Submitted:
17 September 2024
Posted:
18 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Anesthesia and Surgical Preparation
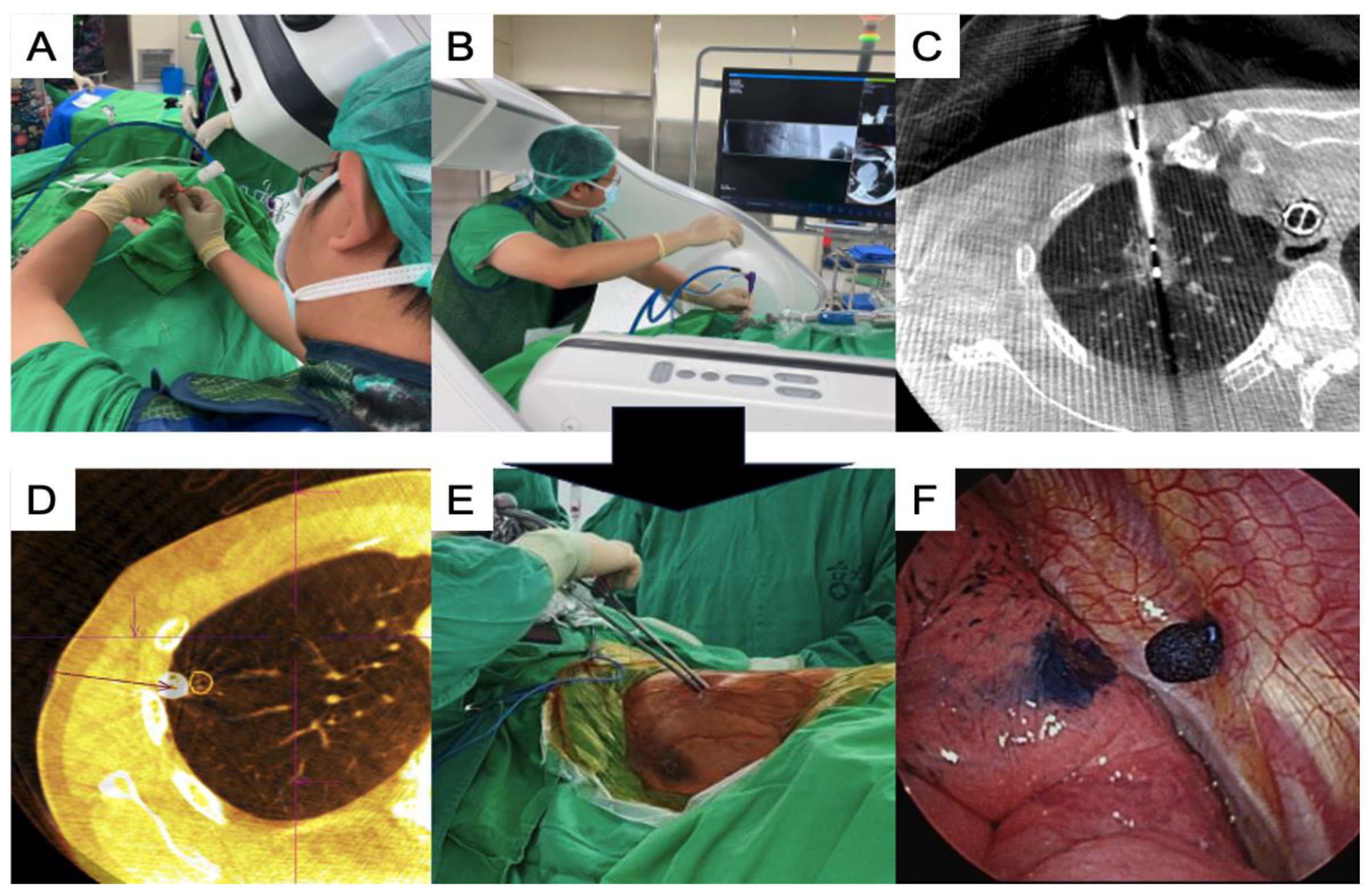
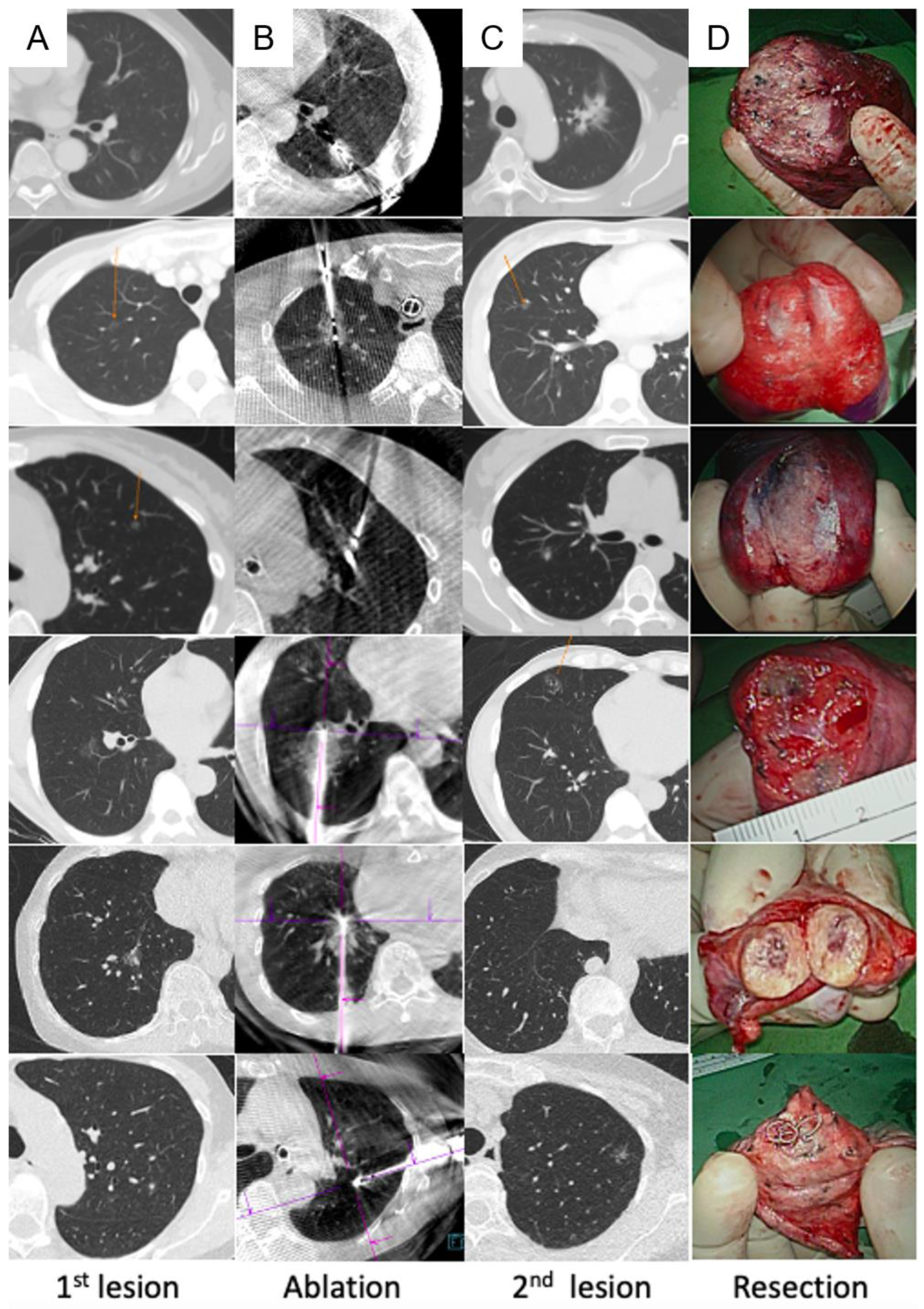
2.3. Image-Guided Lung Ablation
2.4. Image-Guided VATS
2.5. Postoperative Care
2.6. Data Collection
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmid-Bindert, G.; Vogel-Claussen, J.; Gütz, S.; Fink, J.; Hoffmann, H.; Eichhorn, M.E.; Herth, F.J.F. Incidental pulmonary nodules - what do we know in 2022. Respiration 2022, 101, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research, Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; van 't Westeinde, S.; Prokop, M.; Mali, W.P.; Mohamed Hoesein, F.A.A.; van Ooijen, P.M.A.; Aerts, J.G.J.V.; den Bakker, M.A.; Thunnissen, E.; Verschakelen, J.; Vliegenthart, R.; Walter, J.E.; Ten Haaf, K.; Groen, H.J.M.; Oudkerk, M. Reduced lung cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.W.; Chen, J.S. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis 2016, 8, S749–S755. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D'Amico, T.A.; Dilling, T.J.; Dowell, J.; Gettinger, S.; Gubens, M.A.; Hegde, A.; Hennon, M.; Lackner, R.P.; Lanuti, M.; Leal, T.A.; Lin, J.; Loo, B.W. Jr; Lovly, C.M.; Martins, R.G.; Massarelli, E.; Morgensztern, D.; Ng, T.; Otterson, G.A.; Patel, S.P.; Riely, G.J.; Schild, S.E.; Shapiro, T.A.; Singh, A.P.; Stevenson, J.; Tam, A.; Yanagawa, J.; Yang, S.C.; Gregory, K.M.; Hughes, M. NCCN guidelines insights: non-small cell lung cancer, Version 2.2021. J Natl Compr Canc Netw 2021, 19, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Donington, J.; Lynch, W.R.; Mazzone, P.J.; Midthun, D.E.; Naidich, D.P.; Wiener, R.S. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e93S–e120S. [Google Scholar] [CrossRef] [PubMed]
- Moyer, V.A.; U. S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014, 160, 330–338. [Google Scholar] [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; Rubin, G.D.; Schaefer-Prokop, C.M.; Travis, W.D.; Van Schil, P.E.; Bankier, A.A. ; Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Donahoe, L.L.; Nguyen, E.T.; Chung, T.B.; Kha, L.C.; Cypel, M.; Darling, G.E.; de Perrot, M.; Keshavjee, S.; Pierre, A.F.; Waddell, T.K.; Yasufuku, K. ; CT-guided microcoil VATS resection of lung nodules: a single-centre experience and review of the literature. J Thorac Dis 2016, 8, 1986–1994. [Google Scholar] [CrossRef]
- Huang, Y.H.; Chen, K.C.; Chen, J.S. Ultrasound for intraoperative localization of lung nodules during thoracoscopic surgery. Ann Transl Med 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.C.; Lee, Y.F.; Chen, Y.C.; Chien, N.; Huang, Y.S.; Tseng, Y.H.; Lee, J.M.; Hsu, H.H.; Chen, J.S.; Chang, Y.C. CT-guided percutaneous microwave ablation of pulmonary malignant tumors. J Thorac Dis 2016, 8, S659–S665. [Google Scholar] [CrossRef]
- Bhatia, S.; Pereira, K.; Mohan, P.; Narayanan, G.; Wangpaichitr, M.; Savaraj, N. Radiofrequency ablation in primary non-small cell lung cancer: what a radiologist needs to know. Indian J Radiol Imaging 2016, 26, 81–91. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Huang, J. Clinical and pathological research status of multiple pulmonary nodules. J Cancer Ther 2023, 14, 170–181. [Google Scholar] [CrossRef]
- Chockalingam, A.; Konstantinidis, M.; Koo, B.; Moon, J.T.; Tran, A.; Nourouzpour, S.; Lawson, E.; Fox, K.; Habibollahi, P.; Odisio, B.; Loya, M.; Bassir, A.; Nezami, N. Surgical resection, radiotherapy and percutaneous thermal ablation for treatment of stage 1 non-small cell lung cancer: protocol for a systematic review and network meta-analysis. BMJ Open 2022, 12, e057638. [Google Scholar] [CrossRef] [PubMed]
- Palussiere, J.; Catena, V.; Buy, X. Percutaneous thermal ablation of lung tumors - radiofrequency, microwave and cryotherapy: where are we going? Diagn Interv Imaging 2017, 98, 619–625. [Google Scholar] [CrossRef]
- Li, G.; Xue, M.; Chen, W.; Yi, S. Efficacy and safety of radiofrequency ablation for lung cancers: a systematic review and meta-analysis. Eur J Radiol 2018, 100, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Healey, T.T.; March, B.T.; Baird, G.; Dupuy, D.E. Microwave ablation for lung neoplasms: a retrospective analysis of long-term results. J Vasc Interv Radiol 2017, 28, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Knavel, E.M.; Brace, C.L. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol 2013, 16, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.W.; Sydnor, M.K. Jr. Current state of tumor ablation therapies. Dig Dis Sci 2019, 64, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.S.; Dupuy, D.E. Lung cancer ablation: technologies and techniques. Semin Intervent Radiol 2013, 30, 141–150. [Google Scholar] [CrossRef]
- Abtin, F.G.; Eradat, J.; Gutierrez, A.J.; Lee, C.; Fishbein, M.C.; Suh, R.D. Radiofrequency ablation of lung tumors: imaging features of the postablation zone. Radiographics 2012, 32, 947–969. [Google Scholar] [CrossRef]
- Mouli, S.K.; Kurilova, I.; Sofocleous, C.T.; Lewandowski, R.J. The role of percutaneous image-guided thermal ablation for the treatment of pulmonary malignancies. AJR Am J Roentgenol 2017, 209, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e278S–e313S. [Google Scholar] [CrossRef]
- Genshaft, S.J.; Suh, R.D.; Abtin, F.; Baerlocher, M.O.; Dariushnia, S.R.; Devane, A.M.; Himes, E.; Lisberg, A.; Padia, S.; Patel, S.; Yanagawa, J. Society of Interventional Radiology Quality Improvement Standards on percutaneous ablation of non-small cell lung cancer and metastatic disease to the lungs. J Vasc Interv Radiol 2021, 32, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.; Cariati, M.; Marra, P.; Masala, S.; Pereira, P.L.; Carrafiello, G. CIRSE standards of practice on thermal ablation of primary and secondary lung tumours. Cardiovasc Intervent Radiol 2020, 43, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, Y. MDT is still important in the treatment of early-stage lung cancer. J Thorac Dis 2018, 10, S3984–S3985. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Pennathur, A.; Landreneau, R.J.; Luketich, J.D. Radiofrequency and microwave ablation of lung tumors. J Surg Oncol 2009, 100, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Li, X.; Zhang, L.; Jiang, H.; Zhang, J. Comparison of microwave ablation and multipolar radiofrequency ablation in vivo using two internally cooled probes. AJR Am J Roentgenol 2012, 198, W46–W50. [Google Scholar] [CrossRef]
- Andreano, A.; Huang, Y.; Meloni, M.F.; Lee, F.T. Jr.; Brace, C. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys 2010, 37, 2967–2973. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, L.; Bozzi, E.; Faviana, P.; Cioni, D.; Della Pina, C.; Sbrana, A.; Fontanini, G.; Lencioni, R. Thermal ablation of lung tissue: in vivo experimental comparison of microwave and radiofrequency. Cardiovasc Intervent Radiol 2010, 33, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.; Long, Y.J.; Yan, G.W.; Bhetuwal, A.; Zhuo, L.H.; Yao, H.C.; Zhang, J.; Zou, X.X.; Hu, P.X.; Yang, H.F.; Du, Y. Microwave ablation vs. cryoablation for treatment of primary and metastatic pulmonary malignant tumors. Mol Clin Oncol 2022, 16, 62. [Google Scholar] [CrossRef]
- Chaudhry, A.; Grechushkin, V.; Hoshmand, M.; Kim, C.W.; Pena, A.; Huston, B.; Chaya, Y.; Bilfinger, T.; Moore, W. Characteristic CT findings after percutaneous cryoablation treatment of malignant lung nodules. Medicine 2015, 94, e1672. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liang, B.; Li, Y.; Xu, J.; Qian, W.; Lin, M.; Xu, M.; Niu, L. CT-guided percutaneous cryoablation in patients with lung nodules mainly composed of ground-glass opacities. J Vasc Interv Radiol 2022, 33, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.K.; Yang, S.M.; Chien, N.; Chang, C.C.; Fang, H.Y.; Liu, M.C.; Wang, K.L.; Lin, W.C.; Lin, F.C.F.; Chuang, C.Y.; Hsu, P.K.; Huang, T.W.; Chen, C.K.; Chang, Y.C.; Huang, K.W. 2024 multidisciplinary consensus on image-guided lung tumor ablation from the Taiwan Academy of Tumor Ablation. Thorac Cancer 2024, 15, 1607–1613. [Google Scholar] [CrossRef]
- Murphy, M.C.; Wrobel, M.M.; Fisher, D.A.; Cahalane, A.M.; Fintelmann, F.J. Update on image-guided thermal lung ablation: society guidelines, therapeutic alternatives, and Postablation imaging findings. Am J Roentgenol 2022, 219, 471–85. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S.H.; Krimsky, W.S.; Yasufuku, K. The hybrid operating room in modern thoracic surgery. Front Surg 2021, 8, 725897. [Google Scholar] [CrossRef]
- Yang, S.M.; Chung, W.Y.; Ko, H.J.; Chen, L.C.; Chang, L.K.; Chang, H.C.; Kuo, S.W.; Ho, M.C. Single-stage augmented fluoroscopic bronchoscopy localization and thoracoscopic resection of small pulmonary nodules in a hybrid operating room. Eur J Cardiothorac Surg 2022, 63, ezac541. [Google Scholar] [CrossRef] [PubMed]
- Spenkelink, I.M.; Heidkamp, J.; Futterer, J.J.; Rovers, M.M. Image guided procedures in the hybrid operating room: a systematic scoping review. PLoS One 2022, 17, e0266341. [Google Scholar] [CrossRef] [PubMed]
- Harrison, O.J.; Sarvananthan, S.; Tamburrini, A.; Peebles, C.; Alzetani, A. Image-guided combined ablation and resection in thoracic surgery for the treatment of multiple pulmonary metastases: a preliminary case series. JTCVS Tech 2021, 9, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.K.; Yang, S.M.; Chung, W.Y.; Chen, L.C.; Chang, H.C.; Ho, M.C.; Chang, YC.; Yu, C.J. Cone-beam computed tomography image-guided percutaneous microwave ablation for lung nodules in a hybrid operating room: an initial experience. Eur Radiol 2024, 34, 3309–3319. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.S.; Chang, L.K.; Malwade, S.; Chung, W.Y.; Yang, S.M. Cone beam CT derived laser-guided percutaneous lung ablation: minimizing needle-related complications under general anesthesia with lung separation. Acad Radiol 2024, S1076-6332(24)00284-8. [Google Scholar] [CrossRef]
- Yang, S.M.; Malwade, S.; Chung, W.Y.; Wu, W.T.; Chen, L.C.; Chang, L.K.; Change, H.C.; Chan, P.S.; Kuo, S.W. Augmented fluoroscopy-guided dye localization for small pulmonary nodules in hybrid operating room: intrathoracic stamping versus transbronchial marking. Int J Comput Assist Radiol Surg 2024, 1–11. [Google Scholar] [CrossRef]
- Yang, S.M.; Malwade, S.; Chung, W.Y.; Chen, L.C.; Chang, L.K.; Chang, H.C.; Chan, P.S.; Kuo, S.W. Nontraumatic intraoperative pulmonary nodule localization with laser guide stamping in a hybrid operating room. Updates Surg 2024, 1–10. [Google Scholar] [CrossRef]
- Loverdos, K.; Fotiadis, A.; Kontogianni, C.; Iliopoulou, M.; Gaga, M. Lung nodules: a comprehensive review on current approach and management. Ann Thorac Med 2019, 14, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.R.; Barlow, J.; Jaklitsch, M.T.; Schmidlin, E.J.; Hartigan, P.M.; Bueno, R. Image-guided video-assisted thoracoscopic resection (iVATS): translation to clinical practice—real-world experience. J Surg Oncol 2020, 121, 1225–1232. [Google Scholar] [CrossRef]
- Owen, D.; Olivier, K.R.; Mayo, C.S.; Miller, R.C.; Nelson, K.; Bauer, H.; Brown, P.D.; Park, S.S.; Ma, D.J.; Garces, Y.I. . Outcomes of stereotactic body radiotherapy (SBRT) treatment of multiple synchronous and recurrent lung nodules. Radiat Oncol 2015, 10, 43. [Google Scholar] [CrossRef]

| No. | Sex | Age (years) | 1st stage | Localization for VATS | 2nd stage | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Size | Depth | Procedure | Duration | Location | Size | Depth | Procedure | Duration | ||||
| 1 | F | 36 | LUL | 5.6 | 24.6 | MWA | 77 | Transbronchial | RUL | 1.1 | 0.5 | Wedge | 73 |
| 2 | F | 43 | RUL | 7.7 | 27.5 | Cryo | 60 | Transbronchial | RLL | 9.5 | 13.3 | Segmentectomy | 94 |
| 3 | F | 55 | RUL | 6.6 | 18.6 | MWA | 78 | Transbronchial | RUL | 5.8 | 9.4 | Wedge | 65 |
| 4 | F | 54 | RUL | 7.4 | 39.3 | MWA | 21 | Transthoracic | RML | 7.2 | 9.6 | Wedge | 58 |
| 5 | F | 68 | RLL | 15.6 | 61.3 | MWA | 57 | - | RLL | 14.2 | 6.0 | Wedge | 88 |
| 6 | F | 57 | RUL | 5.9 | 20.7 | MWA | 70 | Transthoracic | RUL | 7.3 | 4.2 | Wedge | 95 |
| 7 | M | 60 | RLL | 20.9 | 44.7 | Cryo | 49 | - | RML | 18.3 | 13.1 | Wedge | 89 |
| 8 | M | 42 | RUL | 6.7 | 30.5 | MWA | 71 | Transthoracic | RUL*2 | 12.4/6.3 | 14.5/6.7 | Wedge*2 | 127 |
| 9 | F | 68 | LUL | 6.2 | 21.9 | MWA | 47 | Transthoracic | LUL | 11.6 | 13.9 | Wedge | 75 |
| 10 | F | 58 | RUL | 7.4 | 39.3 | MWA | 24 | - | RUL | 14.8 | 15.5 | Wedge | 105 |
| 11 | F | 54 | RUL | 37.8 | 17.5 | MWA | 42 | - | RML | 8.2 | 4.0 | Wedge | 41 |
| 12 | F | 44 | LLL | 8.4 | 30.1 | MWA | 46 | Transthoracic | LLL | 6.0 | 3.6 | Wedge | 71 |
| 13 | F | 43 | RUL/RLL*2 | 9.4 13.3/11.1 |
35.5 30.8/18.9 |
MWA | 78 | Transthoracic | RLL | 9.4 | 12.2 | Wedge | 120 |
| 14 | F | 63 | RLL | 7.8 | 23.9 | MWA | 61 | Transthoracic | RUL | 5.3 | 3.1 | Wedge | 30 |
| 15 | F | 33 | LUL | 5.1 | 22.2 | MWA | 30 | Transthoracic | LLL*2 | 8.4/5.8 | 4.4/11.1 | Wedge*2 | 118 |
| 16 | M | 57 | RML | 10.3 | 25.2 | MWA | 18 | - | RUL | 36.6 | 9.8 | Lobectomy | 118 |
| 17 | F | 64 | LUL | 8.9 | 31.4 | MWA | 45 | - | LUL/LLL | 14.4/14.1 | 13.1/35.5 | Wedge/Segmentectomy | 172 |
| 18 | F | 43 | RUL | 8.1 | 52.1 | MWA | 68 | Transthoracic | RUL | 6.6 | 4.1 | Wedge | 38 |
| 19 | F | 65 | RUL | 8.1 | 3.4 | Wedge | 50 | Transthoracic | RUL | 4.8 | 27.6 | MWA | 88 |
| 20 | M | 52 | RUL | 10.4 | 40.4 | MWA | 38 | Transthoracic | RLL/RLL | 6.3/10.2 | 12.2/7.2 | Wedge/Segmentectomy | 170 |
| 21 | F | 66 | RUL | 13.3 | 19.2 | MWA | 31 | Transbronchial | LUL | 30.2 | 15.7 | Segmentectomy | 102 |
| 22 | F | 68 | RUL | 11.4 | 25.7 | MWA | 32 | Transbronchial | LLL | 40.8 | 12.8 | Segmentectomy | 110 |
| Median | 56 | 8.2 | 26.6 | 48 | 8.9 | 10.5 | 91.5 | ||||||
| IQR | 21 | 7.1–11.3 | 21.3–37.4 | 32–68 | 6.3–14.2 | 4.4–13.3 | 72–114 | ||||||
| No. | Fluoroscopy duration (min) | Number of Dyna CT scans | Total DAP (uGym2) | Total Ana time | Global OR time | Complications | LOS | Histology | Postoperative follow-up interval (mo) |
Evidence of residual disease or recurrence |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Needle Biopsy | Resection | ||||||||||
| 1 | 2.7 | 14 | 12449 | 210 | 220 | - | 1 | - | Adenocarcinoma | 27 | No |
| 2 | 2.8 | 7 | 17436 | 222 | 232 | - | 2 | - | MIA | 26 | No |
| 3 | 4.7 | 8 | 32943 | 223 | 229 | - | 1 | - | AIS | 22 | No |
| 4 | 1.9 | 6 | 6243 | 157 | 162 | - | 1 | - | AIS | 22 | No |
| 5 | 2.7 | 9 | 14032 | 227 | 240 | - | 3 | AIS at least | Sclerosing pneumocytoma | 20 | No |
| 6 | 2.7 | 9 | 16095 | 233 | 243 | - | 2 | Benign alveolar parenchyma | AIS | 19 | No |
| 7 | 1.2 | 6 | 5416.3 | 202 | 218 | 1 | - | Adenocarcinoma | 18 | No | |
| 8 | 5.1 | 9 | 32305 | 205 | 214 | Hemothorax | 8 | - | AIS*2 | 17 | No |
| 9 | 2.7 | 12 | 13794 | 225 | 235 | - | 2 | - | Adenocarcinoma | 15 | No |
| 10 | 2.2 | 6 | 14119 | 193 | 196 | - | 2 | - | Adenocarcinoma | 15 | No |
| 11 | 1.6 | 5 | 11764 | 162 | 180 | - | 2 | - | Adenocarcinoma | 10 | |
| 12 | 1.6 | 9 | 8871.2 | 169 | 182 | - | 2 | AIS at least | AIS | 11 | No |
| 13 | 2.2 | 14 | 36430 | 264 | 277 | Prolonged air leak | 6 | - | Metastatic colon cancer | 8 | |
| 14 | 3.2 | 12 | 7514.5 | 172 | 192 | - | 2 | Benign alveolar parenchyma | AIS | 10 | No |
| 15 | 2.9 | 10 | 17422 | 196 | 210 | - | 2 | Adenocarcinoma | AIS/AAH | 9 | No |
| 16 | 1.7 | 9 | 30818 | 242 | 253 | - | 2 | AIS at least | Adenocarcinoma | 6 | No |
| 17 | 1.2 | 8 | 22704 | 296 | 302 | - | 2 | - | MIA/MIA | 5 | No |
| 18 | 1.2 | 9 | 11878 | 162 | 185 | - | 1 | AAH | AIS | 4 | No |
| 19 | 1.6 | 10 | 17482 | 216 | 226 | - | 3 | - | AIS | 3 | No |
| 20 | 1.8 | 8 | 13042 | 281 | 291 | - | 2 | - | AIS/MIA | 3 | No |
| 21 | 3.3 | 8 | 5566.8 | 375* | 380* | - | 4 | - | Metastatic colon cancer | 1 | No |
| 22 | 5.2 | 9 | 22354 | 240 | 249 | - | 3 | AIS at least | Adenocarcinoma | 1 | No |
| Median | 2.5 | 9 | 14076 | 219 | 227 | 2 | 10.5 | ||||
| IQR | 1.6–2.9 | 8–10 | 11764–22354 | 193–240 | 196–249 | 2–3 | 5–19 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





