1. Introduction
Magnetic Resonance Imaging (MRI) is indispensable in pediatric healthcare, providing detailed images of anatomical structures without exposure to ionizing radiation. This non-invasive technique is particularly crucial for diagnosing a wide range of pediatric conditions, from neurological issues to soft tissue injuries, offering a safer alternative to CT scans and X-rays for children’s sensitive developing tissues [
1]. Performing MRI on pediatric patients introduces specific challenges, primarily due to their natural inability to remain still for extended periods. The scan durations, often lasting between 30 to 60 minutes, heighten the likelihood of motion artifacts, which can degrade image quality and necessitate repeat scans, thereby increasing the child’s discomfort and the overall procedure time [
2,
3,
4]. Addressing these challenges, effective sedation is critical to minimize movement and reduce stress during MRI procedures, ensuring both the safety and accuracy of the imaging.
Oral Chloral Hydrate (OCH) has been a cornerstone in pediatric sedation, particularly for imaging procedures such as magnetic resonance imaging (MRI) and computed tomography (CT). It is recognized for its efficacy and safety when used within the recommended dosages of 20 to 100 mg/kg, providing sedation for durations of one to two hours, which is typically adequate for most pediatric imaging procedures [
1,
5]. Chloral hydrate’s popularity in pediatric sedation is attributed to its high success rates and its ability to induce sedation with minimal complications. Studies by, Fong et al., (2021), and de Rover I, (2023) highlight its effectiveness with success rates often exceeding 90%, which is notably higher compared to other sedatives like oral promethazine and midazolam [
6,
7] . For instance, Roach et al. (2010) reported a success rate of 97% for chloral hydrate alone in pediatric sedation, while Wilson et al. (2014) noted an increase in success rates from 94.2% to 97.9% with the administration of a second dose [
8,
9]. The administration of chloral hydrate does not require intravenous access, making it less invasive and more cost-effective compared to other sedatives [
10]. This ease of administration is particularly beneficial in a pediatric setting, reducing the stress and discomfort associated with needle-based routes [
11].
Comparatively, other sedatives such as rectal thiopental sodium and midazolam have been used in pediatric imaging but often with varied success. Chloral hydrate is preferred for its quicker onset of sedation and longer duration of action, which are critical factors in achieving stillness necessary for high-quality imaging [
12,
13]. Mataftsi et al. (2017) conducted a meta-analysis demonstrating that chloral hydrate had superior odds ratios for sedation success compared to other agents. This is indicative of its reliable and effective sedative properties in diverse procedural contexts, including both short and longer duration medical procedures [
14]. Moreover, chloral hydrate’s adjustable dosage range allows it to be tailored to the specific needs of the procedure and the patient’s age, which is a significant advantage over other sedatives that might require more stringent dosing regimens. Lower doses have proven particularly effective in infants and young children, achieving adequate sedation without compromising safety [
15]. However, it is noted that its efficacy might decrease with the patient’s age, showing lower success rates in children older than 48 months [
1]. De Rover I, (2023) cited that Oral chloral hydrate demonstrated a high pooled success rate for sedation at 94% (95% CI: 0.91-0.96) [
7]. This high success rate suggests that chloral hydrate is effective in achieving the desired sedative state necessary for MRI procedures without the need for intravenous or intramuscular routes, making it a less invasive option for pediatric patients. In fact, oral chloral hydrate remains a highly favored option for pediatric procedural sedation due to its effective sedative properties, safety profile, and adaptability to various procedural requirements [
16,
17].
In the context of using oral chloral hydrate for procedural sedation in pediatric MRI, various studies have identified a spectrum of side effects. Mataftsi et al. (2017) noted that side effects such as transient vomiting and paradoxical reactions are generally mild and do not typically require medical intervention [
14]. Similarly, Hayrullah Alp et al. (2019) reported an increased incidence of gastrointestinal symptoms like nausea and vomiting [
18], Yang et al. (2014) observed instances of agitation associated with the drug’s use [
19]. However, more severe concerns have also been documented. Vade et al. and Chen et al. identified mild respiratory depression as a significant risk [
5,
20]. Rahim et al. (2023) highlighted critical issues including bradycardia, apnea, and reduced oxygen saturation, compounded by variability in the duration of sedation effects, posing challenges to consistent clinical application and patient safety [
17]. Alotaibi et al. (2014) specifically noted hypoxia as the most common severe adverse event, particularly in infants under two years [
21], and Fong et al. (2021) reported behavioral changes as another serious side effect [
6].
However, while the general efficacy and safety of Chloral Hydrate are well-documented, there appears to be a significant gap in targeted research specifically addressing the median induction time—that critical period from administration to the onset of effective sedation. This phase is pivotal, as it directly impacts the scheduling, efficiency, and overall execution of MRI procedures. Understanding the median induction time is essential for optimizing patient flow and minimizing the duration for which children are required to be under sedation, thereby reducing potential exposure to any sedation-related risks. Despite its prevalent use, detailed explorations into the factors that influence this induction time are sparse. Current literature often clusters outcomes into broad success rates or focuses on post-sedation recovery and side effects, leaving a crucial aspect of sedation—the time to effective sedation—less explored. This oversight can lead to inefficiencies in clinical practice and potential discomfort or increased risk to pediatric patients. Thus, our research aims to fill this gap by methodically evaluating both the efficacy and the specific determinants of median induction time for Oral Chloral Hydrate in pediatric MRI procedural sedation. By identifying these key determinants, we can contribute to refining sedation practices, ensuring they are both safe and precisely timed to enhance the efficacy of pediatric MRI procedures. This study will not only expand the existing knowledge base but also provide a grounded framework for future research and clinical guidelines tailored to pediatric sedation need
2. Materials and Methods
This retrospective study was conducted to assess the efficacy of oral chloral hydrate as procedural sedation for pediatric MRI examinations. Data were collected from electronic medical records (EMRs) of pediatric patients who underwent MRI procedures between October 2018 and December 2022 at the Pediatric ward of Imam Abdulrahman Bin Faisal Hospital in Dammam, Saudi Arabia. The hospital is part of the National Guard Health Affairs and has a total capacity of 100 beds, with the pediatric ward specifically holding 20 beds. This facility records an average annual admission rate of 1,200 pediatric patients, with rates often surpassing the ward’s capacity during the winter months.
Ethical Considerations
The study protocol was reviewed and approved by the Institutional Review Board (IRB) of King Abdullah International Medical Research Center, Riyadh, Saudi Arabia, under the reference number RD20/004/D. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000.
Participants
The study population consisted of pediatric patients who received oral chloral hydrate for sedation during MRI procedures. Inclusion criteria were patients aged between 1 month to 8 years who were electively admitted for MRI scans with oral chloral hydrate procedural sedation. Exclusion criteria included patients with known allergies to chloral hydrate, those who received alternative sedatives, and those with incomplete medical records,
Preparation and Sedation Protocols
The sedation protocol for pediatric MRI involves a comprehensive and systematic approach to ensure patient safety and procedural efficacy. Initially, elective admission is done the previous evening, allowing for thorough preparation. Patients and their caregivers give consent for MRI and procedural sedation, ensuring informed participation. A critical step involves a pre-sedation assessment conducted by a certified provider skilled in procedural sedation, evaluating the patient’s suitability for sedation and identifying any potential risks. To prepare for sedation, patients are required to fast—remaining nil by mouth for at least 8 hours overnight—to minimize the risk of aspiration during the procedure. Oral Chloral Hydrate, dosed between 50-100 mg/kg, is administered to induce sedation.
Monitoring and Safety Precautions
Monitoring of the sedation depth is crucial and is conducted using the Ramsay Sedation Scale along with vital signs to ensure the patient remains in a safe sedative state (typically aiming for a Ramsay score of 3-4). Once the desired sedation level is achieved, the patient is carefully transferred to the MRI table. Continuous monitoring of the patient’s pulse oximetry and blood pressure is maintained throughout the MRI to detect and respond to any physiological changes promptly. The success of the sedation protocol is ultimately judged by the completion of the MRI procedure without any need for re-scans and the production of high-quality images, indicating minimal to no movement from the patient. This process ensures both the safety of the patient and the diagnostic utility of the MRI procedure.
Data Collection
Data for this study were extracted from electronic medical records (EMRs), focusing on specific covariates: Age was categorized into infants (0-12 )months , Child (> 12 to 168 months); Gender was noted as Male or Female; Weight was measured in kilograms at the time of MRI; Diagnosis was classified as neurological (1) or non-neurological (0); Chloral Hydrate Dose (Chdose) was recorded in milligrams to indicate the total dose administered; Multiple Dose was identified as either a single (0) or multiple doses (1); Time to Induce Sleep (TTS) was documented in minutes from medication administration to sleep onset; Duration of MRI (DOMRI) represented the total time taken for the MRI procedure in minutes; Event was documented as successful (1) or failed (0) induction of sleep; and Outcome was defined as either a successful completion of MRI without interruption (Success) or a failure to complete MRI (Fail).
Statistical Analysis
In this study, we employed a comprehensive analytical approach to assess the efficacy and determinants of median induction time for oral Chloral Hydrate in pediatric MRI procedural sedation. Our dataset, comprised of both numerical and categorical variables, enabled a multifaceted exploration of the demographic and clinical characteristics influencing sedation outcomes.
Descriptive and Inferential Statistics
We began with descriptive statistics to summarize the central tendencies and dispersions of all variables. Numerical variables such as Age, Weight (Wt), Chloral Hydrate Dose (Chdose), Time to Sedation induction (TTS), and Duration of MRI (DOMRI) are presented as medians with 25th and 75th percentiles. Categorical variables—Gender, Diagnosis, Multidose administration, and Sedation Outcome—are reported as frequencies and percentages, providing a clear demographic and clinical profile of the study population.
Inferential Analysis
We compared groups with successful and unsuccessful sedation outcomes using non-parametric tests due to the ordinal nature of some data and the non-normal distribution of continuous variables. The Mann-Whitney U test was applied to evaluate differences in age and weight between groups, while the Chi-square test assessed categorical variables such as gender and diagnostic categories.
Time-to-Event Analysis
The efficacy of sedation protocols was further scrutinized using time-to-event (survival) analysis. The Kaplan-Meier method was utilized to estimate the survival function for the time to induce sleep, providing a visual depiction of the decline in wakefulness over time across different diagnostic categories. Log-rank tests evaluated the statistical significance of differences between survival curves. Additionally, we applied Cox proportional hazards regression to model the time to induction of sleep as a function of multiple covariates simultaneously, including age, gender, weight, diagnosis, Chloral Hydrate dose, and MRI duration. This method allowed us to estimate hazard ratios for each predictor, identifying those significantly associated with the timing of sedation.
Robustness Checks
To ensure the reliability of our findings, sensitivity analyses were conducted. The robustness of the median induction time estimate obtained from the Kaplan-Meier analysis was verified through bootstrap resampling techniques. This method provided a 95% confidence interval for the median time to induction, confirming the stability and precision of our estimate.
3. Results
Our analysis includes a broad dataset of numerical and categorical variables that provide insights into the study population’s demographics and clinical characteristics. The individuals’ median age was 30 months (IQR: 11.5 - 48.0), and their median weight was 11.9 kg. Chloral hydrate dosing, which is critical for sedation, had a median of 66.0 mg (IQR: 50.95 - 74.35)/kg/dose, consistent with our pediatric procedures. The median Time to Induce sedative (TTS) was 30 minutes (IQR 23-45), indicating sedative efficiency. Females made up 61.75% of the cohort in categorical variables, which may have an impact on sedative dynamics. Neurological diseases were the most common diagnostic category (75.41%), underscoring the need of MRI in these instances. Multiple doses were rarely necessary, with only 19.23% receiving more than one dose, showing effective initial sedation in most cases. Sedation success was high at 85.79%, with failed outcomes (14.21%) indicating a need for more inquiry. Complications from chloral hydrate sedation were quite rare. 15 patients (8.20%) vomited, 21 patients (11.48%) experienced nausea, and 8 patients (4.37%) were agitated. These findings show that nausea was the most common problem, followed by vomiting, while agitation was the least reported adverse event. (
Table 1).
Moreover, our study examined clinical data to determine characteristics that influence procedure outcomes, comparing groups with successful (Success CS, n=167) and unsuccessful outcomes (Fail CS, n=16) .The Fail CS group had a greater median age (48 months) than the Success CS group (28 months), indicating that younger patients are more likely to succeed, albeit this was not statistically significant (p=0.063). Gender distribution revealed no significant difference (p=0.838). Weight was significantly higher in the Fail CS group (p=0.058), indicating a possible relationship between increased body weight and failure. The chloral hydrate dose and the prevalence of neurological diagnoses were comparable between the groups (p=0.863 and p=0.792, respectively). However, Time to Sleep (TTS) differed considerably, with the Fail CS group requiring a median of 60 minutes against 30 minutes in the Success CS group (p<0.001). This suggests that faster sedation leads to greater success. Multiple dosage treatment resulted in no variation (p=1.000). These findings highlight the role of induction speed, age, and weight in determining procedural success. (
Table 2)
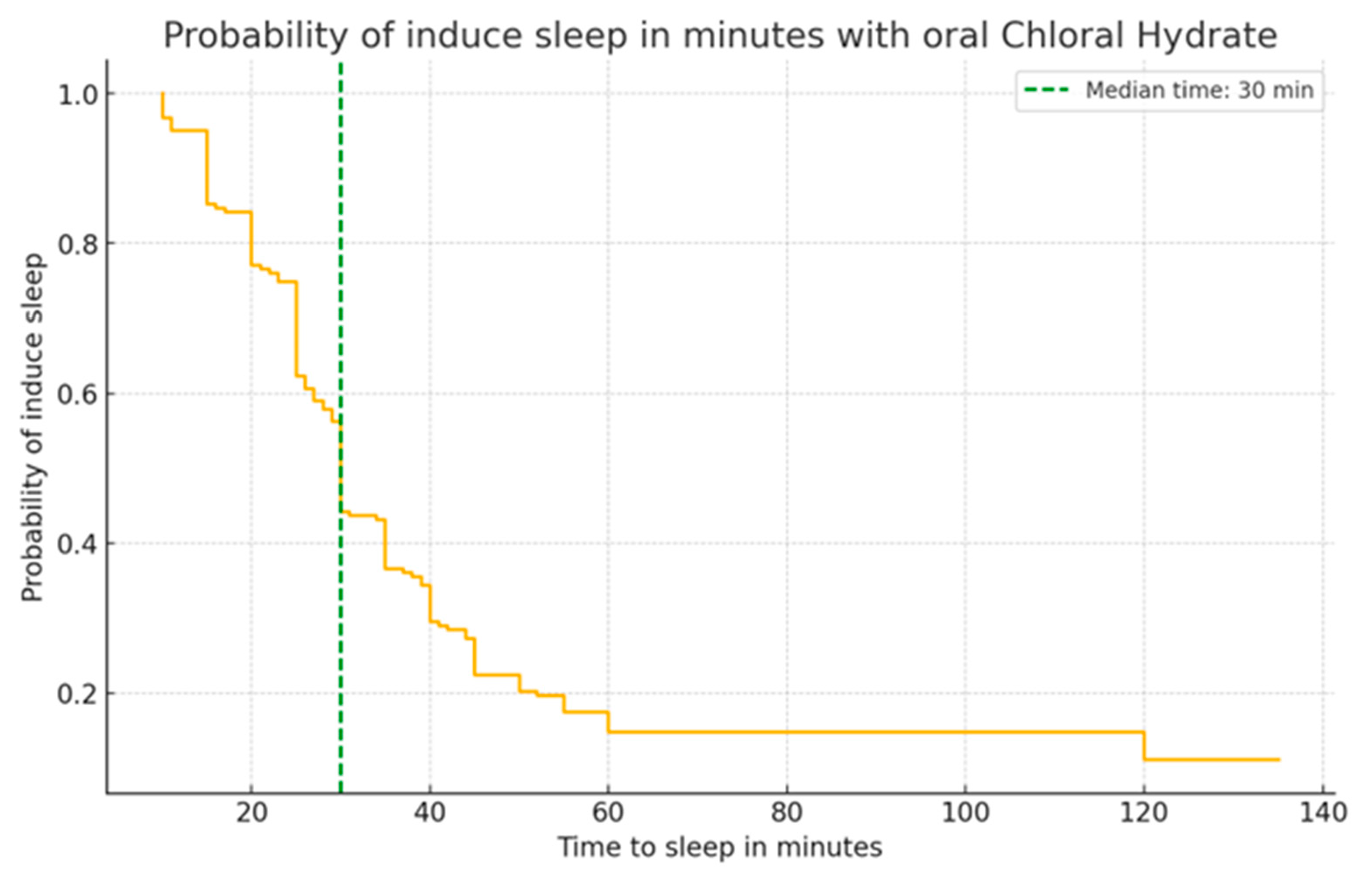
Figure 1 depicts the Kaplan-Meier survival curve for the chance of inducing sleep over time via oral chloral hydrate dosing. The graph shows a dramatic fall in sleep induction probability during the first 30 minutes, with a green dashed line representing the median period when 50% of the population is projected to be sleeping. The continuous fall indicates a decreasing likelihood of sleep induction beyond this stage. The reasonably stable slope after 30 minutes suggests a continuous decline in the likelihood of producing sleep over time, which could be important for evaluating sedation protocols and time-sensitive clinical procedures
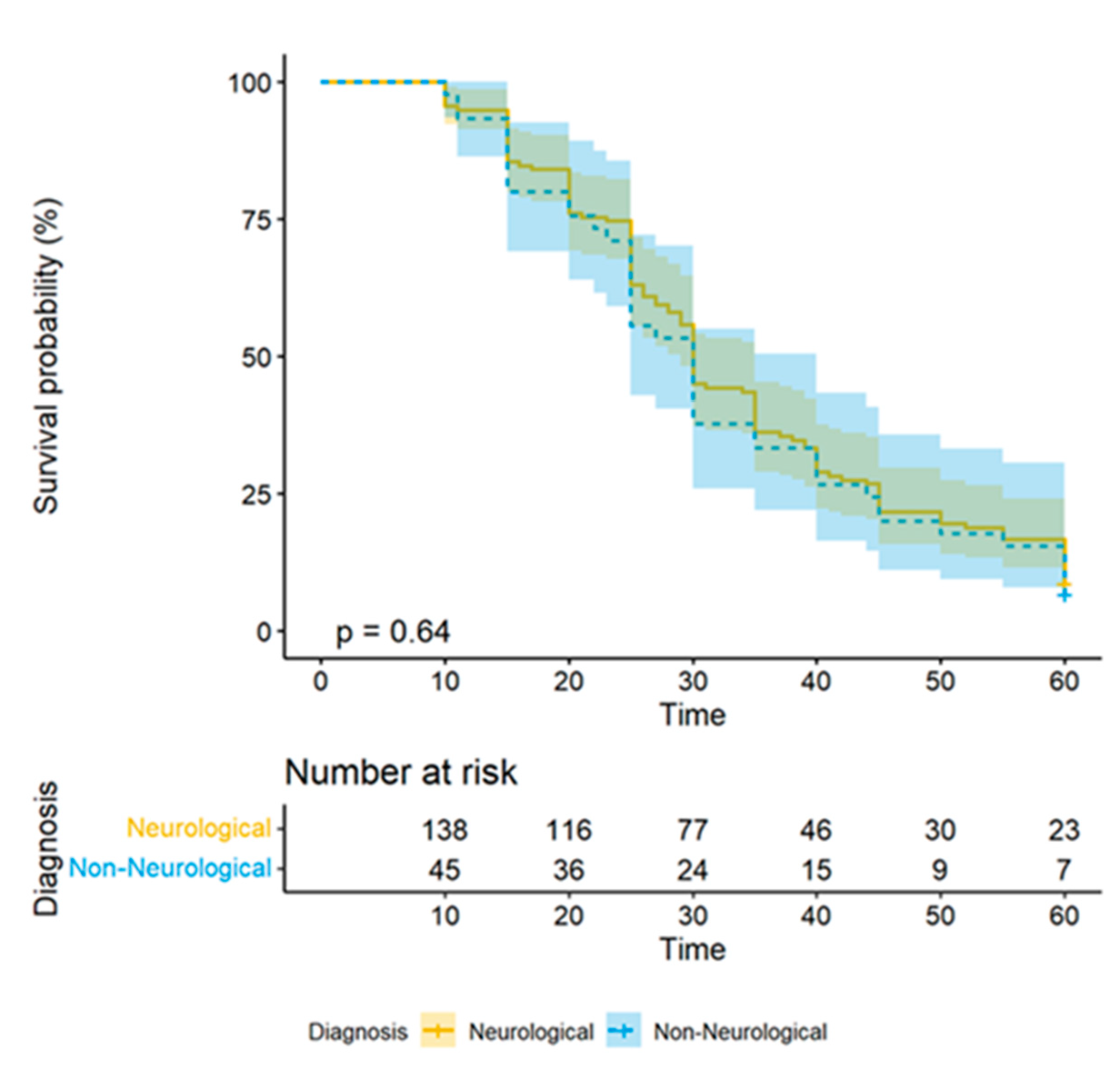
Figure 2 shows Kaplan-Meier curves comparing sleep induction probabilities over 60 minutes for neurological (solid blue) and non-neurological (dashed yellow) juvenile patients. Initially, both groups had a 100% chance of being awake, which declines gradually over time. By the end of the observation period, the survival probabilities have converged, with no significant differences in sleep induction times across groups (p = 0.64). The 95% confidence intervals, given by shaded areas, overlap, implying that sleep induction characteristics are similar across both diagnostic groups. This uniformity is reflected across the timeline, suggesting that neurological status has no substantial influence on chloral hydrate’s efficacy in inducing sleep during the study period.
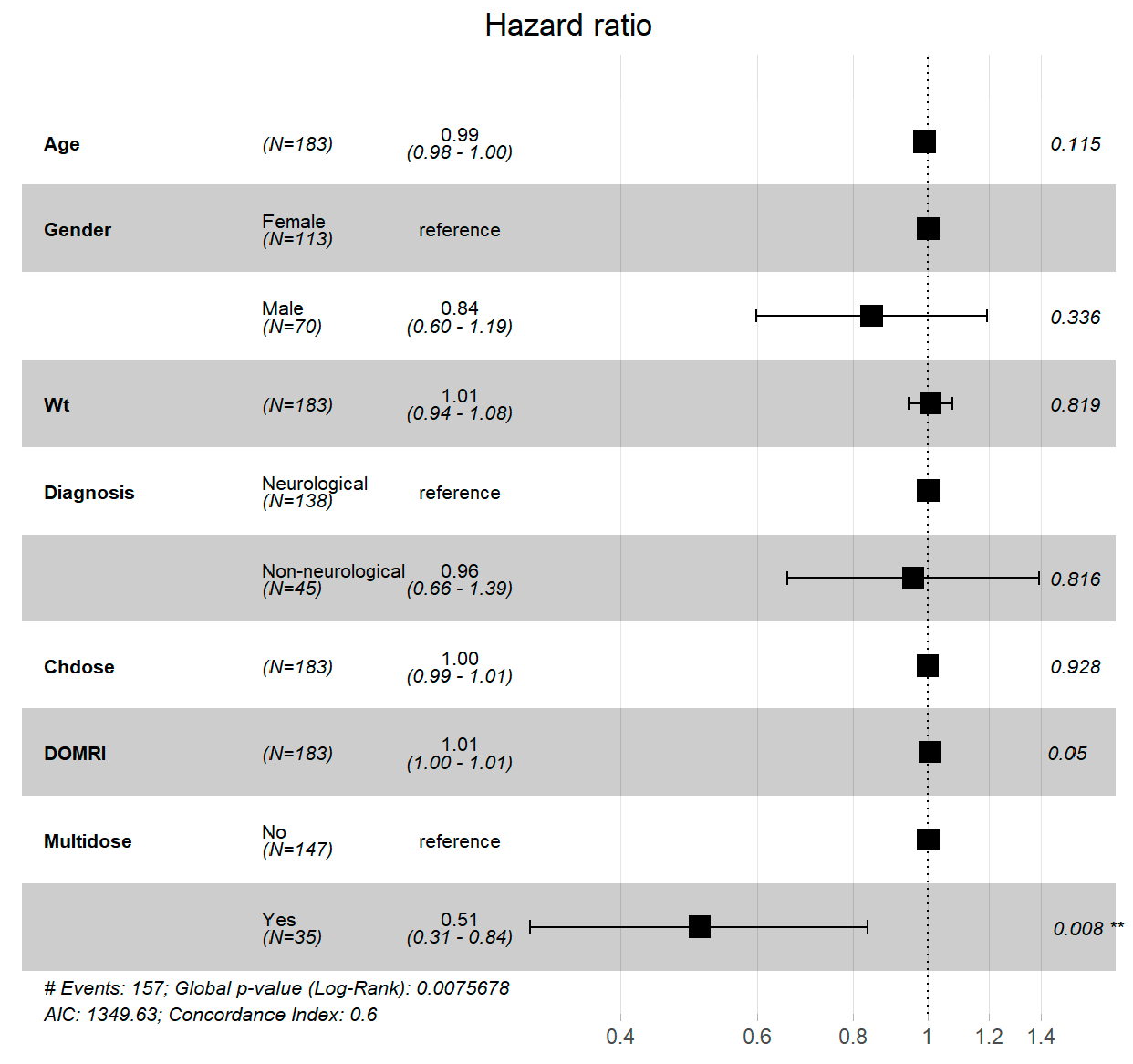
Figure 3 illustrates hazard ratios from a Cox proportional hazards model assessing parameters influencing sleep induction time in children taking oral Chloral Hydrate for procedural sedation, based on 169 patients and 158 events. Age, gender, weight, diagnosis, chloral hydrate dose, and MRI duration were among the key characteristics evaluated. The results show that age, gender, weight, diagnosis, and dose have no significant effects on sedation timing, with p-values greater than 0.05. However, the necessity for numerous doses considerably increases the duration to produce sedation (HR = 0.51, CI: 0.31 - 0.84, p=0.008), indicating that this is a significant role in delay. The model’s global log-rank p-value of 0.007568 and 0.6 concordance index indicate modest model adequacy, emphasizing the significance of dosage repetition in predicting sedation outcomes.
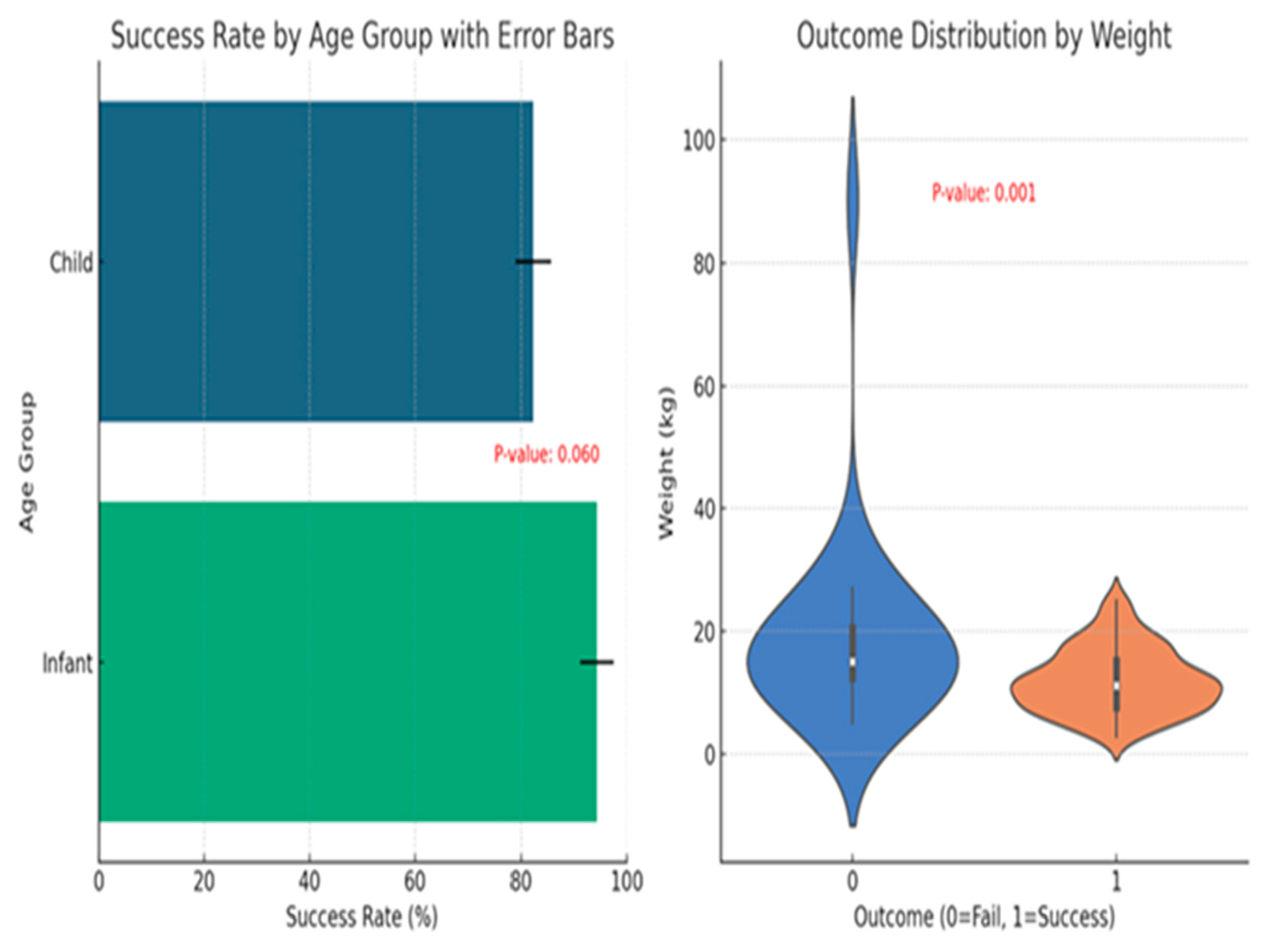
Figure 4 shows a detailed statistical study of sedation success rates by age group and outcome distribution by weight. In the first figure, newborns had a significantly higher success rate in sedation than older children, but the difference is not statistically significant (p-value = 0.060). This implies a pattern in which age influences sedative efficacy, although more data may be required to reach a firm conclusion. The second chart, which displays the result distribution by weight, shows a significant difference in weight between children who had successful versus failed sedation outcomes (p-value = 0.001). This suggests that lighter children are more likely to experience effective sedation outcomes. These figures show how important it is to consider both age and weight when establishing and evaluating pediatric sedation treatments.
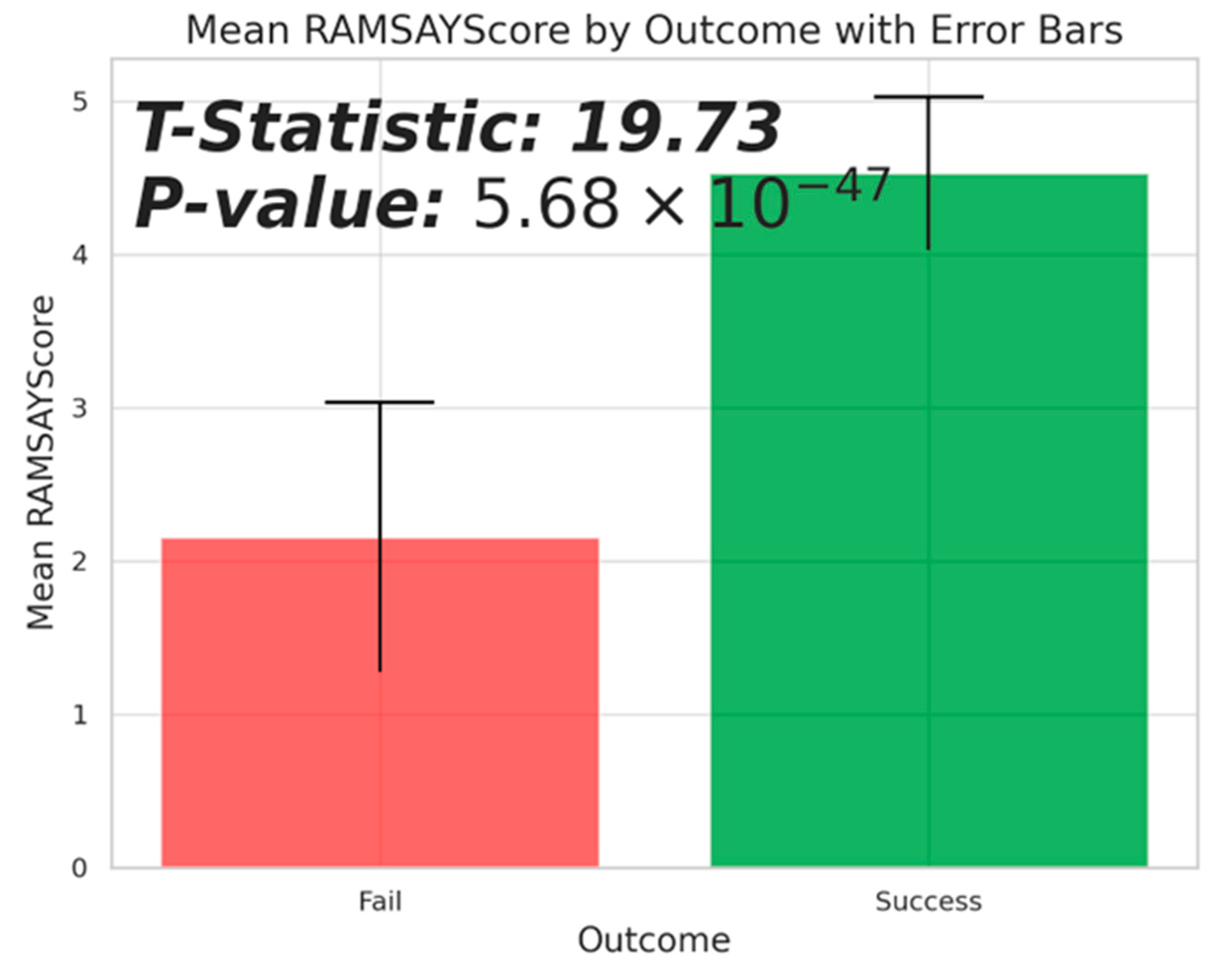
Figure 5 compares mean RAMSAY scores by sedation outcome, revealing a significant difference between successful and unsuccessful sedative attempts. A T-statistic of 19.73 and a P-value of 0.000 indicate that effective sedation outcomes are related with significantly higher RAMSAY scores, around 5, compared to failures, which average around 2. This statistical research strongly validates the RAMSAY scale’s effectiveness in determining sedative level, indicating that higher scores, which indicate deeper sedation, have a substantial correlation with sedation success. These findings highlight the necessity of establishing an acceptable level of sedation, as indicated by the RAMSAY score, to improve the chances of successful sedation in pediatric procedures like MRI scans.
Sensitivity Test
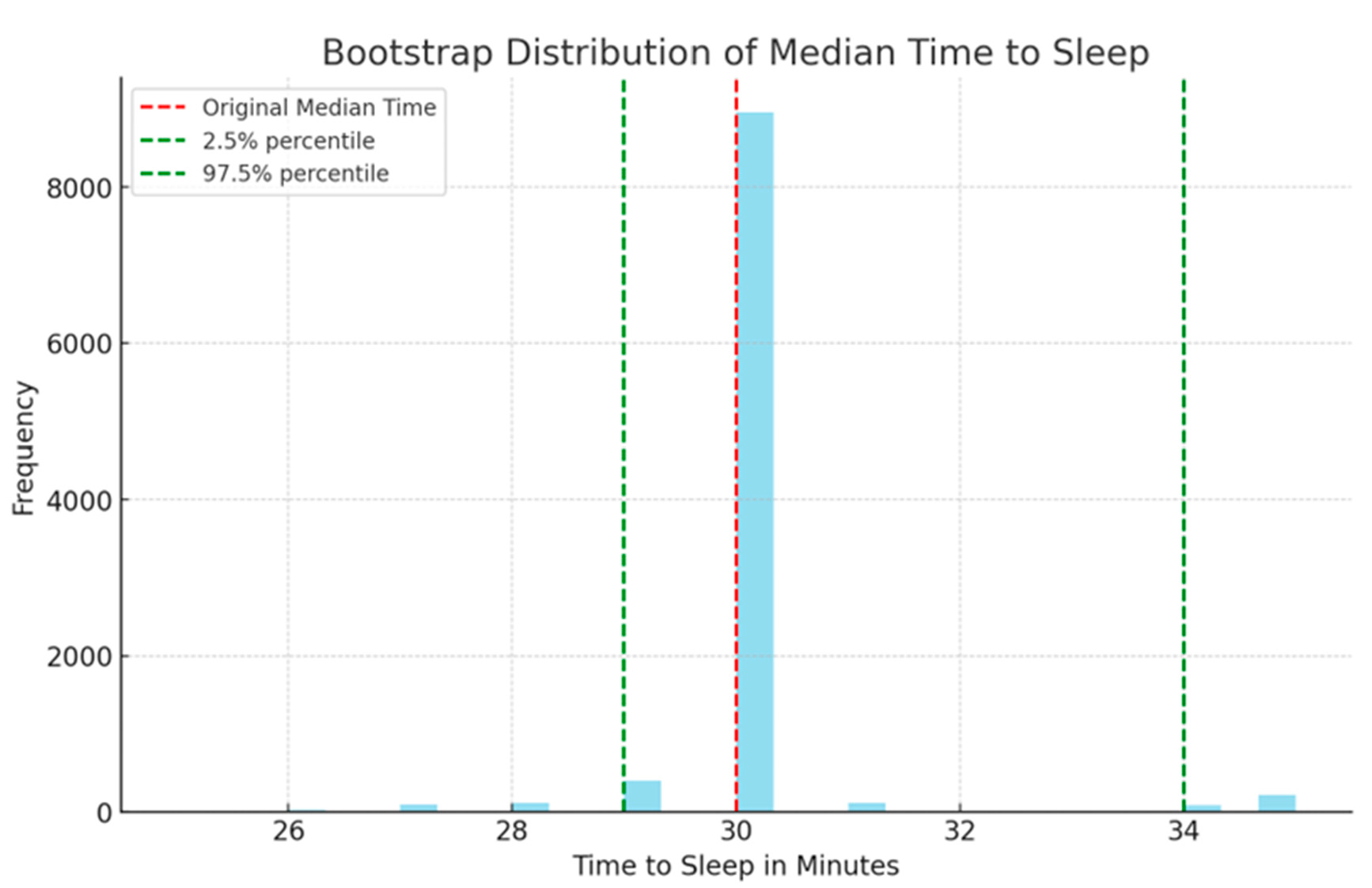
In the context of your study on the efficacy of oral Chloral Hydrate for inducing sleep in pediatric patients, the KM survival curve offers an estimate of the median time to induce sleep, calculated as 30 minutes. This means that half of the patients are expected to fall asleep within 30 minutes of administration. To assess the robustness and validity of this KM estimate, we employed a bootstrap method—a powerful statistical technique that involves repeatedly sampling from the data with replacement to estimate the sampling distribution of a statistic. Here, the statistic of interest is the median time to induce sleep.
The bootstrap analysis you conducted, which involved 10,000 samples, provides a 95% confidence interval (CI) of 29 to 34 minutes for the median time. This confidence interval is particularly valuable because it quantifies the uncertainty around the KM estimated median time. The fact that the original KM estimate of 30 minutes falls comfortably within this range lends credibility to its accuracy. Moreover, the relatively narrow span of the confidence interval indicates a high level of precision in the estimate. Thus, the agreement between the KM estimate and the bootstrap-derived confidence interval enhances the validity of your findings. It confirms that the estimated median time to induce sleep is not an artifact of sample bias or random chance. Instead, it is a reliable statistic likely to be replicated in other samples drawn from the same population. This robust methodological approach enhances the credibility and scientific rigor of your study’s conclusions, making them more compelling for clinical application and further research.(
Figure 6)
4. Discussion:
Efficacy Analysis of Oral Chloral Hydrate for Pediatric MRI Sedation, a Comparative Perspective
Our study on the efficacy of oral Chloral Hydrate (OCH) for pediatric MRI procedural sedation revealed a success rate of 85.79%. This performance aligns closely with other significant findings, though it falls short of the highest rates in the literature. For instance, Ingeborg de Rover et al. (2022) reported a pooled success rate of 94% [
7], while Greenberg SB documented a 91% success rate [
1]. These results underscore OCH’s capability to effectively sedate pediatric patients, a point supported by Malviya S et al. (2000), who observed only a 7% failure rate [
2], and Vade et al., who reported a 100% success rate for specific imaging procedures and age groups [
5].
Comparative assessments further elucidate OCH’s relative effectiveness. Rooks et al. (2003) found no significant differences between OCH and oral pentobarbital sodium [
22], whereas Lyu et al. (2022) noted that dexmedetomidine had a 14% higher success rate than chloral hydrate [
23]. Contrarily, O’Hare et al. (2021) reported higher success rates with OCH compared to both dexmedetomidine and midazolam. Similarly, Chen et al. (2019) compared OCH to rectal sodium thiopental, showing success rates of 82.9% for OCH versus 87.1% for the latter (20). Highlighting another comparative, James D’Agostino (2000) found Chloral Hydrate achieved a 100% success rate in completing intended scans without supplementary dosing, whereas Midazolam had a 50% success rate, often requiring additional doses [
24]. Furthermore, Delgado et al. confirmed Chloral Hydrate’s high efficacy in achieving desired sedation levels for MRI scans in children, comparing favorably or superiorly to Midazolam, which frequently requires additional dosing due to its variable efficacy rates(25). These findings collectively affirm the robust efficacy of oral Chloral Hydrate in pediatric sedation for MRI, positioning it as a viable choice compared to alternative sedatives, tailored to specific clinical needs and contexts.
Insights from Advanced Time to Event Analysis
Our study leverages a Time to Event data analysis, an advanced statistical method not widely used in pediatric sedation research. This approach enables a nuanced analysis of factors influencing sedation onset, providing a higher resolution than traditional average or mean time methods. Additionally, we employed bootstrap techniques on our Kaplan-Meier survival analysis to explore the distribution of sedation induction times in greater detail. From 10,000 bootstrap samples, we identified a median induction time of 30 minutes, with a 95% confidence interval ranging from 29 to 34 minutes. Notably, the 97.5th percentile at 34 minutes serves as a conservative benchmark for clinical practice, suggesting that extending wait times to this duration would likely achieve adequate sedation in most children.
Furthermore, the Kaplan-Meier survival plot underscores a sharp decrease in the probability of sleep induction within the first 30 minutes, reaching a median where approximately half of the children are sedated. Extending this waiting period to 60 minutes ensures near-complete sedation, as evidenced by the curve’s plateau beyond this point. Such an extension represents a prudent strategy to enhance sedation coverage, especially useful in minimizing procedural interruptions and the need for repeat scans. This methodology not only bolsters the reliability of our findings but also enriches our understanding of sedation dynamics, thus aiding the refinement of dosing protocols and timing strategies to boost procedural efficiency and safety in pediatric MRI settings.
Demographics and Major Determinants of Sedation Induction Time
Our findings indicate that infants generally have higher sedation success rates compared to older children, although this difference is not statistically significant (p-value: 0.060). This observation aligns with extensive research indicating that age is a crucial determinant of sedation success in pediatric patients. Notably, Greenberg SB (1993) and Malviya S et al. (2000) all report that older children, particularly those over 48 months, tend to experience higher rates of sedation failure, with older children often requiring increased dosing or encountering more complications during sedation [
1,
2]. Similarly, studies by Hijazi OM (2014) and Chen et al. (2019), underscore that chloral hydrate is significantly more effective in younger patients, particularly infants, where the success rates are notably high [
13,
20] . This pattern is supported by additional research by Rooks et al. (2003) and Fan et al. (2021), which suggest that younger and lighter children exhibit better outcomes, likely due to differences in metabolic and physiological responses to sedation [
22,
29] . Moreover, systematic reviews such as that by Li et al. (2020) reinforce the notion that younger children respond more favorably to sedation, particularly with chloral hydrate, before the age threshold of 48 months, beyond which the effectiveness decreases [
30]. These findings collectively highlight age as a significant and consistent factor influencing the success of pediatric sedation, suggesting a need for tailored sedation strategies that accommodate the unique physiological profiles of younger patients.
Additionally, our analysis and several studies indicate that gender does not significantly influence the efficacy of oral Chloral Hydrate sedation in pediatric patients. This finding is consistent across multiple studies. Chen et al. (2019) found no significant differences in sedation response between male and female pediatric patients across various age groups, suggesting that oral Chloral Hydrate is uniformly effective regardless of gender [
20]. Similarly, Azizkhani et al. (2014) and Frush and Bisset (1995) observed no notable differences in sedation outcomes related to gender, confirming its consistent effectiveness [
10,
31]. Roach et al. (2010) also reported no significant differences in sedation success rates between genders among preschool children, reinforcing that gender does not impact the efficacy of oral Chloral Hydrate in pediatric echocardiographic procedures [
8]. These studies collectively underscore that gender is not a determinant in the success of oral Chloral Hydrate sedation.
Moreover, our study demonstrates that weight significantly impacts sedation success, with successful outcomes more likely in patients with lower median weights (p-value: 0.001). This finding is consistent with other research indicating that lighter weight enhances the efficacy of sedation protocols. Wilson et al. (2014), Lee YJ (2012), and Rooks et al. (2003) all observed that lower body weight in pediatric patients was associated with higher sedation success rates using oral Chloral Hydrate [
9,
22,
32]. These studies underscore the importance of considering weight in the administration of sedation, as variations in body weight can significantly influence both the effectiveness and safety of sedative drugs in pediatric settings.
Furthermore, this study demonstrates that while the dosage of Chloral Hydrate (CH) does not significantly affect the time to induce sedation (HR = 1.000, p = 0.928), multiple doses substantially prolong the induction process (HR = 0.506, p = 0.008). This indicates that additional doses may delay achieving adequate sedation. Supporting this, Glasier et al. (1995) and Alotaibi et al. (2014) note that approximately 15% of patients require a second dose for effective sedation, emphasizing the need for careful management of cumulative doses to optimize sedation effectiveness and minimize risks associated with excessive dosing [
12,
21].Fong et al. (2021) report that subsequent doses of CH typically extend the time needed to reach adequate sedation, complicating sedation management in clinical settings [
33]. Conversely, Litman et al. (2010) suggest that while multiple doses are sometimes necessary, they do not inherently pose additional safety risks [
34]. Similarly, Hijazi et al. (2014) find that CH often requires fewer additional doses than midazolam, indicating a longer-lasting sedative effect [
13]. These findings highlight the importance of strategic dosing in pediatric sedation with CH, particularly when multiple doses are involved, to ensure both efficacy and safety in prolonged or complex procedures.
Our study suggested HR 0.96, p=0.82, no difference in sedation induction time between neurological and non-neurological case. the study by Malviya et al. (2000), those with higher ASA (American Society of Anesthesiologists) statuses, which often include children with more severe neurological conditions, showed higher rates of sedation failure and inadequate sedation [
2].Additionally, Mataftsi et al. (2017), Health status plays a significant role in the administration of CH. The review highlights that severe adverse events are often related to pre-existing comorbidities [
14] .Sandberg KL, (2013) This indicates that CH might be well-tolerated in subjects without pre-existing conditions that could compromise their response to sedatives [
35]. Moreover, author Jung (2020), health status significantly influences sedation choices and outcomes. Children with specific health conditions, such as neurodevelopmental disorders, may respond differently to certain sedatives, exhibiting increased adverse effects or decreased efficacy [
36]. Abdulrahman AN (2024), specific health conditions like mental retardation often require sedation due to their inability to remain still during MRI scans [
37]. These findings suggest that while CH is effective for pediatric sedation, its administration should be carefully tailored to the child’s health and neurological status. Optimizing procedural protocols to accommodate diverse medical needs of children could enhance sedation efficacy and safety. Further research is needed to refine sedation management strategies for pediatric MRI procedures.
Clinical Implications and Future Directions
The findings regarding the use of oral Chloral Hydrate (OCH) for pediatric MRI sedation are highly relevant for clinical practice. The study confirms OCH’s efficacy and safety, aligning with previous research that highlights its success in achieving sedation with minimal complications. This supports continued use of OCH in pediatric settings, emphasizing its advantages over other sedatives like oral promethazine and midazolam. Clinically, this can translate to more efficient MRI procedures with reduced need for re-sedation and minimized patient stress, especially beneficial in pediatric care where non-invasiveness is crucial.
The research points to several areas for future investigation, particularly exploring the variability in median induction times and how they are affected by factors such as patient age and dosage. Future studies could focus on a broader age range and examine the pharmacokinetics and dynamics of OCH in different pediatric subgroups. Additionally, exploring alternative sedation protocols or combination therapies that could reduce the onset time and enhance sedation quality would be beneficial. Such research could lead to more tailored and effective sedation practices, improving both patient experience and procedural efficiency.
Strengths
The study’s methodological rigor represents a notable strength. It employs advanced time-to-event data analysis, offering a detailed and nuanced view of the factors influencing sedation efficacy—this represents a significant advancement over traditional mean-based methods. The comprehensive use of statistical analyses, including Kaplan-Meier and Cox proportional hazards models, allows for an in-depth examination of the sedation process, thus enhancing the reliability and applicability of the findings. Adding to this strength, the incorporation of a sensitivity test with bootstrapping further solidifies the study’s robustness. This technique ensures the stability and reliability of the median induction time estimates by generating a distribution of possible outcomes through repeated sampling. The resulting 95% confidence interval from the bootstrap analysis provides rigorous assessment of the uncertainty around these estimates, bolstering the credibility and scientific integrity of the findings. Collectively, these methodological approaches ensure that the study’s conclusions are well-supported and provide valuable insights for clinical practice, setting a high standard for future research in pediatric sedation protocols.
Weaknesses and Mitigation Strategies
A potential weakness of the study is the limited generalizability due to the specific demographic and clinical settings. The findings are based on a retrospective single-center study, which might not reflect wider pediatric populations or different healthcare settings. To mitigate this, future research should include multi-center trials involving a diverse patient demographic to confirm the findings and enhance the external validity. Additionally, longitudinal studies could provide insights into the long-term effects of OCH sedation and its clinical outcomes across various settings. These summaries provide a comprehensive view of the study’s implications for pediatric sedation practice, highlighting the need for ongoing research and methodological innovations to optimize sedation protocols in pediatric MRI settings. Finally, randomized control trials with multicenter, double blinded study needed to get more scientific insight.
5. Conclusions
This study demonstrates the high efficacy of oral Chloral Hydrate for pediatric MRI sedation, achieving an 85.79% success rate. It highlights that age and weight are crucial determinants of sedation outcomes, with younger, lighter patients experiencing better results. Extending the waiting period to 60 minutes significantly improves success rates. The findings suggest that fine-tuning dosage and timing can optimize sedation effectiveness.
Supplementary Materials
Not avilable
Author Contributions
Conceptualization, methodology, writing—original draft preparation, and data curation, Sara Amer Alomar; data curation, Ahmed Nawfal Alshammari and Fay Fahad Aldenaini; resources, Fahad Alnajin; investigation, Mann Albesair and Mohammed Shahab Uddin; funding acquisition, Amr Esmail and Abdullah Hasan; review and editing, software, validation, formal analysis, investigation, visualization, project administration, and supervision, Mohammed Shahab Uddin. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by all authors.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of King Abdullah International Medical Research Center, Riyadh, Saudi Arabia (protocol code RD20/004/D, date 15/08/2023
Informed Consent Statement
Informed consent was waived by the Institutional Review Board of King Abdullah International Medical Research Center, Riyadh, Saudi Arabia, due to the retrospective nature of this study, where data were collected from existing medical records and no direct patient interaction occurred. All patient data were anonymized, ensuring that no identifiable information was used or disclosed. The study adhered to the highest ethical standards for patient confidentiality and privacy in accordance with institutional guidelines and the Declaration of Helsinki.
Data Availability Statement
Data will be supplied on reasonable request.
Acknowledgments
The authors extend their sincere gratitude to the Department of Pediatrics at Imam Abdulrahman Bin Faisal Hospital for their unwavering support and invaluable contributionsto this study. Special thanks are due to the dedicated healthcare professionals, administrative staff,and research team members for their tireless efforts in patient care, data collection, and analysis.Their commitment to excellence has been instrumental in the successful completion of this research.The authors also acknowledge the children and their families for their participation and cooperation,which made this study possible.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Greenberg SB, Faerber EN, Aspinall CL, Adams RC. High-dose chloral hydrate sedation for children undergoing MR imaging: safety and efficacy in relation to age. AJR. American journal of roentgenology. 1993, 161, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Malviya S, Voepel-Lewis T, Eldevik OP, Rockwell DT, Wong JH, Tait AR. Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. British journal of anaesthesia. 2000, 84, 743–748. [Google Scholar] [CrossRef]
- Avlonitou E, Balatsouras DG, Margaritis E, Giannakopoulos P, Douniadakis D, Tsakanikos M. Use of chloral hydrate as a sedative for auditory brainstem response testing in a pediatric population. International Journal of Pediatric Otorhinolaryngology. 2011, 75, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Ballarín JS, Guixes JS, Botella MG, Palacín JS. Use of a single dose of 70 mg/kg chloral hydrate as a hypnotic in nuclear magnetic resonance. A prospective study of 3132 cases. Revista Española de Anestesiología y Reanimación (English Edition). 2022, 69, 355–359. [Google Scholar]
- Vade A, Sukhani R, Dolenga M, Habisohn-Schuck C. Chloral hydrate sedation of children undergoing CT and MR imaging: safety as judged by American Academy of Pediatrics guidelines. AJR. American journal of roentgenology. 1995, 165, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Fong CY, Lim WK, Li L, Lai NM. Chloral hydrate as a sedating agent for neurodiagnostic procedures in children. Cochrane Database of Systematic Reviews. 2021, 2021. [Google Scholar]
- de Rover I, Wylleman J, Dogger JJ, Bramer WM, Hoeks SE, de Graaff JC. Needle-free pharmacological sedation techniques in paediatric patients for imaging procedures: a systematic review and meta-analysis. British Journal of Anaesthesia. 2023, 130, 51–73. [Google Scholar] [CrossRef] [PubMed]
- Roach CL, Husain N, Zabinsky J, Welch E, Garg R. Moderate sedation for echocardiography of preschoolers. Pediatr Cardiol. 2010, 31, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Wilson ME, Karaoui M, Djasim LA, Edward DP, Shamrani MA, Friedman DS. The safety and efficacy of chloral hydrate sedation for pediatric ophthalmic procedures: a retrospective review. Journal of Pediatric Ophthalmology & Strabismus. 2014, 51, 154–159. [Google Scholar]
- Frush DP, Bisset 3rd GS. Sedation of children in radiology: time to wake up. AJR. American journal of roentgenology. 1995, 165, 913–914. [Google Scholar] [CrossRef]
- Nordt SP, Rangan C, Hardmaslani M, Clark RF, Wendler C, Valente M. Pediatric chloral hydrate poisonings and death following outpatient procedural sedation. Journal of medical toxicology. 2014, 10, 219–222. [Google Scholar] [CrossRef]
- Glasier CM, Stark JE, Brown R, James CA, Allison JW. Rectal thiopental sodium for sedation of pediatric patients undergoing MR and other imaging studies. American journal of neuroradiology. 1995, 16, 111–114. [Google Scholar]
- Hijazi OM, Ahmed AE, Anazi JA, Al-Hashemi HE, Al-Jeraisy MI. Chloral hydrate versus midazolam as sedative agents for diagnostic procedures in children. Saudi Med J. 2014, 35, 123–131. [Google Scholar]
- Mataftsi A, Malamaki P, Prousali E, Riga P, Lathyris D, Chalvatzis NT, Haidich AB. Safety and efficacy of chloral hydrate for procedural sedation in paediatric ophthalmology: a systematic review and meta-analysis. British Journal of Ophthalmology. 2017, 101, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Bracken J, Heaslip I, Ryan S. Chloral hydrate sedation in radiology: retrospective audit of reduced dose. Pediatric radiology. 2012, 42, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Needleman HL, Joshi A, Griffith DG. Conscious sedation of pediatric dental patients using chloral hydrate, hydroxyzine and nitrous oxide-a retrospective study of 382 sedations. Pediatric dentistry. 1995, 17, 424–431. [Google Scholar]
- Dalal PG, Murray D, Cox T, McAllister J, Snider R. Sedation and anesthesia protocols used for magnetic resonance imaging studies in infants: provider and pharmacologic considerations. Anesthesia & Analgesia. 2006, 103, 863–868. [Google Scholar]
- Alp H, Elmacı AM, Alp EK, Say B. Comparison of intranasal midazolam, intranasal ketamine, and oral chloral hydrate for conscious sedation during paediatric echocardiography: results of a prospective randomised study. Cardiology in the Young. 2019, 29, 1189–1195. [Google Scholar] [CrossRef]
- Yang Y, Shin T, Yoo S, Choi S, Kim J, Jeong T. Survey of sedation practices by pediatric dentists. Journal of the Korean academy of pediatric dentistry. 2014, 41, 257–265. [Google Scholar] [CrossRef]
- Chen Z, Lin M, Huang Z, Zeng L, Huang L, Yu D, Zhang L. Efficacy of chloral hydrate oral solution for sedation in pediatrics: a systematic review and meta-analysis. Drug Design, Development and Therapy. 2019, 2643-2653.
- Alotaibi B, Sammons H, Choonara I. G405 (P) Safety and Clinical Effectiveness of Chloral Hydrate for Painless Procedural Sedation in Children. Archives of Disease in Childhood. 2014, 99 (Suppl 1). [Google Scholar]
- Rooks VJ, Chung T, Connor L, Zurakowski D, Hoffer FA, Mason KP, Burrows PE. Comparison of oral pentobarbital sodium (nembutal) and oral chloral hydrate for sedation of infants during radiologic imaging: preliminary results. American Journal of Roentgenology. 2003, 180, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- Lyu X, Tao Y, Dang X. Efficacy and safety of intranasal dexmedetomidine vs. oral chloral hydrate for sedation in children undergoing computed tomography/magnetic resonance imaging: a meta-analysis. Frontiers in Pediatrics. 2022, 10, 872900. [Google Scholar] [CrossRef]
- D’AGOSTINO JA, Terndrup TE. Chloral hydrate versus midazolam for sedation of children for neuroimaging: a randomized clinical trial. Pediatric emergency care. 2000, 16, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Delgado J, Toro R, Rascovsky S, Arango A, Angel GJ, Calvo V, Delgado JA. Chloral hydrate in pediatric magnetic resonance imaging: evaluation of a 10-year sedation experience administered by radiologists. Pediatric radiology. 2015, 45, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ronchera-Oms CL, Casillas C, Marti-Bonmati L, Poyatos C, Tomas J, Sobejano A, Jiménez NV. Oral chloral hydrate provides effective and safe sedation in paediatric magnetic resonance imaging. Journal of clinical pharmacy and therapeutics. 1994, 19, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Park M, Um J, Kim SH, Yoon J, Lee Y, Kwon J, Baek S, Kim DY. Correlation between the actual sleep time 24 hours prior to an examination and the time to achieve chloral hydrate sedation in pediatric patients in South Korea: a prospective cohort study. Child Health Nursing Research. 2023, 29, 51. [Google Scholar] [CrossRef]
- Treluyer JM, Andre C, Carp PF, Chalumeau M, Tonnelier S, Cuq C, Kalifa G, Pons G, Adamsbaum C. Sedation in children undergoing CT scan or MRI: effect of time-course and tolerance of rectal chloral hydrate. Fundamental & clinical pharmacology. 2004, 18, 347–350. [Google Scholar]
- Fan L, Lim Y, Wong GS, Taylor R. Factors affecting successful use of intranasal dexmedetomidine: a cohort study from a national paediatrics tertiary centre. Transl Pediatr. 2021, 10, 765–772. [Google Scholar] [CrossRef]
- Li L, Zhou J, Yu D, Hao X, Xie Y, Zhu T. Intranasal dexmedetomidine versus oral chloral hydrate for diagnostic procedures sedation in infants and toddlers: A systematic review and meta-analysis. Medicine. 2020, 99, e19001. [Google Scholar] [CrossRef] [PubMed]
- Azizkhani R, Kanani S, Sharifi A, Golshani K, Masoumi B, Ahmadi O. Oral chloral hydrate compare with rectal thiopental in pediatric procedural sedation and analgesia; a randomized clinical trial. Emergency. 2014, 2, 85. [Google Scholar]
- Lee YJ, Kwak YH, Kim HB, Park JH, Jung JH. Analysis of the appropriate age and weight for pediatric patient sedation for magnetic resonance imaging. The American journal of emergency medicine. 2012, 30, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Fong CY, Lim WK, Li L, Lai NM. Chloral hydrate as a sedating agent for neurodiagnostic procedures in children. Cochrane database of systematic reviews. 2021. [Google Scholar]
- Litman RS, Soin K, Salam A. Chloral hydrate sedation in term and preterm infants: an analysis of efficacy and complications. Anesthesia & Analgesia. 2010, 110, 739–746. [Google Scholar]
- Sandberg KL, Poole SD, Sundell HW. Cardio-respiratory response to moderate chloral hydrate sedation in young lambs. Acta Paediatrica. 2013, 102, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Jung, SM. Drug selection for sedation and general anesthesia in children undergoing ambulatory magnetic resonance imaging. Yeungnam University Journal of Medicine. 2020, 37, 159–168. [Google Scholar] [CrossRef]
- Abdulrahman AN, Mahmood WA, Abdulrahman ZN. Effective Sedation Strategies for Pediatric MRI: A Comprehensive Analysis. Academia Open. 2024, 9, 10–21070. [Google Scholar]
Figure 1.
Kaplan-Meier survival curve displaying the probability of inducing sleep over time post-administration of oral Chloral Hydrate. The median time to induce sleep (30 minutes) is marked with a green dashed line, demonstrating the point at which 50% of the sample population is expected to be asleep.
Figure 1.
Kaplan-Meier survival curve displaying the probability of inducing sleep over time post-administration of oral Chloral Hydrate. The median time to induce sleep (30 minutes) is marked with a green dashed line, demonstrating the point at which 50% of the sample population is expected to be asleep.
Figure 2.
Kaplan-Meier curves depict survival probabilities for neurological (solid blue) and non-neurological (dashed yellow) patients over 60 minutes, both starting at 100% and declining similarly (p = 0.64). Shaded areas indicate 95% confidence intervals. The x-axis shows time in minutes, and the y-axis displays survival probability (%). A table below details the number of patients at risk at various times.
Figure 2.
Kaplan-Meier curves depict survival probabilities for neurological (solid blue) and non-neurological (dashed yellow) patients over 60 minutes, both starting at 100% and declining similarly (p = 0.64). Shaded areas indicate 95% confidence intervals. The x-axis shows time in minutes, and the y-axis displays survival probability (%). A table below details the number of patients at risk at various times.
Figure 3.
This plot visualizes the hazard ratios for various predictors affecting the time until sleep induction in children administered oral Chloral Hydrate for procedural sedation, analyzed using a Cox proportional hazards model. Data from 169 patients are depicted with 158 events observed. Predictors include Age, Gender, Weight (Wt), Diagnosis (with Neurological as the reference category), Chloral Hydrate Dose (Chdose), and Duration of MRI (DOMRI). The plot illustrates each predictor’s hazard ratio (HR) with corresponding 95% confidence intervals (CIs), demonstrating that none of the individual predictors significantly influence the time to achieve sedation at the p < 0.05 level. Notable metrics include a global log-rank p-value of 0.04354 and a concordance index of 0.58, indicating moderate model adequacy.
Figure 3.
This plot visualizes the hazard ratios for various predictors affecting the time until sleep induction in children administered oral Chloral Hydrate for procedural sedation, analyzed using a Cox proportional hazards model. Data from 169 patients are depicted with 158 events observed. Predictors include Age, Gender, Weight (Wt), Diagnosis (with Neurological as the reference category), Chloral Hydrate Dose (Chdose), and Duration of MRI (DOMRI). The plot illustrates each predictor’s hazard ratio (HR) with corresponding 95% confidence intervals (CIs), demonstrating that none of the individual predictors significantly influence the time to achieve sedation at the p < 0.05 level. Notable metrics include a global log-rank p-value of 0.04354 and a concordance index of 0.58, indicating moderate model adequacy.
Figure 4.
Left Plot: This horizontal bar chart shows the success rate by age group with error bars, including a p-value annotation for age group comparison. It details how the outcomes vary between the two age groups. Right Plot: The violin plot on the right compares the distribution of patient weights across the outcomes, annotated with the Mann-Whitney U test p-value to determine if weight differences significantly impact the success or failure of sedation.
Figure 4.
Left Plot: This horizontal bar chart shows the success rate by age group with error bars, including a p-value annotation for age group comparison. It details how the outcomes vary between the two age groups. Right Plot: The violin plot on the right compares the distribution of patient weights across the outcomes, annotated with the Mann-Whitney U test p-value to determine if weight differences significantly impact the success or failure of sedation.
Figure 5.
Mean RAMSAY Score by Outcome with Error Bars. Bars represent the mean scores for successful (green) and failed (red) pediatric MRI sedations, with error bars showing one standard deviation. Black horizontal lines indicate the upper limits of variability, covering 25% of each bar’s width. A significant difference is evidenced by a T-statistic of 19.73 and a P-value of 0.000, highlighting the disparity in sedation effectiveness between outcomes.
Figure 5.
Mean RAMSAY Score by Outcome with Error Bars. Bars represent the mean scores for successful (green) and failed (red) pediatric MRI sedations, with error bars showing one standard deviation. Black horizontal lines indicate the upper limits of variability, covering 25% of each bar’s width. A significant difference is evidenced by a T-statistic of 19.73 and a P-value of 0.000, highlighting the disparity in sedation effectiveness between outcomes.
Figure 6.
Histogram representing the bootstrap distribution of the median time to induce sleep, derived from 10,000 bootstrap samples. The original median time is indicated by a red dashed line at 30 minutes, with the 95% confidence interval marked by green dashed lines at the 2.5% (29 minutes) and 97.5% (34 minutes) percentiles.
Figure 6.
Histogram representing the bootstrap distribution of the median time to induce sleep, derived from 10,000 bootstrap samples. The original median time is indicated by a red dashed line at 30 minutes, with the 95% confidence interval marked by green dashed lines at the 2.5% (29 minutes) and 97.5% (34 minutes) percentiles.
Table 1.
Patient demographics, sedation characteristics, and outcomes for pediatric MRI sedation using oral chloral hydrate. Data are presented as Median (IQR) for continuous variables and n (%) for categorical variables.
Table 1.
Patient demographics, sedation characteristics, and outcomes for pediatric MRI sedation using oral chloral hydrate. Data are presented as Median (IQR) for continuous variables and n (%) for categorical variables.
| Variable |
Results |
| Age [Median (IQR)] |
30(11.5-48) |
| Weight(kg) [Median (IQR)] |
11.9(8.0-16.7) |
| Chloral Hydrate Dose /kg [Median (IQR)] |
66(50.95-74.35) |
| Time to induce Sleep (Minutes) [Median (IQR)] |
30(23-45) |
| Duration of MRI [Median (IQR)] |
60(50-80) |
| RAMSAY Score |
4(4-5) |
| Gender n (%) |
| Male |
70(38.25%) |
| Female |
113(61.75%) |
| Diagnosis, n (%) |
| Neurological, n (%) |
138(75.41%) |
| Non-neurological, n (%) |
45(24.59%) |
| Multidose, n (%) |
| Yes |
35(19.23%) |
| No |
147(80.77%) |
| Outcome, n (%) |
|
| Success |
157(85.79%) |
| Fail |
26(14.21%) |
| Complications, n (%) |
| Vomiting |
15(8.20%) |
| Nausea |
21(11.48%) |
| Agitation |
8(4.37%) |
Table 2.
Comparison of patient characteristics between failed and successful conscious sedation (CS) outcomes. Continuous variables are presented as median [IQR], and categorical variables are shown as percentages. Statistical significance is indicated by P values. .
Table 2.
Comparison of patient characteristics between failed and successful conscious sedation (CS) outcomes. Continuous variables are presented as median [IQR], and categorical variables are shown as percentages. Statistical significance is indicated by P values. .
| Variable |
Fail CS |
Success CS |
P value |
| N |
16 |
167 |
|
| Age (median [IQR]) |
48[17.5, 72] |
28.00 [10.5, 48] |
0.063 |
| Gender (%) Male |
9 (56.2) |
104 (62.3) |
0.838 |
| Wt (median [IQR]) |
15.30 [9.40, 21.23] |
11.7 [7.93, 15.5] |
0.058 |
| Diagnosis (%), Neurological |
13 (81.2) |
125 (74.9) |
0.792 |
| Chloral dose (median [IQR]) |
67 [54.58, 74.45] |
65.8 [50.61, 74.35] |
0.863 |
| Time to Sleep (median [IQR]) |
60 [60, 60] |
30 [20, 40] |
<0.001 |
| Multiple dose (%), Yes |
3 (18.8) |
32 (19.3) |
1.000 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).