1. Introduction
Helicobacter pylori (H. pylori) is a common human pathogen that affects roughly half of the global population, or about 4.4 billion people, according to estimates. [
1]. H. pylori has been linked to several significant gastrointestinal diseases, including chronic gastritis, stomach ulcers, duodenal ulcers, MALT lymphoma, and gastric adenocarcinoma. Recent studies suggest that patients with unexplained iron deficiency anemia or idiopathic thrombocytopenic purpura may benefit from screening for H. pylori infection. [
2].
To prevent the development of precancerous changes in the gastric mucosa and stomach cancer, which are associated with the presence of H. pylori, eradication therapy has been used worldwide. Unfortunately, the effectiveness of this treatment over the past 35 years has not reached the desired goal of 80-90%, due to the emergence of antibiotic resistance [
3]. In many Asian countries, H. pylori resistance to antibiotics is widespread, and the success rate of standard triple therapy based on clarithromycin is below 80% [
4]. This is due to local patterns of H. pylori resistance, which is why it is essential to analyze local resistance profiles in H. pylori.
The overall antibiotic resistance rate in the United States is 42.1% for metronidazole (95% CI 27.3-58.6%), 37.6% for levofloxacin (95 CI 26.3- 50.4%), 31.5 % for clarithromycin (95% CI 23.6-40.6), 2.6% for amoxicillin (95 1.4-5.0), 0.87 % for tetracycline (95 % CI 0,2-3,8), and 0.17 % for rifabutine (95%CI 0-10,9). Significant variability was observed in the aggregated prevalence rates, with the exception of rifabutin resistance [
5]. In Europe, H. pylori resistance rates are 18-21.4% for clarithromycin, 11-16.3% for levofloxacin, and 39.1-56% for metronidazole. Additionally, antibiotic resistance rates in Central and Southern Europe are higher than in Northern Europe [
6].
Asia, being the most populous continent (with 4.4 billion people), has a high incidence of gastric cancer, with an age-standardized incidence rate of 15.8 per 100,000 people [
7]. In Asia, metronidazole resistance is the highest in the world, at 78.7%, followed by levofloxacin at 41.3%, clarithromycin at 29.9%, and amoxicillin at 11.9%. Minocycline resistance, found in Mongolia, is 0.28% [
8].
In China, initial resistance of H. pylori to clarithromycin, metronidazole, and levofloxacin is notably high, with rates of 28.9%, 63.8%, and 28%, respectively, and an upward trend was observed over the study period [
9]. In South Korea, similar findings were reported, with national study results showing resistance rates of 17.8% for clarithromycin, 29.5% for metronidazole, and 37% for levofloxacin [
10].
The cumulative prevalence of H. pylori antibiotic resistance in the Asia-Pacific region during the analyzed period from 1990 to 2022 was revealed to be 22% (95% CI: 20–23; I² =96%), 52% resistance for clarithromycin (95 % CI: 49
–55), 26 % (95 CI: 95–96), and 4 % resistance for tetracycline (95 % CI: 3-5) and amoxicillin (95%) [
11].
Despite the high prevalence of Helicobacter pylori and widespread levels of antibiotic resistance in various regions of Asia, data on Central Asia is scarce. The aim of this paper is to critically review and analyze published local studies on antibiotic resistance to H. pylori in Central Asia.
2. Search Strategy and Study Selection
We searched various databases, including PubMed, Google Scholar, Embase, Web of Science, and the Cochrane Library, for articles on H. pylori resistance in countries such as Kazakhstan, the Kyrgyz Republic, Tajikistan, Turkmenistan, and Uzbekistan, without any language restrictions. An additional manual search of the documents and summaries from the conference was performed to obtain further information. Articles in English, Russian, Kazakh, Kyrgyz, Uzbek, Tajik, and Turkmen were included, as these are the official languages of the countries in the region. Due to the limited research on this topic, the search for relevant studies was also conducted among children and pregnant women. The study countries were grouped into the Central Asia region based on the UN’s geolocation, which was allocated by the UN Statistics Division.
3. Mechanisms of Resistance in H. pylori Species
The rise in H. pylori resistance is one of the main factors contributing to the ineffectiveness of eradication therapy [
3]. There are both endogenous and exogenous methods for the development of resistant strains of H. pylori [
12]. The endogenous method is related to an individual’s internal characteristics, influenced by the effects of antibiotics and the transmission of resistance genes [
13]. Exogenous acquisition is associated with cross-transmission between individuals or exposure to a common environmental source [
13,
14].
Resistance to H. pylori can result from both isolated and combined drug resistance. Data on heteroresistance, which refers to the presence of H. pylori strains resistant to several antibacterial drugs, have also been published [
15]. Understanding these mechanisms of acquisition is crucial for developing and implementing policies to reduce H. pylori infections [
16]. Endogenous resistance to H. pylori primarily arises from genetic defects that alter drug targets or inhibit drug activation within the cell, thereby leading to resistance to antibacterial drugs [
15]. Three types of endogenous resistance are distinguished in H. pylori: isolated drug resistance (SDR), multidrug resistance (MDR), and heteroresistance (HR) [
3].
3.1. Single Drug Resistance (SDR)
Morphological changes in the H. pylori genome lead to the development of SDR, which results in disturbances in the cellular activity of antibiotics, changes in the drug target, or inhibits the activation of drugs inside the cells. The use of incorrect doses of antibacterial drugs creates ideal conditions for the selection of resistant strains [
10,
17].
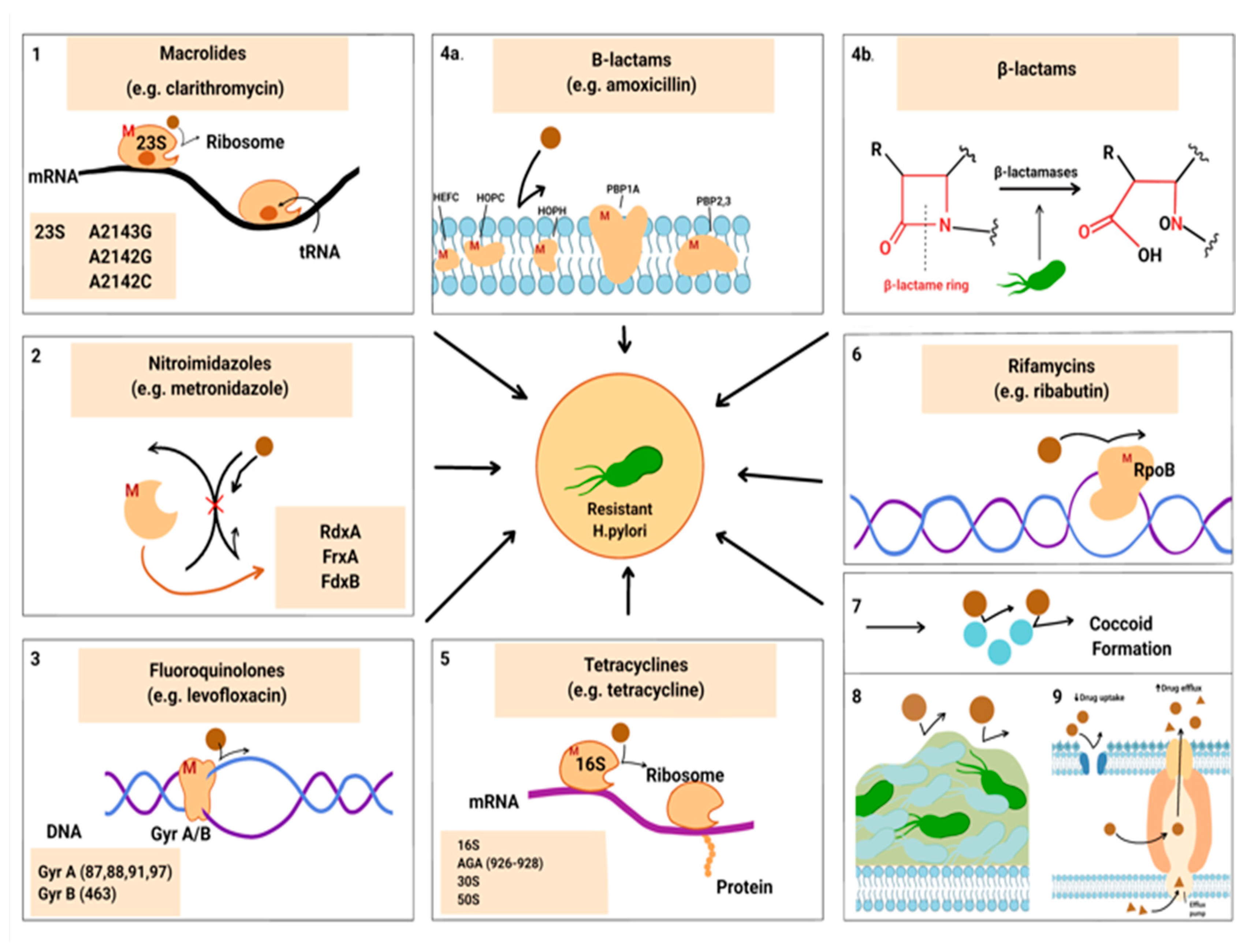
Figure 1 shows the microbiological features that contribute to the development of antibiotic resistance in H. pylori. These transformations change the drug target, or disrupt the activity of antibiotics (1, 3, 4, 5), or inhibit the activation of drugs inside the cells (2). The red letter “M” indicates probable mutations.
Specific alterations in the 23S rRNA V domain can reduce the affinity between clarithromycin and the peptidyltransferase enzyme, leading to resistance in H. pylori. Predominant mutations in this domain include A2143G and A2124G, which are responsible for up to 90% of clarithromycin resistance cases [
18,
19]. Additionally, mutations in the rdxA gene are crucial for metronidazole resistance, as this gene encodes an enzyme that is insensitive to oxygen [
20]. Other mutations in related redox genes, such as frxA and fdxB, can also contribute to resistance [
3]. Furthermore, mutations in the gyrA and gyrB genes can impair the effectiveness of fluoroquinolones in eradication therapies, with a specific mutation in gyrB at position 463 linked to resistance. The most frequent mutations in gyrA occur at positions 87, 88, 91, and 97 [
21].
Beta-lactamase synthesis is associated with amoxicillin resistance in H. pylori. In addition, changes in the efflux pump and reduced cell membrane permeability to amoxicillin may contribute to this resistance. The main mechanism of low-to-moderate levels of amoxicillin resistance is point mutations in PBP1A. However, mutations in other genes, such as PBP2, PBP3, HefC, HopC, and HofH, have also been reported [
22,
23].
Tetracycline binds to the 30S and 50S subunits (5) of the microbial ribosome, and bacteria typically develop resistance through horizontal gene transfer of efflux pumps or ribosomal proteins. It has been found that single and double-base pair mutations are responsible for low-level resistance to tetracycline, while triple-base pair mutation in the AGA region of 16S rRNA (926–928) is associated with high resistance to tetracycline [
24]. Furthermore, a study conducted by Anushiravani et al. (2009) demonstrated proton motive force-dependent efflux mechanisms that may contribute to the development of tetracycline-resistant H. pylori strains [
25].
Morphological mutations in the DNA-dependent RNA polymerase (RpoB) protein result in decreased affinity for the antibiotic rifamycin, thereby safeguarding bacterial DNA replication and transcription from the effects of the antibiotic [
6].
3.2. Multidrug Resistance (MDR)
Resistance to two or more antibiotics from different classes in H. pylori is considered MDR [
26].
Today, the global prevalence of MDR has increased, with 60.8% of strains being doubly or multidrug resistant in China [
27]. In other Asian countries (Cambodia and Korea), the prevalence was estimated at 57.1% and 42.9%, respectively [
28,
29]. The prevalence in Europe is between 10% and 40% [
30]. There is a trend towards increasing MDR levels of H. pylori, which makes eradication difficult worldwide. H. Pylori remains a complex organism [
31].
Factors associated with resistance include national antibiotic use, inappropriate antibiotic prescription, treatment failure, efflux pump activity, genomic mutations, and biofilms [
3].
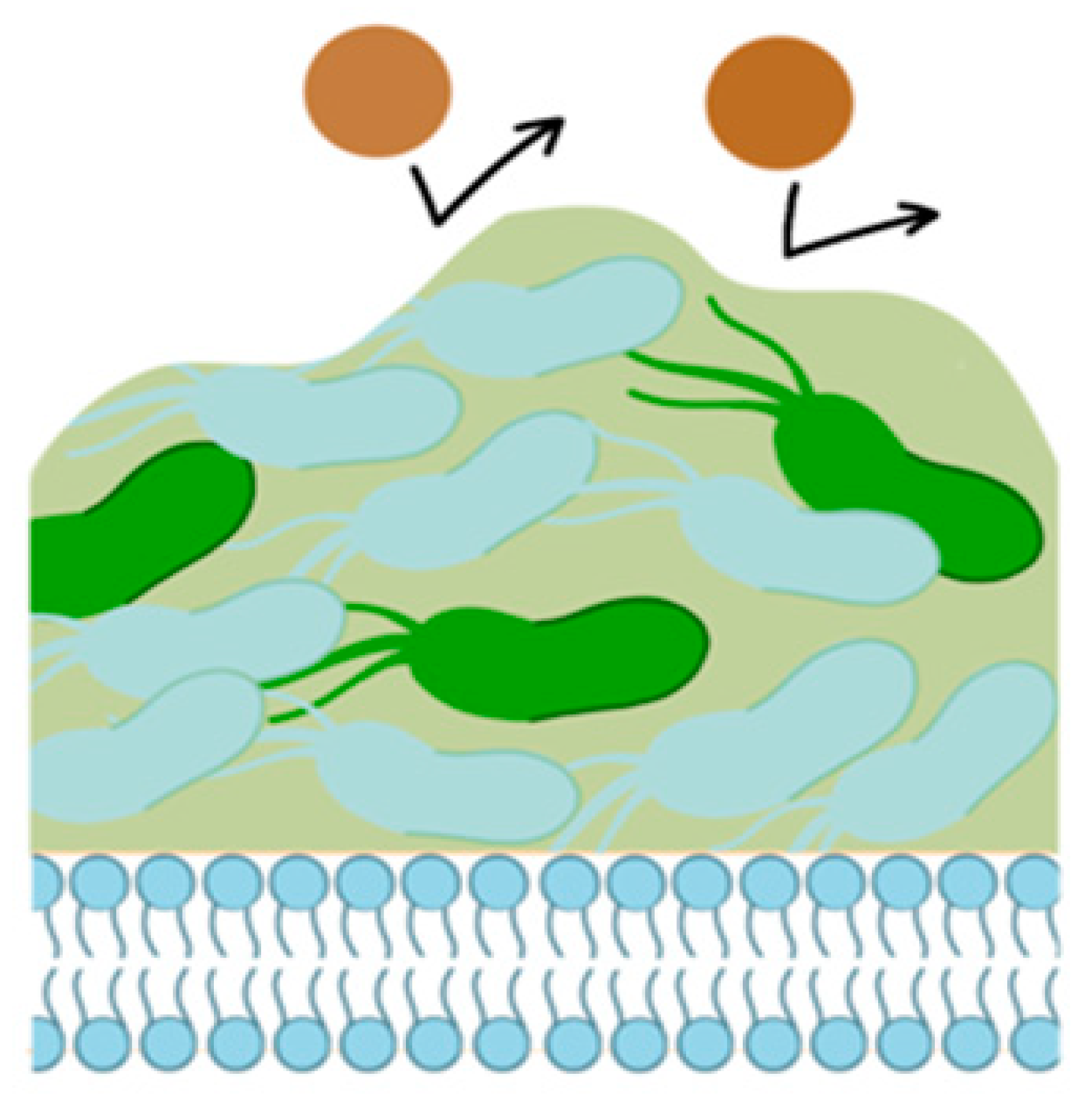
A possible cause for resistance is the formation of biofilms by H. pylori (
Figure 2). Recombination of H. pylori genes, together with drug permeability, probably creates mechanisms of film functioning, with the development of MDR. This hypothesis requires further study. Only a few genes have been studied that are likely associated with biofilm formation in H pylori (SpoT, fucT, jHp_1117, homD, hp0939, hp0497 and hp0471) [
31].
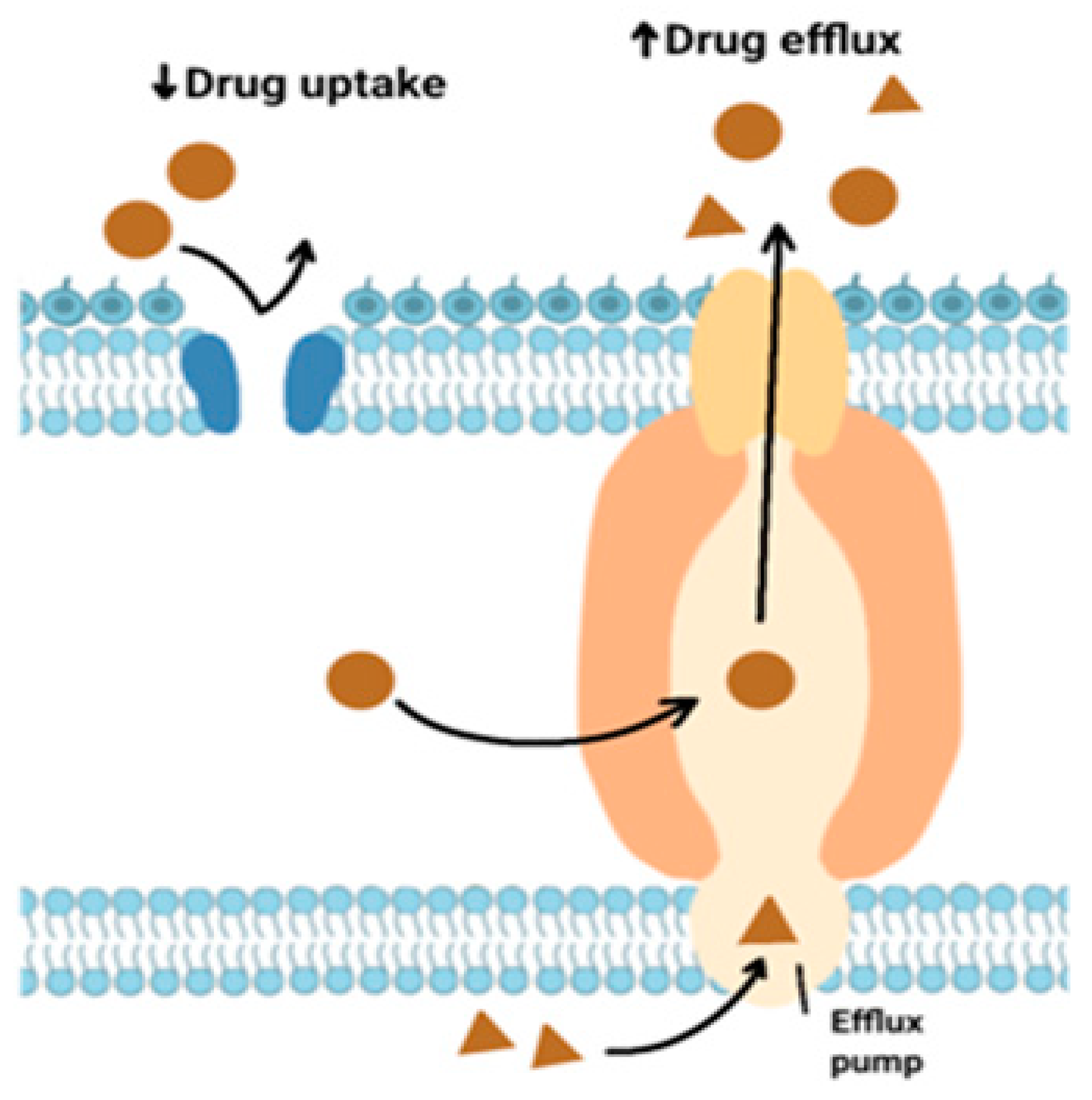
These pumps are protein compounds responsible for the rapid elimination of antibiotics from the cell. As a result, antibacterial agents do not bind to ribosomes of microorganisms [
32,
33]. The ABC, MATE, MFS, SMR, and END protein families enhance this process [
34].
Figure 3 shows efflux pumps in H. pylori.
Analysis of MDR-associated mutations is in the early stages of development. A significant mechanism for MDR development is the A149G mutation in FabH [
35]. With the increasing number of studies using next-generation sequencing, new MDR-related mutations are likely to be discovered [
36].
3.3. Heteroresistance (HR)
The coexistence of H. pylori strains that have both sensitive and resistant strains to antibacterial drugs of the same group is called heteroresistance [
37]. The frequency of heteroresistant strains was 60.1% for clarithromycin, 61.1% for metronidazole, 46.1% for levofloxacin, 3.8% for amoxicillin, and 21.1% overall. During 2001-2022, heteroresistant H. pylori infections were predominantly due to multiple strains in developing countries, while in developed countries, they were attributed to mutations in a single strain [
38,
39]. Biopsies taken from only one area may miss resistant strains [
40]. Literature describes interregional heteroresistance, where both susceptible and resistant strains are colonized in different areas of the stomach [
41]. According to these data, heteroresistance can be caused by the failure of eradication therapy. In a study of patients who did not respond to eradication therapy, clarithromycin-resistant strains exhibited higher minimum inhibitory concentrations (MICs) in the body compared to the antrum, while amoxicillin-resistant strains were more prevalent in the antral region. However, patients with multidrug resistance (MDR) and heterogeneous resistance to sitafloxacin (STFX) showed no difference in the localization of these strains between the antrum and the body of the stomach [
41]. Data from the analysis confirmed that clarithromycin- and amoxicillin-resistant strains are widely distributed throughout different parts of the stomach and can develop following eradication failure [
41]. Recombinant analysis also indicated that mutations at G591K and A480V in PBP1a are associated with amoxicillin heteroresistance [
41].
3.4. Antibiotics Resistance of H. pylori in Central Asia
Due to the high population density in Asia, it has the highest prevalence of gastric cancer, making it a significant area for H. pylori eradication efforts [
7].
In 2021, the overall prevalence of H. pylori in Kazakhstan was 62.7%, similar to the average for the country over the past few decades [
42]. These data are similar to the results of a meta-analysis and systematic review for the period 1980-2022, where the prevalence of H. pylori infection in Kazakhstan was 62.4% (95% CI:59.3-65.3%) [
43].
The prevalence in Kyrgyzstan was found to be 81% using PCR testing in 2013 [
33], highlighting the importance of continued research and efforts to combat this infection.
Higher infection rates were reported in Uzbekistan, with a prevalence of 74.9% for H. pylori in 2010 [
44] and which increased to 80% by 2019 [
45].
Data on the prevalence of H. pylori infection in Turkmenistan and Tajikistan are lacking. As the incidence of stomach cancer is high in these countries, it is expected that the prevalence of H. pylori infections will be high in these regions.
Despite the high prevalence of H. pylori and its burden in the form of gastric cancer, data on antibiotic resistance in Central Asia is limited. In a targeted search of publications on antibiotic resistance in Pylori by authors from Central Asia, we found only 3 relevant articles. In Kazakhstan, only one study has been conducted to determine antibiotic resistance in H. pylori infections.
In the research conducted by Kulmambetova G.N., Bekenova E.E., et al. (2011), PCR was employed to identify potential antibiotic resistance genes, and 16 samples from residents of central Kazakhstan were sequenced to analyze the resistance spectrum [
46]. The analysis revealed that 87.5% of the strains had mutations in the 23S rRNA gene. Among these, 56.25% had the T2182C mutation, while 12.5% exhibited either the A2142G or A2143G mutation. Notably, the mutations associated with clarithromycin resistance in Asia differ from those found in Europe and North America [
47]. In Western countries, about 90% of clarithromycin-resistant strains carry the A2142G or A2142C mutation. In contrast, in Asia, this mutation represents only 23% of resistant strains [
48]. Other mutations, such as T2183C and A2223G, are more prevalent in Asia and align with those observed in the Kazakhstan study [
46]. Additionally, the study identified a mutation in rdxA in 31.25% of cases and a mutation in pbp1A in 18.75% of cases.
In a 2019 study conducted in Uzbekistan, the assessment of point mutations A2143G and A2142G/C in the V-functional domain of the 23S rRNA gene revealed the following results: 13.3% of the samples contained the A2142G mutation, while none had the A2143G mutation [
49]. These percentages were below the 15% threshold set by the Maastricht Agreement. However, given the distribution pattern of the T2183C mutation and the limited scope of the study, these findings should be interpreted with caution.
In 2019, Uzbekistan was identified as one of the regions with a high prevalence of H. pylori infection, accounting for 80% of the cases. Among the population of Uzbekistan, 84% had a mixed genotype of CagA, with IceA1- and IceA2-. In peptic ulcer disease, pathogenic strains such as CagA+VacA s1, VacA m2, and IceA1,2 are prevalent, whereas in chronic gastritis linked to H. pylori, strains such as Cag+VacA s1 and VacA m2 were more common. To assess H. pylori resistance to clarithromycin, mutations in A2142G/C and A2143G were detected using real-time PCR. Of the 30 samples analyzed, the A2142G/C mutation was identified in four (13.3%) [
45].
In Kyrgyzstan, gastric cancer has a high prevalence and mortality rate, accounting for 12.4% and 10.1% of cases, respectively, and it has become the most common cause of cancer-related deaths [
49]. Selective studies have established that the infection rate of H. pylori among patients with peptic ulcer disease in Kyrgyzstan is 74%, according to research by [
50] and [
51]. Data from the National Center for Cardiology and Therapy reported a 100% infection rate in 2007 [
52]. According to these analyses, the prevalence of H. pylori resistance to clarithromycin was 16.2% and to metronidazole was 45% in 2014 [
33]. A drawback of this study is the lack of information on the methods used to detect antibiotic resistance.
Although the availability and quality of data on antibiotic resistance in some regions is limited or non-existent, we can still assume that there is a significant level of resistance to metronidazole and clarithromycin in Central Asia.
4. Conclusion
Antibiotic-resistant H. pylori strains are becoming a significant issue in Central Asia. Current data reveal high levels of resistance to clarithromycin and metronidazole, which are key antibiotics used in eradication therapy. This resistance presents a major challenge to managing H. pylori infections and mitigating the risk of gastric cancer across the region. There is an urgent need for comprehensive surveillance research and enhanced antibiotic stewardship to tackle this escalating problem.
In Kazakhstan, 87.5% of H. pylori strains exhibited mutations in the 23S gene, indicating resistance to clarithromycin. Resistance to amoxicillin was observed in 18.75% of cases, while resistance to metronidazole was found in 31.25%. In Uzbekistan, 13.3% of strains carried the A2142G mutation, which is associated with resistance to both clarithromycin and metronidazole. In Kyrgyzstan, resistance to these antibiotics was 16.2% and 45%, respectively.
Given that eradication therapy is essential for the treatment of gastric cancer, it is crucial to understand the patterns of resistance in order to make informed decisions about the best treatment options.
Author Contributions
Conceptualization, S.A. and Y.Y.; collected the data, S.A., A.O., V.O.; wrote the original draft, S.A.; reviewed and edited the manuscript, Y.Y. and L.Y.; supervised, directed and managed the study, L.A. and Y.Y. All the authors have read and agreed to with the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan, grant No. AR19575049
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, A.V.; Shkarin, V.V.; Kovalishena, O.V. The role of Helicobacter pylori in complex human comorbidity. Russian Journal of Infection and Immunity Infektsiya i immunitet 2022, 12, 21–32. [Google Scholar] [CrossRef]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori infection and antibiotic resistance — from biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; et al. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Ho, J.J.; Navarro, M.; Sawyer, K.; Elfanagely, Y.; Moss, S.F. Helicobacter pylori Antibiotic Resistance in the United States Between 2011 and 2021: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2022, 117, 1221–1230. [Google Scholar] [CrossRef]
- Megraud, F.; Bruyndonckx, R.; Coenen, S.; Wittkop, L.; Huang, T.-D.; Hoebeke, M.; Bénéjat, L.; Lehours, F.; Goossens, H.; Glupczynski, Y. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 2021, 70, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Miftahussurur, M.; Yamaoka, Y. Appropriate First-Line Regimens to Combat Helicobacter pylori Antibiotic Resistance: An Asian Perspective. Molecules 2015, 20, 6068–6092. [Google Scholar] [CrossRef] [PubMed]
- Azzaya, D.; Gantuya, B.; Oyuntsetseg, K.; Davaadorj, D.; Matsumoto, T.; Akada, J.; Yamaoka, Y. High Antibiotic Resistance of Helicobacter pylori and Its Associated Novel Gene Mutations among the Mongolian Population. Microorganisms 2020, 8, 1062. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Lu, N.-H. Primary Antibiotic Resistance of Helicobacter pylori in China. Dig. Dis. Sci. 2017, 62, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ahn, J.Y.; Choi, K.D.; Jung, H.; Kim, J.M.; Baik, G.H.; Kim, B.; Park, J.C.; Jung, H.; Cho, S.J.; et al. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: A prospective multicenter study. Helicobacter 2019, 24, e12592. [Google Scholar] [CrossRef]
- Hong, T.-C.; El-Omar, E.M.; Kuo, Y.-T.; Wu, J.-Y.; Chen, M.-J.; Chen, C.-C.; Fang, Y.-J.; Leow, A.H.R.; Lu, H.; Lin, J.-T.; et al. Primary antibiotic resistance of Helicobacter pylori in the Asia-Pacific region between 1990 and 2022: an updated systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2024, 9, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Coculescu, B. Antimicrobial resistance induced by genetic changes. J Med Life 2009, 2, 114–123. [Google Scholar] [PubMed]
- Malaty, H.M.; Graham, D.Y.; Klein, D.G.; Adam, E.E.; Evans, D.J. Transmission of Helicobacter pylori Infection Studies in Families of Healthy Individuals. Scand. J. Gastroenterol. 1991, 26, 927–932. [Google Scholar] [CrossRef]
- Bamford, K.B.; Bickley, J.; Collins, J.S.; Johnston, B.T.; Potts, S.; Boston, V.; Owen, R.J.; Sloan, J.M. Helicobacter pylori: comparison of DNA fingerprints provides evidence for intrafamilial infection. Gut 1993, 34, 1348–1350. [Google Scholar] [CrossRef] [PubMed]
- Argueta, E.A.; Ho, J.J.C.; Elfanagely, Y.; D’Agata, E.; Moss, S.F. Clinical Implication of Drug Resistance for H. pylori Management. Antibiotics 2022, 11, 1684. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 464–473. [Google Scholar] [CrossRef]
- Gerrits, M.M.; Schuijffel, D.; van Zwet, A.A.; Kuipers, E.J.; Vandenbroucke-Grauls, C.M.J.E.; Kusters, J.G. Alterations in Penicillin-Binding Protein 1A Confer Resistance to β-Lactam Antibiotics in Helicobacter pylori. Antimicrob. Agents Chemother. 2002, 46, 2229–2233. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, J.M.; Lim, C.-H.; Lee, H.A.; Shin, G.-Y.; Choe, Y.; Cho, Y.K.; Choi, M.-G. Types of 23S Ribosomal RNA Point Mutations and Therapeutic Outcomes for Helicobacter pylori. Gut Liver 2021, 15, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Dascălu, R.I.; Bolocan, A.; Păduaru, D.N.; Constantinescu, A.; Mitache, M.M.; Stoica, A.D.; Andronic, O. Multidrug resistance in Helicobacter pylori infection. Front. Microbiol. 2023, 14, 1128497. [Google Scholar] [CrossRef] [PubMed]
- Saranathan, R.; Levi, M.H.; Wattam, A.R.; Malek, A.; Asare, E.; Behin, D.S.; Pan, D.H.; Jacobs, W.R.; Szymczak, W.A., Jr. Helicobacter pylori Infections in the Bronx, New York: Surveying Antibiotic Susceptibility and Strain Lineage by Whole-Genome Sequencing. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Keikha, M.; Askari, P.; Ghazvini, K.; Karbalaei, M. Levofloxacin-based therapy as an efficient alternative for eradicating Helicobacter pylori infection in Iran: a systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2021, 29, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Yoshiyama, H.; Nakazawa, T.; Park, I.-D.; Chang, M.-W.; Yanai, H.; Okita, K.; Shirai, M. A change in PBP1 is involved in amoxicillin resistance of clinical isolates of Helicobacter pylori. J. Antimicrob. Chemother. 2002, 50, 849–856. [Google Scholar] [CrossRef]
- Qureshi, N.N.; Gallaher, B.; Schiller, N.L. Evolution of Amoxicillin Resistance ofHelicobacter pyloriIn Vitro: Characterization of Resistance Mechanisms. Microb. Drug Resist. 2014, 20, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Gerrits, M.M.; Berning, M.; Van Vliet, A.H.M.; Kuipers, E.J.; Kusters, J.G. Effects of 16S rRNA Gene Mutations on Tetracycline Resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 2003, 47, 2984–2986. [Google Scholar] [CrossRef] [PubMed]
- Anoushiravani, M.; Falsafi, T.; Niknam, V. Proton motive force-dependent efflux of tetracycline in clinical isolates of Helicobacter pylori. J. Med Microbiol. 2009, 58, 1309–1313. [Google Scholar] [CrossRef]
- Park, J.Y.; Shin, T.-S.; Kim, J.H.; Yoon, H.J.; Kim, B.J.; Kim, J.G. The Prevalence of Multidrug Resistance of Helicobacter pylori and Its Impact on Eradication in Korea from 2017 to 2019: A Single-Center Study. Antibiotics 2020, 9, 646. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Dong, X.; Teng, G.; Zhang, W.; Cheng, H.; Gao, W.; Dai, Y.; Zhang, X.; Wang, W. The effect of previous eradication failure on antibiotic resistance of Helicobacter pylori: A retrospective study over 8 years in Beijing. Helicobacter 2021, 26, e12804. [Google Scholar] [CrossRef]
- Geng, T.; Yu, Z.-S.; Zhou, X.-X.; Liu, B.; Zhang, H.-H.; Li, Z.-Y. Antibiotic resistance of Helicobacter pylori isolated from children in Chongqing, China. Eur. J. Pediatr. 2022, 181, 2715–2722. [Google Scholar] [CrossRef]
- Shao, Y.F.; Lin, Y.F.; Wang, B.J.; Miao, M.; Ye, G. Antibiotic resistance status of Helicobacter pylori strains isolated from initial eradication patients in Ningbo, China, from 2017 to 2021. Helicobacter 2022, 27(5), e12920. [Google Scholar] [CrossRef]
- Boyanova, L.; Hadzhiyski, P.; Kandilarov, N.; Markovska, R.; Mitov, I. Multidrug resistance inHelicobacter pylori: current state and future directions. Expert Rev. Clin. Pharmacol. 2019, 12, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shao, Y.; Yan, J.; Ye, G. Antibiotic resistance in Helicobacter pylori: From potential biomolecular mechanisms to clinical practice. J. Clin. Lab. Anal. 2023, 37, e24885. [Google Scholar] [CrossRef] [PubMed]
- Megraud, F.; Ducournau, A.; Bénéjat, L.; Sifré, E.; Bessède, E.; Lehours, P. Surveillance of Helicobacter pylori resistance to antibiotics in France in 2014. Helicobacter 2016, 21, 110–114. [Google Scholar] [CrossRef]
- Moldobaeva, M.S., Elistratov, A.A.; Tolombaeva, N.T.; Sharshenalieva G.K. Мodern approaches to the cancer prevention in hp-associated gastritis. Bulletin of KRSU 2014, 14, 5, 102-106.
- Poole, K. Efflux-mediated multiresistance in Gram-negative bacteria. Clin. Microbiol. Infect. 2004, 10, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhong, Z.; Hu, S.; Wang, J.; Deng, Y.; Li, X.; Chen, X.; Li, X.; Tang, Y.; Li, X.; et al. A Survey of Helicobacter pylori Antibiotic-Resistant Genotypes and Strain Lineages by Whole-Genome Sequencing in China. Antimicrob. Agents Chemother. 2022, 66, e0218821. [Google Scholar] [CrossRef] [PubMed]
- Nestegard, O.; Moayeri, B.; Halvorsen, F.-A.; Tønnesen, T.; Sørbye, S.W.; Paulssen, E.; Johnsen, K.-M.; Goll, R.; Florholmen, J.R.; Melby, K.K. Helicobacter pylori resistance to antibiotics before and after treatment: Incidence of eradication failure. PLOS ONE 2022, 17, e0265322. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-Y.; Lee, A.-Y.; Huang, A.-H.; Song, P.-Y.; Yang, Y.-J.; Sheu, S.-M.; Chang, W.-L.; Sheu, B.-S.; Wu, J.-J. Heteroresistance of Helicobacter pylori from the same patient prior to antibiotic treatment. Infect. Genet. Evol. 2014, 23, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Shiotani, A.; Lu, H.; Dore, M.P.; Graham, D.Y. Treating Helicobacter pylori effectively while minimizing misuse of antibiotics. Clevel. Clin. J. Med. 2017, 84, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-Z.; Threapleton, D.E.; Wang, J.-Y.; Xu, J.-M.; Yuan, J.-Q.; Zhang, C.; Li, P.; Ye, Q.-L.; Guo, B.; Mao, C.; et al. Comparative effectiveness and tolerance of treatments forHelicobacter pylori: systematic review and network meta-analysis. BMJ 2015, 351, h4052. [Google Scholar] [CrossRef]
- Keikha, M.; Karbalaei, M. Prevalence of antibiotic heteroresistance associated with Helicobacter pylori infection: A systematic review and meta-analysis. Microb. Pathog. 2022, 170, 105720. [Google Scholar] [CrossRef]
- Islam, J.M.; Yano, Y.; Okamoto, A.; Matsuda, R.; Shiraishi, M.; Hashimoto, Y.; Morita, N.; Takeuchi, H.; Suganuma, N. Evidence of Helicobacter pylori heterogeneity in human stomachs by susceptibility testing and characterization of mutations in drug-resistant isolates. Sci. Rep. 2024, 14, 12066. [Google Scholar] [CrossRef]
- Mežmale, L.; Polaka, I.; Rudzite, D.; Vangravs, R.; Kikuste, I.; Parshutin, S.; Daugule, I.; Tazhedinov, A.; Belikhina, T.; Igissinov, N.; et al. Prevalence and Potential Risk Factors of Helicobacter pylori Infection among Asymptomatic Individuals in Kazakhstan. Asian Pac. J. Cancer Prev. 2021, 22, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Choi, H.; Leung, K.; Jiang, F.; Graham, D.Y.; Leung, W.K. Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Abdiev, S.; Ahn, K.S.; Khadjibaev, A.; Malikov, Y.; Bahramov, S.; Rakhimov, B.; Sakamoto, J.; Kodera, Y.; Nakao, A.; Hamajima, N. Helicobacter pylori infection and cytokine gene polymorphisms in Uzbeks. Nagoya J Med Sci. 2010, 72, 167–72. [Google Scholar] [PubMed]
- Karimov, M.; Sobirova, G.; Saatov, Z.; Islamova, S.; Rustamova, S. Prevalence and Molecular-Genetic Characteristics of Helicobacter pylory in Uzbekistan. Eff. Pharmacother. 2019, 15. [Google Scholar] [CrossRef]
- Kulmambetova, G.; Bekenova, E.; Tuyakova, A.; Logvinenko, A.; Sukashev, A.; Almagambetov, A. Proceedings of the scientific and practical conference “From epidemiology to the diagnosis of current infections. Аntibiotic resistance of helicobacter pylori in the Кazakh population 2011, 76.
- De Francesco, V.; Margiotta, M.; Zullo, A.; Hassan, C.; Troiani, L.; Burattini, O.; Stella, F.; Di Leo, A.; Russo, F.; Marangi, S.; et al. Clarithromycin-Resistant Genotypes and Eradication of Helicobacter pylori. Ann. Intern. Med. 2006, 144, 94–100. [Google Scholar] [CrossRef]
- Oleastro, M.; Ménard, A.; Santos, A.; Lamouliatte, H.; Monteiro, L.; Barthélémy, P.; Mégraud, F. Real-Time PCR Assay for Rapid and Accurate Detection of Point Mutations Conferring Resistance to Clarithromycin in Helicobacter pylori. J. Clin. Microbiol. 2003, 41, 397–402. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Clinical guidelines for the diagnosis and treatment of uncomplicated peptic ulcer disease in the active phase at the primary health care level Kyrgyz Republic. Bishkek: Ministry of Health of the Kyrgyz Republic, 2010, 76.
- Report on the project “Study the resistance of Helicobacter pylori to clarithromycin in Kyrgyzstan and metronidazole to adapt the recommendations of the Maastricht III Consensus (2005) for the treatment of peptic ulcer” / ed. M.S. Moldobaeva. Bishkek: KSMA, 2010, 50.
- Zhumabaev, M.N.; Apushkina, V.V.; Jumanova, R.G.; et al. Prevalence and treatment of gastric and duodenal ulcers associated with Helicobacter Pylori in residents of the Kyrgyz Republic. TsAMZh 2007, 13(1), 127–132. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).