1. Introduction
Canine Morbillivirus, known as canine distemper virus (CDV), belongs to the Morbillivirus genus within the Paramyxoviridae family and is an enveloped negative polarity RNA virus [
1]. Morbilliviruses are highly contagious pathogens that cause outbreaks of systemic, often fatal diseases in animals and humans worldwide. This genus also includes measles virus (MeV), Rinderpest virus (RPV), peste des petits ruminants virus (PPRV), phocine distemper virus (PDV), Cetacean morbillivirus (CeMV), and the recently discovered feline morbillivirus (FeMV) [
1].
CDV infects a wide range of carnivores, including members of Canidae, Procyonidae, Mustelidae, Ursidae, Viverridae, Felidae, Ailruidae, Hyaenidae and Tayassuidae and Cercopithecidae [
2,
3,
4,
5]. It exhibits the second highest fatality rate of infectious diseases in dogs, after rabies [
6]. CDV is a multi-systemic disease affecting the respiratory, gastrointestinal, and nervous systems. Early stages of infection are marked by non-specific signs such as anorexia, depression, conjunctivitis, and hyperkeratosis of digital cushions [
7]. At this stage, viruses can be found in every secretion of the infected animal [
8]. As the disease progresses, a more pronounced and severe clinical picture emerges, including catarrhal inflammation of the bronchi and larynx, along with episodes of vomiting and diarrhea. When the nervous system is affected, apathy, ataxia, and paralysis may ensue, progressing from paraplegia to quadriplegia [
9,
10]. Dogs with nervous system pathologies often do not survive or exhibit lifelong neurological symptoms [
11]. The progression of canine distemper disease is often variable, wherein the manifestation of clinical signs is contingent upon the specific strain of the virus responsible for the infection. This variability of clinical signs depend on the age of the host, its immune status, and virus strain, with more than 50% of cases being probably subclinical [
6,
12].
Rapid and precise diagnosis of CDV facilitates treatment and conservation of infected dogs and prevents further transmission to susceptible hosts. Therefore, it is important to choose a rapid and reliable diagnostic method. Variable signs of distemper and subclinical status of the disease in some cases are the main challenges of diagnosing the disease based on clinical and physical examination. Currently, routine laboratory and clinical findings are helpful for initial diagnosis but not sufficient for confirmation of the infection [
12,
13]. In recent years, advancements in diagnostic methodologies have introduced more sensitive and specific techniques for the accurate detection of CDV, such as reverse transcriptase PCR (RT-PCR), quantitative reverse transcription real-time PCR (RT-qPCR), enzyme-linked immunosorbent assay (ELISA), immunofluorescence assay (IFA), neutralization antibody (NA) test and rapid immunochromatographic assays [
14,
15,
16]. Antibody detection often proves limited in diagnosing CDV due to potential interference from previous vaccination and/or post-infection status. Consequently, diagnostic approaches based on antigen or nucleic acid detection hold greater value and timeliness [
17]. Notably, rapid immunochromatographic (IC) antigen test kits, known for their speed, cost-effectiveness, and user-friendliness, alongside RT-PCR assays, recognized for their heightened sensitivity and specificity, emerge as more practical choices for CDV diagnosis [
12,
14]. Various challenges are inherent in the detection of CDV, involving the identification of the optimal tissue samples, early detection, and detection of target molecules at low concentrations with high sensitivity.
Due to technological advances, various PCR variants have been developed, among which digital droplet PCR (ddPCR) is a novel platform designed to provide greater sensitivity and precision for the detection and quantitation of nucleic acid target molecules [
18,
19]. This technology not only enables detection and allows direct quantification without the need for a standard curve in a sample, but is also highly sensitive, even surpassing qPCR [
20,
21,
22]. Studies have demonstrated that ddPCR can achieve a nucleic acid detection sensitivity of low viral loads (10
–5) [
23]. The heightened sensitivity of the technique is partly attributed to the fragmentation of the sample into a set of nanodroplets, each representing an individual reaction in an oil emulsion. The ability to diagnose CDV even when the agent is excreted in low quantities, either due to the timing of sample collection or partial immunity of the animal, is crucial for accurate diagnosis and management of the disease. This work assessed the diagnostic performance of conventional RT-PCR, RT-qPCR, and ddPCR for CDV detection in diverse samples and compared the results with clinical diagnosis.
2. Materials and Methods
2.1. Samples Collection
In this study, 76 blood, urine, and nasal/ocular secretion samples from dogs exhibiting clinical signs consistent with CDV were included, and 16 negative control samples were acquired from healthy individuals without clinical signs of CDV and evidence of not having been exposed to the virus in the environment or with other pets with possible subclinical disease. The samples were collected between 2021 and 2023 at the Microbiology Laboratory Services, Faculty of Veterinary, Montevideo, Uruguay. Blood samples were collected from the jugular, cephalic, or lateral saphenous vein and placed into sterile EDTA tubes. Urine samples were collected via cystocentesis, and nasal or ocular secretions were taken with a sterile swab and stored in 200 µL DNA/RNA Shield (Zymo Research). Cerebellum (left hemisphere) samples were collected fresh and immediately frozen at necropsy. All samples were stored at -80°C. The right hemisphere was fixed in 10% neutral buffered formalin and processed routinely for histologic examination.
Clinical diagnosis of the dogs was performed by specialized veterinarians, taking into account the presence of some the following symptoms: intermittent or persistent fever, mucous or purulent discharge from the eyes and nose, coughing that may be accompanied by difficulty breathing, vomiting and diarrhea leading to dehydration, loss of appetite resulting in weight loss, lethargy and depression with the dog showing a lack of energy and activity, thickening of the paw pads and nose often referred to as "hyperkeratosis," and neurological symptoms such as seizures, muscle twitches, partial or total paralysis, and abnormal behaviors. The symptomatology used as a reference for the clinical diagnosis of canine distemper virus has been previously described by numerous authors [
7,
11,
24]. For canines euthanized due to severe illness, the brain was sectioned into two hemispheres. The right hemisphere was used for conventional hematoxylin and eosin (H&E) stain, Luxol Fast Blue (LFB) stain; and immunohistochemistry against CDV. For immunohistochemistry, to confirm the presence of CDV antigens, we employed a mouse anti-CDV monoclonal primary antibody (MCA 1893, dilution 1:250; BioRad, UK) and secondary antibody (Mouse-on-Canine HRP-Polymer, Biocare Medical, USA), positive antigen–antibody reactions were observed by incubation with 3,3′-diaminobenzidine tetrahydrochloride (DAB). Histologic lesions were categorized as acute, subacute, or chronic. The lesions were characterized by vacuolation, gliosis, perivascular cuffing, CDV-positive cells in acute stage; patchy demyelination, prominent perivascular cuffing, and CDV-positive cells in subacute stage; and increased neuronal degeneration and necrosis in chronic stage [
25,
26]. The left hemisphere was used for molecular biology techniques to confirm the presence and quantify the viral load in the tissue.
The experimental protocols for the canine studies performed in this paper were approved by Animal Use Ethics Commission (CEUA) from the Universidad de la República Oriental del Uruguay (approval number CEUAFVET-1003/20, Exp. 111900-000900-20).
The Onderstepoort strain of canine distemper virus (CDV-Ond), originally isolated in 1940, was cultured in Vero cells stably expressing canine signaling lymphocytic activation molecules (Vero.DogSLAM cells) [
27]. These cells were kindly provided by Dr. Yusuke Yanagi (Kyushu University, Japan). The virus was propagated and titrated according to standard protocols, and the viral stock was prepared for use in this study.
2.2. RNA Viral Isolation And Reverse Transcription
Viral RNA extraction from urine, nasal and ocular secretions and blood was carried out using the RNA Viral Kit (Zymo Research, Irvine, CA) following the manufacturer's recommendations. For cerebellum samples, TRI Reagent (Zymo Research, Irvine, CA) was used, and total RNA was treated with DNAse I (Invitrogen, Carlsbad, CA) to eliminate any contaminating genomic DNA. Subsequently, first-strand cDNA was synthesized using the Sensifast cDNA kit (Bioline, UK) as per the manufacturer's instructions, and stored at –20 °C prior to use.
2.3. Plasmid Preparation
Plasmid DNA for generating standard curves was prepared by carrying out serial 10-fold dilution steps and was used in both ddPCR and RT-qPCR assays. The target regions between positions 905 and 963 of the N protein-encoding gene were amplified, cloned into pGEM®-T Easy vector (Promega, Medison, WI) and transformed into Competent TOP10 Escherichia coli, which were subsequently spread onto LB/ampicillin/IPTG/X-Gal plates and incubated overnight at 37°C. The white colonies (Escherichia coli with inserted region) were selected for further growth in LB broth. The recombinant plasmid was extracted using the Plasmid Mini Kit (Zymo Research, Irvine, CA) and stored at -20ºC prior to use. PCR and sequencing by Macrogen (South Korea) confirmed the inserted region's presence and direction. The concentration of plasmid DNA was measured using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA) and recalculated to plasmid copies/μL.
2.4. CDV Detection by Conventional Reverse Transcription PCR
A 287 bp fragment of the nucleoprotein (NP), position 1055–1035 (
Table 1), was amplified according to
28. The amplification consisted of an initial denaturation step at 94ºC for 1 minute, followed by 40 cycles consisting of 1 minute denaturation step at 94°C, a 2 minute annealing step at 59.5 °C and a 1 minute extension step at 72°C, and a final extension at 72°C for 5 minutes, which was performed in a thermocycler (C1000 touch cycler; Bio-Rad, Hercules, CA, USA). Subsequently, the PCR product was loaded onto a 2% agarose gel stained with GoodView™ (SBS Genetech). Electrophoresis was carried out at 100 V for a duration of 30 minutes.
2.5. Real-Time Quantitative PCR
RT-qPCR was performed in a final volume of 20 μL. For each amplification reaction, 1 μL of cDNA was added to a reaction mixture containing 10 μL of oasig™ lyophilized 2× qPCR Mastermix (Genesig®, UK), 1,2 μL of primers/FAM labeled probe (600 nm/300 nm) (
Table 1), and PCR-grade H
2O up to the final volume (20 μL). The amplification process was performed using the Corbett Rotor-Gene 3000 (Corbett, Qiagen Rotor gene) according to the following thermal cycling conditions: 2 min at 95ºC for polymerase activation and DNA denaturation, 50 cycles of denaturation at 95ºC for 10 s and annealing /extension + plate reading at 60ºC for 60 s. A non-template control was included in all runs, and every sample was measured in duplicate. Cq values and linear regression analyses of the calibration curves obtained with the tenfold serial dilutions with the standard plasmids were generated by Rotor-Gene AssayManager. For RT-qPCR, linear regression was applied to each of the three independent RT-qPCR experiments, and the correlation coefficient (R²), as well as PCR efficiency (E% = (10(−1/slope) −1) ×100), were calculated.
2.6. Droplet Digital PCR Assay
DdPCR was performed according to the manufacturer's instructions for the QX200 droplet digital PCR system (Bio-Rad, Hercules, CA, USA). The probe and primer sequences (
Table 1) were designed to amplify a partial segment of the NP gene. The primer and probe concentrations and the annealing temperature and duration were optimized. Briefly, the 20 μL reaction mixture contained 10 μL master mix ddPCR for probes, primers (600 nM) and probe (300 nM) and 1 μL cDNA. Initially, 20 μL of mix was transferred to a DG8 (Bio-Rad, Hercules, CA, USA) cartridge and 70 uL of Droplet generation oil was loaded into the DG8. Subsequently, a QX200 Droplet Generator (Bio-Rad, Hercules, CA, USA) was used to generate the partitions. Forty μL of created droplets were loaded into a 96-well plate. The plate was run in a T100 Thermal Cycler (Bio-Rad, Hercules, CA, USA), with 40 cycles of amplification (20 s denaturation at 95 °C, followed by 30 s at 58 °C of annealing and extension). After amplification, droplets were individually read using a QX200 Droplet Reader (Bio-Rad, Hercules, CA, USA). The results were analyzed by QuantaSoft v.1.7.4 (Bio-Rad, Hercules, CA, USA). Reactions with a total number of droplets higher than 10,000 were considered. All samples were analyzed in triplicated and a template-less control (NTC) reaction was also included. For the analysis of data obtained through ddPCR, we employed the Bio-Rad QX Manager™ Software and linear regression analysis. The threshold between positive and negative droplet populations was manually set, guided by per-plate positive controls and no-template controls.
2.7. Diagnostic Performance Evaluation and Data Analysis
Evaluation of analytical sensitivity: The analytical limit of Detection (LoD) of RT-qPCR assays and ddPCR was evaluated through a series of 10-fold serial dilutions of the plasmid containing the NP gene. Each dilution was prepared in triplicate and subjected to testing by each assay on the same day. The LoD was determined as the highest dilution at which all replicates yielded positive, linear regression plots were conducted with GraphPad Prism 7.00, and analysis for LoD was conducted. Additionally, a linear regression analysis was performed to compare the RT-qPCR and ddPCR quantification with the theoretical dilutions of the plasmid.
Precision of RT-qPCR and ddPCR: Three dilutions of plasmid construction were prepared in triplicate and subjected to testing by RT-qPCR and ddPCR on the same day. The results are presented by the coefficients of variation (CV%).
Clinical Performance: The sensitivity and specificity of the molecular techniques (PCR conventional, RT-qPCR and ddPCR) were determined using the two-by-two table [
30,
31] against the clinical diagnosis of the 92 samples. Kappa indices were calculated using WinEpi Software (Working in Epidemiology,
http://www.winepi.net/). Receiver Operating Characteristic (ROC) curves were constructed to evaluate the diagnostic performance of RT-PCR, RT-qPCR, and ddPCR assays. RStudio, with the pROC package, was employed to calculate the Area Under the Curve (AUC) along with the corresponding 95% confidence interval (95% CI).
3. Results
3.1. Description of Clinical Symptoms in Canines Diagnosed with Canine Distemper
Of the 76 samples collected from canines diagnosed with canine distemper, 16% were from dogs aged 0-1 year, 22% from dogs 1-7 years old, and 8% from dogs older than 7 years, with 9% lacking age data. The sex distribution was 43% male and 45% female, with 12% unrecorded. Nine breeds were represented, with mixed breed dogs being most common (42.6%), followed by Poodle (11%), Labrador (7%), French Bulldog (7%), Pug (4.2%), German Shepherd (4.2%), Pitbull (4.2%), Beagle (3%), and Shar-Pei (3%). Additionally, 18% of the cases lacked breed information. Regarding vaccination status, 21% were up-to-date, 49% unvaccinated, and 30% had unknow status.
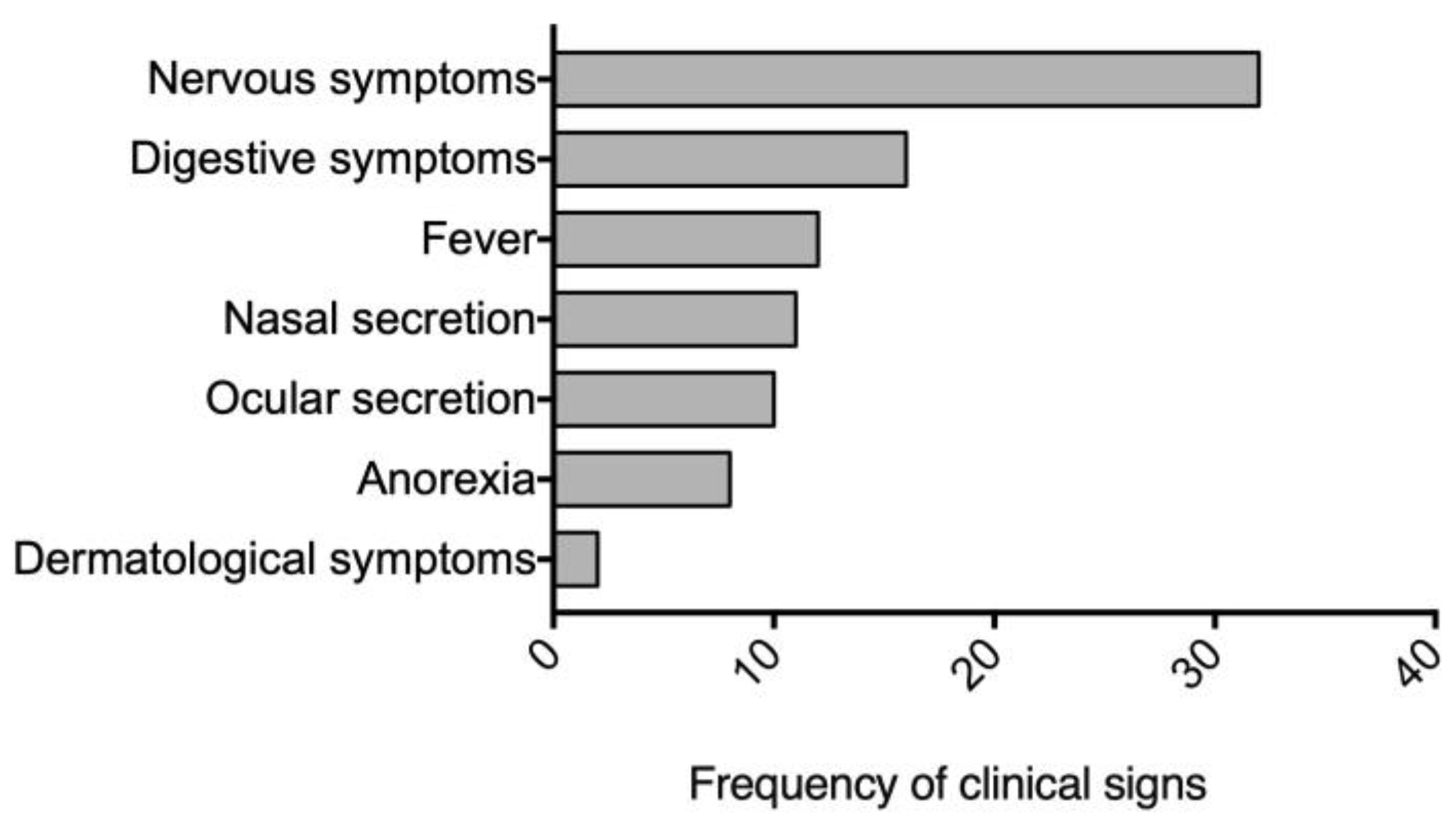
Figure 1 illustrates the frequency of the primary clinical signs observed, highlighting the predominance of nervous and digestive symptoms in dogs diagnosed with canine distemper. Additionally, 22 brain tissue samples were analyzed, with viral presence confirmed through immunohistochemistry. Histological examination of the tissue sections, stained with H&E, and LFB, was performed to assess the damage. We observed that 27% of the cases showed acute lesions, characterized by focal vacuolation, minimal gliosis, minimal perivascular cuffing, the presence of inclusion bodies, and CDV-positive cells. Subacute lesions were observed in 55% of the cases and were characterized by patchy demyelination, extensive gliosis, neuronal necrosis, prominent perivascular cuffing, prominent CDV inclusion bodies, and numerous CDV-positive cells. Chronic lesions represented 18% of the samples included in this study, characterized by lesions like those of the subacute stage but with increased neuronal degeneration (Figure supplementary 1).
3.2. Analytical Sensitivity of RT-qPCR and ddPCR
Serial 10-fold dilutions of the plasmid from 4,22 x1012 copies/μL to 1 X 10-4 copies /μL were used to determine the detection limit of CDV in RT-qPCR and ddPCR. Quantification threshold (Ct) values were measured in triplicate and were plotted against the known copy numbers of plasmid dilution. The generated standard curve showed good linearity (slope=-3.366), with an efficiency (E) was 115%, and a coefficient of linear regression R2= 0.9235. The limit of detection in RT-qPCR was 86 copies/μL.
To assess the detection limit in ddPCR, we employed the same serial dilutions of the plasmid as those in RT-qPCR. Positive droplets were determined by fluorescence intensity using QuantaSoft software. The software provides automated analysis and manual thresholding tools, facilitating the accurate identification of positive and negative droplet populations. Positive droplets were defined based on fluorescence intensity with only droplets that exceeded the threshold value being counted as positive. The analytical curve of the proposed method for quantification of CDV by ddPCR was linear over the entire tested range. Through linear regression analysis (R
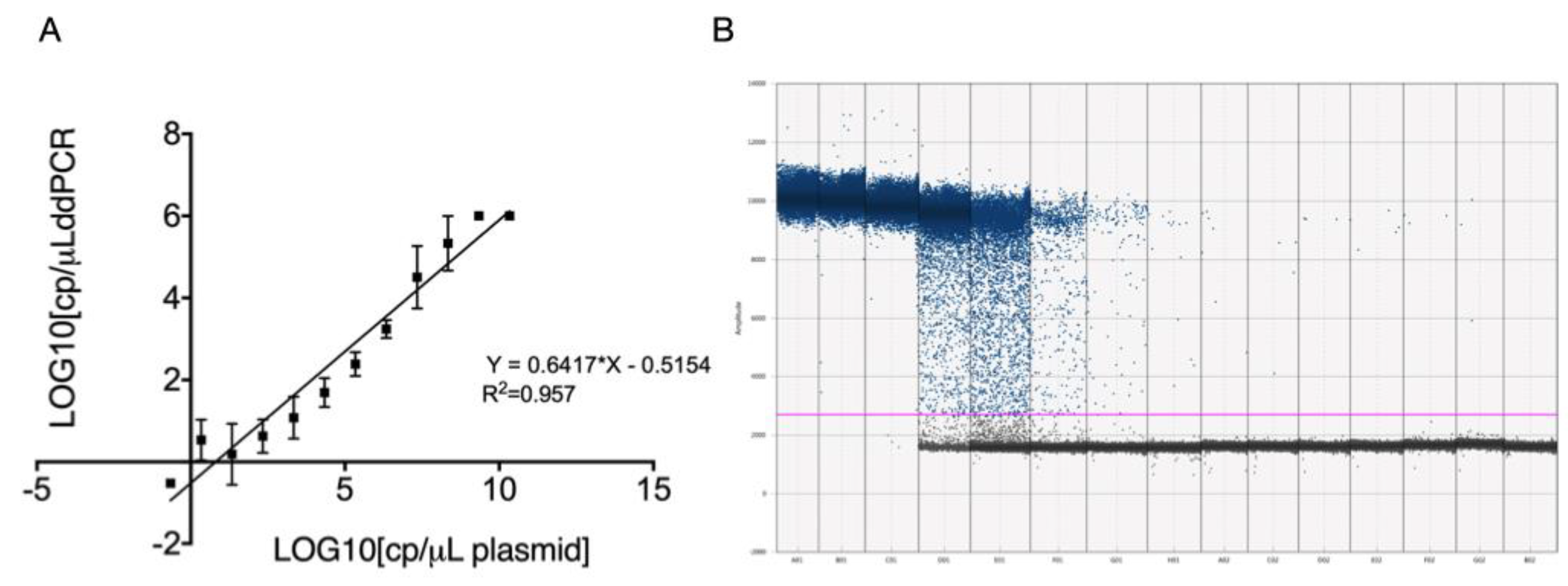
2= 0.953), we calculated a sensitivity (slope) of 0.6417 and an intercept of −0.5154 (
Figure 2). At a 95% confidence level, we calculated a Limit of Detection (LoD) of 3 copies/µL of reaction volume and a Limit of Quantification (LoQ) of 8 copies/µL of reaction volume. For qualitative analysis, samples with a ddPCR result of ≥3 copies/µL of reaction were classified as positive.
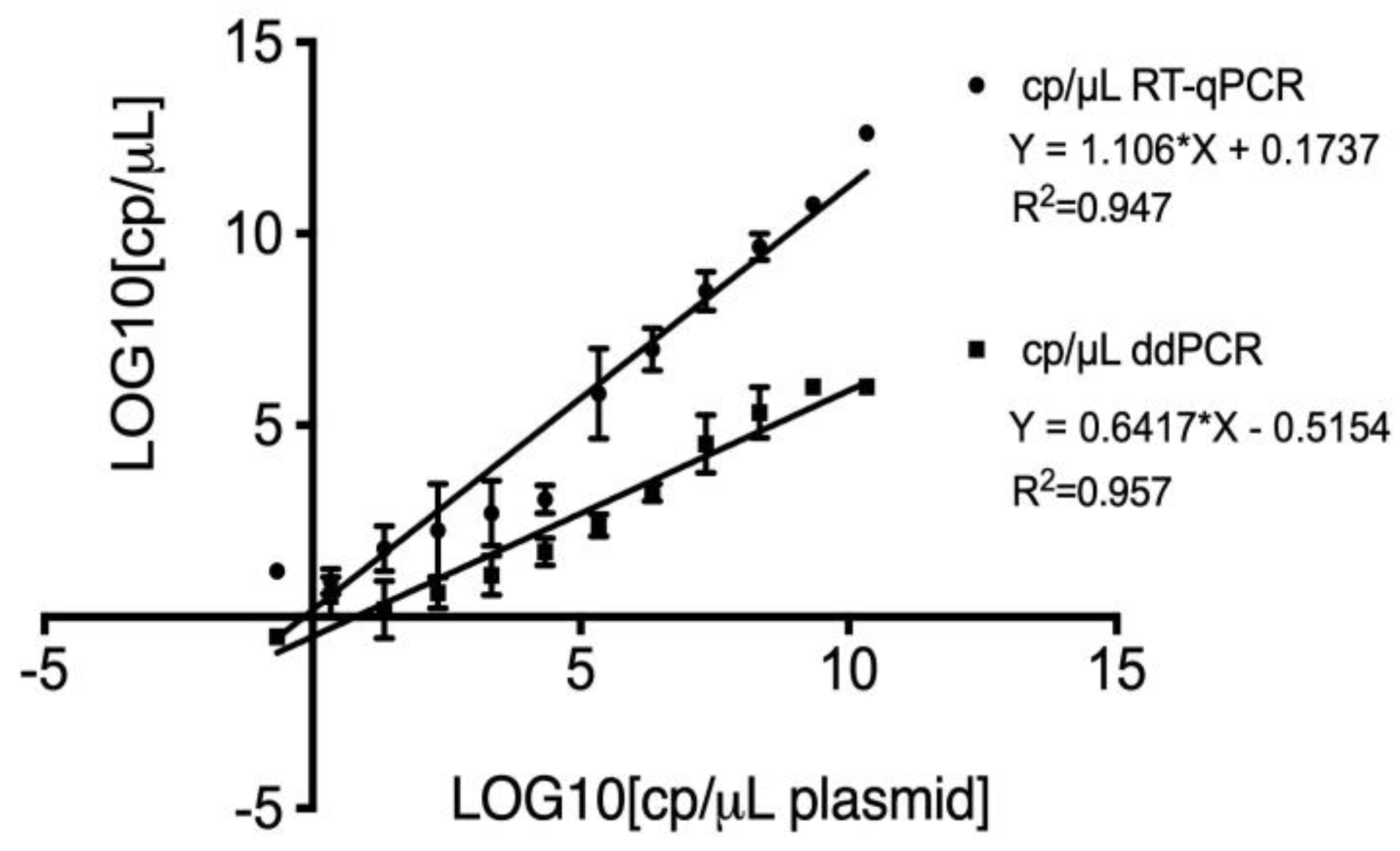
The linear regression analysis comparing plasmid quantification between RT-qPCR and ddPCR against the theoretical dilutions revealed differences in slopes and intercepts. For RT-qPCR, the regression equation was Y = 1.106*X + 0.1737, indicating a slope close to 1, which strongly aligns with the theoretical dilutions. The slight positive intercept of 0.1737 may indicate a minor overestimation in the more diluted samples. In contrast, the ddPCR regression equation was Y = 0.6417*X - 0.5154. Although the slope is less than 1, indicating a slight tendency to underestimate quantification compared to theoretical dilutions, the negative intercept of -0.5154 highlights ddPCR's ability to accurately quantify even at very low concentrations. The coefficient of determination (R²) values were close to 1 for both methods: 0.947 and 0.953 for RT-qPCR and ddPCR, respectively, further supporting the high accuracy of these methods (
Figure 3).
3.3. Precision of RT-qPCR and ddPCR
The precision of RT-qPCR and droplet digital PCR was evaluated using three independent dilutions of a plasmid construction. The coefficient of variation (CV) and standard deviation (SD) were calculated to assess the reproducibility of each method.
The CV values across the dilutions were significantly higher for RT-qPCR, indicating greater variability among technical replicates. Specifically, the CV ranged from 98% to 150%. These results underscore the inherent variability in RT-qPCR, which can impact the accuracy of viral load quantification, especially at low concentrations. In contrast, ddPCR demonstrated markedly improved precision. The CV values for ddPCR were consistently lower, ranging from 8.91% to 77.86% (
Table 2).
3.4. Comparing the Performance of Conventional RT-PCR, RT-qPCR, and ddPCR Against the Clinical Diagnosis
In this study, a total of 92 samples were used. Seventy-six of these samples came from dogs clinically diagnosed with CDV by experienced veterinarians, while 16 samples were from healthy dogs (negative controls). Each sample was tested in duplicate. Our research, which focused on samples with a clinical diagnosis of CDV, compared the performance of conventional RT-PCR, RT-qPCR, and ddPCR. Conventional RT-PCR detected 42.39% of samples as CDV-positive and 57.61% as negative, including the sixteen non-symptomatic CDV (control samples). In contrast, RT-qPCR identified 47.82% as positive and 52.17% as negative, including the control samples. When comparing the clinical diagnosis with ddPCR, we observed that 59.78% of the samples were detected as positive and 40.2% as negative, including the control samples (
Table 3 and 4).
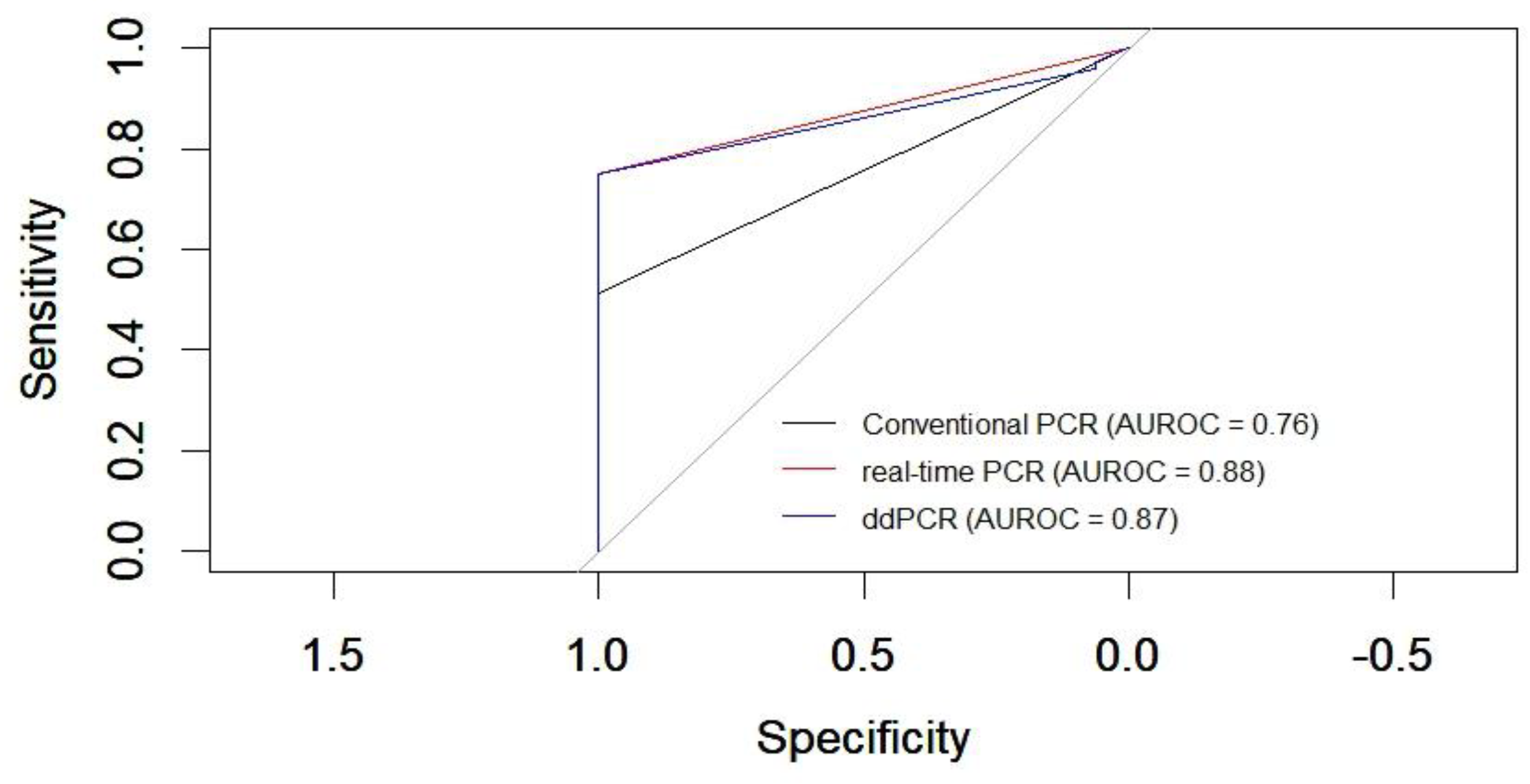
The ROC curve analysis, a reliable method, revealed the following areas under the curve (AUC) for the different PCR techniques compared to the clinical diagnosis of canine distemper: conventional PCR (RT-PCR) showed an AUC of 0.76, indicating moderate diagnostic accuracy. RT-qPCR exhibited a higher AUC of 0.88, reflecting a good level of diagnostic performance. Droplet digital PCR demonstrated an AUC of 0.87, indicating good accuracy and performance comparable to real-time PCR (RT-qPCR) in diagnosing canine distemper based on clinical criteria (
Figure 4).
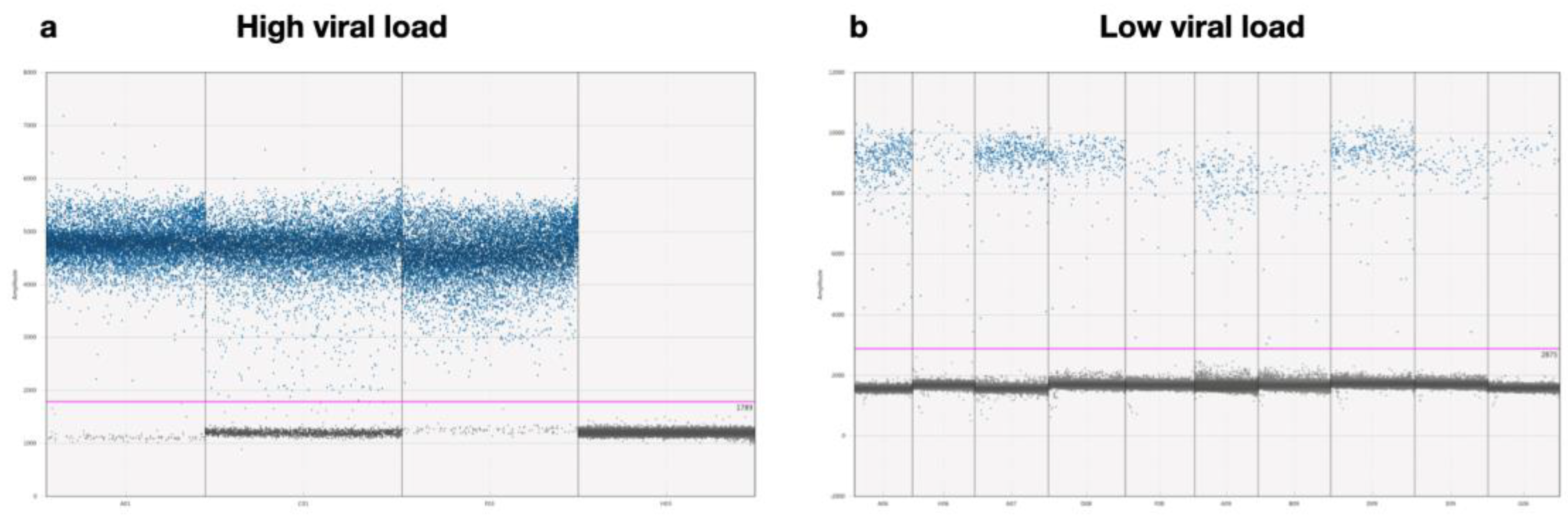
Digital PCR detected the highest number of CDV positives in brain samples, with all 22 samples (100%) testing positive, most with high viral loads (
Figure 5a). Additionally, 60% of blood samples were identified as positive by ddPCR, a higher percentage compared to 33.3% detected by conventional RT-PCR and 50% by RT-qPCR. We identified CDV at low viral loads using ddPCR in eleven blood samples, one nasal secretion, and three nervous tissue samples (
Figure 5b). In contrast, the other PCR methods did not detect the virus in these samples.
4. Discussion
CDV continues to threaten carnivores, particularly domestic dogs, emphasizing the critical necessity for precise and prompt diagnostic methodologies to ensure effective management. The definitive identification of CDV relies primarily on viral detection through PCR analysis of whole blood, serum, and cerebrospinal fluid [
28]. The variability in the viral load excreted by affected animals, contingent upon the progression and stage of the infection, often presents a diagnostic challenge. The discernment of the virus may be hindered by the limited presence of viral material or the interference of inhibitors within the sampled specimens. Against this backdrop, our study explored the diagnostic prowess of ddPCR, contrasting its efficacy with conventional RT-PCR and quantitative real-time RT-PCR. By employing clinical samples from symptomatic dogs, this investigation aimed to shed light on the potential advancements ddPCR offers in overcoming the inherent challenges associated with accurate and timely CDV diagnosis.
In terms of analytical performance, the determination of the LoD for CDV using a plasmid offers crucial insights into the sensitivity of the diagnostic methods, specifically RT-qPCR and ddPCR. Our study established a detection limit of 86 copies/μL for RT-qPCR, which aligns with previous findings in the literature. Elia et al. [
17] reported a detection limit of 10
2 genomic copies/µL, while Sehata et al. [
32] and Brown et al. [
33] calculated a LoD of 50 copies/µL using TaqMan probes targeting the phosphoprotein gene. Halecker et al. [
34] reported a 10
1 genomic copies/µL detection limit, and Geiselhardt et al. [
35] calculated a LoD of 5.47 copies ± 2.49 copies. Although the interlaboratory variability of quantitative viral PCR results in virus diagnosis has not undergone comprehensive analysis, we expected to observe 30 to 40% or more variances when evaluating RT-qPCR results for viral targets. This variability may be influenced by multiple factors, including the choice of quantitative calibrator, detection reagents, nucleic acid extraction methods, and the molecular amplification target gene, among other variables [
36]. In contrast, ddPCR offers a significant advantage by eliminating the need for standard curves, which can introduce variability and require additional preparation. This simplification of the workflow reduces potential sources of error, enhancing the reliability and efficiency of the analysis [
37].
In this study, we developed a novel method of detecting CDV employing ddPCR. This method enables absolute quantification of the virus across various sample types (urine, blood, nasal and eye swabs, cerebellum) with high sensitivity (72.37%) and specificity (100%). Compared to RT-qPCR, our result revealed a lower detection limit in ddPCR at 3 copies/μL and an area under the curve of 0.87. The linear regression equation demonstrated a robust correlation between the observed quantification and the theoretical dilutions, highlighting the method's consistency across a range of concentrations. The slope of 0.6417 suggests a slight underestimation compared to the expected values, which could be attributed to the inherent precision of ddPCR at lower concentrations. These results highlight ddPCR sensitivity and effectiveness in quantifying low-abundance targets. This high sensitivity, as revealed by the comparative data from previous studies, has significant implications for virology. For example, Souto et al. [
38] determined the limit of detection and quantification to be 10 copies per reaction for viral nervous necrosis virus (VNNV), an RNA virus belonging to the family Nodaviridae. Consistent with our results, Hui et al. [
39] established the limit of detection for the RNA Zika virus at 1 copies/μL.
The ddPCR highlighted a significant reduction in the coefficient of variation (CV) when compared to RT-qPCR, with values ranging from 8.91% to 77.86%. This marked improvement demonstrates ddPCR's superior reproducibility, particularly in detecting low viral loads. In the context of diagnosing CDV, this reduced variability is crucial, as it suggests that ddPCR can provide more consistent and reliable results, especially in cases where the viral load is near the detection threshold. The enhanced precision of ddPCR is particularly advantageous for quantifying low-abundance targets, where even minor variations can lead to significant discrepancies in viral load estimation. Multiple researchers have consistently documented this significant finding. For example, Ciesielski et al. [
40], compared the sensitivity, quantification limits, and reproducibility of two similar workflows qPCR and ddPCR by analyzing 60 raw wastewater samples from nine treatment plants over six months. Both methods detected increasing SARS-CoV-2 concentrations, with ddPCR showing greater analytical sensitivity and a lower limit of detection (0.066 copies/μL) compared to qPCR (12 copies/μL). In another study, explored the use of ddPCR for quantifying HIV DNA in patients on antiretroviral therapy [
21]. Their results demonstrate ddPCR's superior precision and accuracy compared to real-time PCR, showing a 5-fold reduction in variability for total HIV DNA. It is important to note that various research groups have been utilizing ddPCR for over a decade to quantify and detect viruses, consistently yielding promising results [
41]. The application of ddPCR for CDV detection aligns with its successful utilization in detecting various other viruses, as evidenced by studies on influenza virus [
42], hepatitis B virus [
43], SARS-CoV-2 [
44,
45], foot-and-mouth disease virus [
46], human parechoviruses (HPeVs) [
47], bovine leukemia virus (BLV) [
48] and cytomegalovirus [
49]. This broader applicability underscores the versatility and reliability of ddPCR across different viral pathogens.
In this study, the use of ddPCR has led to a 17% increase in the detection of positive cases for CDV compared to conventional diagnostic methods. This heightened sensitivity carries significant clinical, diagnostic, and epidemiological implications. Clinically, the improved diagnosis provided by ddPCR gives us confidence in our ability to identify infected animals, reducing the risk of misdiagnosis and ensuring timely treatment. In shelters, this could result in admitting 17% more animals with active infections, posing a severe risk to unvaccinated populations and potentially causing widespread outbreaks. From a diagnostic standpoint, ddPCR's higher sensitivity could be a game-changer in settings where cost efficiency is critical, such as in shelters, by allowing pooled sample testing without compromising accuracy. This approach could significantly reduce the cost per test while maintaining high detection rates. Moreover, the technique's application in environmental sampling, such as in parks and shelters, could provide valuable epidemiological insights, particularly due to its high sensitivity and ability to detect low viral loads. This could enable more effective monitoring of CDV presence in the environment, helping to prevent outbreaks before they occur.
5. Conclusions
Our study demonstrates that ddPCR offers a unique advantage in the early and precise detection of CDV, particularly in cases where the viral load may be low. This heightened sensitivity opens new possibilities for the effective management of CDV, including improved surveillance, earlier intervention, and more targeted control strategies. Furthermore, the high sensitivity of ddPCR could be particularly valuable for monitoring CDV in wildlife populations, where sample collection is often challenging, and viral loads may be low. These findings emphasize the practical implications of our research for veterinary medicine, animal welfare efforts, and wildlife conservation, potentially contributing to a more comprehensive approach to CDV management across both domestic and wild animal populations.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Figure S1: title.
Author Contributions
Conceptualization, V.I, R.P. and J.M.V.; methodology and formal analysis, V.I., GG., C.L. and K.Y.; writing—original draft preparation, V.I.; writing—review and editing, R.P., and J.M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Agency of Research and Innovation (ANII, Fondo María Viñas 2019), Grant FMV-1-2019-1-155934), C.L. was MSc fellowship recipient of National Agency of Research and Innovation (ANII). V.I. is PhD fellowship recipient of Udelar’s Posgraduate Academic Commission (Comisión Académica de Posgrados,CAP-Udelar). J.M.V. and R.P. are Research Careers Member of the National Research System (SNI-ANII), Uruguay, and Program of Development of Basic Sciences (PEDECIBA), Uruguay.
Institutional Review Board Statement
The study was conducted in accordance with Animal Use Ethics Commission (CEUA) from the Universidad de la Repú0blica Oriental del Uruguay (approval number CEUAFVET-1003/20, Exp. 111900-000900-20).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maes, P.; Amarasinghe, G.K.; Ayllón, M.A.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Taxonomy of the Order Mononegavirales: Second Update 2018. Arch Virol 2019, 164, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Roelke-Parker, M.E.; Munson, L.; Packer, C.; Kock, R.; Cleaveland, S.; Carpenter, M.; O’Brien, S.J.; Pospischil, A.; Hofmann-Lehmann, R.; Lutz, H.; et al. A Canine Distemper Virus Epidemic in Serengeti Lions (Panthera Leo). Nature 1996, 379, 441–445. [Google Scholar] [CrossRef] [PubMed]
- <sc>AMUNDSON, T.E.; YUILL, T.M. PREVALENCE OF SELECTED PATHOGENIC MICROBIAL AGENTS IN THE RED FOX (Vulpes Fulva) AND GRAY FOX (Urocyon Cinereoargenteus) OF SOUTHWESTERN WISCONSIN. J Wildl Dis 1981, 17, 17–22. [Google Scholar] [CrossRef]
- Uhl, E.W.; Kelderhouse, C.; Buikstra, J.; Blick, J.P.; Bolon, B.; Hogan, R.J. New World Origin of Canine Distemper: Interdisciplinary Insights. Int J Paleopathol 2019, 24, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Harder, T.; Osterhaus, A. Canine Distemper Virus-a Morbillivirus in Search of New Hosts? Trends Microbiol 1997, 5, 120–124. [Google Scholar] [CrossRef]
- Greene, C.E. Infectious Canine Hepatitis and Canine Acidophil Cell Hepatitis. Infectious diseases of the dog and cat 2006, 3, 41–53. [Google Scholar]
- Beineke, A.; Puff, C.; Seehusen, F.; Baumgärtner, W. Pathogenesis and Immunopathology of Systemic and Nervous Canine Distemper. Vet Immunol Immunopathol 2009, 127, 1–18. [Google Scholar] [CrossRef]
- Avila, M.; Khosravi, M.; Alves, L.; Ader-Ebert, N.; Bringolf, F.; Zurbriggen, A.; Plemper, R.K.; Plattet, P. Canine Distemper Virus Envelope Protein Interactions Modulated by Hydrophobic Residues in the Fusion Protein Globular Head. J Virol 2015, 89, 1445–1451. [Google Scholar] [CrossRef]
- Tipold, A.; Vandevelde, M.; Jaggy, A. Neurological Manifestations of Canine Distemper Virus Infection. Journal of Small Animal Practice 1992, 33, 466–470. [Google Scholar] [CrossRef]
- Martella, V.; Elia, G.; Buonavoglia, C. Canine Distemper Virus. Veterinary Clinics of North America: Small Animal Practice 2008, 38, 787–797. [Google Scholar] [CrossRef]
- Lempp, C.; Spitzbarth, I.; Puff, C.; Cana, A.; Kegler, K.; Techangamsuwan, S.; Baumgärtner, W.; Seehusen, F. New Aspects of the Pathogenesis of Canine Distemper Leukoencephalitis. Viruses 2014, 6, 2571–2601. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.D.B.; Ikuta, N.; Canal, C.W.; Makiejczuk, A.; da Costa Allgayer, M.; Cardoso, C.H.; Lehmann, F.K.; Fonseca, A.S.K.; Lunge, V.R. Detection and Differentiation of Field and Vaccine Strains of Canine Distemper Virus Using Reverse Transcription Followed by Nested Real Time PCR (RT-NqPCR) and RFLP Analysis. J Virol Methods 2013, 194, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.B.; Alfieri, A.A.; Wosiacki, S.R.; Negrao, F.J.; Morais, H.S.A.; Alfieri, A.F. Detection of Canine Distemper Virus by Reverse Transcriptase-Polymerase Chain Reaction in the Urine of Dogs with Clinical Signs of Distemper Encephalitis. Res Vet Sci 2006, 80, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, L.V.; Kantere, M.C.; Kyriakis, C.S.; Pardali, D.; Adamama Moraitou, K.; Polizopoulou, Z.S. Evaluation of a Direct Immunofluorescent Assay and/or Conjunctival Cytology for Detection of Canine Distemper Virus Antigen. Viral Immunol 2018, 31, 272–275. [Google Scholar] [CrossRef]
- Temilade, B.E.; Solomon, O.O.O.; Omotayo, O.E.; Omezuruike, O.I. Seropositivity of Canine Distemper Virus (CDV) in Dogs Presenting at Abeokuta, Nigeria. Public Health Res 2015, 5, 109–119. [Google Scholar]
- Di Francesco, C.E.; Di Francesco, D.; Di Martino, B.; Speranza, R.; Santori, D.; Boari, A.; Marsilio, F. Detection by Hemi-Nested Reverse Transcription Polymerase Chain Reaction and Genetic Characterization of Wild Type Strains of Canine Distemper Virus in Suspected Infected Dogs. Journal of Veterinary Diagnostic Investigation 2012, 24, 107–115. [Google Scholar] [CrossRef]
- Elia, G.; Decaro, N.; Martella, V.; Cirone, F.; Lucente, M.S.; Lorusso, E.; Di Trani, L.; Buonavoglia, C. Detection of Canine Distemper Virus in Dogs by Real-Time RT-PCR. J Virol Methods 2006, 136, 171–176. [Google Scholar] [CrossRef]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C. High-Throughput Droplet Digital PCR System for Absolute Quantitation of DNA Copy Number. Anal Chem 2011, 83, 8604–8610. [Google Scholar] [CrossRef]
- Hindson, C.M.; Chevillet, J.R.; Briggs, H.A.; Gallichotte, E.N.; Ruf, I.K.; Hindson, B.J.; Vessella, R.L.; Tewari, M. Absolute Quantification by Droplet Digital PCR versus Analog Real-Time PCR. Nat Methods 2013, 10, 1003–1005. [Google Scholar] [CrossRef]
- Quan, P.-L.; Sauzade, M.; Brouzes, E. DPCR: A Technology Review. Sensors 2018, 18, 1271. [Google Scholar] [CrossRef]
- Strain, M.C.; Lada, S.M.; Luong, T.; Rought, S.E.; Gianella, S.; Terry, V.H.; Spina, C.A.; Woelk, C.H.; Richman, D.D. Highly Precise Measurement of HIV DNA by Droplet Digital PCR. PLoS One 2013, 8, e55943. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, P.; Liew, J.W.K.; Amir, A.; Ching, X.-T.; Lau, Y.-L. Droplet Digital Polymerase Chain Reaction (DdPCR) for the Detection of Plasmodium Knowlesi and Plasmodium Vivax. Malar J 2020, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Lee, C.-J.; Lee, Y.-M.; Choi, Y.-B.; Mun, S.; Han, K. Rapid Identification of SARS-CoV-2 in the Point-of-Care Using Digital PCR-Based Dr. PCRTM Di20K COVID-19 Detection Kit without Viral RNA Extraction. Genes Genomics 2022, 44, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Summers, B.A.; Greisen, H.A.; Appel, M.J.G. Canine Distemper Encephalomyelitis: Variation with Virus Strain. J Comp Pathol 1984, 94, 65–75. [Google Scholar] [CrossRef]
- Verdes, J.M.; Larrañaga, C.; Varela, B.; Iribarnegaray, V.; Yozzi, V.; Feijóo, G.; Yamasaki, K. Histopathological Analysis of Brains from Dogs Infected with Canine Distemper Virus. In Measles and Related Morbilliviruses: Methods and Protocols; Springer, 2024; pp 177–195.
- Feijóo, G.; Yamasaki, K.; Delucchi, L.; Verdes, J.M. Central Nervous System Lesions Caused by Canine Distemper Virus in 4 Vaccinated Dogs. Journal of Veterinary Diagnostic Investigation 2021, 33, 640–647. [Google Scholar] [CrossRef]
- Seki, F.; Ono, N.; Yamaguchi, R.; Yanagi, Y. Efficient Isolation of Wild Strains of Canine Distemper Virus in Vero Cells Expressing Canine SLAM (CD150) and Their Adaptability to Marmoset B95a Cells. J Virol 2003, 77, 9943–9950. [Google Scholar] [CrossRef]
- Frisk, A.L.; Konig, M.; Moritz, A.; Baumgartner, W. Detection of Canine Distemper Virus Nucleoprotein RNA by Reverse Transcription-PCR Using Serum, Whole Blood, and Cerebrospinal Fluid from Dogs with Distemper. J Clin Microbiol 1999, 37, 3634–3643. [Google Scholar] [CrossRef]
- Panzera, Y. Unpublish.
- Akobeng, A.K. Understanding Diagnostic Tests 1: Sensitivity, Specificity and Predictive Values. Acta Paediatr 2007, 96, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.; Mathai, A.; Parikh, S.; Sekhar, G.C.; Thomas, R. Understanding and Using Sensitivity, Specificity and Predictive Values. Indian J Ophthalmol 2008, 56, 45–50. [Google Scholar] [CrossRef]
- Sehata, G.; Sato, H.; Ito, T.; Imaizumi, Y.; Noro, T.; Oishi, E. Use of Quantitative Real-Time RT-PCR to Investigate the Correlation between Viremia and Viral Shedding of Canine Distemper Virus, and Infection Outcomes in.
- Tomaszewicz Brown, A.; McAloose, D.; Calle, P.P.; Auer, A.; Posautz, A.; Slavinski, S.; Brennan, R.; Walzer, C.; Seimon, T.A. Development and Validation of a Portable, Point-of-Care Canine Distemper Virus QPCR Test. PLoS One 2020, 15, e0232044. [Google Scholar] [CrossRef]
- Halecker, S.; Bock, S.; Beer, M.; Hoffmann, B. A New Molecular Detection System for Canine Distemper Virus Based on a Double-Check Strategy. Viruses 2021, 13, 1632. [Google Scholar] [CrossRef] [PubMed]
- Geiselhardt, F.; Peters, M.; Jo, W.K.; Schadenhofer, A.; Puff, C.; Baumgärtner, W.; Kydyrmanov, A.; Kuiken, T.; Piewbang, C.; Techangamsuwan, S. Development and Validation of a Pan-Genotypic Real-Time Quantitative Reverse Transcription-PCR Assay to Detect Canine Distemper Virus and Phocine Distemper Virus in Domestic Animals and Wildlife. J Clin Microbiol 2022, 60, e02505–21. [Google Scholar] [CrossRef] [PubMed]
- Hayden, R.T.; Yan, X.; Wick, M.T.; Rodriguez, A.B.; Xiong, X.; Ginocchio, C.C.; Mitchell, M.J.; Caliendo, A.M. Factors Contributing to Variability of Quantitative Viral PCR Results in Proficiency Testing Samples: A Multivariate Analysis. J Clin Microbiol 2012, 50, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. Digital PCR Hits Its Stride. Nat Methods 2012, 9, 541–544. [Google Scholar] [CrossRef]
- Souto, S.; Olveira, J.G.; López-Vázquez, C.; Bandín, I.; Dopazo, C.P. Designing and Validation of a Droplet Digital PCR Procedure for Diagnosis and Accurate Quantification of Nervous Necrosis Virus in the Mediterranean Area. Pathogens 2023, 12, 1155. [Google Scholar] [CrossRef]
- Hui, Y.; Wu, Z.; Qin, Z.; Zhu, L.; Liang, J.; Li, X.; Fu, H.; Feng, S.; Yu, J.; He, X. Micro-Droplet Digital Polymerase Chain Reaction and Real-Time Quantitative Polymerase Chain Reaction Technologies Provide Highly Sensitive and Accurate Detection of Zika Virus. Virol Sin 2018, 33, 270–277. [Google Scholar] [CrossRef]
- Ciesielski, M.; Blackwood, D.; Clerkin, T.; Gonzalez, R.; Thompson, H.; Larson, A.; Noble, R. Assessing Sensitivity and Reproducibility of RT-DdPCR and RT-QPCR for the Quantification of SARS-CoV-2 in Wastewater. J Virol Methods 2021, 297, 114230. [Google Scholar] [CrossRef]
- Lei, S.; Chen, S.; Zhong, Q. Digital PCR for Accurate Quantification of Pathogens: Principles, Applications, Challenges and Future Prospects. Int J Biol Macromol 2021, 184, 750–759. [Google Scholar] [CrossRef]
- Schwartz, S.L.; Lowen, A.C. Droplet Digital PCR: A Novel Method for Detection of Influenza Virus Defective Interfering Particles. J Virol Methods 2016, 237, 159–165. [Google Scholar] [CrossRef]
- Huang, J.-T.; Liu, Y.-J.; Wang, J.; Xu, Z.-G.; Yang, Y.; Shen, F.; Liu, X.; Zhou, X.; Liu, S.-M. Next Generation Digital PCR Measurement of Hepatitis B Virus Copy Number in Formalin-Fixed Paraffin-Embedded Hepatocellular Carcinoma Tissue. Clin Chem 2015, 61, 290–296. [Google Scholar] [CrossRef]
- Liu, X.; Feng, J.; Zhang, Q.; Guo, D.; Zhang, L.; Suo, T.; Hu, W.; Guo, M.; Wang, X.; Huang, Z. Analytical Comparisons of SARS-COV-2 Detection by QRT-PCR and DdPCR with Multiple Primer/Probe Sets. Emerg Microbes Infect 2020, 9, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, N.N.; Ritchie, G.; Dong, W.; Cobarrubias, K.D.; Sudderuddin, H.; Lawson, T.; Matic, N.; Montaner, J.S.G.; Leung, V.; Romney, M.G.; et al. SARS-CoV-2 RNA Quantification Using Droplet Digital RT-PCR. The Journal of Molecular Diagnostics 2021, 23, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro-de-Oliveira, T.F.; Fonseca, A.A.; Camargos, M.F.; Laguardia-Nascimento, M.; de Oliveira, A.M.; Cottorello, A.C.P.; Goes-Neto, A.; Barbosa-Stancioli, E.F. Development of a Droplet Digital RT-PCR for the Quantification of Foot-and-Mouth Virus RNA. J Virol Methods 2018, 259, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, Y.; Koyama, A.; Ishihara, T.; Onodera, O.; Saitoh, A. Performance of a Real-Time PCR–Based Approach and Droplet Digital PCR in Detecting Human Parechovirus Type 3 RNA. Journal of Clinical Virology 2016, 84, 27–31. [Google Scholar] [CrossRef] [PubMed]
- De Brun, M.L.; Cosme, B.; Petersen, M.; Alvarez, I.; Folgueras-Flatschart, A.; Flatschart, R.; Panei, C.J.; Puentes, R. Development of a Droplet Digital PCR Assay for Quantification of the Proviral Load of Bovine Leukemia Virus. Journal of Veterinary Diagnostic Investigation 2022, 34, 439–447. [Google Scholar] [CrossRef]
- Trypsteen, W.; Kiselinova, M.; Vandekerckhove, L.; De Spiegelaere, W. Diagnostic Utility of Droplet Digital PCR for HIV Reservoir Quantification. J Virus Erad 2016, 2, 162–169. [Google Scholar] [CrossRef]
Figure 1.
Frequency of clinical signs observed in 76 dogs diagnosed with canine distemper virus (CDV). Nervous symptoms were the most prevalent, followed closely by digestive symptoms. Nervous symptoms included seizures, muscle twitches, partial or total paralysis, and abnormal behaviors. Digestive symptoms encompassed vomiting, diarrhea leading to dehydration, loss of appetite resulting in weight loss, lethargy, and depression. Dermatologic symptoms, primarily characterized by hyperkeratosis of the paw pads and nose, were also observed. This distribution of symptoms highlights the multi-systemic nature of CDV infection, with a particular emphasis on neurological and gastrointestinal manifestations.
Figure 1.
Frequency of clinical signs observed in 76 dogs diagnosed with canine distemper virus (CDV). Nervous symptoms were the most prevalent, followed closely by digestive symptoms. Nervous symptoms included seizures, muscle twitches, partial or total paralysis, and abnormal behaviors. Digestive symptoms encompassed vomiting, diarrhea leading to dehydration, loss of appetite resulting in weight loss, lethargy, and depression. Dermatologic symptoms, primarily characterized by hyperkeratosis of the paw pads and nose, were also observed. This distribution of symptoms highlights the multi-systemic nature of CDV infection, with a particular emphasis on neurological and gastrointestinal manifestations.
Figure 2.
Limit of detection of ddPCR. (A) Log10 copies/μL serial dilutions of the plasmid containing the CDV nucleoprotein (NP) gene. (B) Scatter diagram from droplet digital PCR of plasmid dilutions ranging from 2.18x1010 to 2.18x10-4 (A01–G02) and no-template control (NTC) (B02). Gray dots represent negative events; blue dots represent positive events.
Figure 2.
Limit of detection of ddPCR. (A) Log10 copies/μL serial dilutions of the plasmid containing the CDV nucleoprotein (NP) gene. (B) Scatter diagram from droplet digital PCR of plasmid dilutions ranging from 2.18x1010 to 2.18x10-4 (A01–G02) and no-template control (NTC) (B02). Gray dots represent negative events; blue dots represent positive events.
Figure 3.
Linear regression analysis of plasmid quantification using RT-qPCR (point) and ddPCR (square) compared to theoretical dilutions. The equations for the regression lines were Y = 1.106X + 0.1737 (R² = 0.947) for RT-qPCR and Y = 0.6417X - 0.5154 (R² = 0.953) for ddPCR. The x-axis represents the log10 of theoretical copy numbers, while the y-axis represents the log10 of measured copy numbers.
Figure 3.
Linear regression analysis of plasmid quantification using RT-qPCR (point) and ddPCR (square) compared to theoretical dilutions. The equations for the regression lines were Y = 1.106X + 0.1737 (R² = 0.947) for RT-qPCR and Y = 0.6417X - 0.5154 (R² = 0.953) for ddPCR. The x-axis represents the log10 of theoretical copy numbers, while the y-axis represents the log10 of measured copy numbers.
Figure 4.
Comparison of receiver operating characteristic (ROC) curves of conventional PCR, real-time PCR and ddPCR for CDV. The black curve represents conventional PCR, the red curve corresponds to RT-qPCR, and the blue curve represents digital PCR.
Figure 4.
Comparison of receiver operating characteristic (ROC) curves of conventional PCR, real-time PCR and ddPCR for CDV. The black curve represents conventional PCR, the red curve corresponds to RT-qPCR, and the blue curve represents digital PCR.
Figure 5.
Scatter diagram from droplet digital PCR: (a) ddPCR results for samples with high viral loads. The droplet distribution shows an increase in positive droplets (blue dots) compared to negative ones (gray dots). The high concentration of blue dots reflects a high viral load, with a larger proportion of droplets exceeding the fluorescence threshold. In H03 represents the no template control (NTC). (b) ddPCR results for samples with low viral loads. The droplet distribution is shown with a high number of negative droplets (gray dots) and a small number of positive droplets (blue), indicating the presence of the virus at low concentrations. .
Figure 5.
Scatter diagram from droplet digital PCR: (a) ddPCR results for samples with high viral loads. The droplet distribution shows an increase in positive droplets (blue dots) compared to negative ones (gray dots). The high concentration of blue dots reflects a high viral load, with a larger proportion of droplets exceeding the fluorescence threshold. In H03 represents the no template control (NTC). (b) ddPCR results for samples with low viral loads. The droplet distribution is shown with a high number of negative droplets (gray dots) and a small number of positive droplets (blue), indicating the presence of the virus at low concentrations. .
Table 1.
Primers and probe used in this study.
Table 1.
Primers and probe used in this study.
| Application |
Primer |
Sequence (5´ to 3´) |
| RT-PCR [28] |
CDV-NP_ reverse |
CAAGATAACCATGTACGGTGC |
| CDV-NP_ forward |
ACAGGATTGCTGAGGACCTAT |
| RT-qPCR and ddPCR [29] |
CDV- reverse |
ATGAGTTTTCCGGAGAATTAACAA |
| CDV-forward |
AGCTAGTTTCATCCTAACTATCAAGT |
| CDV probe |
FAM-TGGCATTGAAACTATGTATCCGGCTCT-BHQ1-3 |
Table 2.
Precision of RT-qPCR and ddPCR of three independent dilutions of the plasmid construction. SD (standard deviation); CV (%) (coefficient of variation).
Table 2.
Precision of RT-qPCR and ddPCR of three independent dilutions of the plasmid construction. SD (standard deviation); CV (%) (coefficient of variation).
| |
RT-qPCR assay |
ddPCR assay |
| Plasmid dilution 1
|
10-4
|
10-6
|
10-8
|
10-4
|
10-6
|
10-8
|
| Mean of plasmid copy number 2
|
6,9E+06 |
1,1E+07 |
1,6E+02 |
2,8E+03 |
5,7E+02 |
2,0E+01 |
| SD |
2990 |
864,24 |
28,90 |
84,82 |
50,78 |
59,87 |
| CV (%) |
150,32 |
148,76 |
98,96 |
8,91 |
6,94 |
77,87 |
Table 3.
Comparison of the PCR conventional, RT-qPCR and ddPCR results across 92 different sample types.
Table 3.
Comparison of the PCR conventional, RT-qPCR and ddPCR results across 92 different sample types.
| |
Blood* |
Urine* |
Nasal and eye swabs* |
Brain* |
Total Positive |
Total Negative |
| RT-PCR |
14 |
3 |
3 |
19 |
39 |
53 |
| RT-qPCR |
21 |
4 |
3 |
16 |
44 |
48 |
| ddPCR |
21 |
4 |
3 |
16 |
44 |
48 |
Table 4.
Comparison between clinical diagnostic with RT-PCR, RT-qPCR and ddPCR results of 92 samples. (TPV: true positive value; TNV: true negative value; FNV: false negative value; FP: false positive, FN: false negative).
Table 4.
Comparison between clinical diagnostic with RT-PCR, RT-qPCR and ddPCR results of 92 samples. (TPV: true positive value; TNV: true negative value; FNV: false negative value; FP: false positive, FN: false negative).
| |
True result
(TPV+TNV)
|
False
result
(FPV+FNV)
|
FP (%) |
FN (%) |
Sensitivity (%) |
Specificity (%) |
Kappa (IC 99.5%) |
| RT-PCR |
55 |
37 |
0 |
48.68 |
51.31 |
100 |
0.268 |
| RT-qPCR |
60 |
32 |
0 |
42.10 |
57.89 |
100 |
0.324 |
| ddPCR |
71 |
21 |
0 |
27.63 |
72.37 |
100 |
0.477 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).