Submitted:
16 September 2024
Posted:
17 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
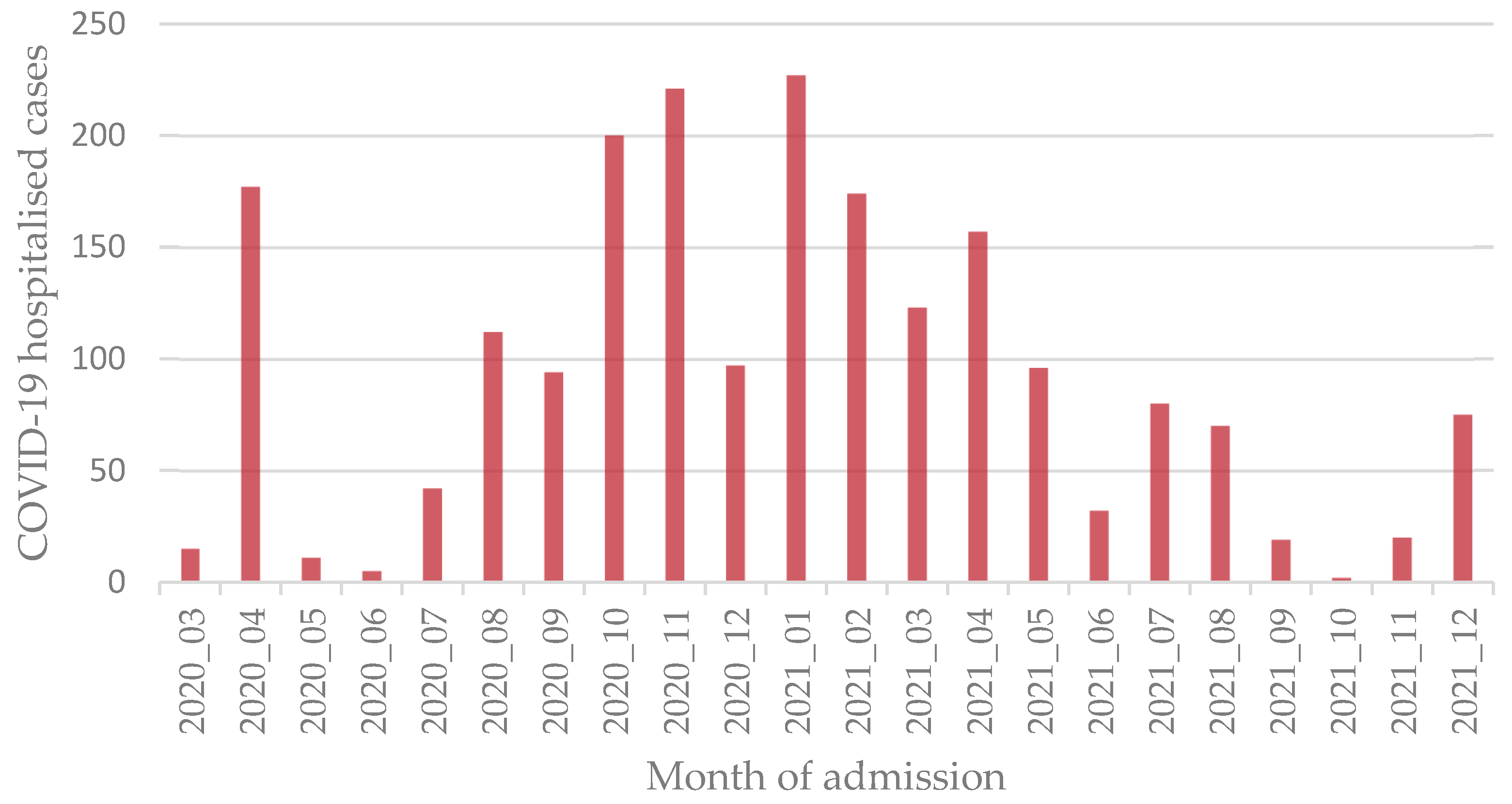
2.1. Study Setting and Design
2.2. Participants
2.3. Laboratory Testing
2.4. Data Collection
2.5. Outcomes of Interest
2.6. Statistical Analysis
2.7. Ethical Approval
3. Results
3.1. Descriptive Analysis
3.2. Bivariate Analysis
3.3. Multivariate Analysis
4. Discussion
4.1. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO COVID-19: Case Definitions Available online: https://iris.who.int/bitstream/handle/10665/360579/WHO-2019-nCoV-Surveillance-Case-Definition-2022.1-eng.pdf?sequence=1 (accessed on 25 July 2024).
- I-MOVE-COVID-19 network. I-MOVE-COVID-19 Available online: https://www.imoveflu.org/i-move-covid-19/ (accessed on 25 July 2024).
- I-MOVE network. I_MOVE Influenza - Monitoring Vaccine Effectiveness in Europe Available online: https://www.imoveflu.org/ (accessed on 25 July 2024).
- Mutch, H.; Young, J.J.; Sadiq, F.; Rose, A.M.; Evans, J.M.; European COVID-19 hospital surveillance analysis writing group Enhanced Surveillance of Hospitalised COVID-19 Patients in Europe: I-MOVE-COVID-19 Surveillance Network, February 2020 to December 2021. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2023, 28, 2200669. [CrossRef]
- Mazagatos, C.; Delgado-Sanz, C.; Monge, S.; Pozo, F.; Oliva, J.; Sandonis, V.; Gandarillas, A.; Quiñones-Rubio, C.; Ruiz-Sopeña, C.; Gallardo-García, V.; et al. COVID-19 Vaccine Effectiveness against Hospitalization Due to SARS-CoV-2: A Test-Negative Design Study Based on Severe Acute Respiratory Infection (SARI) Sentinel Surveillance in Spain. Influenza Other Respir. Viruses 2022, 16, 1014–1025. [CrossRef]
- Richards, F.; Kodjamanova, P.; Chen, X.; Li, N.; Atanasov, P.; Bennetts, L.; Patterson, B.J.; Yektashenas, B.; Mesa-Frias, M.; Tronczynski, K.; et al. Economic Burden of COVID-19: A Systematic Review. Clin. Outcomes Res. CEOR 2022, 14, 293–307. [CrossRef]
- COVID-19 Variants | WHO COVID-19 Dashboard Available online: https://data.who.int/dashboards/covid19/variants (accessed on 25 July 2024).
- Zhang, X.; Tan, Y.; Ling, Y.; Lu, G.; Liu, F.; Yi, Z.; Jia, X.; Wu, M.; Shi, B.; Xu, S.; et al. Viral and Host Factors Related to the Clinical Outcome of COVID-19. Nature 2020, 583, 437–440. [CrossRef]
- Fadl, N.; Ali, E.; Salem, T.Z. COVID-19: Risk Factors Associated with Infectivity and Severity. Scand. J. Immunol. 2021, 93, e13039. [CrossRef]
- Gutiérrez-Gutiérrez, B.; Del Toro, M.D.; Borobia, A.M.; Carcas, A.; Jarrín, I.; Yllescas, M.; Ryan, P.; Pachón, J.; Carratalà, J.; Berenguer, J.; et al. Identification and Validation of Clinical Phenotypes with Prognostic Implications in Patients Admitted to Hospital with COVID-19: A Multicentre Cohort Study. Lancet Infect. Dis. 2021, 21, 783–792. [CrossRef]
- Factsheet on COVID-19 Available online: https://www.ecdc.europa.eu/en/infectious-disease-topics/z-disease-list/covid-19/factsheet-covid-19 (accessed on 25 July 2024).
- Talukder, A.; Razu, S.R.; Alif, S.M.; Rahman, M.A.; Islam, S.M.S. Association Between Symptoms and Severity of Disease in Hospitalised Novel Coronavirus (COVID-19) Patients: A Systematic Review and Meta-Analysis. J. Multidiscip. Healthc. 2022, 15, 1101–1110. [CrossRef]
- Shoaib, N.; Noureen, N.; Munir, R.; Shah, F.A.; Ishtiaq, N.; Jamil, N.; Batool, R.; Khalid, M.; Khan, I.; Iqbal, N.; et al. COVID-19 Severity: Studying the Clinical and Demographic Risk Factors for Adverse Outcomes. PloS One 2021, 16, e0255999. [CrossRef]
- Battaglini, D.; Lopes-Pacheco, M.; Castro-Faria-Neto, H.C.; Pelosi, P.; Rocco, P.R.M. Laboratory Biomarkers for Diagnosis and Prognosis in COVID-19. Front. Immunol. 2022, 13, 857573. [CrossRef]
- Mazzalai, E.; Giannini, D.; Tosti, M.E.; D’Angelo, F.; Declich, S.; Jaljaa, A.; Caminada, S.; Turatto, F.; De Marchi, C.; Gatta, A.; et al. Risk of Covid-19 Severe Outcomes and Mortality in Migrants and Ethnic Minorities Compared to the General Population in the European WHO Region: A Systematic Review. J. Int. Migr. Integr. 2023, 1–31. [CrossRef]
- Charkoftaki, G.; Aalizadeh, R.; Santos-Neto, A.; Tan, W.Y.; Davidson, E.A.; Nikolopoulou, V.; Wang, Y.; Thompson, B.; Furnary, T.; Chen, Y.; et al. An AI-Powered Patient Triage Platform for Future Viral Outbreaks Using COVID-19 as a Disease Model. Hum. Genomics 2023, 17, 80. [CrossRef]
- Rello, J.; Storti, E.; Belliato, M.; Serrano, R. Clinical Phenotypes of SARS-CoV-2: Implications for Clinicians and Researchers. Eur. Respir. J. 2020, 55, 2001028. [CrossRef]
- Lusczek, E.R.; Ingraham, N.E.; Karam, B.S.; Proper, J.; Siegel, L.; Helgeson, E.S.; Lotfi-Emran, S.; Zolfaghari, E.J.; Jones, E.; Usher, M.G.; et al. Characterizing COVID-19 Clinical Phenotypes and Associated Comorbidities and Complication Profiles. PloS One 2021, 16, e0248956. [CrossRef]
- Azoulay, E.; Zafrani, L.; Mirouse, A.; Lengliné, E.; Darmon, M.; Chevret, S. Clinical Phenotypes of Critically Ill COVID-19 Patients. Intensive Care Med. 2020, 46, 1651–1652. [CrossRef]
- Berenguer, J.; Ryan, P.; Rodríguez-Baño, J.; Jarrín, I.; Carratalà, J.; Pachón, J.; Yllescas, M.; Arriba, J.R.; COVID-19@Spain Study Group; Fundación SEIMC-GESIDA; et al. Characteristics and Predictors of Death among 4035 Consecutively Hospitalized Patients with COVID-19 in Spain. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 1525–1536. [CrossRef]
- Rose, A.M.; Kissling, E.; Valenciano, M. European Study of Risk Factors for Severe Disease among Hospitalised COVID-19 Patients: I-MOVE-COVID-19 Protocol. 2021. [CrossRef]
- Rose, A.M.; Kissling, E.; Valenciano, M. European Study of COVID-19 Vaccine Effectiveness against Hospitalised SARI Patients Laboratory-Confirmed with SARS-CoV-2: I-MOVE-COVID-19 Generic Protocol. 2021. [CrossRef]
- Ladbury, G.; Rose, A.M.; Kissling, E.; Valenciano, M. COVID-19 European Hospital Surveillance: I-MOVE-COVID-19 Draft Generic Protocol. 2021. [CrossRef]
- Global Burden of Disease Study 2019 (GBD 2019) Data Resources | GHDx Available online: https://ghdx.healthdata.org/gbd-2019 (accessed on 26 July 2024).
- Tobacco Consumption Statistics Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Tobacco_consumption_statistics (accessed on 26 July 2024).
- Miller, L.J.; Lu, W. These Are the Economies With the Most (and Least) Efficient Health Care. Bloomberg.com 2018.
- Schwab, K. The Gobal Competitiveness Report, 2019; World Economic Forum, 2020;
- Jovell, A.; Blendon, R.J.; Navarro, M.D.; Fleischfresser, C.; Benson, J.M.; DesRoches, C.M.; Weldon, K.J. Public Trust in the Spanish Health-Care System. Health Expect. 2007, 10, 350–357. [CrossRef]
- Wendt, C.; Kohl, J.; Mischke, M.; Pfeifer, M. How Do Europeans Perceive Their Healthcare System? Patterns of Satisfaction and Preference for State Involvement in the Field of Healthcare. Eur. Sociol. Rev. 2010, 26, 177–192. [CrossRef]
- Wu, G.; Yang, P.; Xie, Y.; Woodruff, H.C.; Rao, X.; Guiot, J.; Frix, A.-N.; Louis, R.; Moutschen, M.; Li, J.; et al. Development of a Clinical Decision Support System for Severity Risk Prediction and Triage of COVID-19 Patients at Hospital Admission: An International Multicentre Study. Eur. Respir. J. 2020, 56, 2001104. [CrossRef]
- Wehrfritz, A.; Schmidt, J.; Bremer, F.; Lang, A.; Welzer, J.; Castellanos, I. Ethical Conflicts Associated with COVID-19 Pandemic, Triage and Frailty—Unexpected Positive Disease Progression in a 90-year-old Patient: A Case Report. Clin. Case Rep. 2023, 11, e7710. [CrossRef]
- Tanzadehpanah, H.; Lotfian, E.; Avan, A.; Saki, S.; Nobari, S.; Mahmoodian, R.; Sheykhhasan, M.; Froutagh, M.H.S.; Ghotbani, F.; Jamshidi, R.; et al. Role of SARS-COV-2 and ACE2 in the Pathophysiology of Peripheral Vascular Diseases. Biomed. Pharmacother. 2023, 166, 115321. [CrossRef]
- World Health Organization Clinical Management of Severe Acute Respiratory Infection When COVID-19 Is Suspected. Interim Guidance. Available online: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed on 25 July 2024).
- Zayet, S.; Gendrin, V.; Klopfenstein, T. Natural History of COVID-19: Back to Basics. New Microbes New Infect. 2020, 38, 100815. [CrossRef]
| All 2050 (100%) |
Women 961 (46.8%) |
Men 1089 (53.1%) |
p value | |
|---|---|---|---|---|
| Sociodemographic and clinical factors | ||||
| Age, grouped (0-39; 40-64; 65-84; 85+ years) | <0.001 | |||
| Age 0-39 years | 215 (10.5) | 106 (11.0) | 109 (10.0) | |
| Age 40-64 years | 810 (39.5) | 332 (34.5) | 478 (43.9) | |
| Age 65-84 years | 723 (35.3) | 359 (37.4) | 364 (33.5) | |
| Age 85+ years | 301 (14.7) | 164 (17.1) | 137 (12.6) | |
| Centre (HUVN) | 833 (40.6) | 374 (38.9) | 459 (42.1) | 0.137 |
| Covid-19 vaccinated (only when available) | 215 (23.6) | 91 (22.1) | 124 (24.8) | 0.345 |
| Flu vaccinated | 794 (39.1) | 394 (41.4) | 400 (37.1) | 0.050 |
| Number of previous hospitalizations (last year), mean ± SD | 2.5 ± 3.2 | 2.4 ± 2.1 | 2.7 ± 3.8 | 0.653 |
| Number of previous medical visits (last year), mean ± SD | 6.8 ± 7.1 | 7.7± 7.4 | 6.1± 6.9 | 0.009 |
| Previous medical visits last year >12 | 89 (15.2) | 48 (18.4) | 41 (12.6) | 0.053 |
| Outcomes | ||||
| Mortality | 309 (15.1) | 138 (14.4) | 171 (15.7) | 0.402 |
| ICU stay | 179 (8.8) | 70 (39.1) | 109 (60.9) | 0.031 |
| Need for ventilation | 548 (27.4) | 223 (23.7) | 325 (30.5) | <0.001 |
| Time between events | ||||
| Days from onset to admission, mean ± SD | 6.6 ± 12.4 | 6.2 ± 12.7 | 6.9 ± 12.1 | 0.166 |
| Days from onset to ICU, mean ± SD | 9.9 ± 4.6 | 9.7 ± 5.1 | 9.9 ± 4.2 | 0.750 |
| Days of stay, mean ± SD | 12.2 ± 14.6 | 11.9 ± 16.4 | 12.6 ± 12.7 | 0.263 |
| Days of stay (> 7 days) | 1248 (60.9) | 569 (59.7) | 679 (62.8) | 0.159 |
| Days of ICU stay, mean ± SD | 23.9 ± 20.2 | 23.9 ± 21.9 | 24.0 ± 19.2 | 0.969 |
| Days from admission to ICU, mean ± SD | 3.8 ± 3.3 | 3.8 ± 3.8 | 3.8 ± 2.9 | 0.881 |
| Days from admission to ICU >7 | 14 (7.8) | 6 (8.6) | 8 (7.3) | 0.765 |
| Underlying conditions | ||||
| Anaemia | 241 (11.8) | 138 (14.4) | 103 (9.5) | <0.001 |
| Asthma | 171 (8.4) | 117 (12.2) | 54 (5.0) | <0.001 |
| Body mass index (BMI), mean ± SD | 29.4 ± 5.7 | 29.4 ± 6.1 | 29.4 ± 5.3 | 0.831 |
| Cancer | 161 (7.9) | 70 (7.3) | 91 (8.4) | 0.376 |
| Dementia | 197 (9.6) | 111 (11.6) | 86 (7.9) | 0.005 |
| Diabetes | 487 (23.8) | 220 (22.9) | 267 (24.5) | 0.396 |
| Heart disease | 405 (19.8) | 161 (16.8) | 244 (22.4) | 0.002 |
| Hypertension | 978 (47.8) | 461 (48.1) | 517 (47.6) | 0.818 |
| Ictus | 124 (6.1) | 50 (5.2) | 74 (6.8) | 0.134 |
| Immunodeficiency | 39 (1.9) | 16 (1.7) | 23 (2.1) | 0.464 |
| Kidney disease | 211 (10.3) | 103 (10.8) | 108 (9.9) | 0.550 |
| Lung disease | 216 (10.6) | 69 (7.2) | 147 (13.5) | <0.001 |
| Liver disease | 87 (4.3) | 33 (3.4) | 54 (5.0) | 0.089 |
| Neuromuscular disorders | 110 (5.4) | 50 (5.2) | 60 (5.5) | 0.767 |
| Obesity | 548 (48.4) | 287 (50.7) | 261 (46.0) | 0.115 |
| Rheumatic disease | 501 (24.5) | 290 (30.2) | 211 (19.4) | <0.001 |
| Symptoms | ||||
| Abdominal pain | 59 (2.9) | 34 (3.6) | 25 (2.3) | 0.098 |
| Ageusia | 159 (7.9) | 86 (9.0) | 73 (6.8) | 0.061 |
| Anosmia | 169 (8.4) | 88 (9.3) | 81 (7.5) | 0.061 |
| Chest pain | 263 (12.9) | 147 (15.4) | 116 (10.8) | 0.002 |
| Chills | 67 (3.3) | 30 (3.2) | 37 (3.5) | 0.706 |
| Confusion | 76 (3.7) | 37 (3.9) | 39 (3.6) | 0.774 |
| Coryza | 67 (3.3) | 46 (4.8) | 21 (2.0) | <0.001 |
| Cough | 1435 (70.6) | 685 (71.9) | 750 (69.5) | 0.242 |
| Diarrhoea | 368 (18.1) | 196 (20.5) | 172 (16.0) | 0.008 |
| Dizzy | 92 (4.5) | 47 (4.9) | 45 (4.2) | 0.426 |
| Dyspnoea | 1334 (65.6) | 629 (65.9) | 705 (65.3) | 0.756 |
| Fever | 680 (33.5) | 286 (30.0) | 394 (36.6) | 0.002 |
| Feverish | 1365 (67.1) | 637 (66.6) | 728 (67.5) | 0.666 |
| General deterioration | 671 (33.1) | 346 (36.2) | 325 (30.2) | 0.004 |
| Headache | 327 (16.1) | 171 (17.9) | 156 (14.5) | 0.036 |
| Malaise | 1249 (61.5) | 588 (61.6) | 661 (61.4) | 0.928 |
| Myalgia | 481 (23.7) | 236 (24.7) | 245 (22.8) | 0.298 |
| Nausea | 159 (7.8) | 97 (10.2) | 62 (5.8) | <0.001 |
| Sore throat | 102 (5.0) | 53 (5.6) | 49 (4.6) | 0.307 |
| Tachycardia | 572 (28.1) | 257 (26.8) | 315 (29.2) | 0.225 |
| Vomiting | 130 (6.4) | 83 (8.7) | 47 (4.4) | <0.001 |
| Clinical measures | ||||
| SBP (mm Hg), mean ± SD | 129 ± 21.2 | 126.5± 21.6 | 131.1± 20.5 | <0.001 |
| SBP < 90 mm Hg | 42 (2.2) | 29 (3.3) | 13 (1.3) | 0.004 |
| DBP (mm Hg), mean ± SD | 74.4 ± 13.4 | 73.4± 13.7 | 75.3± 13.1 | 0.002 |
| DBP < 60 mm Hg | 228 (12.2) | 123 (13.9) | 105 (10.7) | 0.033 |
| Heart rate (bpm), mean ± SD | 91 ± 21.2 | 91.1 ± 21.0 | 90.8 ± 21.4 | 0.728 |
| Long QT | 50 (6.5) | 16 (4.3) | 34 (8.5) | 0.020 |
| Oxygen saturation (%), mean ± SD | 92.2 ± 5.7 | 92.4 ± 5.7 | 92.1 ± 5.7 | 0.174 |
| Low oxygen saturation | 1134 (62.2) | 500 (58.8) | 634 (65.2) | 0.005 |
| Respiratory rate (rpm), mean ± SD | 25.8 ± 26.6 | 27.9 ± 37.2 | 23.8 ± 8.1 | 0.142 |
| Biochemical alterations | ||||
| Hypoalbuminaemia (albumin < 3.5 g/dl) | 469 (28.1) | 230 (29.9) | 239 (26.6) | 0.128 |
| ALT > 35 UI/l | 676 (33.7) | 259 (27.3) | 417 (39.3) | <0.001 |
| AST > 35UI/l | 917 (46.9) | 384 (41.7) | 533 (51.5) | <0.001 |
| Hyperbilirubinaemia >1.2 mg/dl | 87 (4.4) | 16 (1.7) | 71 (6.8) | <0.001 |
| C-reactive protein > 1 mg/dl | 1984 (98.6) | 931 (98.5) | 1053 (98.7) | 0.746 |
| CPK > 145 IU/L | 366 (25.5) | 115 (17.9) | 251 (31.6) | <0.001 |
| D-dimer (> 500 μg/ml | 1327 (68.0) | 649 (71.2) | 678 (65.2) | 0.005 |
| Eosinophilia (> 500 eosinophils/μl) | 92 (4.6) | 47 (5.0) | 45 (4.2) | 0.420 |
| Ferritin > 200 ng/ml | 1970 (99.6) | 922 (99.4) | 1048 (99.8) | 0.111 |
| GGT > 38 UI/l | 1126 (57.0) | 467 (50.5) | 659 (62.8) | <0.001 |
| LDH > 247 UI/L | 1462 (77.5) | 679 (77.0) | 783 (78.0) | 0.602 |
| Neutrophilopenia (< 1500 neutrophils/μl) | 40 (2.0) | 21 (2.2) | 19 (1.8) | 0.485 |
| Neutrophilia (> 7700 neutrophils /μl) | 374 (18.4) | 157 (16.5) | 217 (20.2) | 0.030 |
| Thrombocytopenia (< 140 platelets /μl) | 350 (17.3) | 123 (12.9) | 227 (21.2) | <0.001 |
| Thrombocytophilia (> 370 platelets /μl) | 115 (5.7) | 66 (6.9) | 49 (4.6) | 0.022 |
| Prothrombin time > 14.3 seconds | 335 (17.1) | 123 (13.4) | 212 (20.3) | <0.001 |
| Urea in blood > 20 mg/ml | 1902 (94.9) | 858 (91.6) | 1044 (97.8) | <0.001 |
| Data is expressed by absolute (n) and relative (%) frequencies for categorical variables, and by mean (x̅) ± standard deviation (SD) for quantitative variables. Figures in bold point for significant differences (p value ≤ 0.05). Abbreviations: HUVN: Virgen de las Nieves University Hospital, ICU: Intensive Care Unit, SBP: systolic blood pressure, DBP: diastolic blood pressure, ALT: alanine transaminase, AST: aspartate transaminase, CPK: creatinine phosphokinase, GGT: gamma-glutamyl transferase, LDH: lactate dehydrogenase. The reference categoryfor age is 0-40 years. | ||||
| Death Adjusted OR (IC 95%) |
ICU admission Adjusted OR (IC 95%) |
Need for ventilation Adjusted OR (IC 95%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| All | HUMS | HUVN | All | HUMS | HUVN | All | HUMS | HUVN | |
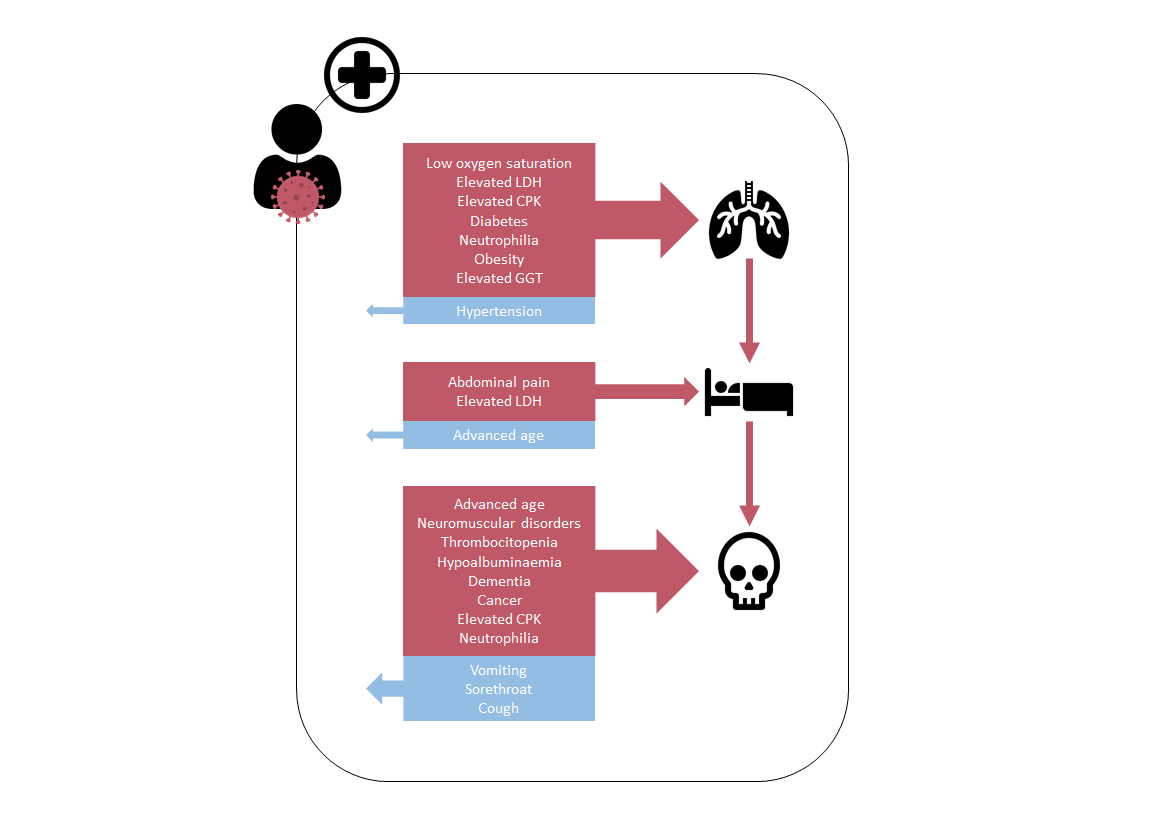
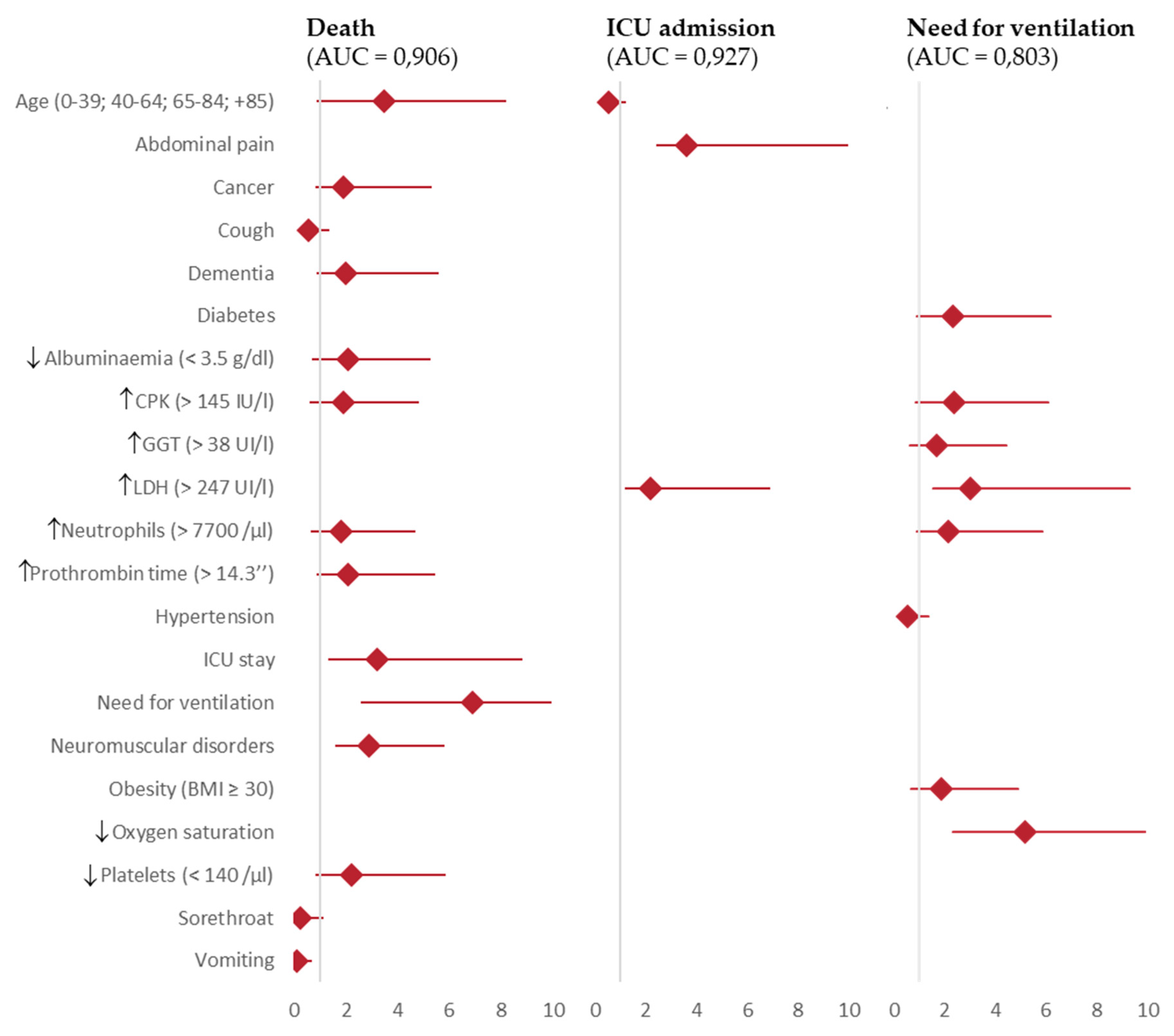
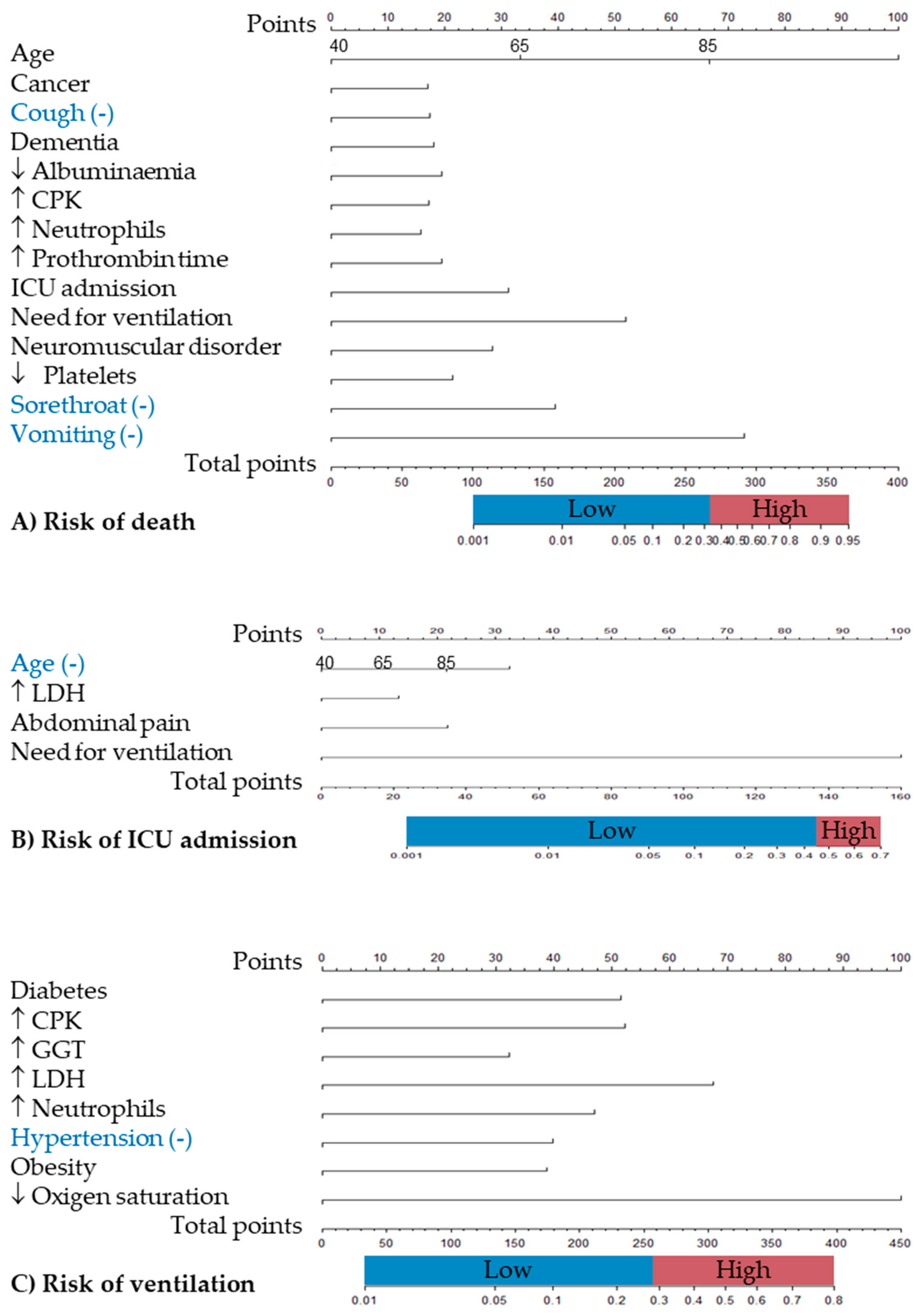
| Age (<40; 40-64; 65-84; +85 years) | 3.5 (2.6 – 4.7)*** | 4.2 (2.4-7.4)*** | 3.1 (2.1-4.6)*** | 0.5 (0.4 – 0.7)*** | 0.6 (0.3-0.9)* | 0.5 (0.4-0.7)*** | |||
| Abdominal pain | 3.6 (1.2 – 10.8)* | 3.2 (0.4-28.2) | 3.8 (1.1-13.6)* | ||||||
| Cancer | 1.9 (1.04 – 3.4)* | 3.0 (1.1-8.5)* | 1.7 (0.8-3.8) | ||||||
| Cough | 0.5 (0.4 – 0.8)** | 0.6 (0.3-1.2) | 0.6 (0.3-1.0)* | ||||||
| Dementia | 2.0 (1.1 – 3.6)* | 5.3 (2.1-13.5)*** | 0.6 (0.2-1.6) | ||||||
| Diabetes | 2.3 (1.4 – 3.9)*** | 2.5 (1.4-4.6)** | 1.9 (0.6-5.6) | ||||||
| Hypoalbuminaemia (< 3.5 g/dl) | 2.1 (1.3 – 3.2)*** | 1.2 (0.6-2.3) | 3.9 (2.0-7.9)*** | ||||||
| Elevated CPK (> 145 IU/l) | 1.9 (1.2 – 2.9)** | 1.7 (0.8-3.4) | 2.1 (1.2-3.8)* | 2.4 (1.5 – 3.7)*** | 2.2 (1.3-3.9)** | 2.4 (0.9-6.6) | |||
| Elevated GGT (> 38 UI/l) | 1.7 (1.1 – 2.7)* | 1.3 (0.8-2.3) | 2.8 (0.9-8.8) | ||||||
| Elevated LDH(> 247 UI/l) | 2.2 (1.0 – 4.7)* | 2.5 (0.4-28.2) | 1.9 (0.7-5.6) | 3.0 (1.5 – 6.3)** | 3.2 (1.4-7.5)** | 0.8 (0.1-5.5) | |||
| Elevated neutrophils (> 7700 /μl) | 1.8 (1.1 – 2.9)* | 4.4 (2.0-9.4)*** | 1.3 (0.7-2.4) | 2.2 (1.3 – 3.7)** | 1.8 (0.9-3.4) | 2.4 (0.7-8.3) | |||
| Elevated prothrombin time (> 14.3’’) | 2.1 (1.2 – 3.3)** | 3.4 (1.6-7.1)*** | 1.2 (0.6-2.4) | ||||||
| Hypertension | 0.5 (0.3 – 0.8)** | 0.7 (0.4-1.2) | 0.3 (0.1-0.8)* | ||||||
| ICU stay | 3.2 (1.8 – 5.6)*** | 4.0 (1.2-12.8)* | 2.5 (1.3-4.9)** | ||||||
| Need for ventilation | 6.9 (4.3-11.1)*** | 11.5 (4.7-28.2)*** | 9.9 (4.5-21.7)*** | 371 (90.8 – 1516.8)*** | 213.4 (50.3-906.3)*** | 5.6e-8 (0-inf) | |||
| Neuromuscular disorders | 2.9 (1.2 – 2.9)** | 6.9 (2.4-19.5)*** | 0.5 (0.1-2.4) | ||||||
| Obesity (BMI ≥ 30) | 1.9 (1.2 – 3.0** | 1.2 (0.7-2.2) | 0.5 (0.1-3.3) | ||||||
| Low oxygen saturation | 5.2 (2.8 – 9.4)*** | 4.9 (2.4-10.0)*** | 4.9 (1.3-19.3)* | ||||||
| Low platelets (< 140 /μl) | 2.2 (1.4 – 3.6)*** | 2.2 (1.0-4.6)* | 2.7 (1.4-5.3)** | ||||||
| Sorethroat | 0.2 (0.1 – 0.8)* | 1.4e-8 (0.0-inf) | 0.5 (0.1-2.0) | ||||||
| Vomiting | 0.1 (0.0 – 0.6)* | 2.9e-8 (0.0-inf) | 0.1 (0.0-1.0)) | ||||||
| Area under ROC curve | 0.906 | 0.939 | 0.896 | 0.927 | 0.944 | 0.887 | 0.803 | 0.789 | 0.755 |
| Nagelkerke’s R2 | 0.471 | 0.571 | 0.462 | 0.520 | 0.522 | 0.480 | 0.294 | 0.231 | 0.266 |
| Accuracy | 0.881 | 0.918 | 0.873 | 0.905 | 0.950 | 0.837 | 0.813 | 0.855 | 0.663 |
| Specificity | 0.967 | 0.973 | 0.961 | 0.984 | 0.996 | 0.963 | 0.956 | 0.989 | 0.605 |
| Sensivity | 0.352 | 0.532 | 0.388 | 0.141 | 0.073 | 0.165 | 0.279 | 0.909 | 0.712 |
| Abbreviations: CPK: creatinine phosphokinase, GGT: gamma-glutamyl transferase, LDH: lactate dehydrogenase ICU: Intensive Care Unit, BMI: body mass index. p value: ***≤0.001; **≤0.01; *≤0.05. The reference for age category is 0-40 years. All data were used for the multivariate model adjustment. | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





