1. Introduction
Although proper pain management in laboratory species is a fundamental aspect of animal welfare, underdiagnosis and undertreatment of pain are considered institutional problems in experimental science [
1,
2]. While increasing analgesic doses or combining different drugs into a multimodal regimen can help close this gap of unmitigated pain in animals, they can also introduce adverse effects that compromise animal well-being and physiological functions. For example, buprenorphine administration (0.05 mg/kg s.c.) has been correlated with pica behavior in rats, particularly in Sprague Dawley [
3,
4]. Furthermore, the genetic differences among inbred laboratory rodent strains result in varied drug metabolism, posing additional challenges to drug dose refinement [
5]. Thus, reports about the side effects of pain management agents are crucial to eliminating suboptimal procedures in experiment-specific regimens. In this context, we report on undesirable symptoms encountered after adapting a carprofen-based analgesia regimen to meet updated recommendations [
6].
Recent recommendation from the German Society for Laboratory Animal Science (GV-SOLAS) for carprofen use in rats is 2–5 mg/kg every 12–24 hours to provide effective post-operative analgesia [
6]. Such recommendations shape experimental protocol development in our facility in collaboration with veterinarians and regulatory authorities. Factors influencing drug dose regimens for lab animals include increases in the stress levels of the animals induced by frequent interventions and practicality for experimenters. To accommodate these factors, our approved experimental procedure (in accordance with the German animal welfare act: TierSchG § 8 sub-section 1) allows for a range of 1 –2 carprofen applications per day. While internal recommendations favored twice daily analgesia to prevent periods of insufficient pain mitigation, adverse effects prompted veterinary consult and a shift to once daily dosing as previously used in our lab.
This study aims to investigate the adverse effects observed with the current carprofen-based analgesia regimen, within the context of multimodal drug administration and surgical interventions in neuroscientific experiments. By examining these side effects, we aim to refine analgesia protocols to enhance animal welfare and reduce confounding variables that may affect experimental outcomes. Our findings underscore the need for further research into optimizing analgesic strategies to balance efficacy and safety for laboratory animals.
2. Materials and Methods
Ethical Statement
All results reported here are side products of neuroscientific studies aimed at advancing our understanding of cognitive action control, with potential implications for improving movement disorder therapy. Rats are relevant translational models for such experiments due to cognitive homologies with humans, especially in relation to the prefrontal cortex [
7,
8]. At no time were medical problems deliberately induced through drug administration. All animal-related procedures were in accordance with directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes and were approved by the local authorities (Regierungspraesidium Freiburg).
Animal Care and Monitoring
Wild-type Sprague Dawley rats (Janvier Labs, France) were group-housed in individually ventilated cages (480 x 375 x 210 mm, Eurostandard Type IV S, 1500U; TECNIPLAST, Hohenpeissenberg, Germany), with a maximum of 4 rats (up to 400 g), or 3 rats (up to 600 g) per cage. Cages were supplied with aspen wood bedding, unbleached egg cartons, paper towels, and aspen wood gnawing sticks for nesting and enrichment. Rats were maintained in a reversed 12-hour light cycle and had ad libitum access to food (#1314, 10 mm breeding diet pellets; Altromin International, Lage, Germany) and water, except for specific rats undergoing behavioral training (Groups 3 and 4) that were water-deprived during training sessions and carefully monitored to maintain healthy body weight. No water deprivation occurred during surgical or recovery periods. Animals were habituated to experimenters via handling for 1–5 days before surgery to minimize stress during procedures.
Stereotactic Surgery
All rats underwent stereotactic cranial surgery. Uniform drug administration was employed across all cohorts on the surgery day (
Table 1). Rats were briefly anesthetized with 5% isoflurane and received i.p. injections of 80 mg/kg ketamine and 0.06 mg/kg medetomidine. Prior to surgery, surgical analgesia was pre-emptively applied with a subcutaneous injection of 0.05 mg/kg buprenorphine and local application of xylocaine gel to the incision site. A concluding s.c. injection of carprofen was administered immediately following the end of surgery as pre-emptive analgesia for post-surgical pain. Cohorts 1 and 2 underwent unilateral injection of an optogenetic virus (University of North Carolina Vector Core, USA) and implantation with a low-profile optical fiber (200 µm-wide; Doric Lenses, Canada). Cohorts 3 and 4 underwent silicone electrode implantation (E32+R-100-S2-L6-200 NT, Atlas Neuroengineering, Belgium). Cohort 5 received only viral injections without implantation. The latter were by far the shortest surgeries, did not involve complicated recoveries, and generally involved the least amount of stress to the animals (
Table 2). For a more detailed description of surgical techniques, please refer to previous studies from our group [
9,
10].
Post-Operative Care
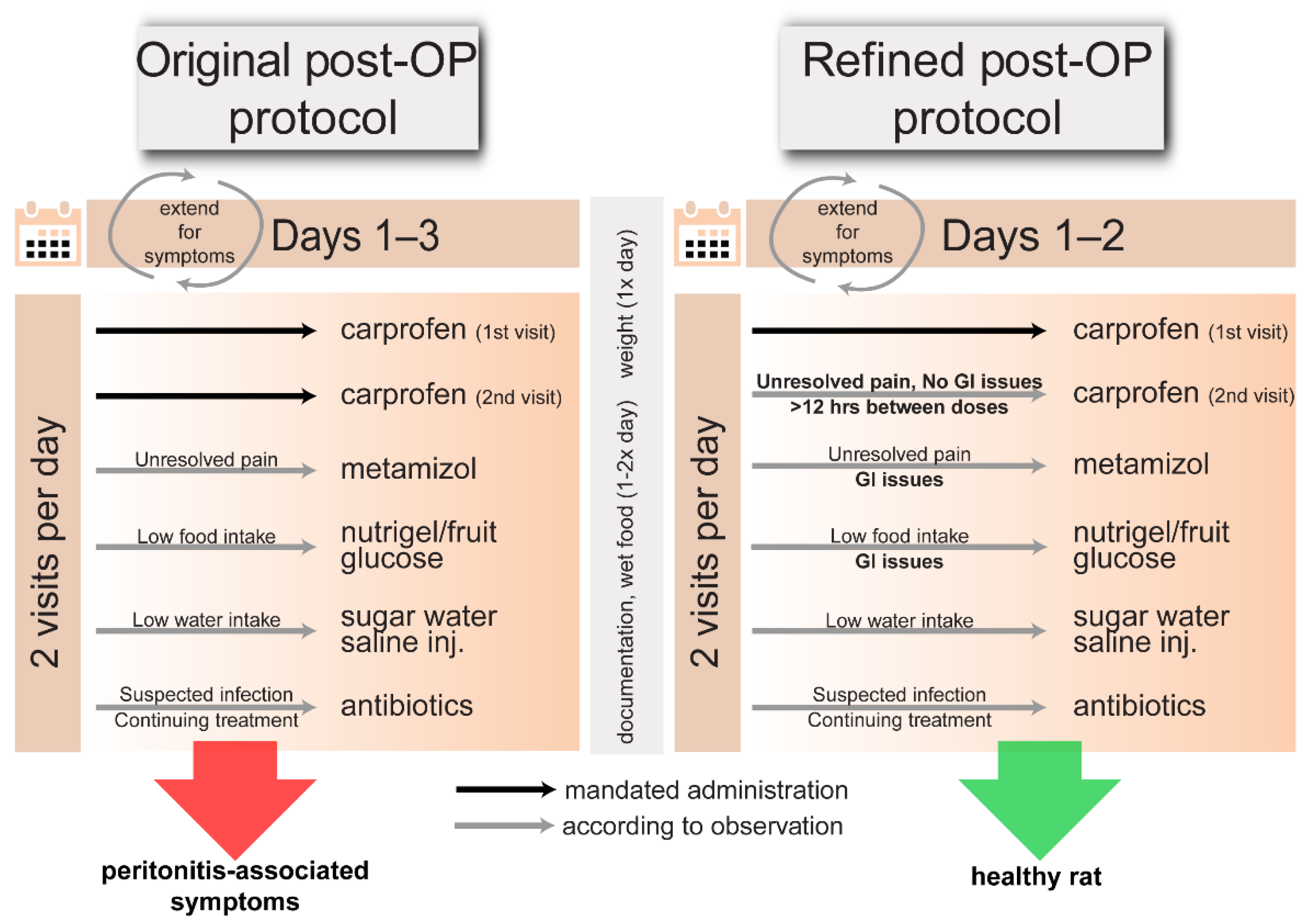
In order to reduce post-surgical pain, 5 mg/kg carprofen (Zoetis Deutschland GmbH, Germany) was given pre-emptively 1-2 times daily for 1-3 post-operative days (
Table 1 and
Table 2); dose-frequency administration was decided following current recommendations [
6]. To facilitate food intake, wet food was given 1-2 times daily to all rats for up to 1.5 weeks post-surgery. The post-operative care window and carprofen administration time period was extended for rats that displayed pain or discomfort, with particular attention for abnormal or high-severity symptoms. Suspected infections prompted a 20 mg/kg s.c. injection of an antibiotic (Sulphix; bela-pharm GmbH & Co. KG, Germany) once daily for 3–5 days (Table 3). Abnormal food intake was addressed by motivating consumption with nutrigel (Nutriplus Gel; Virbac, Germany), fruit, and sweetened water; if unsuccessful, 5% glucose (1 mL) was administered s.c. for blood sugar maintenance. For low water intake, saline (1-2 mL) was injected s.c. to prevent dehydration. Encouraging natural food and drink intake was prioritized over injection.
After encountering several instances of negative outcomes, we suspected high-frequency carprofen administration to be exacerbating symptoms. We therefore modulated analgesia to lower-frequency carprofen administration with ongoing documentation and veterinary consultation (within monitoring and diagnostic limitations) to best improve outcomes.
Protocol Modulation between Rat Cohorts
The rats in this study belonged to separate cohorts varying in age, sex, or surgical procedure conducted for different neuroscientific experiments (
Table 2). We here report on the unintended side effects stemming from similar protocols in our animal model. We compiled post-OP observational data from separate neuroscientific experiments to investigate common factors, potentially contributing to negative symptoms. All included observational data were collected over a 1-year timeframe from post-OP care following primary surgeries conducted on rats by experimenters with at least 6 months of experience in stereotactic surgery. Operations which did not meet these criteria and non-survival surgeries were excluded. Post-OP analgesia protocols (
Table 1) were modified between surgeries due to high incidence of adverse symptoms. As such, the treatments and post-OP observations were not randomized or blinded. To assess effects from the frequency of carprofen administration on rat health, rats were categorized
post hoc into two groups: a high-frequency carprofen group (Protocols A–D), which received carprofen twice daily for at least two days, and a low-frequency carprofen group (Protocols E–F), which received doses once daily, except one animal of protocol E, which received two doses on the first day only and was switched to single doses afterwards.
Cohort 1 was first treated with the high-frequency carprofen protocol (Protocols A & C). After several cases of suspected peritonitis, possible risk factors were examined before commencing procedures on further animals. As elevated pica behavior was observed in several rats post-surgery, the consumption of frequently chewed, non-essential cage items were suggested risk factors for bezoar formation and ulceration through mechanical damage to the GI tract. Consequently, the cage environment was adjusted for the next cohorts (Cohorts 2 –5) by removing enrichment items, such as cardboard and paper towels, during the post-surgery week and restricting access to plastic vents extending into the cage. Additionally, i.p. injections were supervised by the faculty veterinarian to rule out incorrect injection techniques as a source of ulceration. Cohort 2 and Cohort 3 initially received high-frequency carprofen analgesia (Protocols A–D). In response to additional cases of suspected peritonitis, administration frequency was reduced (Protocol E-F) for the remaining animals of Cohort 2 and 3, as well as subsequent cohorts 4 and 5, aiming to mitigate adverse symptoms suspected to be linked with high-frequency carprofen application.
Termination and Post-Mortem Investigation
We established humane endpoints to minimize suffering in rats. Termination criteria included a ≥15% weight loss from the initial weight. The initial weight, adjusted over time for regained weight, ensured that the termination criteria accounts for the post-recovery phase and normal growth changes. Other criteria encompassed persisting symptoms such as cramps, paralysis, abnormal breathing, irregular thermoregulation, vocalizing pain, reduced grooming, limited exploration, self-harm, and food refusal. Seven rats that did not recover after high-frequency carprofen treatment were euthanized via 5% isoflurane anesthesia followed by an overdose of i.p. ketamine and xylazine (Rompun 2%; Elanco GmbH, Germany). Post-mortem investigation was performed on six of these rats; this involved ventral abdominal access with surgical scissors, removal of the greater omentum and dissection of the abdominal organs.
Analysis
We utilized custom MATLAB scripts to categorize rats into groups according to sex, carprofen protocol, and health outcome data. We investigated effects of carprofen treatment and sex on frequency of negative health outcome (peritonitis-indicative symptoms) with Fisher's exact test. Groups with significant effects were also checked for any differences in baseline weight (independent t-test), which could play a role in robustness to surgical intervention and drug therapy, potentially affecting health outcome. We further examined the association between three different surgical interventions and symptomatic presentation using Fisher's exact test [
11].
3. Results
3.1. High-Frequency Carprofen Effect on Pertonitis-Like Symptoms
All rats in Cohort 1 received high-frequency carprofen treatment. Twenty percent (2/10) exhibited symptoms such as weight loss, hunched posture, poor balance, reduced food and water intake, pica behavior, piloerection, and abdominal bloating. Following veterinary recommendations, carprofen and antibiotic administration was extended beyond the standard post-OP care timeframe for these two rats to manage pain symptoms, along with antibiotics to treat suspected infections underlying the observed disease progression. In both cases, symptoms worsened, with additional symptoms such as low movement, a lack of feces in the cage, and increased abdominal bloating, prompting humane termination.
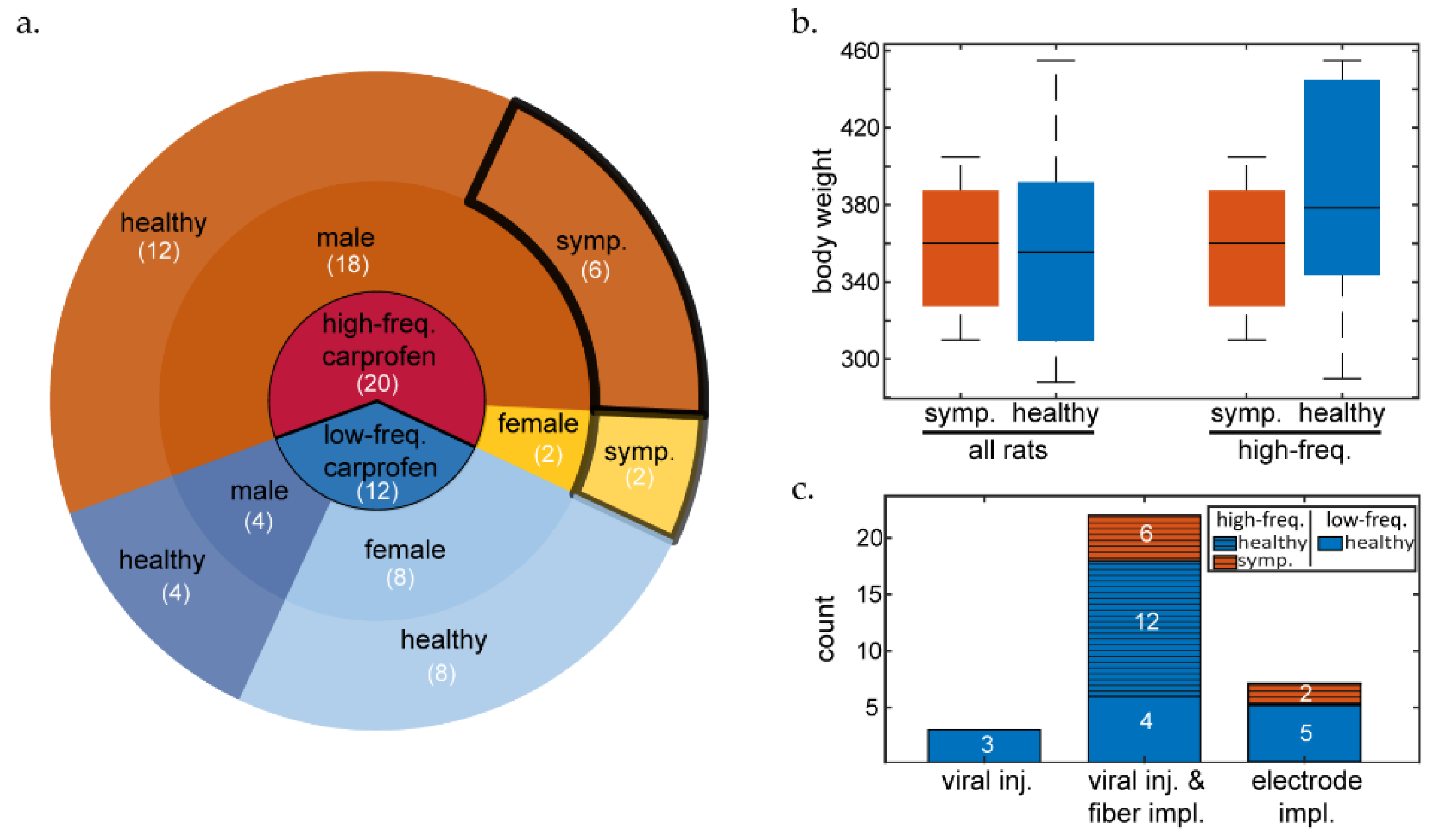
Although post-surgical pica behavior was restricted in the following cohorts, peritonitis-like symptoms persisted. Fifty percent of Cohort 2 rats (4/8) and 100% of Cohort 3 rats (2/2) from the high-frequency carprofen group, exhibited similar symptoms (
Table 2). Among these, five received extended carprofen treatment and supplementary antibiotics, leading to termination due to exacerbating symptoms. One of these six rats was treated with metamizole instead of prolonged carprofen treatment and antibiotic administration and recovered. No adverse symptoms were observed in rats that were treated with low-frequency carprofen (0/11).
In summary, 40% (8/20) of all high-frequency carprofen-treated rats became symptomatic, with 35% (7/20) terminated due to peritonitis-like symptoms (initially observed 3-6 days post-surgery). Out of the female rats from this group, 100% (2/2) were affected by similar symptoms and were terminated. For males undergoing high-frequency carprofen treatment, 33.33% (6/18) presented suspected peritonitis and 27.7% (5/18) were terminated due to the negative symptoms. Low-frequency carprofen group rats presented zero events of suspected peritonitis (
Figure 1).
We observed a significant effect of carprofen treatment (
Figure 1A) on symptom presentation (Fisher's exact test [two-tailed]: p = 0.0135). There was no significant effect between sex and health outcome (Fisher's exact test [two-tailed]: p > 0.05). An independent t-test did not reveal significantly different initial body weights (
Figure 1B) between symptomatic or healthy rats overall (mean difference: 1.8 g, p = 0.93, 95% CI: [-44.1889, 40.6056]), or within the high-frequency carprofen group (mean difference: 28.5 g, p = 0.22, 95% CI: [-75.534, 18.6174]). In addition, we found no effect between surgery type and symptom presentation (
Figure 1C, Fisher's exact test [two-tailed]: p = 0.84069). While the sample sizes in this study were generally low, the surgery type groups were particularly small and imbalanced, so we recommend caution when generalizing these findings.
3.2. Post-Mortem Analysis of High-Frequency Carprofen-Treated Rats Reveals GI Complications
Peritonitis-like symptoms occurred in eight animals (all belonging to high-frequency carprofen protocols), six of which underwent post-mortem examination. Each rat presented signs of gastric defect and various signs of inflammatory or suppurative processes. Abdominal examination revealed abnormalities such as fluid-filled abdomen, discolored omental tissue, fecal obstructions up to 1.5 cm diameter in the large intestine, blood traces, intestinal discoloration, or intestinal ulceration.
4. Discussion
The 3R principle aspires replacing animal experiments with alternative methods, reducing animal use, and refining procedures to minimize animal suffering. While adequate analgesia is crucial, its value diminishes if adverse effects from its administration lead to increased morbidity or suffering. Effective doses in animals, as in humans, may induce adverse effects [
4], and careful monitoring for symptoms is essential, since animals cannot communicate discomfort directly. If drug protocols are not optimized, unnecessary suffering, increased animal usage, and repeated experiments may occur, thereby contradicting the 3Rs principle.
In our rat model, high-frequency carprofen treatment led to a substantial increase in peritonitis-like symptoms, with an unacceptably high (40%) percentage of animals showing signs of adverse effects. Antibiotics did not ameliorate symptoms, consistent with previous reports of negative interactions resulting from the co-administration of carprofen and antibiotics [
12,
13]. In one case, metamizole successfully provided alternative analgesia while allowing gastric issues to subside.
The underlying mechanisms of frequent carprofen-induced gastric dysfunction in our study remain unclear. However, our results suggest that a high-frequency carprofen regimen (5 mg/kg, twice daily) within a multimodal anesthesia and analgesia protocol (involving isoflurane, ketamine, medetomidine, buprenorphine, lidocaine, and carprofen) may predispose animals to abnormal gastric function and higher morbidity rates. In contrast, reducing the carprofen administration frequency under the same multimodal drug protocol for cranial surgery resulted in a 100% recovery rate and healthy post-OP presentation, suggesting that less frequent carprofen is safer in our experimental context. These findings are supported by studies showing that carprofen interferes with anastomotic wound repair in the rat ileum [
14] and that buprenorphine exacerbates ethanol-induced gastric lesions [15]; considering their individual adverse effects on the gastric system, combination therapy including both carprofen and buprenorphine could elevate the risk of peritonitis. Indeed, a recent study revealed heightened drug sensitivity under multimodal therapy [16]. Multimodal drug-therapy (0.1 mg/kg buprenorphine every 8 hours, 5 mg/kg carprofen every 24 h and local irrigation during surgery with 10 mg/ml lidocaine and 2.5 mg/ml bupivacaine) led to peritonitis, while single-drug therapy with the same dose-frequency administration of carprofen or buprenorphine did not reflect such effects, nor did multimodal therapy with lower-dose buprenorphine [16]. Another recent study failed to find desired synergies from multimodal drug application, potentially due to high carprofen doses (well-suited for monotherapy). They suggest that potential synergies of drug combinations in perioperative management should be assessed with lower carprofen doses [
14]. Thus, current recommendations for high-frequency administration may be suboptimal under specific strain-, experiment-, and multimodal-specific conditions. Additionally, we argue for alternative analgesic strategies, particularly in the presence of gastric issues, to prevent symptom exacerbation. Effective analgesia and increased awareness for potential adverse effects from multimodal drug administration is crucial for optimizing animal care and aligning with the 3R principle.
5. Conclusions
Our findings suggest that high-frequency carprofen administration in multimodal anesthesia and analgesia protocols may lead to significant adverse effects in rats, such as peritonitis-like symptoms and increased mortality. Reducing the frequency of carprofen administration resulted in better outcomes, emphasizing the need for careful consideration of drug protocols in animal models to prevent unnecessary suffering. Future research should further investigate the underlying mechanisms of drug interactions in multimodal settings and explore alternative analgesic options to enhance animal welfare and adhere to the 3R principle.
Author Contributions
Conceptualization, Z.J., A.A., S.H, B.K., R.S., and I.D.; methodology, Z.J. and A.A.; validation, Z.J., A.A.; formal analysis, Z.J., A.A., B.K; investigation, Z.J., A.A.; resources, I.D.; data curation, Z.J. and A.A.; writing—original draft preparation, Z.J., writing—review and editing, P.C. and I.D.; visualization, Z.J.; supervision, I.D.; project administration, I.D.; funding acquisition, I.D. All authors have read and agreed to the published version of the manuscript.
Funding
The data reported of herein was obtained from projects funded as part of BrainLinks-BrainTools, which is funded by the Federal Ministry of Economics, Science and Arts of Baden-Württemberg within the sustainability programme for projects of the Excellence Initiative II; as well as the Research Unit 5159 “Resolving Prefrontal Flexibility” (Grant DI 1908/11-1), and the Innovative Training Network (ETN) of the Marie Skłodowska-Curie Actions - European School of Network Neuroscience (euSNN), all to I.D.
Institutional Review Board Statement
The animal study protocols (TVA G-20-65, TVA G-20-26, L-20-12) were approved by the local authorities (Regierungspraesidium Freiburg) in accordance with the directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.
Data Availability Statement
Detailed experimental methods, post-OP and post-mortem documentation, as well as analyses are available upon reasonable request.
Acknowledgments
We thank Christine Zeschnigk for support in post-OP care.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Foley, P.L.; Kendall, L.V.; Turner, P.V. Clinical Management of Pain in Rodents. Comp Med 2019, 69, 468–489. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.E.; Pavek, T.J.; Schwark, W.S.; et al. Comparison of Nociceptive Effects of Buprenorphine, Firocoxib, and Meloxicam in a Plantar Incision Model in Sprague–Dawley Rats. J Am Assoc Lab Anim Sci JAALAS 2021, 60, 539–548. [Google Scholar] [CrossRef]
- Reifenrath, J.; Heider, M.; Kempfert, M.; et al. Buprenorphine in rats: potent analgesic or trigger for fatal side effects? Acta Vet Scand 2022, 64, 37. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.C.; Kristal, M.B.; Sallaj, A.; et al. Analgesic Efficacy of Orally Administered Buprenorphine in Rats: Methodologic Considerations. Comp Med 2004, 54, 293–300. [Google Scholar] [PubMed]
- Champy, M.-F.; Selloum, M.; Zeitler, V.; et al. Genetic background determines metabolic phenotypes in the mouse. Mamm Genome Off J Int Mamm Genome Soc 2008, 19, 318–331. [Google Scholar] [CrossRef]
- Arras, M.; Becker, K.; Bergadano, A.; et al. Pain management for laboratory animals. 2020. Available online: https://www.gv-solas.de/wp-content/uploads/2021/08/2021-04_Pain_Management_for_laboratory_animals.pdf.
- Kesner, R.P.; Churchwell, J.C. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem 2011, 96, 417–431. [Google Scholar] [CrossRef]
- Robbins, T.W. From Rodent Behavioral Models to Human Disorders. In Translational Neuroscience: Toward New Therapies; Nikolich, K., Hyman, S.E., Eds.; MIT Press: Cambridge (MA), 20 June 2015; Available online: http://www.ncbi.nlm.nih.gov/books/NBK569708/ (accessed on 5 June 2023).
- Hardung, S.; Epple, R.; Jäckel, Z.; et al. A Functional Gradient in the Rodent Prefrontal Cortex Supports Behavioral Inhibition. Curr Biol 2017, 27, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Karvat, G.; Schneider, A.; Alyahyay, M.; et al. Real-time detection of neural oscillation bursts allows behaviourally relevant neurofeedback. Commun Biol 2020, 3, 72. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, G. MyFisher. Mathworks. 2010. Available online: https://de.mathworks.com/matlabcentral/fileexchange/26883-myfisher (accessed on 20 April 2023).
- Jones, S.M.; Gaier, A.; Enomoto, H.; et al. The effect of combined carprofen and omeprazole administration on gastrointestinal permeability and inflammation in dogs. J Vet Intern Med 2020, 34, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Burch, M.-A.; Keshishian, A.; Wittmann, C.; et al. The non-steroidal anti-inflammatory drug carprofen negatively impacts new bone formation and antibiotic efficacy in a rat model of orthopaedic-device-related infection. Eur Cell Mater 2021, 41, 739–755. [Google Scholar] [CrossRef]
- Munk, A.; Philippi, V.; Buchecker, V.; et al. Refining pain management in mice by comparing multimodal analgesia and NSAID monotherapy for neurosurgical procedures. Sci Rep 2024, 14, 1–17. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).