal.
Squitti R. et al. 2017 [
19] study observed a total of 56 diabetic patients compared to 28 healthy controls. It demonstrated that type 2 diabetes (T2D) patients have a higher risk of contracting AD. In fact, AD and T2D patients had very similar values of non-Cp-Cu (2,1µmol for AD and 2.0µmol for T2D), way higher than controls. The cause of this copper dysregulation might be attributed to the fact that both AD and T2D share a common pathway.
4. Discussion
AD is a neurodegenerative condition that causes permanent damage to several processes such as thinking, memory and linguistic ability. AD is the most prevalent type of dementia among elderly patients (> 65 years old). The main risk factors for AD are genetic influences (mutations in the amyloid precursor or APOE gene, ATP7B gene), immune system dysfunction, inflammation, and oxidative stress from metal ions. Cu toxicosis, or its accumulation, has mostly been disregarded up until now, because, while in WD it causes motor problems in children, in AD it produces cognitive problems in adults over 65 years. [
12]
Squitti et al. analyzed the correlation between ATP7B and Cu dyshomeostasis in patients with AD, as this gene is involved in regulating the homeostasis of this metal. Specifically, in hepatocytes, ATP7B donates Cu to ceruloplasmin. This study showed that AD patients with ATP7B mutations had higher serum non-Cp Cu concentrations compared to healthy controls. [
12] These data led to the conclusion that gene variants of ATP7B could trigger an increase in free Cu in the serum, activating APP and increasing susceptibility to developing AD. [
20] Therefore, the authors hypothesized that ATP7B, like APOE, could represent genetic risk factors for the development of AD, although they share independent pathogenic pathways. [
12,
19] Recent research has suggested that two Cu chaperones (ATOX1 and MURR1) might trigger mutations in the ATP7B gene [
21,
22], playing a key role both in sabotaging Cu homeostasis and in the age of onset of AD. It also appears that Cu dysfunction caused by ATP7B is a causative rather than an associative risk factor for AD. [
12,
19]
Interestingly, these genes also interact with other essential ions in our body such as Fe, Zn, Mn, Mg, and Se. Post-mortem studies of brain tissue in AD patients have shown the presence of high concentrations of Cu, Zn and Fe around amyloid-β (Aβ) plaques. Negahdar et al. hypothesized that under oxidative stress conditions, amyloid-β plaques exert a chelating role towards Cu as a compensatory action. A reduction in serum Cu correlated with the severity of AD was observed; patients with MCI II had higher Cu concentrations compared to patients with MCI I and healthy subjects. [
23] Conversely, other studies have observed a reduction in serum Zn and Fe concentrations and an increase in Cu concentrations. [
15,
17,
24,
25] The APOE protein has also shown a particular affinity with Cu and less with Zn. Elevated Cu concentrations are also detectable in other cognitive disorders such as MCI and SMC [
17], as well as in other conditions like type 2 diabetes (T2D) and dyslipidemias. Tissue damage caused by hyperglycemia leads to the impairment of copper homeostasis. As demonstrated by numerous authors, patients with T2D have high serum levels of both total Cu and non-Cp Cu, a situation also observable in the serum of AD patients. [
19,
26] Furthermore, the formation of amyloid-β chains induced by oxidative stress has been detected in the pancreatic tissue of patients with T2D [
19] Evidence suggests that AD and diabetes share common pathways. Moreover, it has been observed that the multi-ligand receptor AGEs are responsible for various inflammatory processes by transporting circulating amyloid-β chains that induce oxidative stress common to diabetes, obesity, and AD. [
27,
28] Studies on murine models have shown that Cu stimulates the deposition of amyloid- β plaques in the brains of mice fed with cholesterol. [
29] Although the role of cholesterol in cognitive decline is still unclear, differences in the lipid profile of AD patients and those at high cardiac risk have been observed, suggesting a correlation between atherosclerosis and AD with high serum cholesterol concentrations as a common risk factor, [
30] implying that a condition of hypercholesterolemia in young adults could influence the onset of AD in later life. [
31] However, there is not enough evidence to define the lipid profile as a risk factor for AD; probably, multiple factors, including vascular damage caused by hyperlipidemia, contribute to neurovegetative disorders. At the same time, in vivo laboratory studies aiming to vary the diet have found an inverse relationship between copper and cholesterol, suggesting that cholesterol reduces hepatic copper concentrations. A study conducted by Singh et al. [
32] in vivo and in vitro found that small amounts of copper ingested through water could reach the brain via the bloodstream, causing oxidative stress and contributing to the malfunction of the protein 1 responsible for clearing amyloid-β plaques from the brain. However, Al-Khateeb et al. [
11] analyzed Cu concentrations in water and serum of patients involved in the study, finding that Cu levels in water did not significantly impact serum Cu levels.
All these observations highlight that genetic changes, lifestyle, and exposure have different effects on copper metabolism and its involvement in AD. However, it is complex to define a physiological range of Cu as its concentrations can vary from individual to individual based on age and health status. It is evident that in various studies analyzed in this review, serum Cu concentrations were higher in AD patients compared to controls.
To our knowledge, this work is the first review conduct to provide updated evidence of literature since 2013 on the role of the Cu on pathophysiology of AD, as well as the mechanisms involved in neurotoxicity and cognitive decline. However, the effect of updating literature search of this review has its limitations. Firstly, the literature search is focused only on case-control studies on serum of AD patients; therefore, additional relevant studies might have been missed. Secondly, we excluded articles published in preprint databases due to lack of peer-review.
Conclusion
This review summarizes the updated 10-years findings on the relationships between AD and Cu which may provide a new perspective and direction for future scientific research, preventive measures against AD such as changes in the style of life and/or diet; as well as developing of new therapies.
Future researches are required to provide more robust evidence to characterizing the relationships between AD and Cu as well as the balance and interaction between different metal ion in etiopathogenesis of disease.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Text of the protocol S1: Our protocol based on the Cochrane Handbook for Systematic Reviews of Interventions.
Author Contributions
Research activity planning: G.S. and R.M. Data/evidence collection: A.S and V.M. Original draft of the manuscript: A.S and V.M. Review and editing of the manuscript: A.S., V.M., G.S. and R.M.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grimm A, Friedland K, Eckert A. Mitochondrial dysfunction: the missing link between aging and sporadic Alzheimer’s disease. Biogerontology 2016, 17, 281–96. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L. E. , Scherr, P. A., Beckett, L. A., Albert, M. S., Pilgrim, D. M., Chown, M. J.,... & Evans, D. A. Age-specific incidence of Alzheimer’s disease in a community population. Jama 1995, 273, 1354–1359. [Google Scholar]
- Masters, C. , Bateman, R., Blennow, K. et al. Alzheimer’s disease. Nat Rev Dis Primers 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- 2022 Alzheimer’s disease facts and figures. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 2022, 18, 700–789. [CrossRef]
- Majeed, A. , Marwick, B., Yu, H., Fadavi, H., & Tavakoli, M. Ophthalmic Biomarkers for Alzheimer’s Disease: A Review. Frontiers in aging neuroscience 2021, 13, 720167. [Google Scholar] [CrossRef] [PubMed]
- Hart, N. J. , Koronyo, Y., Black, K. L., & Koronyo-Hamaoui, M. Ocular indicators of Alzheimer’s: exploring disease in the retina. Acta neuropathologica 2016, 132, 767–787. [Google Scholar] [CrossRef]
- Adlard, P. A. , & Bush, A. I. Metals and Alzheimer’s Disease: How Far Have We Come in the Clinic? Journal of Alzheimer’s disease : JAD 2018, 62, 1369–1379. [Google Scholar] [CrossRef]
- Davies, K. M. , Hare, D. J., Cottam, V., Chen, N., Hilgers, L., Halliday, G., Mercer, J. F., & Double, K. L. Localization of copper and copper transporters in the human brain. Metallomics : integrated biometal science 2013, 5, 43–51. [Google Scholar] [CrossRef]
- Liu, Z. , Wang, M., Zhang, C., Zhou, S., & Ji, G. Molecular Functions of Ceruloplasmin in Metabolic Disease Pathology. Diabetes, metabolic syndrome and obesity : targets and therapy 2022, 15, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R. , Ventriglia, M., Gennarelli, M., Colabufo, N. A., El Idrissi, I. G., Bucossi, S., Mariani, S., Rongioletti, M., Zanetti, O., Congiu, C., Rossini, P. M., & Bonvicini, C. Non-Ceruloplasmin Copper Distincts Subtypes in Alzheimer’s Disease: a Genetic Study of ATP7B Frequency. Molecular neurobiology 2017, 54, 671–681. [Google Scholar] [CrossRef]
- Al-khateeb, E. , Al-zayadneh, E., Al-dalahmah, O., Alawadi, Z., khatib, F., Naffa, R., & Shafagoj, Y. Relation between copper, lipid profile, and cognition in elderly Jordanians. Journal of Alzheimer’s disease: JAD 2014, 41, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R. , Polimanti, R., Siotto, M., Bucossi, S., Ventriglia, M., Mariani, S., Vernieri, F., Scrascia, F., Trotta, L., & Rossini, P. M. ATP7B variants as modulators of copper dyshomeostasis in Alzheimer’s disease. Neuromolecular medicine 2013, 15, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated 23). Cochrane, 2023. 20 August.
- Page M J, McKenzie J E, Bossuyt P M, Boutron I, Hoffmann T C, Mulrow C D et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Yadav, J. , Verma, A. K., Ahmad, M. K., Garg, R. K., Shiuli, Mahdi, A. A., & Srivastava, S. Metals toxicity and its correlation with the gene expression in Alzheimer’s disease. Molecular biology reports 2021, 48, 3245–3252. [Google Scholar] [CrossRef] [PubMed]
- Negahdar H, Hosseini SR, Parsian H, et al. Homocysteine, trace elements and oxidant/antioxidant status in mild cognitively impaired elderly persons: a cross-sectional study. Rom J Intern Med. 2015, 53, 336–342. [Google Scholar] [CrossRef]
- Paglia G, Miedico O, Cristofano A, et al. Distinctive Pattern of Serum Elements During the Progression of Alzheimer’s Disease. Sci Rep. 2016, 6, 22769. [Google Scholar] [CrossRef]
- Squitti R, Ventriglia M, Gennarelli M, et al. Non-Ceruloplasmin Copper Distincts Subtypes in Alzheimer’s Disease: a Genetic Study of ATP7B Frequency [published correction appears in Mol Neurobiol. 2017 Jan;54(1):682-683]. Mol Neurobiol. 2017, 54, 671–681. [Google Scholar] [CrossRef]
- Squitti, R. , Mendez, A. J., Simonelli, I., & Ricordi, C. Diabetes and Alzheimer’s Disease: Can Elevated Free Copper Predict the Risk of the Disease? Journal of Alzheimer’s disease : JAD 2017, 56, 1055–1064. [Google Scholar] [CrossRef]
- Bush, A. I. , & Tanzi, R. E. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics 2008, 5, 421–432. [Google Scholar] [PubMed]
- Simon I, Schaefer M, Reichert J, Stremmel W. Analysis of the human Atox 1 homologue in Wilson patients. World J Gastroenterol 2008, 14, 2383–2387. [Google Scholar] [CrossRef]
- Gupta A, Chattopadhyay I, Mukherjee S, Sengupta M, Das SK, Ray K. A novel COMMD1 mutation Thr174Met associated with elevated urinary copper and signs of enhanced apoptotic cell death in a Wilson Disease patient. Behav Brain Funct 2010, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C. J. , Bush, A. I., Masters, C. L., Cappai, R., & Li, Q. X. Metals and amyloid-beta in Alzheimer’s disease. International journal of experimental pathology 2005, 86, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markes- bery WR. Copper, iron, and zinc in Alzheimer’s disease senile plaques. J Neurol Sci 1998, 158, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wang ZX, Tan L, Wang HF, Ma J, Liu J, Tan MS, Sun JH, Zhu XC, Jiang T, Yu JT. Serum iron, zinc, and copper levels in patients with Alzheimer’s disease: a replication study and meta- analyses. J Alzheimers Dis 2015, 47, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Pal A, Siotto M, Prasad R, Squitti R. Towards a unified vision of copper involvement in Alzheimer’s disease: A review connecting basic, experimental, and clinical research. J Alzheimers Dis 2015, 44, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Perrone L, Grant WB. Observational and ecological studies of dietary advanced glycation end products in national diets and Alzheimer’s disease incidence and prevalence. J Alzheimers Dis 2015, 45, 965–979. [Google Scholar] [CrossRef]
- Puqazhenthi S, Qin L, Reddy PH. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta. 2016. [CrossRef]
- Lu J, Wu DM, Zheng YL, Sun DX, Hu B, Shan Q, Zhang ZF, Fan SH. Trace amounts of copper exacerbate beta amyloid-induced neurotoxicity in the cholesterol-fed mice through TNF-mediated inflammatory pathway. Brain Behav Immunol 2009, 23, 193–203. [Google Scholar] [CrossRef]
- Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, van Duijn CN, Van Broeckhoven C, Grobbee DE. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam study. Lancet 1997, 349, 151–154. [Google Scholar] [CrossRef]
- Kivipelto, M. , Helkala, E. L., Hänninen, T., Laakso, M. P., Hallikainen, M., Alhainen, K., Soininen, H., Tuomilehto, J., & Nissinen, A. Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology 2001, 56, 1683–1689. [Google Scholar] [CrossRef]
- Singh, I. , Sagare, A. P., Coma, M., Perlmutter, D., Gelein, R., Bell, R. D., Deane, R. J., Zhong, E., Parisi, M., Ciszewski, J., Kasper, R. T., & Deane, R. Low levels of copper disrupt brain amyloid-β homeostasis by altering its production and clearance. Proceedings of the National Academy of Sciences of the United States of America 2013, 110, 14771–14776. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).