1. Introduction
In the context of multimodal treatment for esophageal carcinoma, surgical resection currently represents the only curative intent therapy [
1]. Specifically, in cases of distal esophageal neoplasms, Ivor-Lewis Esophagectomy (IL-E) with gastric conduit reconstruction is the preferred surgical procedure [
2]. However, this surgery is burdened with a series of short- and long-term complications with significant morbidity, including anastomotic leaks (AL), conduit necrosis, anastomotic strictures, and delayed gastric conduit emptying (DGCE)[
3], [
4]. The pathophysiology of DGCE remains unclear, although increasing evidence demonstrates a multifactorial etiology involving multiple factors. The primary factors include dysfunctions in peristalsis and pyloric release (vagotomy), an unfavorable pressure gradient (negative pressure in the chest and positive pressure in the abdomen), conduit angulation that may also be redundant, reduced esophageal hiatus width, and altered function/release of GUT hormones[
4], [
5].
The limitations of the current literature on DGCE have primarily been associated with the absence of shared diagnostic criteria and symptom grading tools, which the International Society for Diseases of the Esophagus (ISDE) recently proposed and published, identifying an Early form (E-DGCE) and a Late form (L-DGCE), aiming to overcome these limitations (
Table 1) [
6].
To further standardize, the management of DGCE, a diagnostic protocol utilizing Upper Gastrointestinal (UGI) contrast study has recently been advocated and incorporated within the dedicated ERAS protocol[
7].
DGCE has an incidence ranging from 10 to 50% and it can lead to short-term complications (AL, aspiration pneumonia) or long-term complications (malnutrition) that impact the patient's quality of life[
5]. Current evidence has not demonstrated a clear clinical benefit derived from prophylactic intraoperative pyloric drainage (IPD). On the contrary, such procedures, including finger fracture, pyloroplasty, and pyloromyotomy, have been highlighted to be associated, in some cases, with complications such as duodenal leak, dumping syndrome, and bile reflux[
8]. More robust data on this topic will likely come from ongoing randomized controlled trials (RCTs) [
8]. Since prokinetic drugs in this setting have proven to be ineffective, endoscopy thus plays an important role. The IntraPyloric injection of Botulinum Toxin (IPBT), through its inhibitory effect on gastric and pyloric smooth muscle, is moderately effective in the short-term postoperative period. However, in the long term, it is characterized by a considerable recurrence rate of symptoms requiring endoscopic or surgical interventions[
9], [
10]. Pneumatic balloon dilation of the pylorus (PBD) represents a viable alternative both in the short-term postoperative and long-term[
11]. Recently, the possibility of simultaneously performing IPBT and PBD (BTPD) has been proposed to optimize the myorelaxant effect with the mechanical effect of dilation[
12]. The currently available evidence presents several limitations regarding patient selection, the definition of clinical outcomes, and the lack of comparative data. Based on data from our tertiary care center, our study aims to show comparative data on the efficacy and safety of the endoscopic treatments currently most widely used in managing DGCE after esophagectomy.
2. Materials and Methods
2.1. Study Population and Ethical Approval
This retrospective single-center cohort study was conducted at the Gastroenterology and Gastrointestinal Endoscopy Division, of IRCCS San Raffaele Hospital and University in Milan, following the Declaration of Helsinki (as revised in 2013). We performed a retrospective analysis of prospectively collected data into a prospective registry active at our center, approved by the local Ethical Committee (Reg/EGDSColon), including all data about diagnostic and operative luminal endoscopic procedures. We recorded demographic, clinicopathological, surgical, endoscopic, and follow-up data in an electronic endoscopic database and patient hospital electronic medical records.
2.2. Patients
To analyze and compare the efficacy and safety of the three endoscopic procedures for DGCE management (IPBT, PBD, and BTBD), we enrolled all consecutive adult patients (>18 years old) affected by DGCE refractory to prokinetic drugs that were referred to our Endoscopy Unit from the Gastrointestinal Surgery Unit of our University Hospital (IRCCS San Raffaele Hospital, Milan, Italy), which underwent endoscopic DGCE management, from January 2014 to November 2023. For the DGCE diagnosis and classification, we referred to recently published DGCE diagnostic criteria by
Konradsson et al in 2020 (
Table 1) [
6]. For patients treated before the publication of these diagnostic criteria, we applied them retrospectively and included only patients with a confirmed DGCE diagnosis.
To assess the quality and consistency of feeding tolerated by patients we used the Gastric Outlet Obstruction Score (GOOS), which gives a point of which provides a score from 0 to 3 depending on whether the patient does not eat (0), eats only a liquid diet (1), soft diet (2) or free diet (3) [
13]. According to oncological and surgical indications, the GOOS was collected at diagnosis, at discharge, and the last clinical follow-up.
2.3. Surgical Interventions
All surgical interventions were performed by the same surgical team, experienced in esophagogastric and thoracic surgery. All surgical data were collected, including types of surgery (Ivor-Lewis Esophagectomy, McKeown Esophagectomy, total esophagectomy), the approach (Open, Laparoscopic, Hybrid), the site of the esophagogastric anastomosis (cervical, thoracic), IPD (finger fracture, pyloroplasty, pyloromyotomy) and eventually other surgery-related complications (e.g. AL, anastomotic stricture, conduit ischemia/necrosis).
2.4. Endoscopic Procedures
All endoscopic procedures were performed by expert endoscopists under deep sedation with anesthesiologic assistance, with the patient positioned supine on the left side in an operative endoscopy room equipped with fluoroscopy. If the patient had a nasogastric tube (NGT), it was removed immediately before starting the endoscopic procedure. A therapeutic gastroscope was used to provide a larger operative channel for the potential aspiration of food residues and secretions. Before the therapeutic endoscopic maneuver, a diagnostic esophagogastroduodenoscopy (EGD) was conducted to rule out other complications. IPBT was performed using a 25-gauge endoscopic injection needle. A vial of 100 units (U) of BT was mixed with 4 cc of normal saline, and 25 U of BT/ml was injected into the four quadrants of the pylorus. PBD was always performed with the same through-the-scope pneumatic balloon (Boston Scientific, CRE PRO Wireguided). Before dilation, a guidewire preloaded in the balloon catheter was advanced into the duodenum. Once the balloon was positioned across the pylorus, it was progressively inflated to 18, 19, and 20 mm, with the balloon being kept inflated for 60 seconds at each size. All these procedures were performed under endoscopic control, and fluoroscopy was never necessary. A final endoscopic check was always conducted to rule out any possible adverse events (AEs). In the combined procedure (BTPD), IPBT was performed first, followed by PBD.
2.5. Definitions
Technical success (TS) was defined as the completion of the scheduled endoscopic procedure. On the other hand, clinical success (CS) was defined as the resolution of DGCE symptoms (those included in the recently published diagnostic criteria) with the resumption of feeding (at least GOOS 2, soft solid) at discharge OR expanding dietary intake at any rate (always taking as reference GOOS score), without the need to replace the NGT [
6]. For patients who achieved CS, recurrence was defined as relapse of DGCE symptoms after more than 15 days of well-being requiring intervention (nasogastric tube/endoscopic procedure/surgery). Endoscopic procedure-related AEs were classified according to the recent classification for Adverse events Gastrointestinal Endoscopy (AGREE) [
14].
2.6. Statistical Analysis
All the statistical analyses were performed by MedCalc Version 18 (MedCalc Software, Ostend, Belgium). The normality of continuous variable distribution was tested with the Shapiro-Wilk test. Mean ± standard deviation (SD) and median and interquartile range (IQR) were used to express normally and non-normally distributed variables respectively. Categorical parameters were compared using Pearson Chi-square test and Fisher exact test, along with Bonferroni correction for plus 2 groups within a variable, while continuous variables were compared using Pearson or Spearman correlation according to normality distribution, while by Student t-test or ANOVA test between 2 plus groups for normally distributed data, Mann-Whitney test or Kruskar Wallis test between 2 groups, for non-normally distributed data. All reported P values were two-sided, with statistical significance set at 0.05. Time to refeed and time to discharge were analyzed by using the Kaplan-Meier method, in which patients were censored at recurrence, death, and last follow-up visit, whichever came first. A comparison of time-to-event curves was performed by using the log-rank test.
3. Results
3.1. Baseline Characteristics
During the study period, 64 patients affected by DGCE were referred to our endoscopy unit and enrolled. Most patients are male (81.2%, 52/64), with a median age of 62 years (IQR 55-70). About comorbidities at the time of esophageal surgery, most patients presented an American Society of Anesthesiologist Score (ASA) of 2 (54.7%, 35/64). The most common indication for surgery was a malignant reason (96.9%, 62/64), and esophageal adenocarcinoma (EAC) represented the most common one (77.4%, 48/62), followed by squamous cell carcinoma (21.0%, 13/62) and leiomyoma (1.6%, 1/62). Concerning benign indication, all two cases underwent esophageal surgery for perforations not amenable endoscopically. According to oncological staging at the diagnosis, most of the patients were classified as locally advanced (75.8%, 47/62). 47 (75.8%) patients underwent neoadjuvant treatments at diagnosis, more in detail: 40.3% (25/62) chemotherapy (CT), 33.9% (21/62) chemo-radiotherapy (CRT), and one patient (1.6%) radiotherapy (RT). Ivor-Lewis Esophagectomy (ILE) was the most performed surgical intervention (90.6%, 58/64), followed by McKeown Esophagectomy (MKE) (4.7%, 3/64) and total esophagectomy (4.7%, 3/64). In 87.5% (56/64) of cases, a minimally invasive approach (laparoscopic + thoracoscopic) was selected to perform surgery. According to the recent ISDE classification, almost two-thirds (67.2%, 43/64) of the patients included in the study were suffering from late DGCE (L-DGCE) [
6]. Baseline characteristics of the entire study population are resumed in
Table 2.
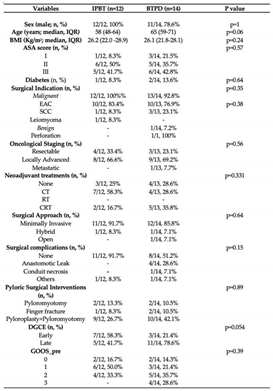
3.2. Comparison
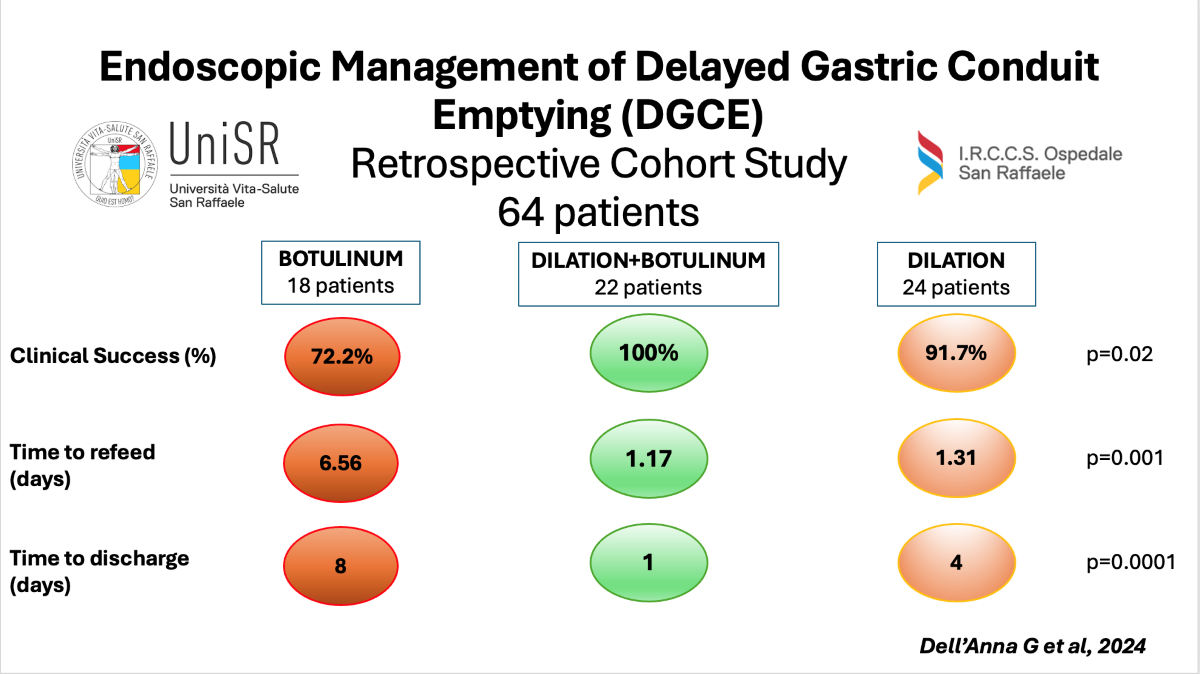
In the entire cohort, 24 patients (37.5%) underwent PBD, 22 patients (34.4%) underwent BTPD, and 18 patients (28.1%) underwent IPBT. There were no significant differences between the groups in terms of sex (p=0.61) or median age (p=0.08). The three groups also appeared homogeneous regarding ASA score (p=0.16) and comorbidities, particularly diabetes (p=0.68), a known cause of gastroparesis [
15]. Additionally, no differences were found between the groups concerning surgical indications (malignant vs. benign cause; p=0.53), histology (esophageal adenocarcinoma, squamous cell carcinoma, leiomyoma; p=0.58), staging (resectable, locally advanced, metastatic; p=0.39), and neoadjuvant treatments (CT, RT, CRT; p=0.17). A minimally invasive surgical approach was the most common in all three groups (88.8% in the IPTB group, 83.3% in the PBD group, and 90.9% in the BTPD group), with no statistically significant differences among the groups (p=0.78). An important surgical aspect concerns IPD, where no significant differences were found between the treatment groups (p=0.10). The combination of same-session pyloroplasty and pyloromyotomy was most common in the IPTB group (55.5%, 10/18) and the BTPD group (45.5%, 10/22). Regarding other surgical complications, such as AL, gastric conduit necrosis, or cardiopulmonary complications, no differences were observed between the groups (p=0.12). Most patients in all three endoscopic groups did not experience complications, apart from DGCE (88.8% in the IPTB group, 66.7% in the PBD group, and 54.5% in the BTPD group). When comparing the timing of DGCE onset among the three groups, the rate of early DGCE was significantly higher in the IPTB group compared to the other two treatment groups (77.7% vs. 20.83% vs. 9.09%; p<0.0001). In contrast, no differences were found between the groups when comparing the GOOS score before endoscopic treatment (p=0.33). In both the IPTB (44.4%, 8/18) and PBD (50.0%, 12/24) groups, most patients had a GOOS score of 1 (liquid diet) before endoscopy. Conversely, in the BTPD group, many patients (36.36%, 8/22) had a GOOS score of 2 (soft solid diet). The results of the baseline characteristic comparisons between the three groups are reported in
Table 3.
3.3. Outcomes
Despite a 100% TS rate across all three groups, the BTPD group demonstrated a significantly higher CS rate compared to the PBD and IPTB groups (100.0% BTPD vs. 91.6% PBD vs. 72.22% IPTB; p=0.01). No significant differences were observed in the median follow-up times (days) among the groups (p=0.19), with follow-ups of 374 days (IQR 208-739) for the IPTB group, 230 days (IQR 144-589) for the BTPD group, and 184 days (IQR 35-710) for the PBD group. Among patients who achieved CS after endoscopic treatment, the IPTB group had the highest rate of symptom recurrence compared to the other two groups, though this difference was not statistically significant [23.08% (3/13) in the IPTB group, 22.72% (5/22) in the PBD group, 9.09% (2/22) in the BTPD group; p=0.4126]. Regarding rescue management, the three recurrent cases in the IPTB group were treated with Gastric-Peroral Endoscopic Myotomy (G-POEM), BTPD, and surgical pyloromyotomy with pyloroplasty, respectively. The five recurrent cases in the PBD group were managed with two BTPD, two PBD, and one IPTB. Finally, the two recurrent cases in the BTPD group were treated with G-POEM and surgical pyloromyotomy with pyloroplasty, respectively. No AEs related to endoscopic procedures were recorded during the study period.
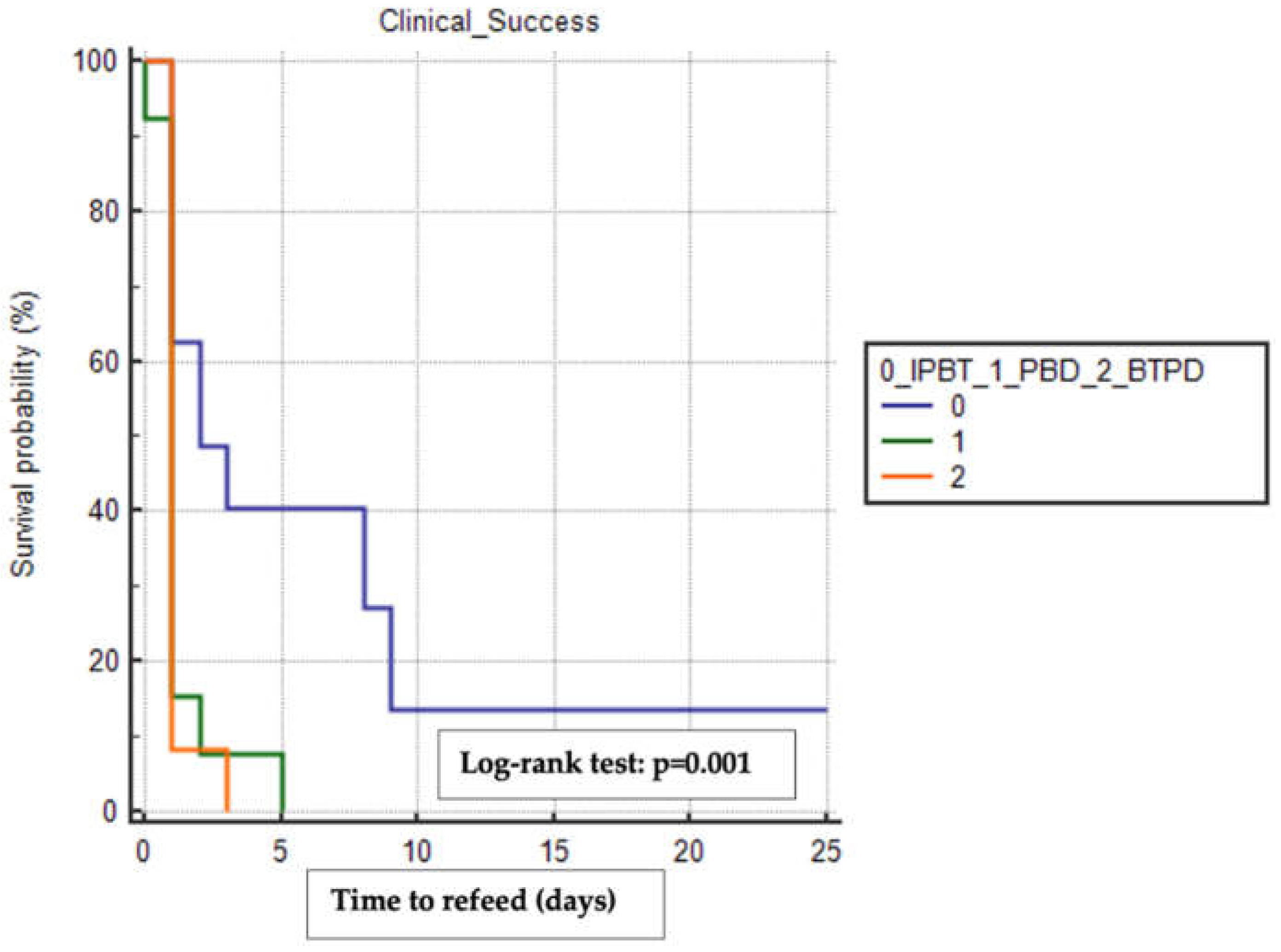
We used the Kaplan-Meier method to estimate the mean time to refeeding and discharge among the treatment groups, including only patients who achieved clinical success and excluding those who experienced other surgical complications (e.g., anastomotic leak, conduit necrosis, pulmonary disorders, anastomotic stricture). The BTPD group had the shortest mean time to refeeding (1.17 days, 95% CI 0.84-1.49) compared to the PBD group (1.31 days, 95% CI 0.66-1.95) and the IPTB group (6.56 days, 95% CI 1.79-11.33), with a statistically significant difference observed when comparing the time-to-event curves using the Log-rank test (p=0.001) (
Figure 1).
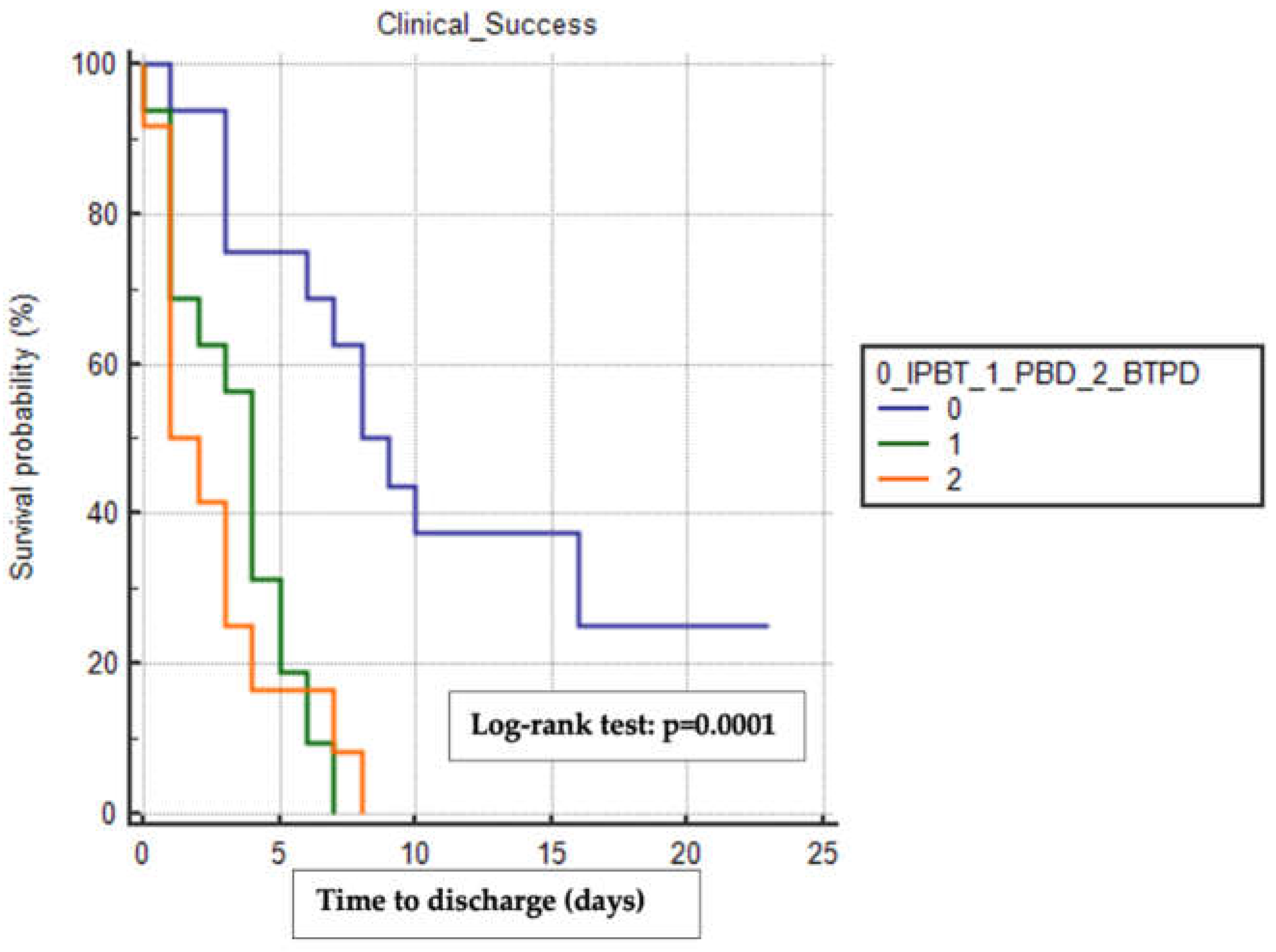
Similarly, the BTPD group had the shortest median time to discharge (1 day, 95% CI 1-3) compared to the PBD group (4 days, 95% CI 1-5) and the IPTB group (8 days, 95% CI 6-16), with a statistically significant difference observed (Log-rank test p=0.0001) (
Figure 2).
The results of outcomes comparison between the three groups are reported in
Table 4.
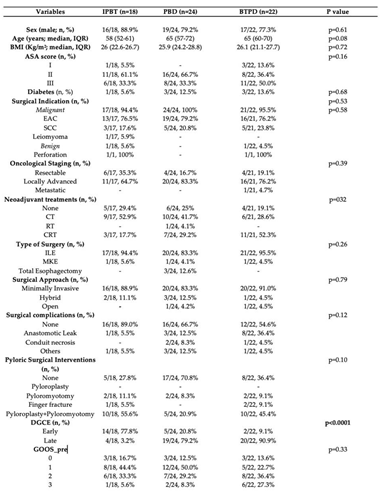
3.4. Subgroup Analysis
We also performed a subgroup analysis to reduce the risk of bias by comparing baseline characteristics and outcomes of patients treated with IPTB (n=12) or BTPD (n=14), specifically including only those who underwent ILE with IPD. The two subgroups did not differ in baseline characteristics (comorbidities, indication for surgery, neoadjuvant treatments, oncological staging), surgical aspects (surgical approach, types of IPD, surgical complications), or the timing of DGCE onset and subsequent disease classification (
Table 5).
Data from the subgroup analysis further indicated that the BTPD subgroup had a significantly higher CS rate compared to the IPTB subgroup, with rates of 100% vs. 75%, respectively (p=0.047). There was no difference in median follow-up between the two subgroups [313 days (IQR 208-608) for the IPTB subgroup vs. 197 days (IQR 65-499) for the BTPD subgroup; p=0.12]. The IPTB subgroup also had a higher rate of symptom relapse compared to the BTPD subgroup (33.3% vs. 7.1%; p=0.11) (
Table 6).
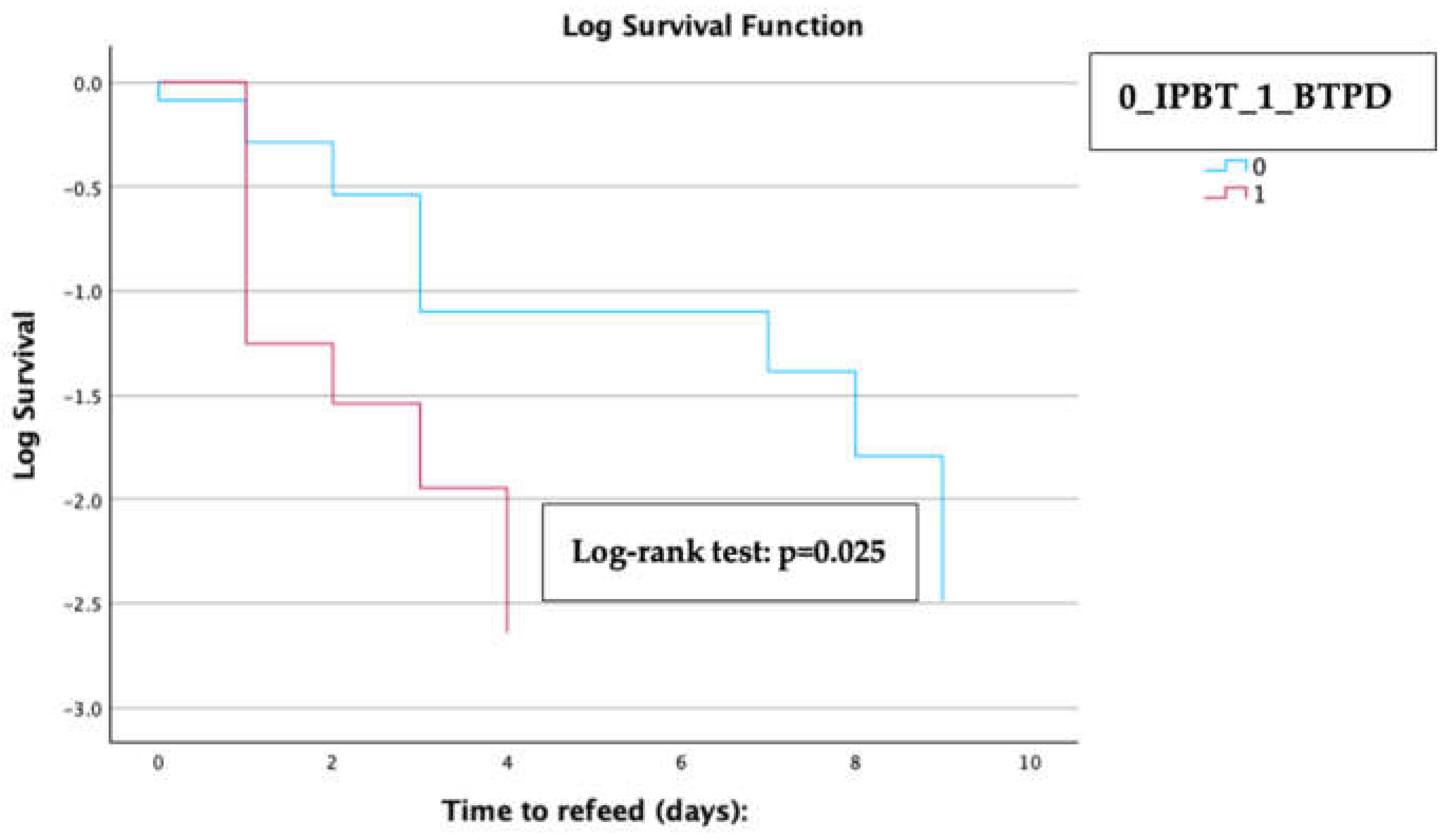
Kaplan-Meier analysis with a log-rank test revealed that patients in the BTPD subgroup experienced a significantly shorter median time to refeeding compared to those in the IPTB subgroup [1 day (IQR 0.3-1.2) for the BTPD subgroup vs. 3 days (IQR 1.9-4.1) for the IPTB subgroup; log-rank test p=0.025] (
Figure 3).
4. Discussion
DGCE. is a common complication following esophagectomy, significantly impacting both short- and long-term outcomes. Various surgical and endoscopic IPD strategies have been attempted to prevent DGCE, but the results have been inconsistent[
4].
Hajibandeh S et al., in their recent meta-analysis, found that intraoperative IPBT did not reduce the risk of DGCE or the need for endoscopic pyloric interventions compared to no intraoperative IPBT or pyloroplasty [
16]. Similarly,
Arya S et al., in a systematic review, did not find any difference in DGCE risk between patients who underwent various types of IPD (e.g., IPBT, pyloroplasty, pyloromyotomy, finger fracture) and those who did not [
17]. Conversely,
Loo JH et al., in their meta-analysis, demonstrated that IPD, including pyloroplasty, pyloromyotomy, and IPBT, was associated with a reduced risk of developing DGCE [
18]. Supporting this, a meta-analysis by
Abdelrahman M et al. showed that intraoperative PBD significantly reduces the rate of early DGCE and AL following esophagectomy [
19].
The evidence for the endoscopic treatment of DGCE is currently limited to small retrospective series that lack standardization in inclusion criteria and treatment protocols. For example,
Mertens A et al. evaluated PBD in managing early DGCE (<14 post-operative days) in a retrospective single-center series of 12 patients, reporting a CS rate of 58% [
11].
Bhutani MS et al. recently published results from a series of 21 patients on the efficacy and safety of same-session combination BTPD in treating DGCE, showing a clinical efficacy rate of 85% [
12]. G-POEM has also shown promise in this setting, but the evidence is limited to case reports and small case series [
20], [
21].
These studies are primarily limited by a lack of standardization in the definition and evaluation of DGCE, outcomes assessment, and the absence of comparative data. Diagnostic criteria for DGCE were recently published by ISDE to assist clinicians in better diagnosing and classifying this complication and to provide researchers with a valuable tool for standardizing their results. In this context, our single-center cohort study aimed to evaluate the safety and efficacy of the most common endoscopic procedures for treating DGCE: IPBT, PBD, and same-session BTPD. For the first time, our study applied the ISDE Diagnostic Criteria for DGCE diagnosis and classification to minimize selection bias among included patients. Our results demonstrated that the BTPD group not only had significantly higher clinical efficacy but also experienced shorter times to refeeding and hospital discharge, with no differences in safety outcomes. We also performed a subgroup analysis to reduce selection bias due to the retrospective nature of the study, including only patients who underwent Ivor-Lewis esophagectomy with surgical IPD. In this analysis, the BTPD group again demonstrated higher clinical efficacy and was associated with a shorter time to refeeding compared to the IPBT group.
Another important strength of our study is the use of the GOOS as a tool for evaluating the clinical efficacy of endoscopic procedures. The GOOS score is a validated tool for assessing GOO, a syndrome caused by a mechanical obstruction in the gastro-duodenal outflow. To our knowledge, its use in the clinical evaluation of DGCE has not been reported until now. We chose the GOOS score because it is a quick and simple tool that allows us to assess the feeding capacity of patients with a condition like DGCE, which is primarily characterized by the slowed passage of gastric contents into the duodenum.
To the best of our knowledge, no similar comparative studies have been published on this topic, making it difficult to directly compare our results with existing evidence, which has been severely limited by a lack of standardization in DGCE diagnosis, management, and outcomes assessment. Only the study by
Bhutani MS et al. evaluated BTPD for DGCE management, reporting a lower CS rate (85%) compared to ours (100%) [
12]. We hypothesize that this difference may be due to several factors. First,
Bhutani MS and colleagues did not use a standardized protocol for PBD, employing balloons of both 12mm and 20mm diameters, whereas in our series, we consistently performed progressive dilation from 18mm to 20mm. Additionally, they did not use a standardized protocol for clinical assessment and included in the success group patients who required more than one procedure to achieve symptom relief. Furthermore, in the
Bhutani MS et al. series, most patients did not undergo surgical IPD (52%), whereas, in our series, 63.6% of the BTPD group received surgical IPD. Moreover, 67% of patients in the
Bhutani MS and colleagues' series underwent open surgery, which is associated with higher morbidity, while in our study, only 4.5% of cases in the BTPD group involved open surgery.
Although ours is the first comparative study on the endoscopic management of DGCE, it does have some limitations. First, the retrospective and single-center nature of the study limits its generalizability, despite being conducted in a tertiary referral center specializing in both luminal digestive endoscopy and esophageal surgery. Another important limitation is that we included all patients affected by DGCE in our comparative study, regardless of the time of onset (early vs. late according to ISDE Classification), which could have influenced the results. To mitigate the impact of the time of onset on clinical outcomes, we included only patients without other surgical complications when performing the Kaplan-Meier analysis with the log-rank test. Additionally, although we used the GOOS score as a tool for clinical evaluation, this score is not currently validated for use in DGCE.
In conclusion, the endoscopic management of DGCE remains challenging for gastroenterologists for several reasons. In addition to the highly variable factors that contribute to this condition (e.g., anatomical, hormonal), there are no specific guidelines or standardized indications available. As a result, the choice between different treatment options is largely dependent on the experience of the endoscopist and the resources available locally. Results from our retrospective comparative cohort study conducted at a tertiary referral center suggest that the same-session combination of BTPD may provide better and faster symptom relief for patients with both early and late DGCE compared to the individual techniques (IPBT, PBD). More robust data from large prospective controlled comparative studies are needed, particularly considering factors such as the type of surgery, IPD, the timing of DGCE onset, and clinical evaluation. The goal should be the standardization of DGCE diagnosis and treatment, always within a multidisciplinary approach.
Author Contributions
Conceptualization, G.D..; methodology, G.D., and F.V.M; formal analysis, G.D.; data curation, J.F., E.F., R.B., A.B., S.B., and G.D..; writing—original draft preparation, G.D.; writing—review and editing, E.V., F.A., L.F., F.P., A.C., S.B., U.E., R.R., L.F., V.A., A.M., S.D., F.V.M., and G.D. .; supervision, L.F., V.A., R.R., A.M., S.D., F.V.M. and G.D..; project administration, S.D., F.V.M., and G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of IRCCS San Raffaele Hospital (Reg/EGDSColon).
Informed Consent Statement
Written informed consent has been obtained from the patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
S.D. has served as a speaker, consultant, and advisory board member for Schering-Plough, AbbVie, Actelion, Alphawasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson and Johnson, Millenium Takeda, MSD, Nikkiso Europe GmbH, NovoNordisk, Nycomed, Pfizer, Pharmacosmos, UCB Pharma, and Vifor. The other authors declare no conflicts of interest.
References
- M. Sheikh, G. Roshandel, V. McCormack, and R. Malekzadeh, “Current Status and Future Prospects for Esophageal Cancer.,” Cancers (Basel), vol. 15, no. 3, Jan. 2023. [CrossRef]
- B. J. van der Wilk et al., “Outcomes after totally minimally invasive versus hybrid and open Ivor Lewis oesophagectomy: results from the International Esodata Study Group.,” Br J Surg, vol. 109, no. 3, pp. 283–290, Feb. 2022. [CrossRef]
- D. E. Low et al., “International Consensus on Standardization of Data Collection for Complications Associated with Esophagectomy,” Ann Surg, vol. 262, no. 2, pp. 286–294, Aug. 2015. [CrossRef]
- H. C. Yang, J. H. Choi, M. S. Kim, and J. M. Lee, “Delayed Gastric Emptying after Esophagectomy: Management and Prevention,” Korean J Thorac Cardiovasc Surg, vol. 53, no. 4, pp. 226–232, Aug. 2020. [CrossRef]
- J. C. Tham et al., “Gut hormones profile after an Ivor Lewis gastro-esophagectomy and its relationship to delayed gastric emptying,” Diseases of the Esophagus, vol. 35, no. 10, Oct. 2022. [CrossRef]
- M. Konradsson et al., “Diagnostic criteria and symptom grading for delayed gastric conduit emptying after esophagectomy for cancer: international expert consensus based on a modified Delphi process,” Diseases of the Esophagus, vol. 33, no. 4, Apr. 2020. [CrossRef]
- F. Klevebro et al., “ERAS guidelines-driven upper gastrointestinal contrast study after esophagectomy can detect delayed gastric conduit emptying and improve outcomes,” Surg Endosc, vol. 37, no. 3, pp. 1838–1845, Mar. 2023. [CrossRef]
- N. Okada et al., “PYloroplasty versus No Intervention in GAstric REmnant REconstruction after Oesophagectomy: study protocol for the PYNI-GAREREO phase III randomized controlled trial,” Trials, vol. 24, no. 1, p. 412, Jun. 2023. [CrossRef]
- S. M. Eldaif et al., “Intrapyloric Botulinum Injection Increases Postoperative Esophagectomy Complications,” Ann Thorac Surg, vol. 97, no. 6, pp. 1959–1965, Jun. 2014. [CrossRef]
- R. Bagheri et al., “Botulinum toxin for prevention of delayed gastric emptying after esophagectomy.,” Asian Cardiovasc Thorac Ann, vol. 21, no. 6, pp. 689–92, Dec. 2013. [CrossRef]
- Mertens et al., “Treating Early Delayed Gastric Tube Emptying after Esophagectomy with Pneumatic Pyloric Dilation,” Dig Surg, vol. 38, no. 5–6, pp. 337–342, 2021. [CrossRef]
- M. S. Bhutani et al., “Endoscopic Intrapyloric Botulinum Toxin Injection with Pyloric Balloon Dilation for Symptoms of Delayed Gastric Emptying after Distal Esophagectomy for Esophageal Cancer: A 10-Year Experience,” Cancers (Basel), vol. 14, no. 23, p. 5743, Nov. 2022. [CrossRef]
- G. Vanella et al., “EUS-guided gastroenterostomy for management of malignant gastric outlet obstruction: a prospective cohort study with matched comparison with enteral stenting.,” Gastrointest Endosc, vol. 98, no. 3, pp. 337-347.e5, Sep. 2023. [CrossRef]
- K. J. Nass et al., “Novel classification for adverse events in GI endoscopy: the AGREE classification.,” Gastrointest Endosc, vol. 95, no. 6, pp. 1078-1085.e8, Jun. 2022. [CrossRef]
- F. V. Mandarino et al., “Imaging in Gastroparesis: Exploring Innovative Diagnostic Approaches, Symptoms, and Treatment.,” Life (Basel), vol. 13, no. 8, Aug. 2023. [CrossRef]
- S. Hajibandeh et al., “Effect of intraoperative botulinum toxin injection on delayed gastric emptying and need for endoscopic pyloric intervention following esophagectomy: a systematic review, meta-analysis, and meta-regression analysis.,” Dis Esophagus, vol. 36, no. 11, Oct. 2023. [CrossRef]
- S. Arya, S. R. Markar, A. Karthikesalingam, and G. B. Hanna, “The impact of pyloric drainage on clinical outcome following esophagectomy: a systematic review.,” Dis Esophagus, vol. 28, no. 4, pp. 326–35, 2015. [CrossRef]
- J. H. Loo, A. D. R. Ng, K. S. Chan, and A. M. Oo, “Outcomes of Intraoperative Pyloric Drainage on Delayed Gastric Emptying Following Esophagectomy: A Systematic Review and Meta-analysis.,” J Gastrointest Surg, vol. 27, no. 4, pp. 823–835, Apr. 2023. [CrossRef]
- M. Abdelrahman et al., “Systematic review and meta-analysis of the influence of prophylactic pyloric balloon dilatation in the prevention of early delayed gastric emptying after oesophagectomy.,” Dis Esophagus, vol. 35, no. 6, Jun. 2022. [CrossRef]
- F. V. Mandarino et al., “Gastric emptying study before gastric peroral endoscopic myotomy (G-POEM): can intragastric meal distribution be a predictor of success,” Gut, vol. 72, no. 5, pp. 1019–1020, May 2023. [CrossRef]
- F. Azzolini, S. G. Testoni, D. Esposito, G. F. Bonura, G. Pepe, and P. A. Testoni, “Gastric peroral endoscopic myotomy (G-POEM) for refractory gastroparesis: 3-month follow-up results.,” Dig Liver Dis, vol. 52, no. 10, pp. 1215–1218, Oct. 2020. [CrossRef]
Figure 1.
Kaplan-Meier analysis with the log-rank test of the difference in time to refeed between the three treatment groups. In this analysis were included only patients who reached clinical success and who did not experience other surgical complications. IPBT, intra-pyloric botulinum toxin injection. PBD, pneumatic balloon dilation. BTPD, Botulinum Toxin Pneumatic Dilation. The copyright of the image belongs to the authors.
Figure 1.
Kaplan-Meier analysis with the log-rank test of the difference in time to refeed between the three treatment groups. In this analysis were included only patients who reached clinical success and who did not experience other surgical complications. IPBT, intra-pyloric botulinum toxin injection. PBD, pneumatic balloon dilation. BTPD, Botulinum Toxin Pneumatic Dilation. The copyright of the image belongs to the authors.
Figure 2.
Kaplan-Meier analysis with the log-rank test of the difference in time to discharge between the three treatment groups. This analysis included only patients who reached clinical success and did not experience other surgical complications. IPBT, intra-pyloric botulinum toxin injection. PBD, pneumatic balloon dilation. BTPD, Botulinum Toxin Pneumatic Dilation. The copyright of the image belongs to the authors.
Figure 2.
Kaplan-Meier analysis with the log-rank test of the difference in time to discharge between the three treatment groups. This analysis included only patients who reached clinical success and did not experience other surgical complications. IPBT, intra-pyloric botulinum toxin injection. PBD, pneumatic balloon dilation. BTPD, Botulinum Toxin Pneumatic Dilation. The copyright of the image belongs to the authors.
Figure 3.
Kaplan-Meier analysis with the log-rank test of the difference in time to refeed between the two treatment groups included in the subgroup analysis. The copyright of the image belongs to the authors.
Figure 3.
Kaplan-Meier analysis with the log-rank test of the difference in time to refeed between the two treatment groups included in the subgroup analysis. The copyright of the image belongs to the authors.
Table 1.
Diagnostic Criteria of Delayed Gastric Conduit Emptying (DGCE) [
6]. *Early DGCE (E-DGCE): within 14 days of surgery; **Late DGCE (L-DGCE): later than 14 days after surgery.
Table 1.
Diagnostic Criteria of Delayed Gastric Conduit Emptying (DGCE) [
6]. *Early DGCE (E-DGCE): within 14 days of surgery; **Late DGCE (L-DGCE): later than 14 days after surgery.
| Diagnostic Criteria of DGCE [6] |
|
|
|
| E-DGCE* |
>500mL diurnal nasogastric tube output measured on the morning of postoperative day five or later (but within 14 days of surgery) |
OR
|
>100% increased gastric tube width on frontal chest X-ray projection (in comparison to baseline chest-X-ray taken on the day of surgery) together with the presence of an air-fluid level |
| L-DGCE** |
The patient should have “quite a bit” or “very much” of at least two of the following symptoms: early satiety/fullness, vomiting, nausea, regurgitation, inability to meet caloric needs by oral intake |
AND
|
Delayed contrast passage on upper GI water-soluble contrast radiogram or on timed barium swallow (until precise evaluation criteria are available, relying on the verdict “delayed contrast passage” by an expert radiologist |
Table 2.
Baseline characteristics of the entire study cohort. BMI, body mass index. ASA score, American Society of Anesthesiologists score. EAC, esophageal adenocarcinoma. SCC, squamous cell carcinoma. CT, chemotherapy. RT, radiotherapy. CRT, Chemo-radiotherapy. ILE, Ivor-Lewis Esophagectomy. MKE, McKeown Esophagectomy. DGCE, Delayed Gastric Conduit Emptying.
Table 2.
Baseline characteristics of the entire study cohort. BMI, body mass index. ASA score, American Society of Anesthesiologists score. EAC, esophageal adenocarcinoma. SCC, squamous cell carcinoma. CT, chemotherapy. RT, radiotherapy. CRT, Chemo-radiotherapy. ILE, Ivor-Lewis Esophagectomy. MKE, McKeown Esophagectomy. DGCE, Delayed Gastric Conduit Emptying.
| Study cohort (n=64) |
|
Sex (male; n, %)
Age (years; median, IQR)
BMI (Kg/m2; median, IQR)
ASA score (n, %)
I
II
III
Surgical Indication (n, %)
Malignant
EAC
SCC
Leiomyoma
Benign
Perforation
Oncological Staging (n, %)
Resectable
Locally Advanced
Metastatic
Neoadjuvant treatments (n, %)
None
CT
RT
CRT
Type of Surgery (n, %)
ILE
MKE
Total Esophagectomy
Surgical Approach (n, %)
Minimally Invasive
Hybrid
Open
DGCE (n, %)
Early
Late |
52/64, 81.2%
62 (IQR 55-70)
26 (IQR 23.1-27.8)
4/64, 6.2%
35/64, 54.7%
25/64, 39.1%
62/64, 96.9%
48/62, 77.4%
13/62, 21.0%
1/62, 1.6%
2/64, 3.1%
2/2, 100%
14/62, 22.6%
47/62, 75.8%
1/62, 1.6%
15/62, 24.2%
25/62, 40.3%
1/62, 1.6%
21/62, 33.9%
58/64, 90.6%
3/64, 4.7%
3/64, 4.7%
56/64, 87.5%
6/64, 9.4%
2/64, 3,1%
21/64, 32.81%
43/64, 67.2% |
Table 3.
Comparison of baseline characteristics between the three study groups. IPBT, intra-pyloric botulinum toxin injection. PBD, pneumatic balloon dilation. BTPD, Botulinum Toxin Pneumatic Dilation. BMI, body mass index. ASA score, American Society of Anesthesiologists score. EAC, esophageal adenocarcinoma. SCC, squamous cell carcinoma. CT, chemotherapy. RT, radiotherapy. CRT, Chemo-radiotherapy. ILE, Ivor-Lewis Esophagectomy. MKE, McKeown Esophagectomy. DGCE, Delayed Gastric Conduit Emptying. GOOS, Gastric Outlet Obstruction Score.
Table 3.
Comparison of baseline characteristics between the three study groups. IPBT, intra-pyloric botulinum toxin injection. PBD, pneumatic balloon dilation. BTPD, Botulinum Toxin Pneumatic Dilation. BMI, body mass index. ASA score, American Society of Anesthesiologists score. EAC, esophageal adenocarcinoma. SCC, squamous cell carcinoma. CT, chemotherapy. RT, radiotherapy. CRT, Chemo-radiotherapy. ILE, Ivor-Lewis Esophagectomy. MKE, McKeown Esophagectomy. DGCE, Delayed Gastric Conduit Emptying. GOOS, Gastric Outlet Obstruction Score.
Table 4.
Outcomes comparison between the three groups. IPBT, intra-pyloric botulinum toxin injection. PBD, pneumatic balloon dilation. BTPD, Botulinum Toxin Pneumatic Dilation. G-POEM, Gastric-Peroral Endoscopic Myotomy. IQR, inter-quartile range.
Table 4.
Outcomes comparison between the three groups. IPBT, intra-pyloric botulinum toxin injection. PBD, pneumatic balloon dilation. BTPD, Botulinum Toxin Pneumatic Dilation. G-POEM, Gastric-Peroral Endoscopic Myotomy. IQR, inter-quartile range.
| Variables |
IPBT (n=18) |
PBD (n=24) |
BTPD (n=22) |
P value |
| Technical Success (n, %) |
18/18, 100% |
24/24, 100% |
22/22, 100% |
p=0.65 |
| Clinical Success (n, %) |
13/18, 72.2% |
22/24, 91.7% |
22/22, 100% |
p=0.02 |
| PBD |
3/5, 60% |
- |
- |
|
| BTPD |
2/5, 40% |
2/2, 100% |
- |
|
| Recurrence (n, %) |
3/13, 23.1% |
5/22, 22.7% |
2/22, 9.1% |
p=0.41 |
| IPBT |
- |
1/5, 20% |
- |
|
| PBD |
- |
2/5, 40% |
- |
|
| BTPD |
- |
2/5, 40% |
- |
|
| G-POEM |
3/3, 100% |
- |
1/2, 50% |
|
| Pyloromyotomy+ |
- |
- |
1/2, 50% |
|
| pyloroplasty |
|
|
|
|
| Follow up |
374 (208-739) |
184 (35-710) |
230 (144-589) |
p=0.19 |
| (months; median/IQR) |
|
|
|
|
Table 5.
Comparison of baseline characteristics for subgroup analysis. IPBT, intra-pyloric botulinum toxin injection. BTPD, Botulinum Toxin Pneumatic Dilation. BMI, body mass index. ASA score, American Society of Anesthesiologists score. EAC, esophageal adenocarcinoma. SCC, squamous cell carcinoma. CT, chemotherapy. RT, radiotherapy. CRT, Chemo-radiotherapy. DGCE, Delayed Gastric Conduit Emptying. GOOS, Gastric Outlet Obstruction score.
Table 5.
Comparison of baseline characteristics for subgroup analysis. IPBT, intra-pyloric botulinum toxin injection. BTPD, Botulinum Toxin Pneumatic Dilation. BMI, body mass index. ASA score, American Society of Anesthesiologists score. EAC, esophageal adenocarcinoma. SCC, squamous cell carcinoma. CT, chemotherapy. RT, radiotherapy. CRT, Chemo-radiotherapy. DGCE, Delayed Gastric Conduit Emptying. GOOS, Gastric Outlet Obstruction score.
Table 6.
Outcomes comparison of the subgroup analysis. IPBT, intra-pyloric botulinum toxin injection. PBD, pneumatic balloon dilation. BTPD, Botulinum Toxin Pneumatic Dilation. G-POEM, Gastric-Peroral Endoscopic Myotomy. IQR, inter-quartile range.
Table 6.
Outcomes comparison of the subgroup analysis. IPBT, intra-pyloric botulinum toxin injection. PBD, pneumatic balloon dilation. BTPD, Botulinum Toxin Pneumatic Dilation. G-POEM, Gastric-Peroral Endoscopic Myotomy. IQR, inter-quartile range.
| Variables |
IPBT (n=12) |
BTPD (n=14) |
P value |
| Technical Success (n, %) |
12/12, 100% |
14/14, 100% |
p=0.65 |
| Clinical Success (n, %) |
9/12, 72.2% |
14/14, 100% |
p=0.04 |
| PBD |
1/3, 33.3%% |
- |
|
| BTPD |
2/3, 66.7% |
- |
|
| Recurrence (n, %) |
3/9, 33.3% |
1/14, 7.1% |
p=0.11 |
| BTPD |
1/3, 33.3% |
- |
|
| G-POEM |
1/3, 33.3% |
- |
|
| Pyloromyotomy+ |
1/3, 33.3% |
1/1, 100% |
|
| pyloroplasty |
|
|
|
| Follow up |
313 (208-608) |
197 (65-499) |
p=0.12 |
| (months; median/IQR) |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).