Submitted:
17 September 2024
Posted:
18 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. FGFR2 Expression in Skin and cSCC
3. Genetic Diversity and Genomic Alterations of FGFR2 Connected with cSCC
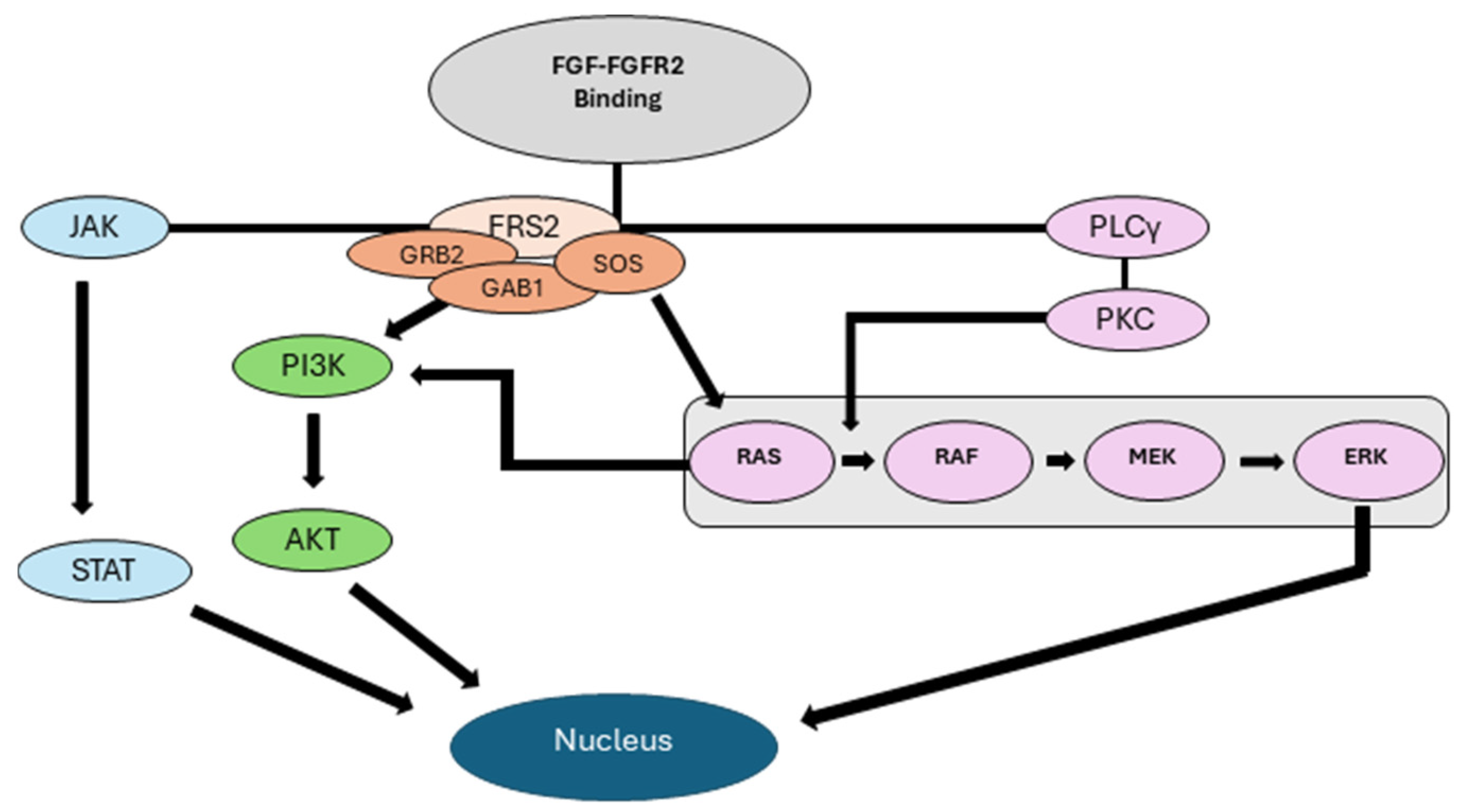
4. FGF-FGFR2 Interactions in the Development and Progression of cSCC
4.1. FGF-FGFR2 Interactions in the Development and Progression of cSCC
4.2. Role of FGFR2 in Epithelial Mesenchymal Transition (EMT) and Enrichment of Cancer Stem Cells (CSCs)
4.3. FGFR2’s role in Promoting Angiogenesis and Metastatic Spread
4.4. FGFR2 in the Development of Therapeutic Resistance Against Current Treatments
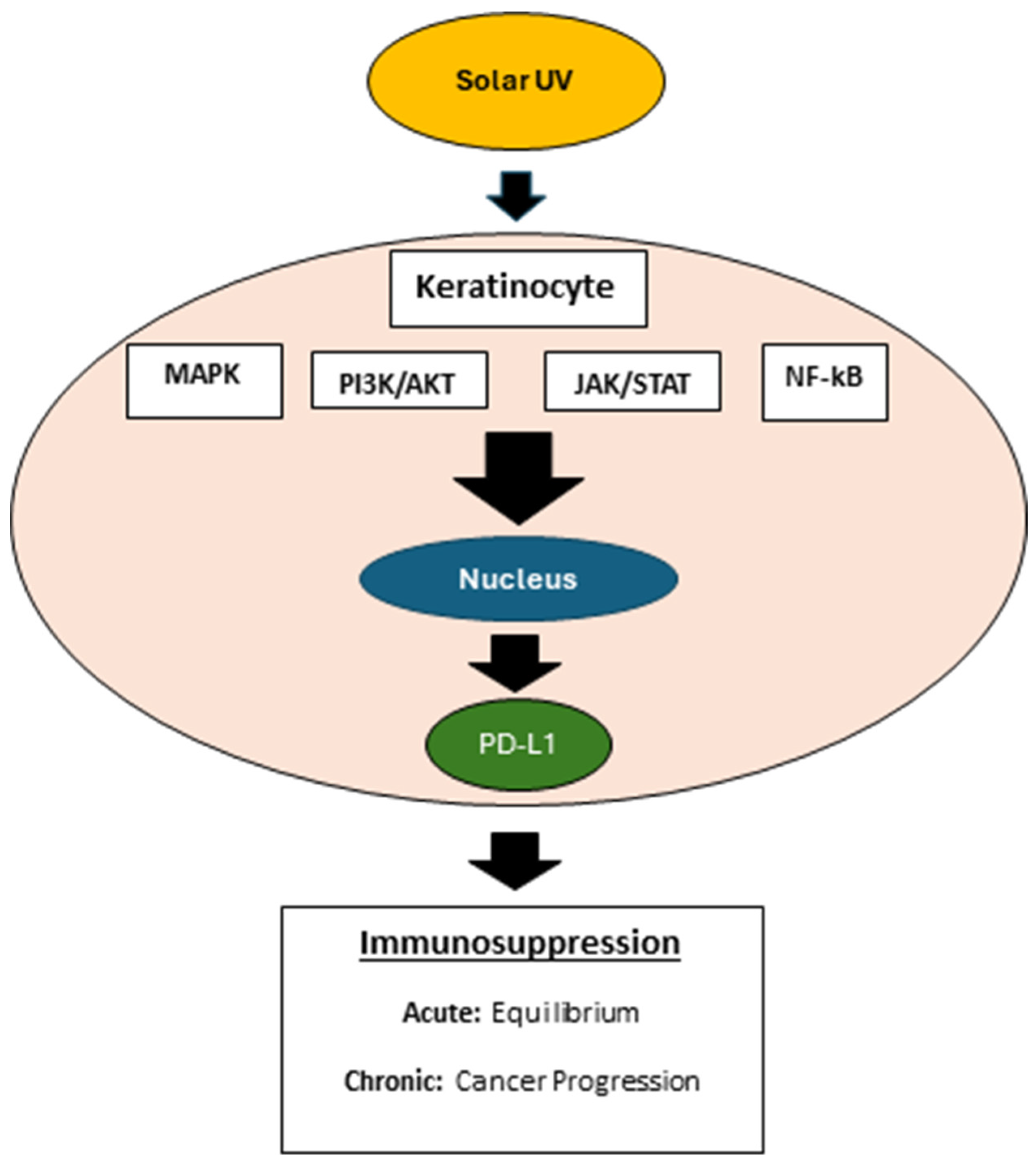
5. Deregulated FGFR2 Activity in Immune Cell Function and Adverse Immune Outcomes in cSCC
6. Current Progress in the Development of FGFR2-Oriented Therapies
7. The Scope of Immune Targeting of cSCC Together with FGFR2 Inhibition Therapy
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Waldman, A.S., C, Cutaneous Squamous Cell Carcinoma. Hematology/Oncology Clinics of North America, 2019. 33(1): p. 1-12.
- Schmults, C.D., et al., NCCN Guidelines® Insights: Squamous Cell Skin Cancer, Version 1.2022. Journal of the National Comprehensive Cancer Network, 2021. 19(12): p. 1382-1394.
- Schmults, A.W.a.C., Cutaneous Squamous Cell Carcinoma. Hematology/Oncology Clinics of North America, 2019. 33(1): p. 1-12.
- Corchado-Cobos, R., et al., Cutaneous Squamous Cell Carcinoma: From Biology to Therapy. International Journal of Molecular Sciences, 2020. 21(8): p. 2956. [CrossRef]
- Schmults, C.D., et al., NCCN Guidelines® Insights: Squamous Cell Skin Cancer, Version 1.2022. Journal of the National Comprehensive Cancer Network, 2021. 19(12): p. 1382-1394.
- Porta, R., et al., FGFR a promising druggable target in cancer: Molecular biology and new drugs. Critical Reviews in Oncology/Hematology, 2017. 113: p. 256-267. [CrossRef]
- Grose, R. and C. Dickson, Fibroblast growth factor signaling in tumorigenesis. Cytokine Growth Factor Rev, 2005. 16(2): p. 179-86. [CrossRef]
- De Luca, A., et al., FGFR Fusions in Cancer: From Diagnostic Approaches to Therapeutic Intervention. International Journal of Molecular Sciences, 2020. 21(18): p. 6856.
- Ornitz, D.M. and N. Itoh, The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol, 2015. 4(3): p. 215-66. [CrossRef]
- Thakur, M.A., et al., Inhibition of Fibroblast Growth Factor Receptor Attenuates UVB-Induced Skin Carcinogenesis. J Invest Dermatol, 2022. 142(11): p. 2873-2884 e7. [CrossRef]
- Yang, J., et al., Fibroblast growth factor receptors 1 and 2 in keratinocytes control the epidermal barrier and cutaneous homeostasis. J Cell Biol, 2010. 188(6): p. 935-52.
- Grose, R., et al., The role of fibroblast growth factor receptor 2b in skin homeostasis and cancer development. The EMBO Journal, 2007. 26(5): p. 1268-1278. [CrossRef]
- Thakur, M., et al., Inhibition of Fibroblast Growth Factor Receptor Attenuates UVB-Induced Skin Carcinogenesis. Journal of Investigative Dermatology, 2022. 142: p. 2873-2884. [CrossRef]
- Khandelwal, A.R., et al., Fibroblast growth factor receptor promotes progression of cutaneous squamous cell carcinoma. Molecular Carcinogenesis, 2019. 58(10): p. 1715-1725. [CrossRef]
- Thakur, M., et al., Inducible Keratinocyte Specific FGFR2 Deficiency Inhibits UVB-Induced Signaling, Proliferation, Inflammation, and Skin Carcinogenesis. J Invest Dermatol, 2024. 144(2): p. 341-350 e7. [CrossRef]
- Zilberg, C., et al., Analysis of clinically relevant somatic mutations in high-risk head and neck cutaneous squamous cell carcinoma. Modern Pathology, 2018. 31(2): p. 275-287. [CrossRef]
- Helsten, T., et al., The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clinical Cancer Research, 2016. 22(1): p. 259-267. [CrossRef]
- Babina, I.S. and N.C. Turner, Advances and challenges in targeting FGFR signalling in cancer. Nature Reviews Cancer, 2017. 17(5): p. 318-332. [CrossRef]
- Czyz, M., Fibroblast Growth Factor Receptor Signaling in Skin Cancers. Cells, 2019. 8(6): p. 540. [CrossRef]
- Thakur, M., et al., Inducible Keratinocyte Specific FGFR2 Deficiency Inhibits UVB-Signaling, Proliferation, Inflammation, and Skin Carcinogensis. Journal of Investigative Dermatology, 2024. 144: p. 341-350.
- Carr, T.D., et al., Inhibition of mTOR Suppresses UVB-Induced Keratinocyte Proliferation and Survival. Cancer Prevention Research, 2012. 5(12): p. 1394-1404. [CrossRef]
- Thiery, J.P., Epithelial–mesenchymal transitions in tumour progression. Nature Reviews Cancer, 2002. 2(6): p. 442-454. [CrossRef]
- Kalluri, R. and R.A. Weinberg, The basics of epithelial-mesenchymal transition. Journal of Clinical Investigation, 2009. 119(6): p. 1420-1428. [CrossRef]
- Wells, A., Epithelial and mesenchymal phenotypic switchings modulate cell motility in metastasis. Frontiers in Bioscience, 2011. 16(1): p. 815. [CrossRef]
- Warzecha, C.C., et al., ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol Cell, 2009. 33(5): p. 591-601. [CrossRef]
- Baraniak, A.P., J.R. Chen, and M.A. Garcia-Blanco, Fox-2 mediates epithelial cell-specific fibroblast growth factor receptor 2 exon choice. Mol Cell Biol, 2006. 26(4): p. 1209-22. [CrossRef]
- Baraniak, A.P., et al., A stem structure in fibroblast growth factor receptor 2 transcripts mediates cell-type-specific splicing by approximating intronic control elements. Mol Cell Biol, 2003. 23(24): p. 9327-37. [CrossRef]
- Belleudi, F., V. Purpura, and M.R. Torrisi, The Receptor Tyrosine Kinase FGFR2b/KGFR Controls Early Differentiation of Human Keratinocytes. PLoS ONE, 2011. 6(9): p. e24194. [CrossRef]
- Zhang, Y., et al., Growth inhibition by keratinocyte growth factor receptor of human salivary adenocarcinoma cells through induction of differentiation and apoptosis. Proceedings of the National Academy of Sciences, 2001. 98(20): p. 11336-11340. [CrossRef]
- Haugsten, E.M., et al., Roles of Fibroblast Growth Factor Receptors in Carcinogenesis. Molecular Cancer Research, 2010. 8(11): p. 1439-1452. [CrossRef]
- Ranieri, D., et al., Expression of the FGFR2 mesenchymal splicing variant in epithelial cells drives epithelial-mesenchymal transition. Oncotarget, 2016. 7(5): p. 5440-5460. [CrossRef]
- Shih, W. and S. Yamada, N-cadherin-mediated cell–cell adhesion promotes cell migration in a three-dimensional matrix. Journal of Cell Science, 2012. 125(15): p. 3661-3670. [CrossRef]
- Ranieri, D., et al., Expression of the FGFR2c mesenchymal splicing variant in human keratinocytes inhibits differentiation and promotes invasion. Molecular Carcinogenesis, 2018. 57(2): p. 272-283. [CrossRef]
- Ranieri, D., et al., Role of PKCε in the epithelial-mesenchymal transition induced by FGFR2 isoform switch. Cell Communication and Signaling, 2020. 18(1). [CrossRef]
- Isakov, N., Protein kinase C (PKC) isoforms in cancer, tumor promotion and tumor suppression. Semin Cancer Biol, 2018. 48: p. 36-52. [CrossRef]
- Gorin, M.A. and Q. Pan, Protein kinase C epsilon: an oncogene and emerging tumor biomarker. Mol Cancer, 2009. 8: p. 9.
- Gupta, P.B., C.L. Chaffer, and R.A. Weinberg, Cancer stem cells: mirage or reality? Nature Medicine, 2009. 15(9): p. 1010-1012.
- Maehara, O., et al., FGFR2 maintains cancer cell differentiation via AKT signaling in esophageal squamous cell carcinoma. Cancer Biology & Therapy, 2021. 22(5-6): p. 372-380. [CrossRef]
- Majidpoor, J. and K. Mortezaee, Angiogenesis as a hallmark of solid tumors - clinical perspectives. Cell Oncol (Dordr), 2021. 44(4): p. 715-737. [CrossRef]
- Brantsch, K.D., et al., Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol, 2008. 9(8): p. 713-20. [CrossRef]
- Brougham, N.D., et al., The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol, 2012. 106(7): p. 811-5. [CrossRef]
- Nelson, T.G. and R.E. Ashton, Low incidence of metastasis and recurrence from cutaneous squamous cell carcinoma found in a UK population: Do we need to adjust our thinking on this rare but potentially fatal event? J Surg Oncol, 2017. 116(6): p. 783-788.
- Cherpelis, B.S., C. Marcusen, and P.G. Lang, Prognostic factors for metastasis in squamous cell carcinoma of the skin. Dermatol Surg, 2002. 28(3): p. 268-73.
- Ramsey, M.R., et al., FGFR2 signaling underlies p63 oncogenic function in squamous cell carcinoma. J Clin Invest, 2013. 123(8): p. 3525-38. [CrossRef]
- Takano, S., Glioblastoma angiogenesis: VEGF resistance solutions and new strategies based on molecular mechanisms of tumor vessel formation. Brain Tumor Pathology, 2012. 29(2): p. 73-86. [CrossRef]
- Cross, M.J. and L. Claesson-Welsh, FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci, 2001. 22(4): p. 201-7. [CrossRef]
- Gillis, P., et al., Keratinocyte growth factor induces angiogenesis and protects endothelial barrier function. Journal of Cell Science, 1999. 112(12): p. 2049-2057. [CrossRef]
- Dudka, A.A., S.M. Sweet, and J.K. Heath, Signal transducers and activators of transcription-3 binding to the fibroblast growth factor receptor is activated by receptor amplification. Cancer Res, 2010. 70(8): p. 3391-401.
- Quintero-Fabián, S., et al., Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Frontiers in Oncology, 2019. 9.
- Finch, P.W. and J.S. Rubin, Keratinocyte Growth Factor⧸Fibroblast Growth Factor 7, a Homeostatic Factor with Therapeutic Potential for Epithelial Protection and Repair. 2004, Elsevier. p. 69-136.
- Feng, S., et al., Cancer-associated fibroblast-secreted FGF7 as an ovarian cancer progression promoter. Journal of Translational Medicine, 2024. 22(1). [CrossRef]
- Huang, T., et al., FGF7/FGFR2 signal promotes invasion and migration in human gastric cancer through upregulation of thrombospondin-1. International Journal of Oncology, 2017. 50(5): p. 1501-1512.
- Wang, T.N., et al., Thrombospondin-1 (TSP-1) promotes the invasive properties of human breast cancer. J Surg Res, 1996. 63(1): p. 39-43. [CrossRef]
- Wang, T.N., et al., The effect of thrombospondin on oral squamous carcinoma cell invasion of collagen. Am J Surg, 1995. 170(5): p. 502-5. [CrossRef]
- Housman, G., et al., Drug resistance in cancer: an overview. Cancers (Basel), 2014. 6(3): p. 1769-92. [CrossRef]
- Szymczyk, J., et al., FGF/FGFR-Dependent Molecular Mechanisms Underlying Anti-Cancer Drug Resistance. Cancers (Basel), 2021. 13(22). [CrossRef]
- Zhang, Y., et al., Resistance to Cetuximab in EGFR-Overexpressing Esophageal Squamous Cell Carcinoma Xenografts Due to FGFR2 Amplification and Overexpression. Journal of Pharmacological Sciences, 2014. 126(1): p. 77-83. [CrossRef]
- Turczyk, L., et al., FGFR2-Driven Signaling Counteracts Tamoxifen Effect on ERα-Positive Breast Cancer Cells. Neoplasia, 2017. 19(10): p. 791-804. [CrossRef]
- McDermott, S.C., et al., FGFR signaling regulates resistance of head and neck cancer stem cells to cisplatin. Oncotarget, 2018. 9(38): p. 25148-25165.
- Byron, S.A., et al., The N550K/H mutations in FGFR2 confer differential resistance to PD173074, dovitinib, and ponatinib ATP-competitive inhibitors. Neoplasia, 2013. 15(8): p. 975-88. [CrossRef]
- Goyal, L., et al., Polyclonal Secondary <i>FGFR2</i> Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion–Positive Cholangiocarcinoma. Cancer Discovery, 2017. 7(3): p. 252-263.
- Katoh, M., FGFR2 Abnormalities Underlie a Spectrum of Bone, Skin, and Cancer Pathologies. Journal of Investigative Dermatology, 2009. 129(8): p. 1861-1867. [CrossRef]
- Blazanin, N., et al., Activation of a protumorigenic IFNγ/STAT1/IRF-1 signaling pathway in keratinocytes following exposure to solar ultraviolet light. Mol Carcinog, 2019. 58(9): p. 1656-1669.
- Dickinson, S.E., et al., Inhibition of UV-Induced Stress Signaling and Inflammatory Responses in SKH-1 Mouse Skin by Topical Small-Molecule PD-L1 Blockade. JID Innov, 2024. 4(2): p. 100255. [CrossRef]
- Vaishampayan, P., C. Curiel-Lewandrowski, and S.E. Dickinson, Review: PD-L1 as an emerging target in the treatment and prevention of keratinocytic skin cancer. Mol Carcinog, 2023. 62(1): p. 52-61. [CrossRef]
- Adams, A.C., et al., Solar Simulated Light Induces Cutaneous Squamous Cell Carcinoma in Inbred Mice: A Clinically Relevant Model to Investigate T-Cell Responses. J Invest Dermatol, 2021. 141(12): p. 2990-2993.e6.
- Huang, S.J., et al., Imiquimod enhances IFN-gamma production and effector function of T cells infiltrating human squamous cell carcinomas of the skin. J Invest Dermatol, 2009. 129(11): p. 2676-85.
- Gambichler, T., et al., Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother, 2017. 66(9): p. 1199-1204. [CrossRef]
- Borden, E.S., et al., Neoantigen Fitness Model Predicts Lower Immune Recognition of Cutaneous Squamous Cell Carcinomas Than Actinic Keratoses. Front Immunol, 2019. 10: p. 2799. [CrossRef]
- Palakurthi, S., et al., The Combined Effect of FGFR Inhibition and PD-1 Blockade Promotes Tumor-Intrinsic Induction of Antitumor Immunity. Cancer Immunol Res, 2019. 7(9): p. 1457-1471. [CrossRef]
- Pettersen, J.S., et al., Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. J Invest Dermatol, 2011. 131(6): p. 1322-30. [CrossRef]
- Li, Y., et al., FGFR2 upregulates PAI-1 via JAK2/STAT3 signaling to induce M2 polarization of macrophages in colorectal cancer. Biochim Biophys Acta Mol Basis Dis, 2023. 1869(4): p. 166665.
- Huang, T.X., et al., Targeting cancer-associated fibroblast-secreted WNT2 restores dendritic cell-mediated antitumour immunity. Gut, 2022. 71(2): p. 333-344. [CrossRef]
- Javle, M., et al., Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol, 2018. 36(3): p. 276-282.
- Gozgit, J.M., et al., Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models. Mol Cancer Ther, 2012. 11(3): p. 690-9.
- Konecny, G.E., et al., Second-line dovitinib (TKI258) in patients with FGFR2-mutated or FGFR2-non-mutated advanced or metastatic endometrial cancer: a non-randomised, open-label, two-group, two-stage, phase 2 study. Lancet Oncol, 2015. 16(6): p. 686-94. [CrossRef]
- Du, S., Y. Zhang, and J. Xu, Current progress in cancer treatment by targeting FGFR signaling. Cancer Biol Med, 2023. 20(7): p. 490-9. [CrossRef]
- SenthilKumar, G., et al., FGFR Inhibition Enhances Sensitivity to Radiation in Non-Small Cell Lung Cancer. Mol Cancer Ther, 2020. 19(6): p. 1255-1265. [CrossRef]
- Weeden, C.E., et al., Cisplatin Increases Sensitivity to FGFR Inhibition in Patient-Derived Xenograft Models of Lung Squamous Cell Carcinoma. Mol Cancer Ther, 2017. 16(8): p. 1610-1622. [CrossRef]
- Wu, Q., et al., EGFR Inhibition Potentiates FGFR Inhibitor Therapy and Overcomes Resistance in FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discov, 2022. 12(5): p. 1378-1395. [CrossRef]
- Peng, M., et al., Dual FGFR and VEGFR inhibition synergistically restrain hexokinase 2-dependent lymphangiogenesis and immune escape in intrahepatic cholangiocarcinoma. J Gastroenterol, 2023. 58(9): p. 908-924. [CrossRef]
- Shen, G., et al., Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol, 2018. 11(1): p. 120. [CrossRef]
- Dickinson, S.E., et al., Increased PD-L1 Expression in Human Skin Acutely and Chronically Exposed to UV Irradiation. Photochem Photobiol, 2021. 97(4): p. 778-784. [CrossRef]
- Garcia-Diaz, A., et al., Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep, 2017. 19(6): p. 1189-1201. [CrossRef]
- Mimura, K., et al., PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci, 2018. 109(1): p. 43-53. [CrossRef]
- Rana, S., et al., Ultraviolet B suppresses immunity by inhibiting effector and memory T cells. Am J Pathol, 2008. 172(4): p. 993-1004. [CrossRef]
- Schuler, M., et al., Rogaratinib in patients with advanced cancers selected by FGFR mRNA expression: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol, 2019. 20(10): p. 1454-1466. [CrossRef]
- Subbiah, V., et al., FIGHT-101, a first-in-human study of potent and selective FGFR 1-3 inhibitor pemigatinib in pan-cancer patients with FGF/FGFR alterations and advanced malignancies. Ann Oncol, 2022. 33(5): p. 522-533. [CrossRef]
- Van Cutsem, E., et al., A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann Oncol, 2017. 28(6): p. 1316-1324. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




