1. Introduction
Diabetes mellitus (DM) has emerged as a significant global public health challenge, ranking as the third leading cause of death worldwide. Recent statistical data reveal a troubling rise in the prevalence of diabetes [
1]. The growth acceleration persisted after COVID-19, as suboptimal management became a potent indicator of more severe consequences in SARS-CoV-2 infections among individuals with type 2 DM (T2DM) [
2]. In 2021, Romania documented roughly 1.5 million diagnosed diabetes cases, consistent with global trends. Additionally, it was observed that one out of every twelve adults in Romania had diabetes [
3]. DM has numerous chronic complications, comorbidities including liver steatosis and fibrosis [
4], cataracts, glaucoma [
5], heart failure (HF) [
6,
7], psychiatric disorders [
7,
8,
9,
10], and a high risk of solid malignancies [
11,
12,
13,
14,
15]. The biological consequences of this issue are significant, as evidenced by a decrease in life expectancy and health expectancy. This is primarily due to the negative effect on the quality of life caused by both acute and chronic complications. Between 2014 and 2021, the most common complications of DM in Romania were due to strokes and polyneuropathies, followed by retinopathies. The least common complications were acute myocardial infarction [
16]. However, data about the prevalence of HF in Romanian patients is lacking.
In recent years, the guidelines of the professional associations responsible for diabetes care have recommended the personalization of T2DM therapy according to the presence of comorbidities such as atherosclerotic cardiovascular disease, HF, or chronic kidney disease, in which the indicated molecules are from the class of glucose co-transporter-2 inhibitors (iSGLT2) or glucagon-like-peptide-1 (GLP-1) receptors agonist with clinical evidence in reducing the risk of these complications [
17]. However, according to the new guidelines, only a few patients are currently undergoing treatment. One reason may be the underdiagnosis of these comorbidities, such as HF.
HF is a medical disorder characterized by diverse underlying etiologies and pathophysiological processes [
18]. HF, which affects over 56 million individuals globally, presents a considerable burden on health worldwide, resulting in decreased quality of life, recurrent hospitalizations, elevated healthcare expenditures, and a substantial incidence of premature death [
18,
19,
20]. HF has gained considerable attention in diabetology in recent years, primarily due to the critical role of iSGLT2 in preventing and treating cardiovascular disease (CVC) and HF. It is widely recognized that HF is a common complication associated with diabetes [
21].
HF is a heterogeneous syndrome composed of various clinical entities and differing stages. It can be classified into three primary categories, including HF with reduced ejection fraction (HFrEF), which is characterized by a left ventricle ejection fraction (LVEF) of 40% or less; HF with mildly reduced ejection fraction (HFmrEF), where the LVEF ranges from 41% to 49%; and HF with preserved ejection fraction (HFpEF), which is defined by an LVEF of 50% or higher. Furthermore, distinct phenotypes of ventricular dysfunction in systole (left ventricular systolic dysfunction) and diastole (left ventricular diastolic dysfunction (LVDD) can be identified through echocardiography [
22]. The following text describes phenotypes that display ventricular dysfunction without any noticeable symptoms of HF [
23]. Among the categories mentioned, LVDD and HFpEF are currently considered the most prevalent phenotypes of HF in T2DM. However, there is no universally accepted estimate of the precise prevalence of the various HF subtypes [
24,
25].
A comprehensive analysis of the occurrence and likelihood of HF diagnosis and outlook in T2DM patients unveiled that the occurrence of LVDD enhanced by up to 43%, which fluctuates depending on the category. Grade I LVDD is more widespread, accounting for up to 40% of the cases [
26].
It is essential to accurately diagnose and identify individuals at risk for HF, as the pathophysiology, treatment, and prognosis vary depending on the specific subtype of HF. A timely diagnosis is crucial in this regard [
27].
A systematic review recently carried out has revealed a strong connection between nonalcoholic fatty liver disease (NAFLD) and its link to LVDD, indicating that NAFLD is a multisystemic disorder with close ties to CVDs. Globally, NAFLD is a widespread metabolic disease affecting approximately a quarter of the population [
28], an independent risk factor for CVDs [
29,
30]. A recently introduced definition is a metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as NAFLD [
31]. The implications of this alteration on cardiac structural and functional abnormalities remain unclear [
32,
33,
34].
The recent epidemiological data indicates that MASLD is linked to not only accelerated coronary artery disease but also several other detrimental effects on the heart, such as an elevated risk of LVDD and hypertrophy, as well as cardiac valvular calcification and arrhythmias, particularly persistent atrial fibrillation [
35,
36,
37,
38,
39]. Furthermore, a comprehensive meta-analysis of 11 longitudinal cohort studies, which incorporated data from over 11 million middle-aged individuals from recent times, revealed that MASLD significantly increases the long-term risk of developing HF by 1.5-fold, regardless of the presence of hypertension, T2DM, and other common cardiometabolic risk factors [
40].
However, it is crucial to establish whether MASLD is merely an association in patients with LVDD owing to shared metabolic risk factors or whether it is an independent risk factor for LVDD. This issue necessitates in-depth investigation through high-quality randomized clinical trials [
41].
The study analyzed the relationship between the right and left ventricular function, epicardiac adipose tissue thickness (EAT), liver steatosis, and fibrosis evaluated through fibromax tests.
2. Materials and Methods
2.1. Study Design and Patients
In this single-center, cross-sectional study, patients were prospectively enrolled between October 10, 2022, and June 01, 2024, from the Diabetes Clinic and outpatient Diabetes Center of the University Emergency County Hospital Pius Brînzeu Timisoara, Romania. The hospital ethics committee approved this research (332/October 10, 2022) as it met the requirements of the Helsinki Declaration (version 2013), respecting the confidentiality of patient data, according to the General Data Protection Regulation (GDPR) Compliance. All participants signed a written informed consent form before enrollment in the study.
Patients were eligible for study enrollment if the following criteria were present: T2DM patients over 18 years of age, without cardiac valvulopathies or significant cardiac disease, without chronic viral or alcoholic hepatitis, with no alcohol misuse, left ventricular ejection fraction (LVEF) > 40%, and an estimated glomerular filtration rate (eGFR) >30 mL/min. The exclusion criteria were as follows: LVEF< 40% and significant structural heart disease, ongoing or planned pregnancy, lactation, other types than T2DM, eGFR < 30 mL/min, underweight with a body mass index (BMI) < 23 kg/m2, history of pancreatitis, or refusal to participate in this clinical trial.
2.2. Laboratory Tests and Clinical Examinations
Under fasting conditions (minimum 12 hours of food rest), the patients underwent routine laboratory tests (glycated hemoglobin (HbA1c), high-density lipoprotein cholesterol (HDLc), low-density lipoprotein cholesterol (LDLc), estimated glomerular filtration rate (eGFR), urinary albumin/creatinine ratio (UACr), total bilirubin (TB), alkaline phosphatase (FAL)), and fibromax, with the prior signing of a responsibility assumption form regarding compliance with the pre-analytical conditions established by the external lab and the provision of correct height and weight data. Fibromax is a medical laboratory analysis tool developed by BioPredictive, in which the following five non-invasive tests are combined: FIBROTEST (degree of liver fibrosis), ACTITEST (necroinflammatory activity), STEATOTEST (degree of hepatic steatosis), NASHTEST (presence of nonalcoholic steatohepatitis in patients with dyslipidemia, insulin resistance, diabetes, or obesity), and ASHTEST (degree of liver damage in patients with excessive alcohol consumption). The Fibromax score for evaluating the degree of liver fibrosis was obtained using a calculation algorithm that combined the results of medical laboratory analyses with the patient's age, sex, weight, and height. Medical laboratory analyses determined for Fibromax included ɑ2 macroglobulin, apolipoprotein A1, total bilirubin, haptoglobin, gamma-glutamyl-transferase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), glycemia, total cholesterol, and triglycerides. Interpretation of Fibromax results was obtained from medical laboratory analyses entered on the BioPredictive website, where a report was generated with the scores of fibrosis, necroinflammatory activity, hepatic steatosis, nonalcoholic steatohepatitis, and alcoholic steatohepatitis.
We assessed the anthropometric characteristics of the patients by calculating their body mass index (BMI) and visceral adipose tissue (VAT). The abdominal waist circumference was measured with a measuring tape midway between the lower rib and iliac crest, and the proximal thigh circumference (proximal thigh C) was measured 15 cm proximal to the superior pole of the patella. The VAT was calculated using the formula VAT (women) = 2.15 × waist circumference - 3.63 × proximal thigh circumference + 1.46 × age + 6.22 × BMI - 92.713, respectively VAT (men)= 6 × waist circumference - 4.41 × proximal thigh circumference + 1.19 × age - 213.65 [
42].
2.3. Echocardiac Ultrasound Protocol
Two expert cardiologists evaluated cardiac function and epicardiac adipose tissue (EAT) by performing 2D echocardiography and tissue and spectral color Doppler. The echocardiographic parameters studied were: left atrium (LA) diameter (mm) in the parasternal incidence, long-axis view, left ventricle ejection fraction (LVEF), EAT measured in the subcostal view, parasternal long axis as the hypoechoic distance between the liver and the right ventricular wall at the end of systole, tricuspid annular plane systolic excursion (TAPSE), global longitudinal strain (GLS), and the peak velocities of the early (E) and late (A) phases of mitral valve (MV) inflow during diastole (cm/s). We calculated the E/A ratio; E-wave deceleration time (DTE); MV a`, s`, e` lateral wall velocities; MV a`, s`, e` septum velocities; calculated average E/e` ratio; ascending aorta (AO);
The 10-year primary risk of atherosclerotic cardiovascular disease (ASCVD) was estimated using the Pooled Cohort Equation (PCE) calculator. The risk was categorized as low (<5%), borderline (5 to <7.5%), intermediate (≥7.5% to <20%), or high (≥20%) [
43,
44].
2.4. Definitions and Outcomes
We defined grade 1 LVDD according to the diastolic dysfunction grading algorithm from the guide for evaluating left ventricular diastolic function by echocardiography developed by the American Society of Echocardiography and the European Association of Echocardiography [
45], using the following criteria E/A < 0.8, DTE> 200ms, average E/e' ≤8. In the data analysis, we coded the presence of LVDD as 1 and its absence or the presence of other diastolic dysfunction as 0.
MASLD was defined in studied T2DM patients with liver steatosis and at least one of five cardiometabolic risk factors [
46].
The FIBROTEST assesses liver fibrosis and is classified as no fibrosis (F0), minimal fibrosis (F1), moderate fibrosis (F2), advanced fibrosis (F3), and severe fibrosis (F4). The liver steatosis assessed with STEATOTEST classified as S0, no steatosis (<5% fat overload), S1, mild steatosis (5-33% fat overload), S2S3 clinically significant moderate to severe steatosis (34-100% fat overload). Compared to a burn, liver inflammation assessed with ACTITEST is classified as A0, A1, A2, or A3, meaning no, minimal, significant, or severe active inflammation. The alcoholic inflammation was verified with ASHTEST from H0 to H3 alcoholic inflammation. NASHTEST assessed the inflammation as an over-reaction of the body to an accumulation of fat in the liver, present in metabolic diseases such as diabetes, overweight, and dyslipidemia and classified as N0, no NASH, N1, mild NASH, N2, moderate NASH, respectively N3, severe NASH. According to the new nomenclature, we present the data as MASLD instead of NASH in the present study of T2DM patients who also present with obesity and dyslipidemia.
The main objective of this study was to evaluate the possible predictors of grade 1 LVDD in patients with T2DM.
2.5. Statistical Analysis
The data were statistically analyzed using MedCalc® Statistical Software version 22.016 (MedCalc Software Ltd, Ostend, Belgium; 2023). To meet the desired statistical constraints for the study outcome, we applied a Shapiro-Wilk test to determine whether the continuous numerical variables were normally distributed. Normal-distributed variables are presented as mean and standard deviation, while non-parametric variables are presented as median and IQR values. The number of individuals in the class and the percentage of the total subgroup represent the qualitative/nominal variables. To assess disparities in central tendency indicators between groups, we utilized t-tests (paired/unpaired) to compare the arithmetic means of parametric variables between two groups and Mann-Whitney-U tests to assess medians.
Additionally, we employed chi-square tests to determine the statistical significance of differences in proportions between groups. We conducted multivariate and logistic regression analyses to evaluate the strength of the relationships between numerical variables. The coefficient of determination (R2) was calculated to verify the proportion of variation in the independent variable that generated the variation in the dependent variable. To verify the statistical significance of the analyzed correlations and, consequently, the possibility of generalizing the association in the population, we used the distribution test of t-values, which considers the value of the correlation coefficient and the size of the studied sample. To evaluate the association of LVDD grade I with the factors included in the analysis measured on a continuous scale, we built univariate, then multivariate logistic regression models, with potential predictors as of the variables mentioned above, respectively outcome as a dichotomous event, grade I LVDD. The variation of the risk of occurrence of the outcome was interpreted by the exponent means of B (Exp (β) coefficient of the regression equation, equivalent to the percentage change in the relative value of the risk relative to an increase with a unit of measure in the predictor scale. With the help of Nagelkerke's pseudo-coefficient of determination, we showed how the regression model built explains the occurrence of the dichotomous event.
To assess the predictive ability of grade I LVDD based on a continuous variable's value, we conducted "Receiver-Operating Characteristics" (ROC) analyses. The predictive performance was measured using sensitivity and specificity. The optimal threshold value for the predictor was determined to be the Youden index. To evaluate the statistical significance of the predictive capability, we compared the area under the ROC (AUROC) curves of the model with the non-discriminant model. For 90% study power, the required sample size for the comparison of the area under the ROC curve with a null hypothesis value was 50 patients based on an α-level of 0.05, two-sided, β-level of 0.10, with 0.760 AUC expected to be found in the study, a null hypothesis AUC of 0.5, and the ratio of sample sizes in negative/positive groups ½.
In our analysis, we determined the 95% confidence interval (CI) and considered a p-value of less than 0.05 statistically significant.
3. Results
The study included 50 patients, 46% (23/50) men. Overall, the group had a mean age of 58±11.3 years, median diabetes duration of 7 (4;15) years, abdominal obesity (mean BMI 34.4±5.9 kg/m
2, mean waist 116±11 cm), who had a mean HbA1c of 7.9±1.5%. Women tended to have a higher BMI. However, men had more VAT than women (median 55.7 vs. 95.7 cm², p= 0.01). As for lipid profile, women presented higher values of apo A1, TG, and LDLc than men (p< 0.05); all patients were on statin therapy with atorvastatin or rosuvastatin. However, men had lower median GLS values than women (14.2 vs. 17.4%, p= 0.02). The 2D echocardiography and tissue and spectral color Doppler revealed significantly higher values of MV a' lateral wall and septum velocities and ascending AO in men than in women. FIBROTEST, ACTITEST, and ASHTEST results were also significantly higher in men than women. The comparison of general characteristics by gender is presented in
Table 1.
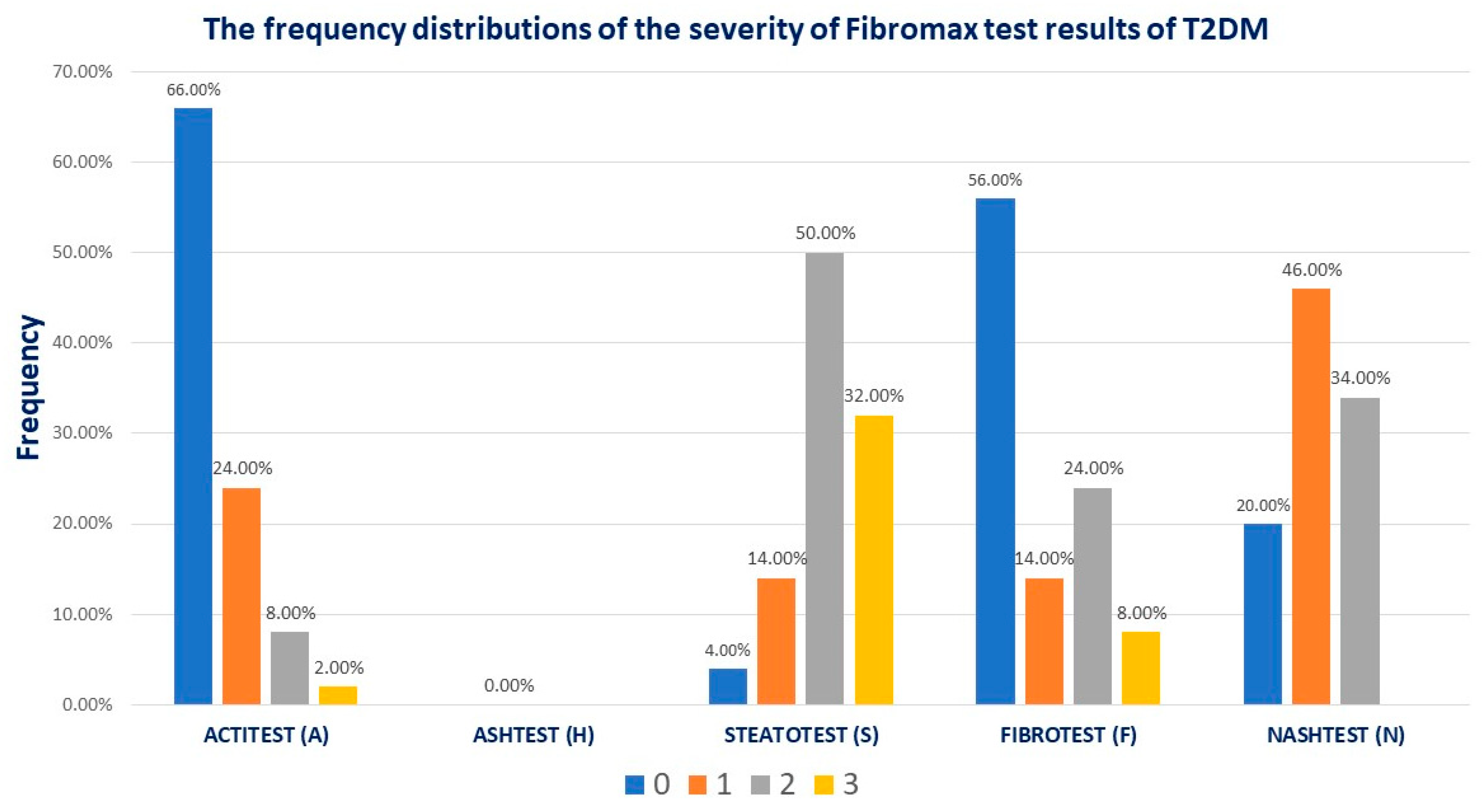
The Fibromax scores revealed that 44% had different grades of fibrosis, 14% (7/50) had mild steatosis, 80% (40/50) had moderate to severe liver steatosis, 43% (23/50) had mild MASLD, and 34% (17/50) had moderate MASLD (
Figure 1).
Interpretation of the fibromax results as follows: FIBROTEST no fibrosis (F0), mild fibrosis (F1), moderate fibrosis (F2), advanced fibrosis (F3), and severe fibrosis (F4); STEATOTEST S0, no steatosis (<5% fat overload), S1, mild steatosis (5-33% fat overload), S2S3 clinically significant moderate to severe steatosis (34-100% fat overload); ACTITEST A0, no activity, A1, minimal activity, A2, significant activity, and A3, sever activity inflammation; ASHTEST from H0 to H3 alcoholic inflammation; NASHTEST N0, no NASH, N1, mild NASH, N2, moderate NASH, respectively N3, severe NASH.
All patients had normal FEVS, TAPSE, and mean GLS of 16.1±2.5%. 54% (27/50) of the patients had grade I LVDD. Patients with grade I LVDD were older, had higher values in mean VAT (86.2±46.9 cm² vs. 57.7±33.9 cm², p= 0.04), mean EAT (4.6±1.5 mm vs. 3.4±1.6 mm, p= 0.01), median LA diameter (3.9 cm vs. 3.3 cm, p= 0.009), mean ascending AO (2.9 ± 0.3 cm vs. 2.6 ± 0.3 cm, p= 0.005), median DTE (219.5 ms vs. 183 ms, p< 0.001), median average E/e` (7.6 ms vs. 6.7 ms, p<0.0001), lower values in median MV e` septum velocities (7 cm/s vs. 10.5 cm/s, p= 0.0001), and MV e` lateral wall velocities (8 cm/s vs. 12 cm/s, p= 0.0001), compared with patients without LVDD. Notably, patients with grade I LVDD had a significantly higher PCE score of 22.8 % vs. 10.8 %, p= 0.02. Regarding fibromax results, the FIBROTEST score was significantly higher in patients with grade I LVDD than those without, 0.19 vs. 0.11, p= 0.003. After applying the chi-squared test across the severity of different fibromax scores, there were no statistical differences in the frequency of grade I LVDD. ɑ2 macroglobulin was significantly higher in patients with grade I LVDD (2.2 g/L vs. 1.6 g/L, p= 0.002).
Table 2 presents the differences between the studied factors by the presence of grade I LVDD.
In regression analysis, the strength of the associations between the numerical variables analyzed was evaluated with the help of regression coefficients: In the case of bivariate regressions, we calculated the coefficient of determination (R
2) to verify in what proportion the variation of the independent variable (studied factors) generates the variation of the dependent variable (grade I LVDD). To verify the statistical significance of the analyzed correlations and, consequently, the possibility of generalizing the association in the population, we used the distribution test of t-values that considers the value of the correlation coefficient and the size of the studied sample.
Table 3 presents the results of the regression analysis. There were significant direct associations between the outcome (grade I LVDD) and age, VAT, ɑ2 macroglobulin, EAT, TAPSE, LA, DTE, MV s` lateral wall velocities, ascending AO, MV A wave velocity, PCE risk, and FIBROTEST score, respectively negative associations with MV e wave velocity, average E/e,` MV e` septum and lateral wall velocities.
According to the univariate logistic regression model presented in
Table 4, age is a statistically significant predictive factor (Exp (β) = 1.25, p = 0.001). This result suggests that for every 1-year-old increase, the risk of grade 1 LVDD increases by 25%. For every 1 cm
2 VAT and every 1 mm EAT increase, the risk of grade 1 LVDD increases by 1% (p= 0.04), respectively, by 94% (p= 0.03). Similarly, for every 1% increase in PCE risk, the risk of grade 1 LVDD increases by 12%. Increases in MV s` lateral wall velocities, E wave velocities, e` septum, and lateral wall velocities decrease the likelihood of grade I LVDD. Increases in MV A wave velocities, DTE, average E/e`, ɑ2 macroglobulin, and FIBROTEST score increase the likelihood of grade I LVDD.
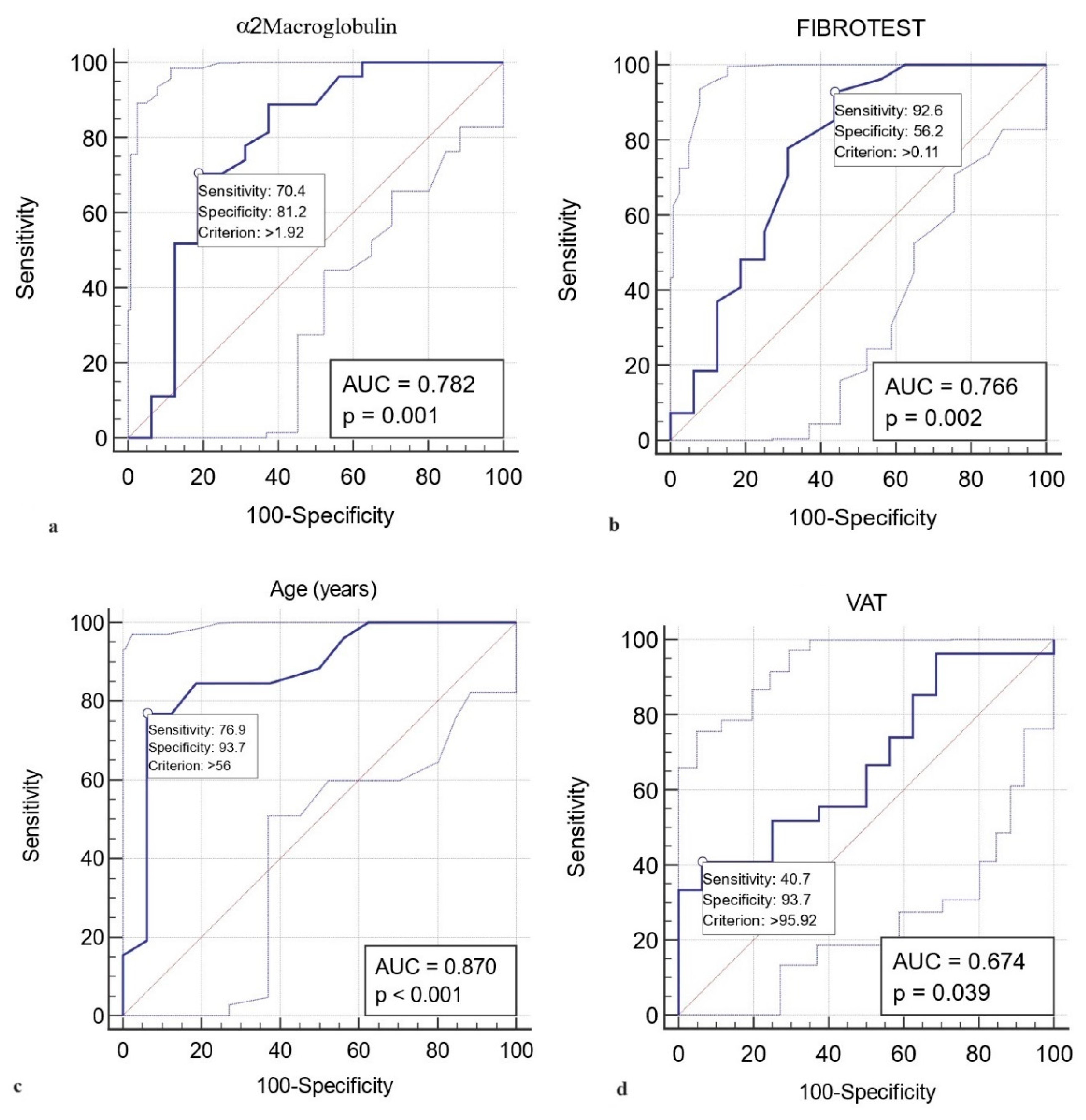
A value higher than 1.92 g/L in ɑ2 macroglobulin represented a statistically significant predictive value (AUROC curve = 0.782, sensitivity 70.4 %, specificity 81.2 %, p= 0.001) for grade 1 LVDD (
Figure 2a). Similarly, FIBROTEST scores above 0.11 predict the LVDD grade 1 with a sensitivity of 92.6 %, specificity of 56.2 %, AUROC curve = 0.766, p= 0.002. The results indicate that FIBROTEST scores above 0.11 correctly report 56.2% of patients without grade 1 LVDD as test negative (true negatives), but 43.8% of patients without grade 1 LVDD are incorrectly identified as test positive (false positives) (
Figure 2b). Younger T2DM patients than 56 years old are unlikely to present grade 1 LVDD (AUROC curve = 0.870, sensitivity 76.9 %, specificity 93.7 %, p< 0.001) (
Figure 2c). A VAT> 95.9 cm
2 predicts the LVDD grade 1 with a sensitivity of 40.7 %, specificity of 93.7 %, AUROC curve = 0.674, p= 0.03, indicating that patients with VAT lower than 95.9 cm
2 will rule out grade 1 LVDD in 93.7% of the cases (
Figure 2d).
4. Discussion
T2DM patients are more likely to develop HF, which often occurs following asymptomatic abnormalities. The present study aimed to analyze the interrelation between right and left ventricular function, EAT thickness, and liver function assessed by fibromax in patients with T2DM to identify associated factors with grade 1 LVDD, the first abnormality in the evolution of HF.
We identified in the study group that 80% of the patients presented MASLD, and 54% of the study group had grade 1 LVDD, with no statistical differences in the frequency of grade I LVDD across the different stages of MASLD. The FIBROTEST score was significantly higher in patients with grade I LVDD than those without LVDD. A recent investigation disclosed that 73.3% of cases exhibited grade 1 LVDD upon cardiac ultrasound evaluation. After a year of dapagliflozin treatment, 24.4% of these cases demonstrated remission of grade 1 LVDD. A significant predictor of a favorable therapy response was the decrease in liver fibrosis. Although the study's design did not enable the establishment of causality, it did not delve into the underlying mechanism linking the remission of LVDD and the reduction of liver fibrosis [
47,
48].
In our study, patients with grade I LVDD demonstrated a significantly higher PCE score of 22.8% compared to 10.8%. Researchers have been exploring distinct phenogroups within HF to enable more targeted interventions: younger, cardiometabolic phenogroup (predominantly female gender with a high prevalence of cardiometabolic and coronary disease), frail phenogroup, which comprises patients with lung disease or atrial fibrillation, respectively inflammatory phenogroup comprised of patients characterized by systemic inflammation with high rates of diabetes and renal dysfunction [
49]. The results of this study delineate a portrait of patients with grade 1 LVDD who concurrently present with MASLD, substantial visceral adipose tissue, and surpass the age of 56, accompanied by α2-macroglobulin concentrations higher than 1.92 g/L and liver fibrosis. It is essential to conduct additional research to explore the correlation between these factors and the potential for implementing targeted therapy for α2-macroglobulin to mitigate the likelihood of grade 1 LVDD.
Patients with grade I LVDD were older and had an EAT thickness and VAT higher than those without LVDD. Notably, ɑ2 macroglobulin was significantly higher in patients with grade I LVDD. α2-macroglobulin, a significant plasma glycoprotein primarily produced in the liver, is a large protein synthesized in the body [
50], and it is believed that it may be linked to inflammatory responses and properties that promote blood clotting, which could lead to a lack of blood flow. Endothelial dysfunction contributes to the progression of plaque atherosclerosis and the emergence of cardiovascular disease [
51]. α2-macroglobulin is a protein with diverse inhibitory functions, including the suppression of endopeptidases. It has been linked to various physiological processes, such as cell growth, inflammation, and coagulation. Research in the Moli-sani cohort study has demonstrated a relationship between α2-macroglobulin and cardiovascular events. Specifically, individuals with high levels of α2-macroglobulin were found to have an increased risk of developing coronary heart disease. Interestingly, this association was observed regardless of the subject's age, gender, or lifestyle [
52].
Cardiovascular proteomics has made impressive strides in recent years, with substantial progress in identifying novel biomarkers and elucidating the molecular mechanisms underlying cardiovascular diseases [
53].
The data revealed a substantial relationship between α2-macroglobulin and grade I LVDD, a finding that has not been previously documented. However, this research was not without its limitations. First, the study was based on a cross-sectional analysis, which allowed the assessment of only association rather than causal relationships. Second, this single-center study with a small sample size may have resulted in selection bias. Moreover, it is important to account for the potential concomitant effects of medications that target endothelial function, even if they were not administered during the examination.
5. Conclusions
HF is a complicated illness that involves damage to multiple organ systems, such as nonalcoholic MASLD. Although researchers have not yet fully understood the reasons for this link, there are some new and straightforward biomarkers, like α2-macroglobulin or fibrotest, that may indicate both liver stiffness and the likelihood of having grade I LVDD, an early and symptom-free stage of HF in individuals with T2DM.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, AB and RT; methodology, AB, VI, MMB, LD, BT; software, MMB, VI; validation, AB, BT, and RT; formal analysis, AB and BT; investigation, AB, VI, MMB, and LD; resources, VI and BT; data curation, AB, MMB, and LD; writing—original draft preparation, AB, BT, and RT; writing—review and editing, VI, MMB, and LD; visualization, AB, BT, VI, MMB, LD, and RT; supervision, BT and RT; project administration, AB; funding acquisition, AB, and BT. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to acknowledge the Victor Babes University of Medicine and Pharmacy of Timisoara for covering the publication costs for this research paper and the Association Center for Strategies for Public Health Policies in Diabetes for covering the costs for fibromax tests.
Institutional Review Board Statement
The study was conducted following the principles outlined in the Declaration of Helsinki and received ethical approval from the Ethical Committee at Emergency County Hospital Timisoara (332/October 10, 2022).
Informed Consent Statement
Patients signed written informed consent forms to provide their consent for the study.
Data Availability Statement
Participants in this study did not grant permission for the public release of their data, which is why supporting information is currently unavailable due to the confidential nature of the research.
Acknowledgments
The authors express their gratitude to the researchers from the Diabetes, Nutrition, Metabolic Diseases, and Systemic Rheumatology University Clinic at the "Pius Brinzeu" Emergency Clinical County University Hospital in Timisoara, Romania, for their invaluable help in obtaining the data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Organization WHO. WHO mortality database. 2016.
- Albai O, Braha A, Timar B, Sima A, Deaconu L, Timar R. Assessment of the Negative Factors for the Clinical Outcome in Patients with SARS-CoV-2 Infection and Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2024;17:271-82.
- Diabet FRd. Barometrul privind diabetul zaharat în România Romania2023 [Available from: România (forumdiabet.ro).
- Sima A, Sporea I, Timar R, Vlad M, Braha A, Popescu A, et al. NON-INVASIVE ASSESSMENT OF LIVER STEATOSIS AND FIBROSIS USING TRANSIENT ELASTOGRAPHY AND CONTROLLED ATTENUATION PARAMETER IN TYPE 2 DIABETES PATIENTS. Acta Endocrinologica (Bucharest). 2018;14(3):394.
- Braha A, Simion A, Timar R, Timar B. Factors Associated with Increased Intraocular Pressure in Type 2 Diabetes Patients. J Clin Med. 2024;13(3). [CrossRef]
- Braha A, Timar B, Diaconu L, Lupusoru R, Vasiluta L, Sima A, et al. Dynamics of Epicardiac Fat and Heart Function in Type 2 Diabetic Patients Initiated with SGLT-2 Inhibitors. Diabetes Metab Syndr Obes. 2019;12:2559-66. [CrossRef]
- Gonzalez-Manzanares R, Anguita-Gámez M, Muñiz J, Barrios V, Gimeno-Orna JA, Pérez A, et al. Prevalence and incidence of heart failure in type 2 diabetes patients: results from a nationwide prospective cohort-the DIABET-IC study. Cardiovasc Diabetol. 2024;23(1):253. [CrossRef]
- Shuvo SD, Hossen MT, Riazuddin M, Hossain MS, Mazumdar S, Parvin R, et al. Prevalence of comorbidities and its associated factors among type-2 diabetes patients: a hospital-based study in Jashore District, Bangladesh. BMJ Open. 2023;13(9):e076261.
- Messina R, Mezuk B, Rosa S, Iommi M, Fantini MP, Lenzi J, et al. Age of type 2 diabetes onset as a risk factor for dementia: A 13-year retrospective cohort study. Diabetes Res Clin Pract. 2024;213:111760. [CrossRef]
- Albai O, Timar B, Braha A, Timar R. Predictive Factors of Anxiety and Depression in Patients with Type 2 Diabetes Mellitus. J Clin Med. 2024;13(10). [CrossRef]
- Lindblad P, Chow WH, Chan J, Bergström A, Wolk A, Gridley G, et al. The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia. 1999;42(1):107-12. [CrossRef]
- Balasubramanyam, M. Diabetic oncopathy--one more yet another deadly diabetic complication! Indian J Med Res. 2014;140(1):15-8.
- Roman D, Saftescu S, Timar B, Avram V, Braha A, Negru Ș, et al. Diabetes Mellitus and Other Predictors for the Successful Treatment of Metastatic Colorectal Cancer: A Retrospective Study. Medicina (Kaunas). 2022;58(7). [CrossRef]
- Săftescu S, Popovici D, Oprean C, Negru A, Croitoru A, Zemba M, et al. Endurance of erythrocyte series in chemotherapy. Exp Ther Med. 2020;20(6):214. [CrossRef]
- Popovici D, Stanisav C, Pricop M, Dragomir R, Saftescu S, Ciurescu D. Associations between Body Mass Index and Prostate Cancer: The Impact on Progression-Free Survival. Medicina (Kaunas). 2023;59(2). [CrossRef]
- Cristea, DC. EVIDENŢA EVOLUŢIEI DIABETULUI ZAHARAT ÎN PERIOADA 2012-2021. MINISTERUL SĂNĂTĂŢII. INSTITUTUL NAŢIONAL DE SĂNĂTATE PUBLICĂ CENTRUL NAŢIONAL DE STATISTICĂ ÎN SĂNĂTATE PUBLICĂ; 2022.
- American Diabetes Association Professional Practice, C. Summary of Revisions: Standards of Care in Diabetes—2024. Diabetes Care. 2023;47(Supplement_1):S5-S10.
- Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30-41. [CrossRef]
- Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140(7):e294-e324. [CrossRef]
- Yan T, Zhu S, Yin X, Xie C, Xue J, Zhu M, et al. Burden, Trends, and Inequalities of Heart Failure Globally, 1990 to 2019: A Secondary Analysis Based on the Global Burden of Disease 2019 Study. J Am Heart Assoc. 2023;12(6):e027852. [CrossRef]
- Gómez-Perez AM, Damas-Fuentes M, Cornejo-Pareja I, Tinahones FJ. Heart Failure in Type 1 Diabetes: A Complication of Concern? A Narrative Review. J Clin Med. 2021;10(19). [CrossRef]
- Ng LL, Sandhu JK, Squire IB, Davies JE, Jones DJ. Vitamin D and prognosis in acute myocardial infarction. Int J Cardiol. 2013;168(3):2341-6. [CrossRef]
- Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23(3):352-80.
- Bouthoorn S, Gohar A, Valstar G, den Ruijter HM, Reitsma JB, Hoes AW, et al. Prevalence of left ventricular systolic dysfunction and heart failure with reduced ejection fraction in men and women with type 2 diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol. 2018;17(1):58. [CrossRef]
- Bouthoorn S, Valstar GB, Gohar A, den Ruijter HM, Reitsma HB, Hoes AW, et al. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis. Diab Vasc Dis Res. 2018;15(6):477-93. [CrossRef]
- Hoek AG, Dal Canto E, Wenker E, Bindraban N, Handoko ML, Elders PJM, et al. Epidemiology of heart failure in diabetes: a disease in disguise. Diabetologia. 2024;67(4):574-601. [CrossRef]
- Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263-71.
- Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol. 2022;19(1):60-78. [CrossRef]
- Abdallah LR, de Matos RC, YPDM ES, Vieira-Soares D, Muller-Machado G, Pollo-Flores P. Nonalcoholic Fatty Liver Disease and Its Links with Inflammation and Atherosclerosis. Curr Atheroscler Rep. 2020;22(1):7. [CrossRef]
- Chang W, Wang Y, Sun L, Yu D, Li Y, Li G. Evaluation of left atrial function in type 2 diabetes mellitus patients with nonalcoholic fatty liver disease by two-dimensional speckle tracking echocardiography. Echocardiography. 2019;36(7):1290-7. [CrossRef]
- Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73(1):202-9. [CrossRef]
- Eslam M, Sanyal AJ, George J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158(7):1999-2014.e1. [CrossRef]
- Younossi ZM, Rinella ME, Sanyal AJ, Harrison SA, Brunt EM, Goodman Z, et al. From NAFLD to MAFLD: Implications of a Premature Change in Terminology. Hepatology. 2021;73(3):1194-8. [CrossRef]
- Peng D, Yu Z, Wang M, Shi J, Sun L, Zhang Y, et al. Association of Metabolic Dysfunction-Associated Fatty Liver Disease With Left Ventricular Diastolic Function and Cardiac Morphology. Front Endocrinol (Lausanne). 2022;13:935390. [CrossRef]
- Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2018;15(7):425-39. [CrossRef]
- Yong JN, Ng CH, Lee CW, Chan YY, Tang ASP, Teng M, et al. Nonalcoholic fatty liver disease association with structural heart, systolic and diastolic dysfunction: a meta-analysis. Hepatol Int. 2022;16(2):269-81.
- Di Minno MN, Di Minno A, Ambrosino P, Songia P, Tremoli E, Poggio P. Aortic valve sclerosis as a marker of atherosclerosis: Novel insights from hepatic steatosis. Int J Cardiol. 2016;217:1-6. [CrossRef]
- Mantovani A, Dauriz M, Sandri D, Bonapace S, Zoppini G, Tilg H, et al. Association between nonalcoholic fatty liver disease and risk of atrial fibrillation in adult individuals: An updated meta-analysis. Liver Int. 2019;39(4):758-69. [CrossRef]
- Cai X, Zheng S, Liu Y, Zhang Y, Lu J, Huang Y. Nonalcoholic fatty liver disease is associated with increased risk of atrial fibrillation. Liver Int. 2020;40(7):1594-600. [CrossRef]
- Mantovani A, Petracca G, Csermely A, Beatrice G, Bonapace S, Rossi A, et al. Nonalcoholic fatty liver disease and risk of new-onset heart failure: an updated meta-analysis of about 11 million individuals. Gut. 2022. [CrossRef]
- Gohil NV, Tanveer N, Makkena VK, Jaramillo AP, Awosusi BL, Ayyub J, et al. Nonalcoholic Fatty Liver Disease and Its Association With Left Ventricular Diastolic Dysfunction: A Systematic Review. Cureus. 2023;15(8):e43013. [CrossRef]
- Samouda H, Dutour A, Chaumoitre K, Panuel M, Dutour O, Dadoun F. VAT=TAAT-SAAT: innovative anthropometric model to predict visceral adipose tissue without resort to CT-Scan or DXA. Obesity (Silver Spring). 2013;21(1):E41-50. [CrossRef]
- Goff DC, Jr., Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49-73.
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646.
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277-314.
- Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542-56. [CrossRef]
- Braha A, Albai A, Timar R, Diaconu L, Vasiluță L, Cipu D, et al. Factors Associated with the Remission of Type 1 Diastolic Dysfunction after Dapagliflozin Treatment in Patients with Type 2 Diabetes. J Clin Med. 2020;9(11). [CrossRef]
- Braha A, Albai A, Timar B, Cipu D, Vasiluță L, Potre O, et al. Predictors of Epicardial Fat Volume Decrease after Dapagliflozin Treatment in Patients with Type 2 Diabetes. Medicina (Kaunas). 2021;58(1). [CrossRef]
- Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, et al. Clinical Phenogroups in Heart Failure With Preserved Ejection Fraction: Detailed Phenotypes, Prognosis, and Response to Spironolactone. JACC Heart Fail. 2020;8(3):172-84.
- Andus T, Gross V, Tran-Thi TA, Schreiber G, Nagashima M, Heinrich PC. The biosynthesis of acute-phase proteins in primary cultures of rat hepatocytes. Eur J Biochem. 1983;133(3):561-71. [CrossRef]
- Shimomura R, Nezu T, Hosomi N, Aoki S, Sugimoto T, Kinoshita N, et al. Alpha-2-macroglobulin as a Promising Biological Marker of Endothelial Function. J Atheroscler Thromb. 2018;25(4):350-8. [CrossRef]
- de Laat-Kremers R, Costanzo S, Yan Q, Di Castelnuovo A, De Curtis A, Cerletti C, et al. High alpha-2-macroglobulin levels are a risk factor for cardiovascular disease events: A Moli-sani cohort study. Thromb Res. 2024;234:94-100. [CrossRef]
- Mokou M, Lygirou V, Vlahou A, Mischak H. Proteomics in cardiovascular disease: recent progress and clinical implication and implementation. Expert Review of Proteomics. 2017;14(2):117-36. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).