Submitted:
17 September 2024
Posted:
17 September 2024
You are already at the latest version
Abstract
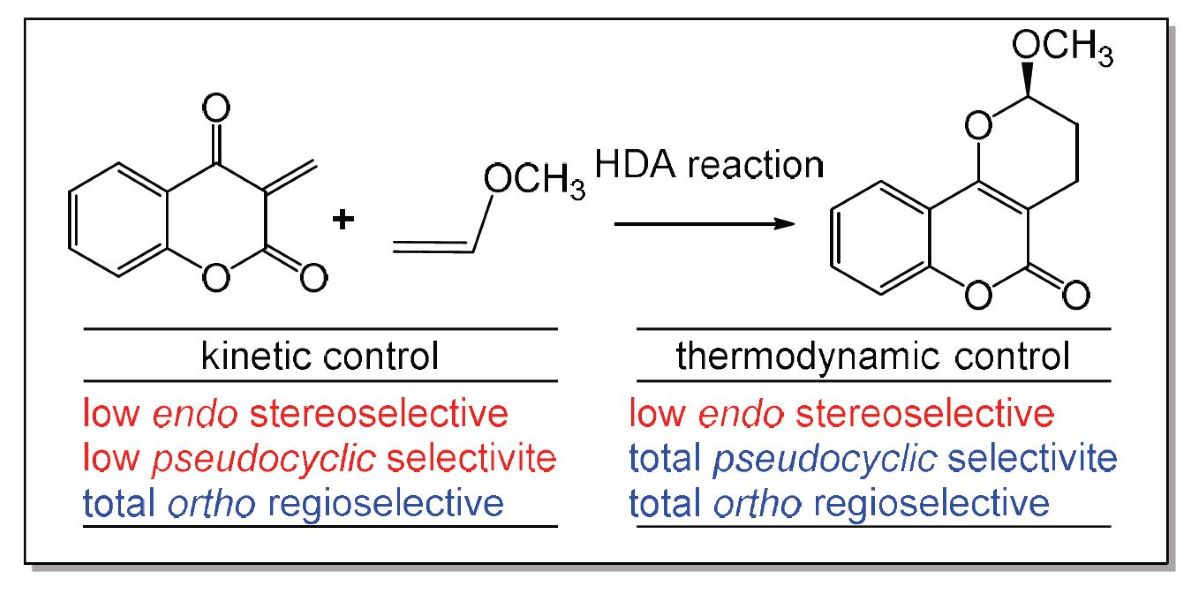
Keywords:
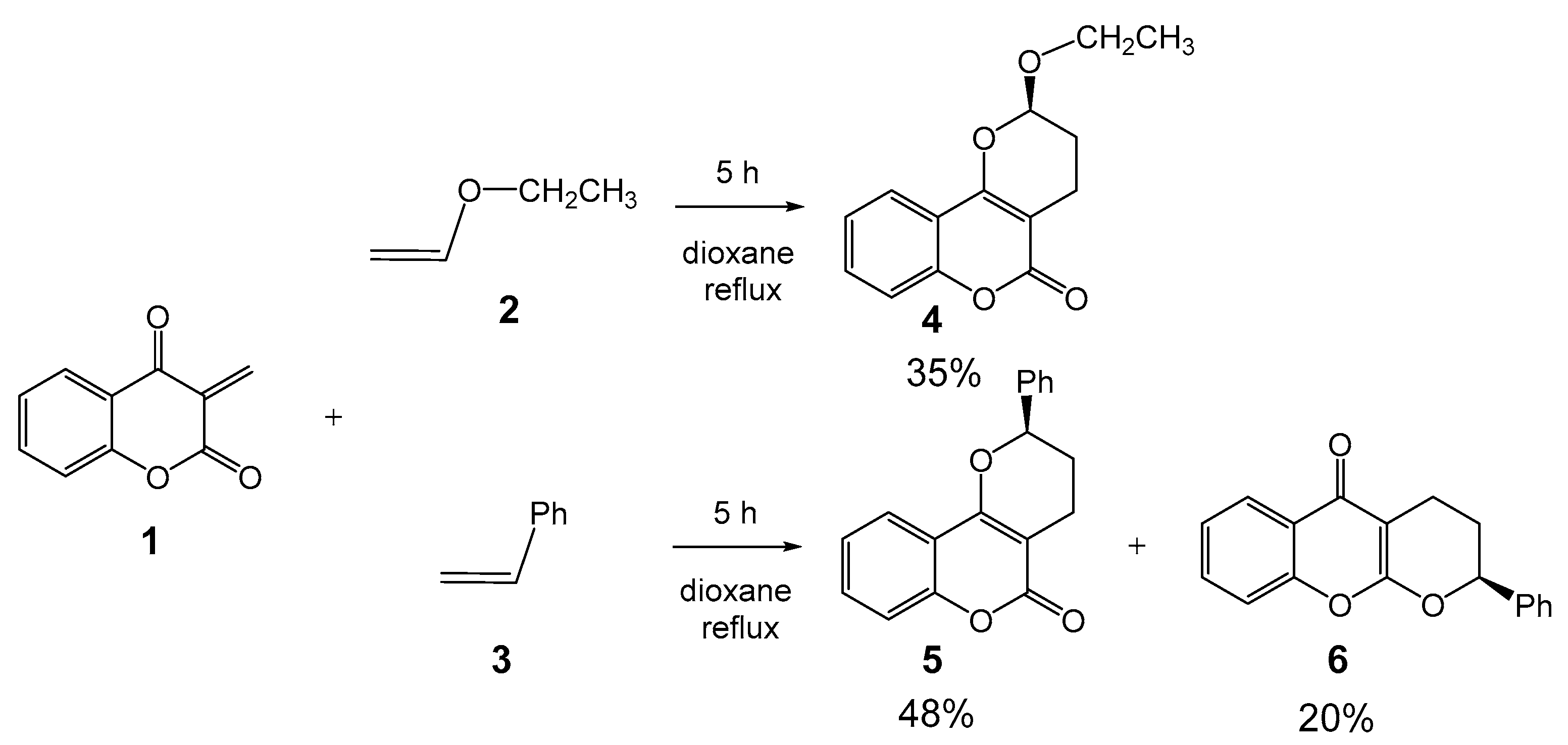
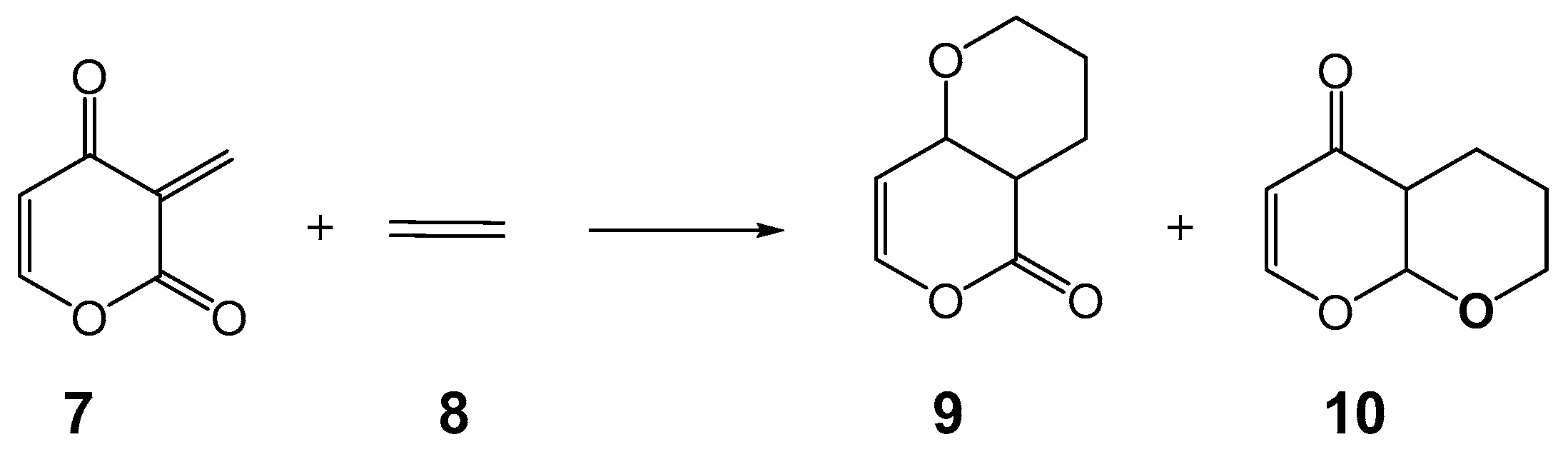
1. Introduction
2. Results and Discussion
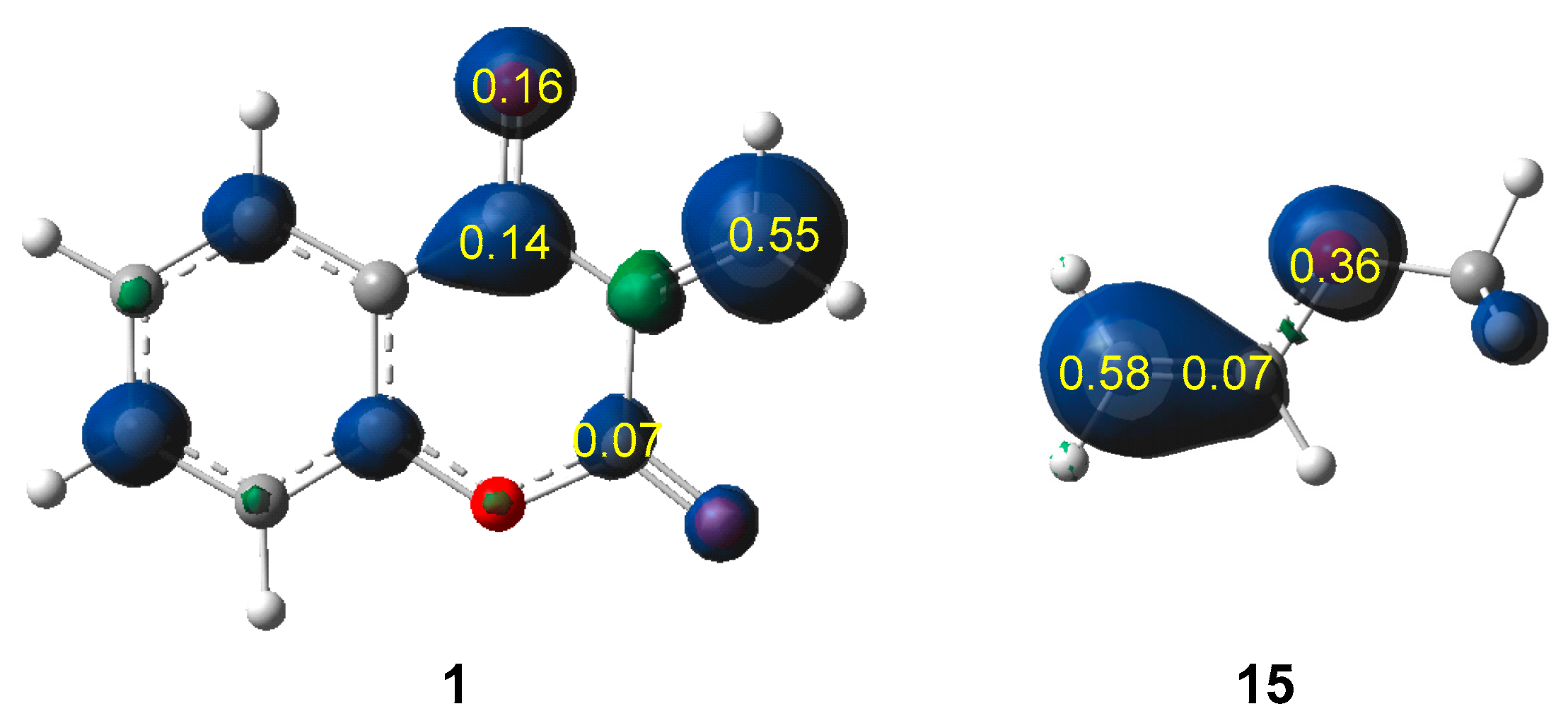
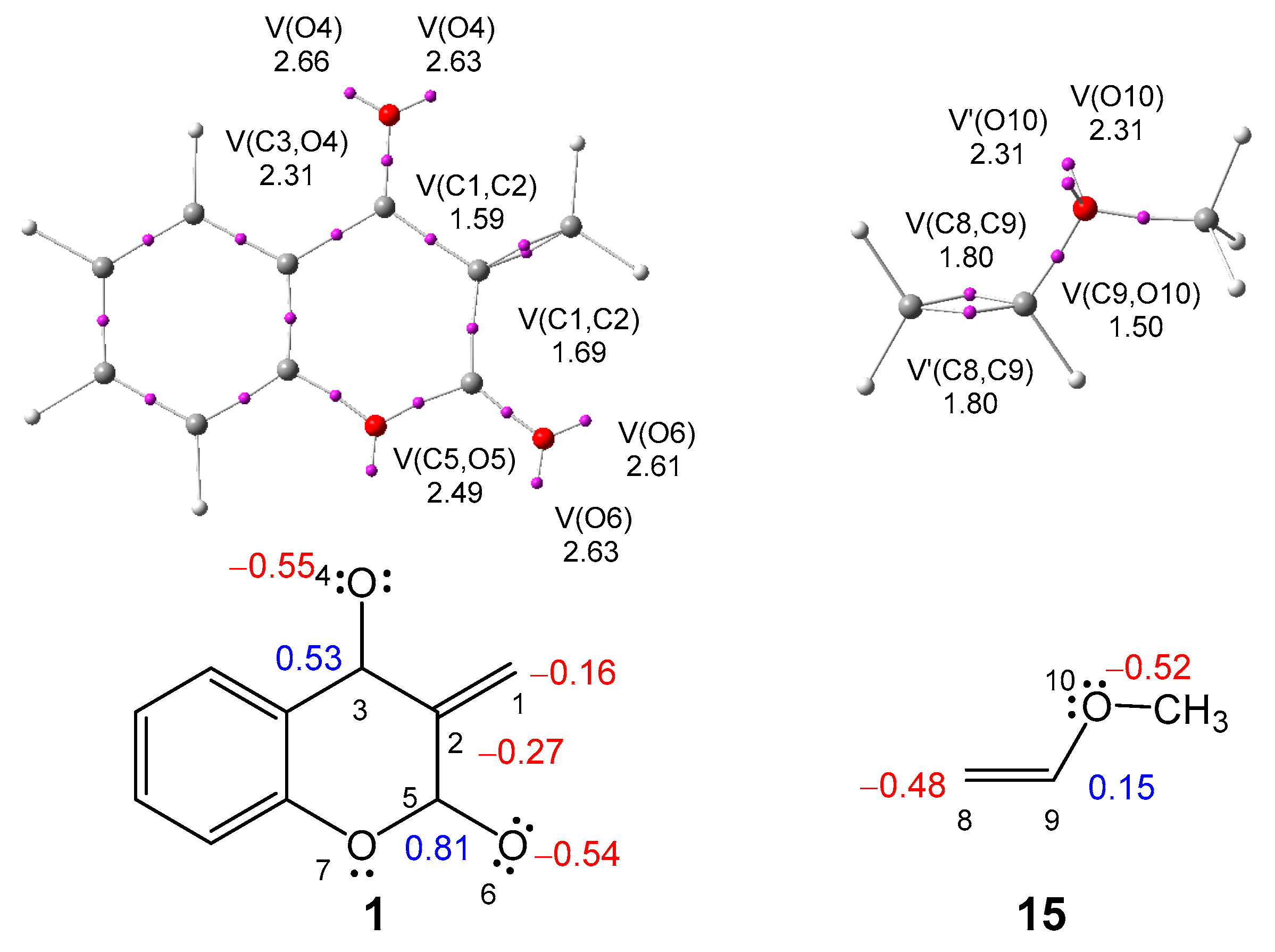
2.1. ELF Topological Analysis of the ELF of MCDO 1 and MVE 15
2.2. Analysis of the DFT-Based Reactivity Indices in the Ground State of the Reagents
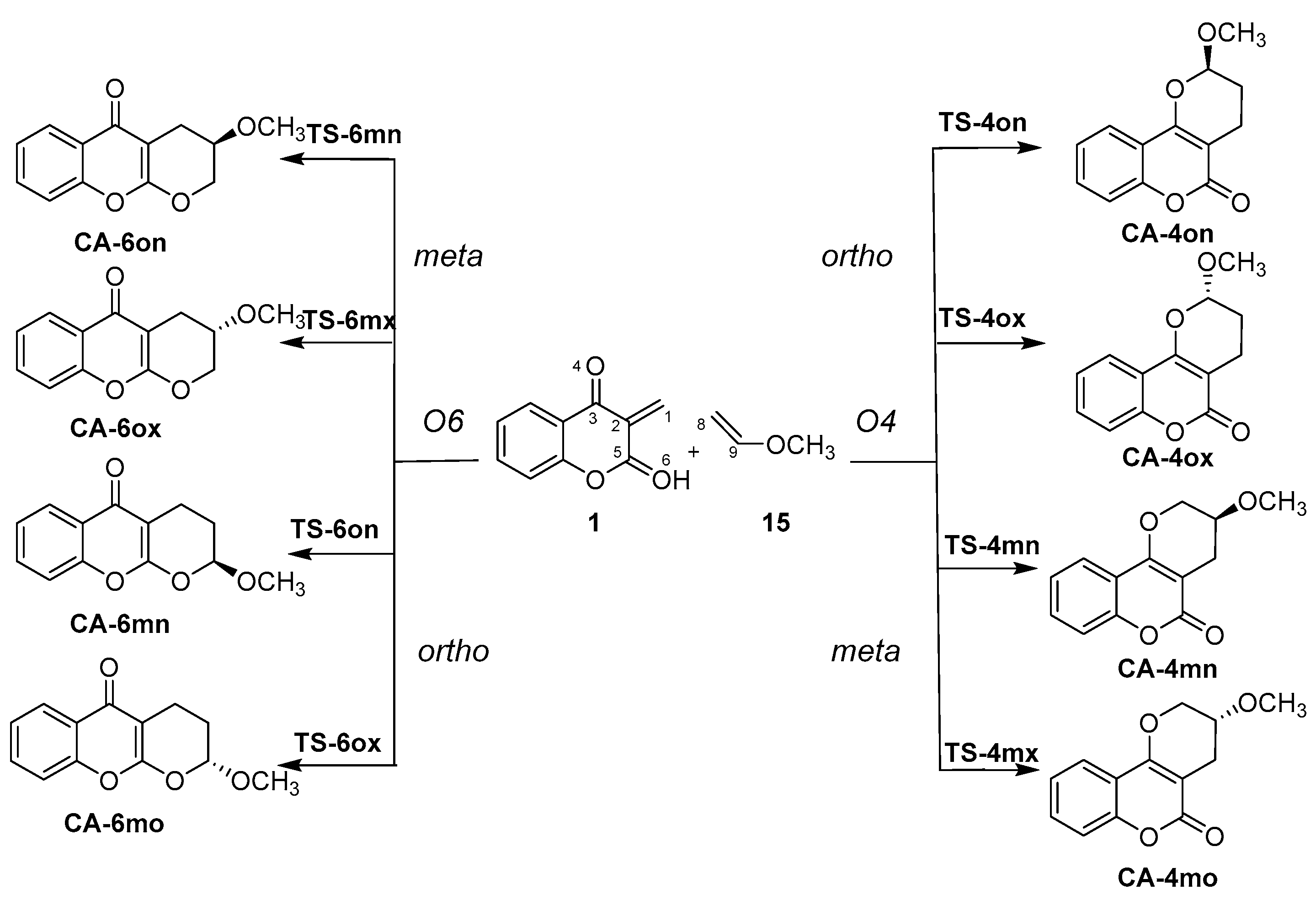
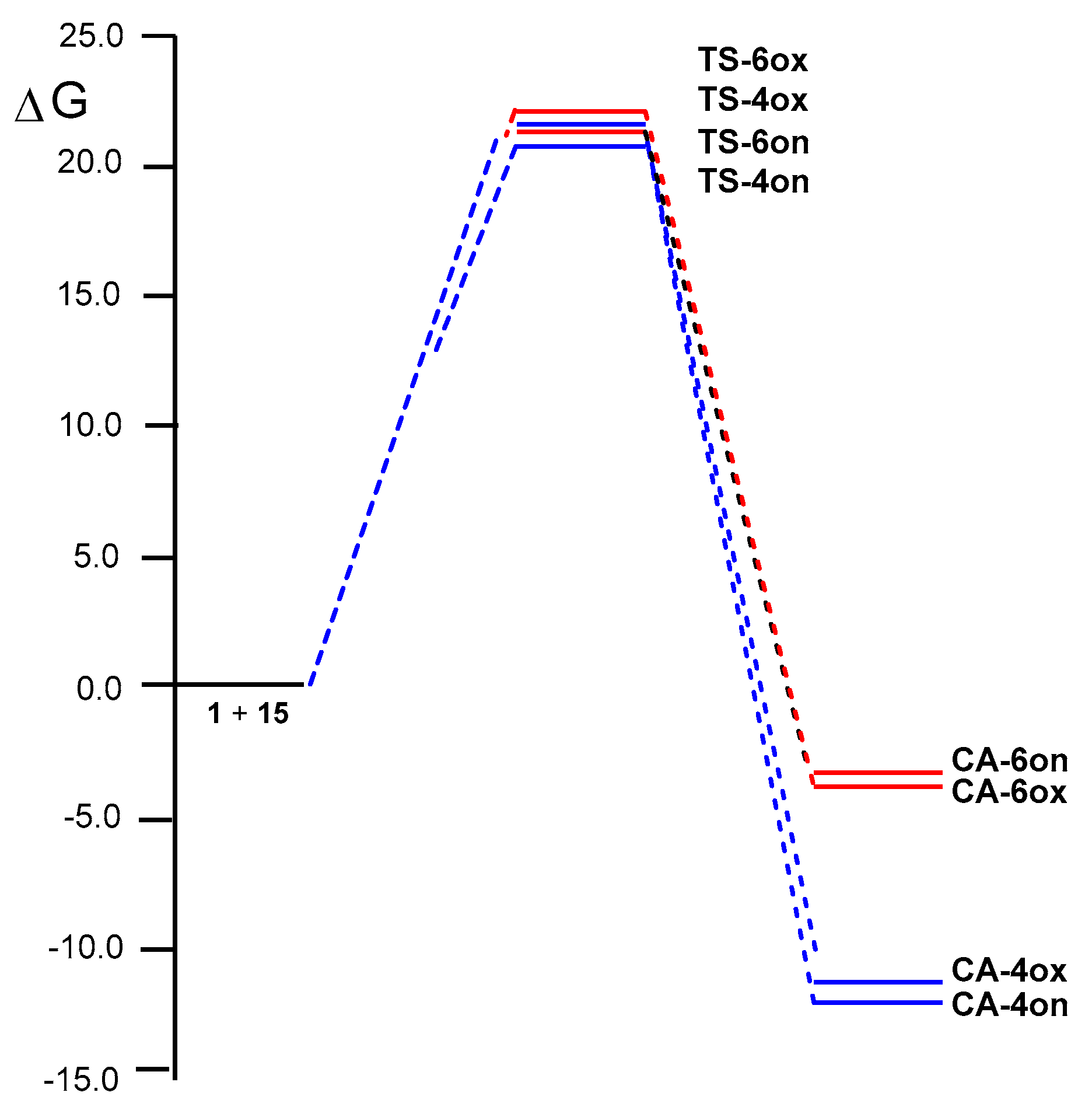
2.3. Analysis of Competitive Reaction Paths Associated with the HDA Reaction of MCDO 1 with MVE 15
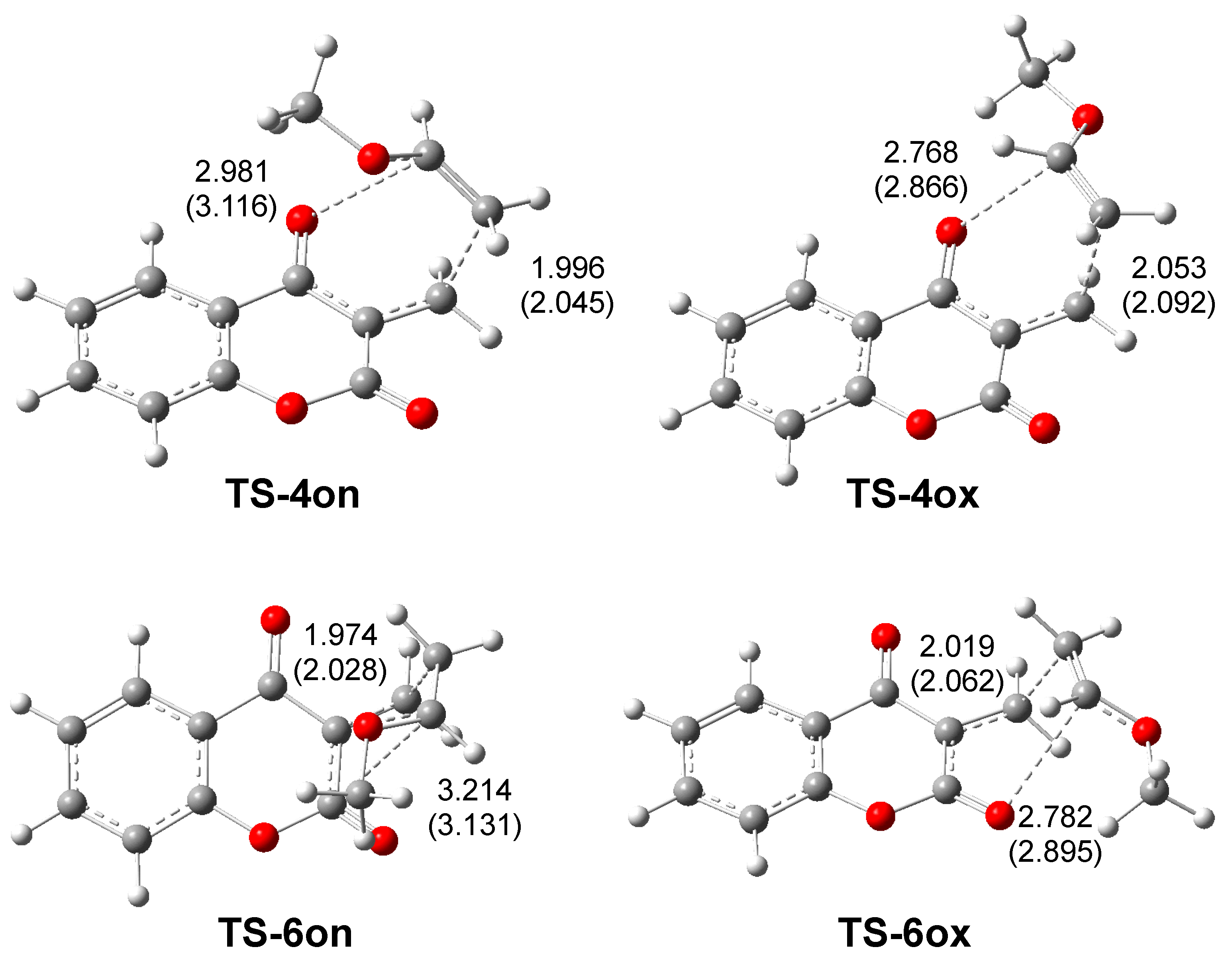
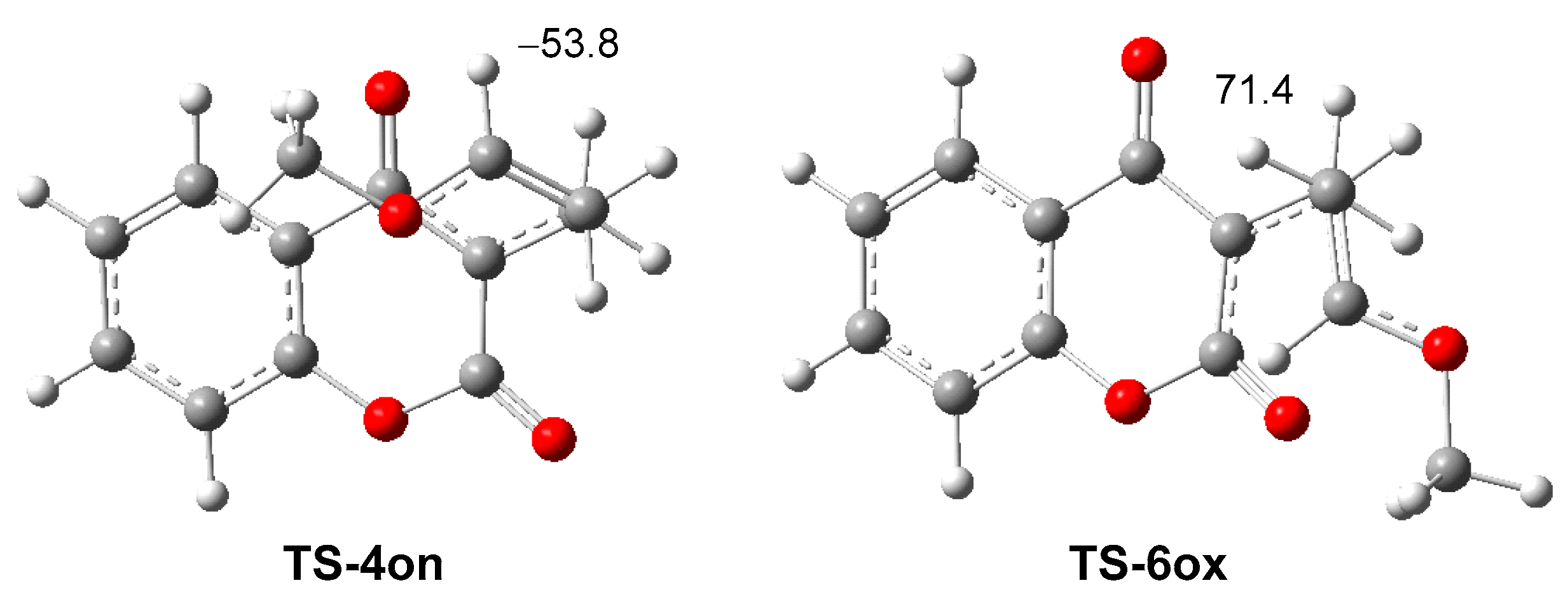
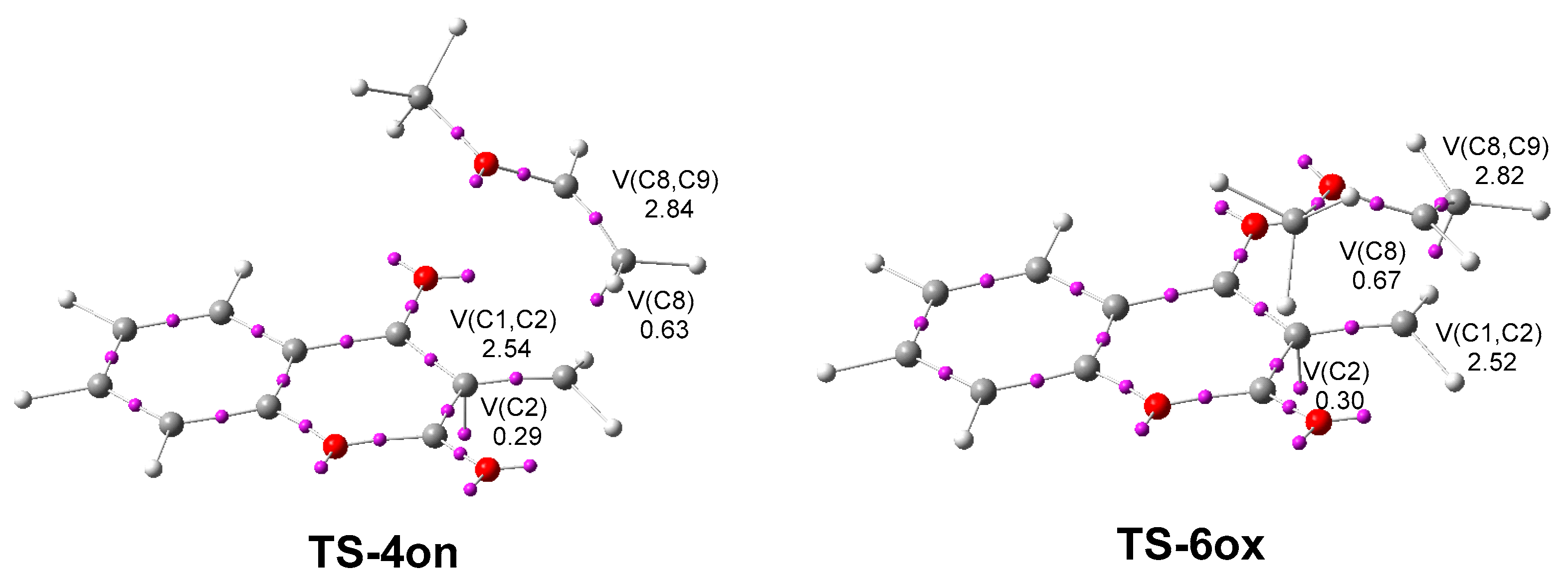
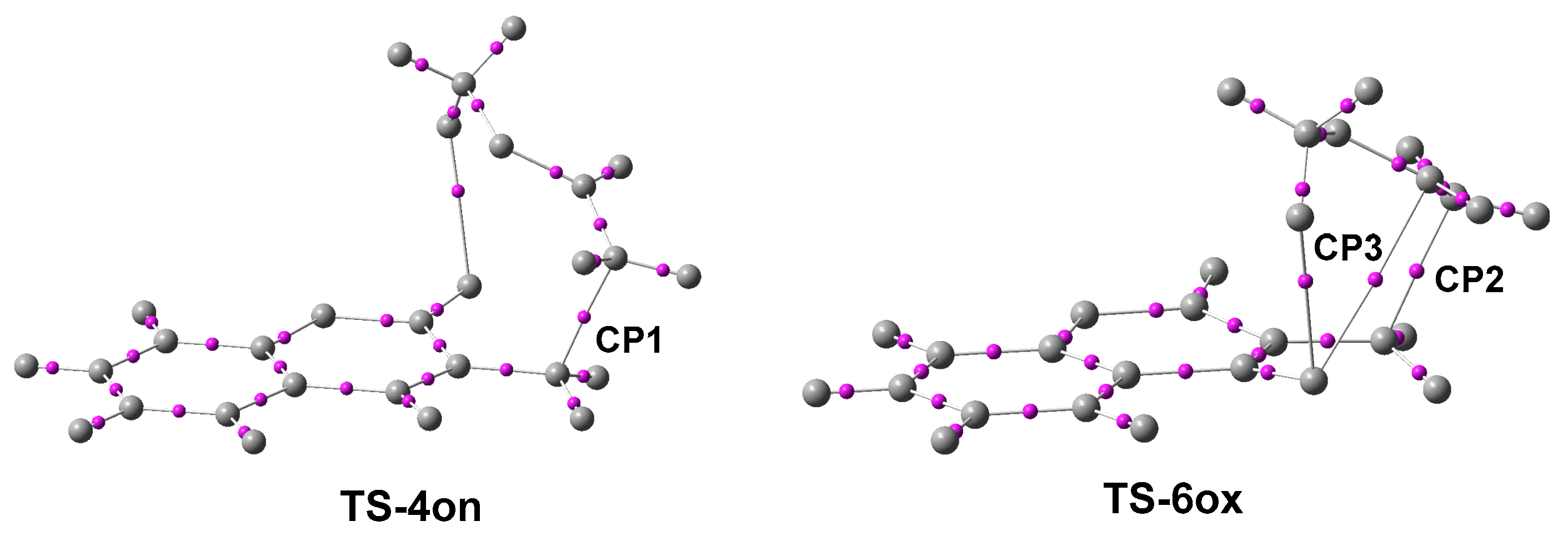
2.4. ELF and QTAIM Analyses of the of the Structure Electronic of the Stereoisomeric TS-4on and TS-6on
2.5. RIAE Analysis of the HDA Reactions of MCDO 1 with MVE 15 and with Ethylene 8.
3. Conclusion
Supplementary Materials
Author Contributions
Funding
Declaration of Competing Interest
Acknowledgments
References
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe. Justus Liebigs Ann. Chem. 1928, 460, 98–122. [Google Scholar] [CrossRef]
- Carruthers, W. Some Modern Methods of Organic Synthesis, 2nd ed.; Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Carruthers, W. Cycloaddition Reactions in Organic Synthesis; Pergamon: Oxford, UK, 1990. [Google Scholar]
- Tietze, L.F.; Kettschau, G. In Stereoselective Heterocyclic Synthesis I. (Eds.: Metz, P.), Springer-Verlag Berlin Heidelberg, 1997, 2, 1–120.
- Waldmann, H. Asymmetric Hetero Diels-Alder Reactions. Synthesis 1994, 535–551. [Google Scholar] [CrossRef]
- Schaus, S.E.; Brånalt, J.; Jacobsen, E.N. Asymmetric hetero-Diels-Alder reactions catalyzed by chiral (salen)chromium(III) complexes. J. Org. Chem. 1998, 63, 403–405. [Google Scholar] [CrossRef]
- Maruoka, K.; Itoh, T.; Shirasaka, T.; Yamamoto, H. Asymmetric Hetero-Diels-Alder Reaction Catalyzed by Organo-Aluminum Reagents. J. Am. Chem. Soc. 1988, 110, 310–312. [Google Scholar] [CrossRef]
- Juhl, K.; Jorgensen, K.A. The first organocatalytic enantioselective inverse-electron-demand hetero-Diels-Alder reaction. Angew. Chem., Int. Ed. Engl. 2003, 42, 1498–1501. [Google Scholar] [CrossRef]
- Oehlenschlaeger, K.K.; Mueller, J.O.; Brandt, J.; Hilf, S.; Lederer, A.; Wilhelm, M.; Graf, R.; Coote, M.L.; Schmidt, F.G.; Barner-Kowollik, C. Adaptable Hetero Diels-Alder Networks for Fast Self-Healing under Mild Conditions. Adv. Mater. 2014, 26, 3561–3566. [Google Scholar] [CrossRef]
- Lozynskyi, A.; Zimenkovsky, B.; Karkhut, A.; Polovkovych, S.; Gzella, A.K.; Lesyk, R. Application of the 2(5H)furanone Motif in the Synthesis of New Thiopyrano[2,3-d]thiazoles via the Hetero-Diels-Alder reaction and Related Tandem Processes. Tetrahedron Lett. 2016, 57, 3318–3321. [Google Scholar] [CrossRef]
- Hossain, M. A Review on Heterocyclic: Synthesis and Their Application in Medicinal Chemistry of Imidazole Moiety. Sci. J. Chem. 2018, 6, 83. [Google Scholar] [CrossRef]
- Shukla, P.K.; Verma, A.; Mishra, P. Significance of Nitrogen Heterocyclic Nuclei in the Search of Pharmacologically Active Compounds. New Perspective. Agric. Hum. Med. 2017, 100–126. [Google Scholar]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 2020, 21, 1–83. [Google Scholar] [CrossRef]
- Bouhaoui, A.; Eddahmi, M.; Dib, M.; Khouili, M.; Aires, A.; Catto, M.; Bouissane, L. Synthesis and Biological Properties of Coumarin Derivatives. A Review. ChemistrySelect 2021, 6, 5848–5870. [Google Scholar] [CrossRef]
- Soliman, A.Y.; Mohamed, F.K.; Abdel-Motaleb, R.M.; Abdel-Rahman, R.M.; Abdel-Mohsen, A.M.; Fouda, M.M.G.; Mohamed, A.S. Synthesis of New Coumarin Derivatives Using Diels-Alder Reaction. Life Sci. J. 2013, 10, 846–850. [Google Scholar]
- Appendino, G.; Cravotto, G.; Toma, L.; Annunziata, R.; Palmisano, G. The Chemistry of Coumarin Derivatives. Part VI. Diels-Alder Trapping of 3-Methylene-2,4-chromandione. A New Entry to Substituted Pyrano[3,2-c]coumarins. J. Org. Chem. 1994, 59, 5556–5564. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C-C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A. Understanding the mechanism of polar Diels-Alder reactions. Org. Biomol. Chem. 2009, 7, 3576–3583. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Yang, W. Density functional theory of atoms and molecules. Oxford University Press, New York, 1989.
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the conceptual density functional indices to organic chemistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M. In Application of Reactivity Indices in the Study of Polar Diels–Alder Reactions. Conceptual Density Functional Theory: Towards a New Chemical Reactivity Theory, Ed. Shubin Liu. WILEY-VCH GmbH. 2022, Vol. 2, pp. 481–502.
- Domingo, L.R. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization n atomic and molecular-systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Krokidis, X.; Noury, S.; Silvi, B. Characterization of elementary chemical processes by catastrophe theory J. Phys. Chem. A 1997, 101, 7277–7282. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Tang, Y.H.; Tal, Y.; Biegler-König, F.W. Properties of atoms and bonds in hydrocarbon molecules. J. Am. Chem. Soc. 1982, 104, 946–952. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory. Oxford University Press, Oxford, New York, 1994.
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.A.; Martín Pendás, A.; Francisco, E. Interacting Quantum Atoms: A Correlated Energy Decomposition Scheme Based on the Quantum Theory of Atoms in Molecules. J. Chem. Theory Comput. 2005, 1, 1096–1109. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. An Interacting Quantum Atoms Analysis of the Electronic Effects of Lewis Acid Catalysts in Accelerating Polar Diels-Alder Reactions. J. Org. Chem. 2024, 89, 12349–12359. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Pérez, P.; Ríos-Gutiérrez, M.; Aurell, M.J. A Molecular Electron Density Theory Study of Hydrogen Bond Catalysed Polar Diels–Alder Reactions of α,β-unsaturated Carbonyl Compounds. Tetrahedron Chem. 2024, 10, 100064. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. Revealing the Decisive Role of Global Electron Density Transfer in the Reaction Rate of Polar Organic Reactions within Molecular Electron Density Theory. Molecules 2024, 29, 1870. [Google Scholar] [CrossRef]
- Domingo, L.R. 1999 – 2024, a Quarter Century of the Parr’s Electrophilicity ω Index. Sci. Rad. 2024, 3, 157–186. [Google Scholar] [CrossRef]
- Berski, S.; Andrés, J.; Silvi, B.; Domingo, L.R. New Finding on the Diels-Alder Reactions. An Analysis Based on the Bonding Evolution Theory. J. Phys. Chem. A 2006, 110, 13939–13947. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A Molecular Electron Density Theory Study of the Competitiveness of Polar Diels-Alder and Polar Alder Ene Reactions. Molecules 2018, 23, 1913. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Chamorro, E.; Pérez, P. Aromaticity in Pericyclic Transition State Structures? A Critical Rationalisation Based on the Topological Analysis of Electron Density. ChemistrySelect 2016, 1, 6026–6039. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions Nature 1994, 371, 683–686.
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. A Useful Classification of Organic Reactions Bases on the Flux of the Electron Density. Sci. Rad. 2023, 2, 1. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.v.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef]
- Aurell, M.J.; Domingo, L.R.; Pérez, P.; Contreras, R. A theoretical study on the regioselectivity of 1,3-dipolar cycloadditions using DFT-based reactivity indexes. Tetrahedron 2004, 60, 11503–11509. [Google Scholar] [CrossRef]
- Domingo, L. R.; Pérez, P.; Sáez, J. A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Understanding the regioselectivity in hetero Diels-Alder reactions. An ELF analysis of the reaction between nitrosoethylene and 1-vinylpyrrolidine. Tetrahedron 2013, 69, 107–114. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Sáez, J.A. Understanding the Origin of the Asynchronicity in Bond-Formation in Polar Cycloaddition Reactions. A DFT Study of the 1,3-Dipolar Cycloaddition Reaction of Carbonyl Ylides with 1,2-Benzoquinones. RSC Adv. 2012, 2, 1334–1342. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A. , Zaragozà, J.R.; Arnó, M. Understanding the Participation of Quadricyclane as Nucleophile in Polar [2σ+2σ+2π] Cycloadditions toward Electrophilic π Molecules. J. Org. Chem. 2008, 73, 8791–8799. [Google Scholar] [CrossRef]
| Reactants | μ | η | ω | N |
| MCDO 1 | −4.88 | 4.16 | 2.87 | 2.16 |
| ethylene 8 | −3.37 | 7.77 | 0.73 | 1.86 |
| MVE 15 | −2.43 | 6.98 | 0.42 | 3.20 |
| Gas phase | 1,4-Dioxane | |
| TS-4on | 4.21 | 3.48 |
| TS-4ox | 4.88 | 4.65 |
| TS-4mn | 19.76 | 20.05 |
| TS-4mx | 23.09 | 23.03 |
| TS-6on | 4.26 | 3.55 |
| TS-6ox | 5.44 | 5.15 |
| TS-6mn | 22.19 | 22.49 |
| TS-6mx | 25.69 | 25.60 |
| CA-4on | -34.12 | -33.62 |
| CA-4ox | -35.39 | -34.79 |
| CA-4mn | -27.26 | -26.61 |
| CA-4mx | -26.64 | -26.35 |
| CA-6on | -19.02 | -18.26 |
| CA-6ox | -18.46 | -18.07 |
| CA-6mn | -26.31 | -25.64 |
| CA-6mx | -27.28 | -26.62 |
| TS-4on | TS-6on | ||
| CP1 | CP2 | CP3 | |
| Density ρ(r) | 0.0850 | 0.0810 | 0.0078 |
| Laplacian ∇2ρ(r) | 0.0128 | 0.0194 | 0.0343 |
| G(r) | 0.0309 | 0.0300 | 0.0074 |
| K(r) | 0.0277 | 0.0251 | 0.0012 |
| V(r) | -0.0587 | -0.0552 | -0.0062 |
| E(r) | -0.0277 | -0.0251 | 0.0012 |
| ƒ(X) | |||||
|---|---|---|---|---|---|
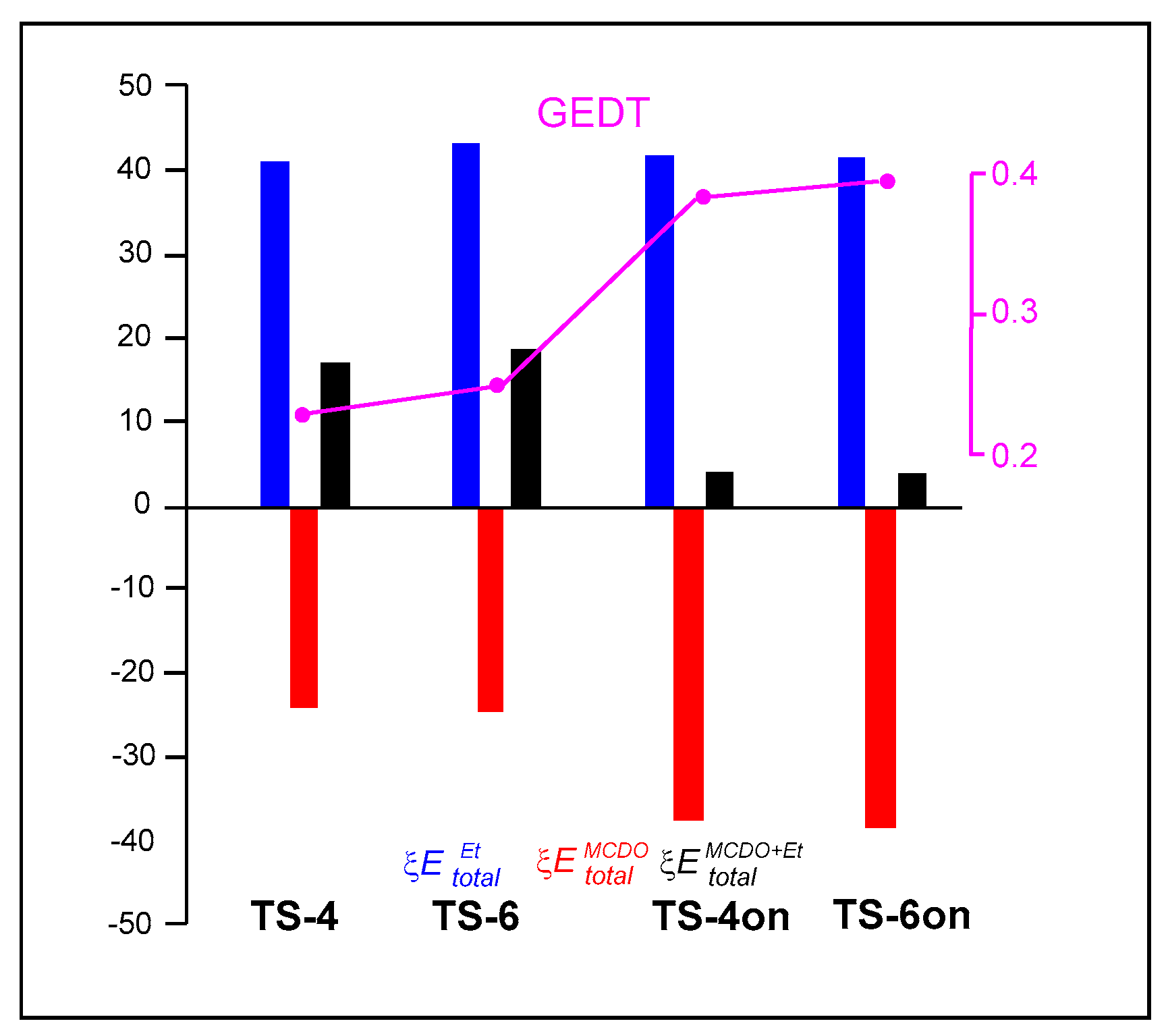
| TS-4 | MCDO | −83.78 | 59.90 | −23.87 | 17.19 |
| ethylene | 44.92 | −3.87 | 41.06 | ||
| TS-6 | MCDO | −84.44 | 59.89 | −24.55 | 19.02 |
| ethylene | 47.25 | −3.68 | 43.57 | ||
| TS-4on | MCDO | −66.20 | 28.60 | −37.60 | 3.96 |
| MVE | 71.48 | −29.92 | 41.56 | ||
| TS-6on | MCDO | −67.42 | 29.43 | −37.99 | 4.02 |
| MVE | 71.61 | &#;29.59 | 42.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





