Submitted:
16 September 2024
Posted:
17 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Expression and Purification of TS Recombinant Antigen
2.2. Mice
2.3. Immunization and Infection Protocol
2.4. Determination of Specific Antibodies in Plasma and Nasal Lavages
2.5. Delayed Type Hypersensitivity Test
2.6. Lymphocyte Culture and Flow Cytometry
2.7. Quantification of Plasma Cytokines
2.8. NALT and Heart Cytokine Profiling by RT-qPCR
2.9. Clinical Score
2.10. Determination of Muscle and Liver Damage in the Acute and Chronic Phases
2.11. Detection of Parasite Burden in Target Tissues
2.12. Histopathology
2.13. Electrocardiograms
2.14. Statistical Analysis
3. Results
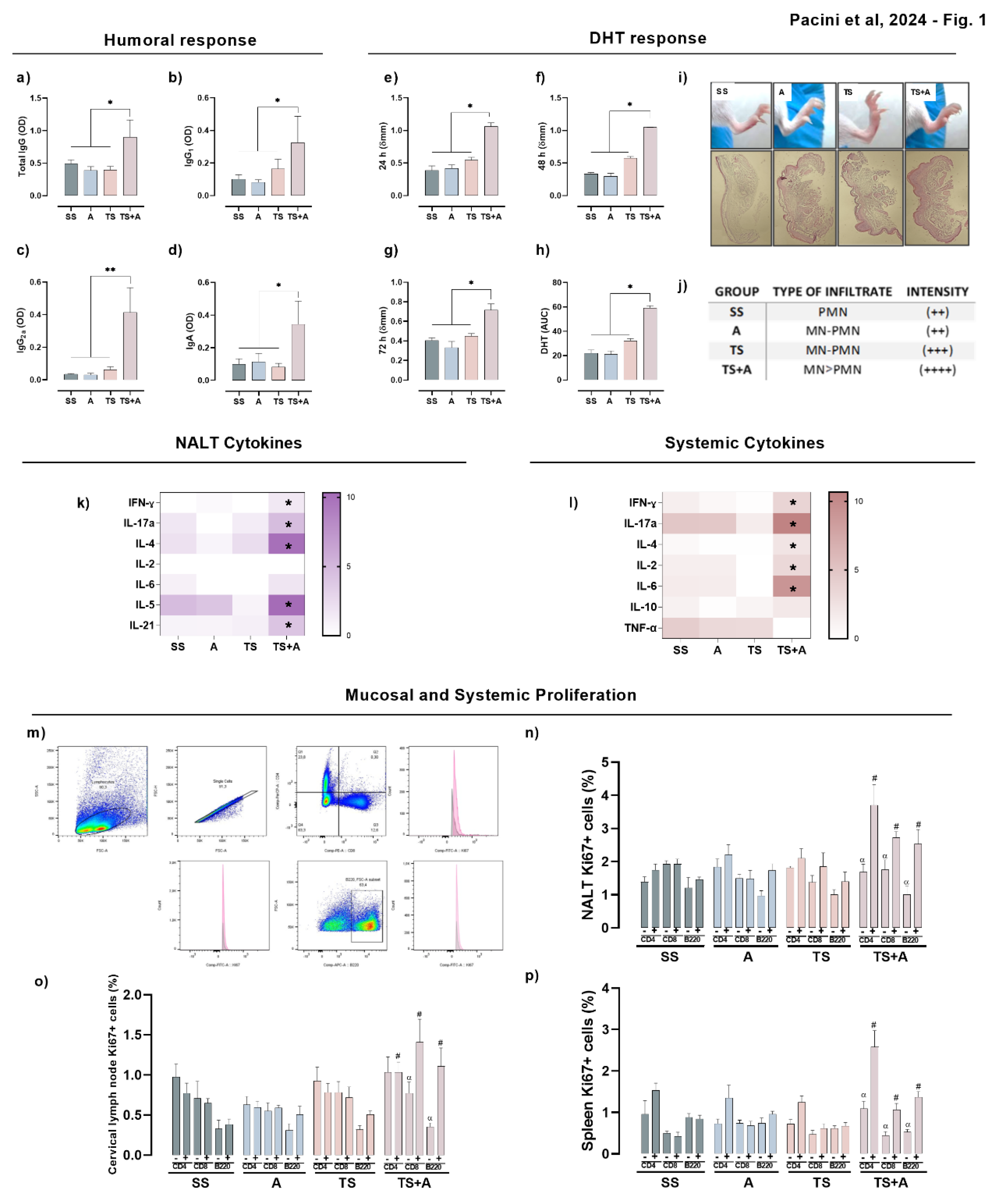
3.1. TS+A Recombinant Vaccine Elicits Both Mucosal and Systemic Immunogenicity
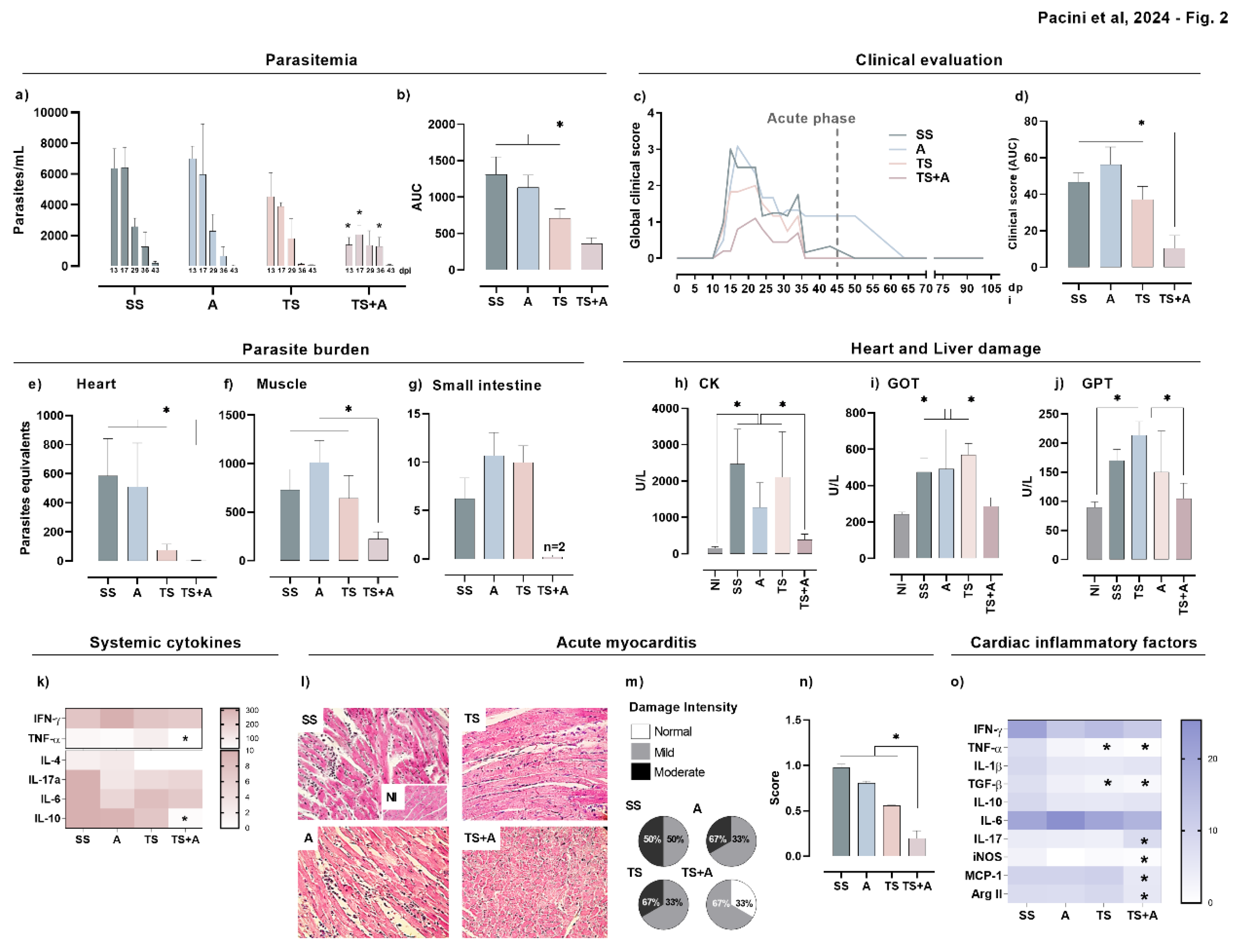
3.2. Prophylactic TS+A Immunization Confers Protection during Acute Oral T. cruzi Infection
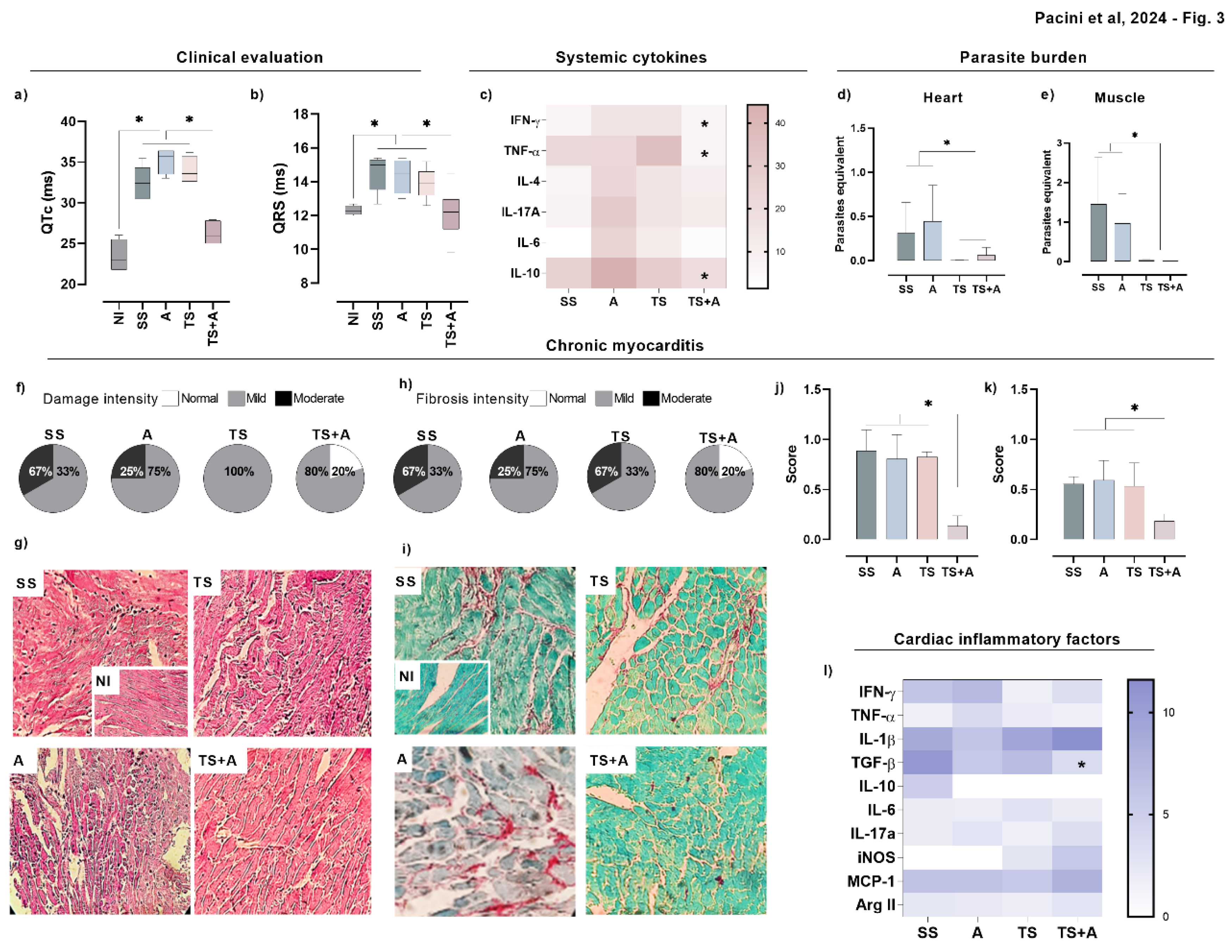
3.3. Prophylactic Immunization with TS+A Formulation Attenuates Chronic Chagasic Myocarditis and Heart Functional Abnormalities
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization World Health Organization (2023) Chagas Disease (American Trypanosomiasis). Available online: https://www.who.int/chagas/en/.
- Marin Neto, J.A.; Simões, M. V.; Sarabanda, A. V Chagas’ Heart Disease. N. Engl. J. Med. 2000, 20, 825–924. [Google Scholar]
- H S Rassr, R.; Rassi, A.; L, W.C. Chagas’ Heart Disease. Clin. Cardiol 2000, 23, 883–889. [Google Scholar]
- Rassi, A.; Rassi, A.; Marin-Neto, J.A. Chagas Disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Bonney, K.M.; Luthringer, D.J.; Kim, S.A.; Garg, N.J.; Engman, D.M. Pathology and Pathogenesis of Chagas Heart Disease. Annu. Rev. Pathol. Mech. Dis. 2019. [CrossRef]
- Coura, J.R. Transmission of Chagasic Infection by Oral Route in the Natural History of Chagas Disease. Rev. Soc. Bras. Med. Trop. 2006. [Google Scholar]
- Rueda, K.; Trujillo, J.E.; Carranza, J.C.; Vallejo, G.A. Oral Transmission of Trypanosoma Cruzi: A New Epidemiological Scenario for Chagas’ Disease in Colombia and Other South American Countries. Biomedica 2014. [Google Scholar]
- Pereira, K.S.; Schmidt, F.L.; Guaraldo, A.M.A.; Franco, R.M.B.; Dias, V.L.; Passos, L.A.C. Chagas’ Disease as a Foodborne Illness. J. Food Prot. 2009. [CrossRef] [PubMed]
- Filigheddu, M.T.; Górgolas, M.; Ramos, J.M. Orally-Transmitted Chagas Disease. Med. Clínica (English Ed. [CrossRef]
- Prata, A. Evolution of the Clinical and Epidemiological Knowledge about Chagas Disease 90 Years after Its Discovery. Mem. Inst. Oswaldo Cruz 1999. [Google Scholar] [CrossRef] [PubMed]
- de Noya, B.A.; González, O.N. An Ecological Overview on the Factors That Drives to Trypanosoma Cruzi Oral Transmission. Acta Trop. 2015. [Google Scholar] [CrossRef] [PubMed]
- Silva-dos-Santos, D.; Barreto-de-Albuquerque, J.; Guerra, B.; Moreira, O.C.; Berbert, L.R.; Ramos, M.T.; Mascarenhas, B.A.S.; Britto, C.; Morrot, A.; Serra Villa-Verde, D.M.; et al. Unraveling Chagas Disease Transmission through the Oral Route: Gateways to Trypanosoma Cruzi Infection and Target Tissues. PLoS Negl. Trop. Dis. 2017. [Google Scholar] [CrossRef]
- Giddings, O.K.; Eickhoff, C.S.; Sullivan, N.L.; Hoft, D.F. Intranasal Vaccinations with the Trans-Sialidase Antigen plus CpG Adjuvant Induce Mucosal Immunity Protective against Conjunctival Trypanosoma Cruzi Challenges. Infect. Immun. 2010. [Google Scholar] [CrossRef] [PubMed]
- Pacini, M.F.; González, F.B.; Dinatale, B.; Bulfoni Balbi, C.; Villar, S.R.; Farré, C.; Lupi, G.; Espariz, M.; Blancato, V.S.; Magni, C.; et al. Nasal Immunization with a L. Lactis-Derived Trans-Sialidase Antigen plus c-Di-AMP Protects against Acute Oral T. Cruzi Infection. Vaccine 2022, 40, 2311–2323. [Google Scholar] [CrossRef] [PubMed]
- Pacini, M.F.; Perdomini, A.; Bulfoni Balbi, C.; Dinatale, B.; Herrera, F.E.; Perez, A.R.; Marcipar, I. The High Identity of the Trypanosoma Cruzi Group-I of Trans-Sialidases Points Them as Promising Vaccine Immunogens. Proteins Struct. Funct. Bioinforma. 2023. [Google Scholar] [CrossRef] [PubMed]
- Bontempi, I.; Fleitas, P.; Poato, A.; Vicco, M.; Rodeles, L.; Prochetto, E.; Cabrera, G.; Beluzzo, B.; Arias, D.; Racca, A.; et al. Trans-Sialidase Overcomes Many Antigens to Be Used as a Vaccine Candidate against Trypanosoma Cruzi. Immunotherapy 2017, 9, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Quintana, I.; Espariz, M.; Villar, S.R.; González, F.B.; Pacini, M.F.; Cabrera, G.; Bontempi, I.; Prochetto, E.; Stülke, J.; Perez, A.R.; et al. Genetic Engineering of Lactococcus Lactis Co-Producing Antigen and the Mucosal Adjuvant 3’ 5’- Cyclic Di Adenosine Monophosphate (c-Di-AMP) as a Design Strategy to Develop a Mucosal Vaccine Prototype. Front. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pacini, M.F.; Balbi, C.B.; Dinatale, B.; González, F.B.; Prochetto, E.; De Hernández, M.A.; Cribb, P.; Farré, C.; Espariz, M.; Blancato, V.S.; et al. Intranasal Trans-Sialidase-Based Vaccine against Trypanosoma Cruzi Triggers a Mixed Cytokine Profile in the Nasopharynx-Associated Lymphoid Tissue and Confers Local and Systemic Immunogenicity. Acta Trop. 2023. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.A.; Ramos, S.G.; Bestetti, R.B. Chagas’ Heart Disease: Clinical-Pathological Correlation. Front. Biosci. 2003. [CrossRef] [PubMed]
- Álvarez, J.M.; Fonseca, R.; Borges Da Silva, H.; Marinho, C.R.F.; Bortoluci, K.R.; Sardinha, L.R.; Epiphanio, S.; D’Império Lima, M.R. Chagas Disease: Still Many Unsolved Issues. Mediators Inflamm. 2014. [CrossRef]
- Higuchi, M. de L.; De Brito, T.; Martins Reis, M.; Barbosa, A.; Bellotti, G.; Pereira-Barreto, A.C.; Pileggi, F. Correlation between Trypanosoma Cruzi Parasitism and Myocardial Inflammatory Infiltrate in Human Chronic Chagasic Myocarditis: Light Microscopy and Immunohistochemical Findings. Cardiovasc. Pathol. 1993, 2, 101–106. [CrossRef]
- Higuchi, M.D.L.; Reis, M.M.; Aiello, V.D.; Benvenuti, L.A.; Gutierrez, P.S.; Bellotti, G.; Pileggi, F. Association of an Increase in CD8+ T Cells with the Presence of Trypanosoma Cruzi Antigens in Chronic, Human, Chagasic Myocarditis. Am. J. Trop. Med. Hyg. 1997. [Google Scholar] [CrossRef]
- Bartekova, M.; Radosinska, J.; Jelemensky, M.; Dhalla, N.S. Role of Cytokines and Inflammation in Heart Function during Health and Disease. Heart Fail. Rev. 2018. [CrossRef] [PubMed]
- Koh, C.C.; Neves, E.G.A.; de Souza-Silva, T.G.; Carvalho, A.C.; Pinto, C.H.R.; Sobreira Galdino, A.; Gollob, K.J.; Dutra, W.O. Cytokine Networks as Targets for Preventing and Controlling Chagas Heart Disease. Pathogens 2023. [CrossRef]
- Burdette, D.L.; Monroe, K.M.; Sotelo-Troha, K.; Iwig, J.S.; Eckert, B.; Hyodo, M.; Hayakawa, Y.; Vance, R.E. STING Is a Direct Innate Immune Sensor of Cyclic Di-GMP. Nature 2011. [Google Scholar] [CrossRef]
- Lirussi, D.; Ebensen, T.; Schulze, K.; Trittel, S.; Duran, V.; Liebich, I.; Kalinke, U.; Guzmán, C.A. Type I IFN and Not TNF, Is Essential for Cyclic Di-Nucleotide-Elicited CTL by a Cytosolic Cross-Presentation Pathway. EBioMedicine 2017. [Google Scholar] [CrossRef]
- Ebensen, T.; Libanova, R.; Schulze, K.; Yevsa, T.; Morr, M.; Guzm??n, C.A. Bis-(3’,5’)-Cyclic Dimeric Adenosine Monophosphate: Strong Th1/Th2/Th17 Promoting Mucosal Adjuvant. Vaccine 2011, 29, 5210–5220. [Google Scholar] [CrossRef]
- Madhun, A.S.; Haaheim, L.R.; Nøstbakken, J.K.; Ebensen, T.; Chichester, J.; Yusibov, V.; Guzman, C.A.; Cox, R.J. Intranasal C-Di-GMP-Adjuvanted Plant-Derived H5 Influenza Vaccine Induces Multifunctional Th1 CD4+ Cells and Strong Mucosal and Systemic Antibody Responses in Mice. Vaccine 2011. [Google Scholar] [CrossRef] [PubMed]
- Prochetto, E.; Bontempi, I.; Rodeles, L.; Cabrera, G.; Vicco, M.; Cacik, P.; Pacini, M.F.; Pérez Gianeselli, M.; Pérez, A.R.; Marcipar, I. Assessment of a Combined Treatment with a Therapeutic Vaccine and Benznidazole for the Trypanosoma Cruzi Chronic Infection. Acta Trop. 2022, 229. [Google Scholar] [CrossRef]
- Cho, S.H.; Oh, S.Y.; Zhu, Z.; Lee, J.; Lane, A.P. Spontaneous Eosinophilic Nasal Inflammation in a Genetically-Mutant Mouse: Comparative Study with an Allergic Inflammation Model. PLoS One 2012. [Google Scholar] [CrossRef] [PubMed]
- Cisney, E.D.; Fernandez, S.; Hall, S.I.; Krietz, G.A.; Ulrich, R.G. Examining the Role of Nasopharyngeal-Associated Lymphoreticular Tissue (NALT) in Mouse Responses to Vaccines. J. Vis. Exp. 2012. [Google Scholar] [CrossRef]
- Cummings, K.L.; Tarleton, R.L. Rapid Quantitation of Trypanosoma Cruzi in Host Tissue by Real-Time PCR. Mol. Biochem. Parasitol. 2003. [Google Scholar] [CrossRef]
- Mitchell, P.H.; Ferketich, S.; Jennings, B.M. Quality Health Outcomes Model. J. Nurs. Scholarsh. 1998. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.R.; Mucci, J.; Meira, M.A.; Bogliotti, Y.; Musikant, D.; Leguizamón, M.S.; Campetella, O. Trypanosoma Cruzi Trans-Sialidase Prevents Elicitation of Th1 Cell Response via Interleukin 10 and Downregulates Th1 Effector Cells. Infect. Immun. 2015. [Google Scholar] [CrossRef]
- Nardy, A.F.F.R.; Freire-de-Lima, C.G.; Pérez, A.R.; Morrot, A. Role of Trypanosoma Cruzi Trans-Sialidase on the Escape from Host Immune Surveillance. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Cai, C.W.; Eickhoff, C.S.; Meza, K.A.; Blase, J.R.; Audette, R.E.; Chan, D.H.; Bockerstett, K.A.; DiPaolo, R.J.; Hoft, D.F. Th17 Cells Provide Mucosal Protection against Gastric Trypanosoma Cruzi Infection. Infect. Immun. 2021, 89. [Google Scholar] [CrossRef] [PubMed]
- De Meis, J.; Morrot, A.; Farias-de-Oliveira, D.A.; Villa-Verde, D.M.S.; Savino, W. Differential Regional Immune Response in Chagas Disease. PLoS Negl. Trop. Dis. 2009. [CrossRef] [PubMed]
- Morales-Sánchez, A.; Fuentes-Pananá, E.M. Human Viruses and Cancer. Viruses 2014. [CrossRef]
- Ning, H.; Zhang, W.; Kang, J.; Ding, T.; Liang, X.; Lu, Y.; Guo, C.; Sun, W.; Wang, H.; Bai, Y.; et al. Subunit Vaccine ESAT-6:C-Di-AMP Delivered by Intranasal Route Elicits Immune Responses and Protects Against Mycobacterium Tuberculosis Infection. Front. Cell. Infect. Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Eto, D.; Lao, C.; DiToro, D.; Barnett, B.; Escobar, T.C.; Kageyama, R.; Yusuf, I.; Crotty, S. IL-21 and IL-6 Are Critical for Different Aspects of B Cell Immunity and Redundantly Induce Optimal Follicular Helper CD4 T Cell (Tfh) Differentiation. PLoS One 2011. [Google Scholar] [CrossRef]
- Matos, M.N.; Cazorla, S.I.; Schulze, K.; Ebensen, T.; Guzm??n, C.A.; Malchiodi, E.L. Immunization with Tc52 or Its Amino Terminal Domain Adjuvanted with C-Di-AMP Induces Th17+Th1 Specific Immune Responses and Confers Protection against Trypanosoma Cruzi. PLoS Negl. Trop. Dis. 2017, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Cui, R.; Li, X.; Ning, H.; Kang, J.; Lu, Y.; Zhou, S.; Huang, X.; Peng, Y.; Zhang, J.; et al. Ag85B with C-Di-AMP as Mucosal Adjuvant Showed Immunotherapeutic Effects on Persistent Mycobacterium Tuberculosis Infection in Mice. Brazilian J. Med. Biol. Res. 2024, 57, 1–10. [Google Scholar]
- Acevedo, G.R.; Girard, M.C.; Gómez, K.A. The Unsolved Jigsaw Puzzle of the Immune Response in Chagas Disease. Front. Immunol. 2018. [CrossRef] [PubMed]
- Sanchez Alberti, A.; Bivona, A.E.; Cerny, N.; Schulze, K.; Weißmann, S.; Ebensen, T.; Morales, C.; Padilla, A.M.; Cazorla, S.I.; Tarleton, R.L.; et al. Engineered Trivalent Immunogen Adjuvanted with a STING Agonist Confers Protection against Trypanosoma Cruzi Infection. npj Vaccines 2017, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Alberti, A.; Bivona, A.E.; Matos, M.N.; Cerny, N.; Schulze, K.; Weißmann, S.; Ebensen, T.; González, G.; Morales, C.; Cardoso, A.C.; et al. Mucosal Heterologous Prime/Boost Vaccination Induces Polyfunctional Systemic Immunity, Improving Protection Against Trypanosoma Cruzi. Front. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, D.A.; Jackson, S.W.; Gorosito-Serran, M.; Acosta-Rodriguez, E.V.; Amezcua Vesely, M.C.; Sather, B.D.; Singh, A.K.; Khim, S.; Mucci, J.; Liggitt, D.; et al. Trypanosoma Cruzi Trans-Sialidase Initiates a Program Independent of the Transcription Factors RORγt and Ahr That Leads to IL-17 Production by Activated B Cells. Nat. Immunol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.R.; Berbert, L.R.; Lepletier, A.; Revelli, S.; Bottasso, O.; Silva-Barbosa, S.D.; Savino, W. TNF-α Is Involved in the Abnormal Thymocyte Migration during Experimental Trypanosoma Cruzi Infection and Favors the Export of Immature Cells. PLoS One 2012. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.R.; Waghabi, M.C.; Bailly, S.; Feige, J.J.; Hasslocher-Moreno, A.M.; Saraiva, R.M.; Araujo-Jorge, T.C. The Search for Biomarkers and Treatments in Chagas Disease: Insights From TGF-Beta Studies and Immunogenetics. Front. Cell. Infect. Microbiol. 2022. [CrossRef] [PubMed]
- Saraiva, R.M.; Waghabi, M.C.; Vilela, M.F.; Madeira, F.S.; Da Silva, G.M.S.; Xavier, S.S.; Feige, J.J.; Hasslocher-Moreno, A.M.; Araujo-Jorge, T.C. Predictive Value of Transforming Growth Factor-Β1in Chagas Disease: Towards a Biomarker Surrogate of Clinical Outcome. Trans. R. Soc. Trop. Med. Hyg. 2013. [Google Scholar] [CrossRef]
- Eickhoff, C.S.; Lawrence, C.T.; Sagartz, J.E.; Bryant, L.A.; Labovitz, A.J.; Gala, S.S.; Hoft, D.F. ECG Detection of Murine Chagasic Cardiomyopathy. J. Parasitol. 2010. [Google Scholar] [CrossRef]
- Guhl, F.; Ramírez, J.D. Retrospective Molecular Integrated Epidemiology of Chagas Disease in Colombia. Infect. Genet. Evol. 2013. [Google Scholar] [CrossRef]
- Zingales, B.; Andrade, S.G.; Briones, M.R.S.; Campbell, D.A.; Chiari, E.; Fernandes, O.; Guhl, F.; Lages-Silva, E.; Macedo, A.M.; Machado, C.R.; et al. A New Consensus for Trypanosoma Cruzi Intraspecific Nomenclature: Second Revision Meeting Recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz 2009, 104, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Burgos, J.M.; Diez, M.; Vigliano, C.; Bisio, M.; Risso, M.; Duffy, T.; Cura, C.; Brusses, B.; Favaloro, L.; Leguizamon, M.S.; et al. Molecular Identification of Trypanosoma Cruzi Discrete Typing Units in End-Stage Chronic Chagas Heart Disease and Reactivation after Heart Transplantation. Clin. Infect. Dis. 2010. [Google Scholar] [CrossRef] [PubMed]
- Barreto-de-Albuquerque, J.; Silva-dos-Santos, D.; Pérez, A.R.; Berbert, L.R.; de Santana-van-Vliet, E.; Farias-de-Oliveira, D.A.; Moreira, O.C.; Roggero, E.; de Carvalho-Pinto, C.E.; Jurberg, J.; et al. Trypanosoma Cruzi Infection through the Oral Route Promotes a Severe Infection in Mice: New Disease Form from an Old Infection? PLoS Negl. Trop. Dis. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, V.L.T.; Piotto, M.R.; Esper, H.R.; Nakanishi, E.Y.S.; Fonseca, C. de A.; Assy, J.G.P.L.; Berreta, O.C.P.; França, F.O. de S.; Lopes, M.H. Detection of Trypanosoma Cruzi DTUs TcI and TcIV in Two Outbreaks of Orally-Transmitted Chagas Disease in the Northern Region of Brazil. Rev. Inst. Med. Trop. Sao Paulo. [CrossRef]
- Ramírez, J.D.; Montilla, M.; Cucunubá, Z.M.; Floréz, A.C.; Zambrano, P.; Guhl, F. Molecular Epidemiology of Human Oral Chagas Disease Outbreaks in Colombia. PLoS Negl. Trop. Dis. 2013. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, W.M.; Magalhães, L.K.C.; de Sá, A.R.N.; Gomes, M.L.; Toledo, M.J. de O.; Borges, L.; Pires, I.; Guerra, J.A. de O.; Silveira, H.; Barbosa, M. das G.V. Trypanosoma Cruzi IV Causing Outbreaks of Acute Chagas Disease and Infections by Different Haplotypes in the Western Brazilian Amazonia. PLoS One 2012. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




