1. Introduction
The main organs affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease (Covid-19) are lungs where tissue hypoxia occurs, causing diffuse alveolar damage with severe capillary congestion as seen on postmortem examinations [
1,
2,
3,
4]. Alveolocapillary microthrombi and specific vascular angiogenesis are more frequent in the lungs of patients with Covid-19 than in those with influenza A (H1N1) [
5]. In parallel, post-mortem studies have also shown the presence of tracheal injuries. The autopsy findings of 21 patients with Covid-19 revealed severe mucous tracheitis in a third of them, also describing lymphoid infiltrates in the trachea [
1].
Autophagosomes with viral aggregates may be present in tracheal epithelial cells and within extracellular mucus in the tracheal lumen [
6]. In another post-mortem study with 14 cases, mild inflammatory changes of the submucosa, oedema with small lymphocytic aggregates, and focal acute tracheitis were observed [
2]. In situ expression of SARS-CoV-2 was detected in tracheal sections in 4 of 7 autopsies using reverse transcriptase-polymerase chain reaction (RT-PCR) [
7]. In a multi-institutional study involving 68 autopsies, small white aphthous ulcers were described in the SARS-CoV-2 inflammation of the trachea and virus was observed in airway epithelium by RNAscope® technology [
8].
Similar findings could be found in the trachea of the Covid-19 tracheostomized patients. The aim of this study is to show the histological findings in the tracheal tissue of Covid-19 tracheostomized patients and to determine if there is an association between these findings and the SARS-COV-2 infection.
2. Materials and Methods
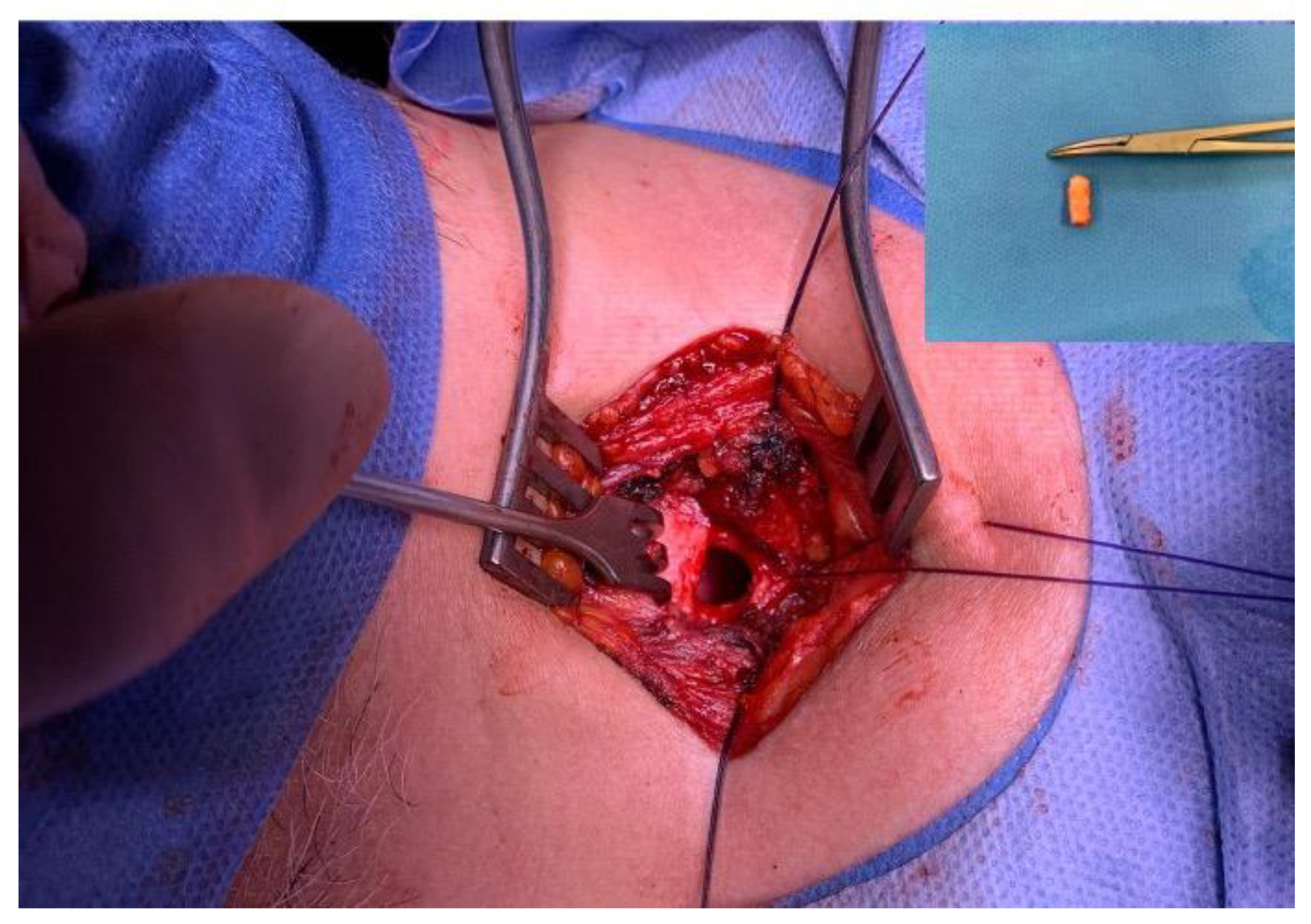
Data collection period was between May 2020-May 2022. We examined 80 tracheal samples obtained during tracheostomy (
Figure 1) from patients admitted to the intensive care unit undergoing long-term endotracheal intubation. The tracheal tissues were studied using standard microscopy techniques.
The diagnosis of positive covid was confirmed by nasal antigen test and polymerase chain reaction. None of these patients had been vaccinated against SARS-CoV-2 infection.
The following variables were collected: sex, age, Covid-19 disease (yes/no), comorbidities (yes/no), histopathological findings (yes/no), Positive End-Expiratory Pressure (PEEP), ratio of the partial pressure of oxygen in arterial blood (PaO2) to the inspired oxygen fraction (FiO2) (PAFI) at the time of intubation and at the day of tracheostomy.
The characteristics of the patients were described and compared between the positive SARS-CoV-2 group and the negative SARS-CoV-2 group to study the homogeneity and comparability of both samples. The hypothesis of independence between suffering SARS-CoV-2 infection and presenting relevant histologic findings was also studied by Chi-Square test, Fisher’s exact test and Cramer V as association measurement index. Finally, some risk indices such as the Relative Risk index (RR), the Odds Ratio (OR) or the Percentage of Attributable Risk (PAR) were estimated. Differences in quantitative variables between the different groups compared were performed using the Mann-Whitney U test and R Wilcoxon (r) as size effect index.
The Type I Error probability used was 5%. Statistical analyses on the data collected were carried out using R software (R Core Team, 2020) [
9].
3. Results
3.1. Characteristics and Differences between Patients with and without SARS-CoV-2 Infection
The mean age (SD) was 67.1 (6.9) years in the positive SARS-CoV-2 group and 67.8 (9.6) years in the negative SARS-CoV-2 group (p=0.3). The number of men in each group was 30 (75.0%) in the first group versus 27 (67.5%) in the second group, respectively (p=0.5).
The most frequent diagnosis presented by the Covid-19 group patients was pneumonia. Patients without Covid-19 disease (control group) presented with diagnoses that also required prolonged orotracheal intubation, including stroke, multiorgan failure, pancreatitis, cerebral hemorrhage, complication of cardiac surgery, head trauma, abdominal abscesses, pneumonia, status epilepticus, pneumomediastinum.
There appeared to be no differences (p>0.05) between the positive SARS-CoV-2 group and negative SARS-CoV-2 group in the values and percentages of days until tracheostomy, PEEP at day of tracheostomy, PAFI at day of tracheostomy and comorbidities (
Table 1).
However, the following variables appeared to be statistically unbalanced between the groups and of moderate to high magnitude: PEEP at the day of intubation (p<0.001, R=0.671) and PAFI at the day of intubation (p=0.041, r=0.318)
3.2. Histological Findings
Altered histology was founded in 14 cases (17.5%). No relevant histological alterations were found in 82.5% of samples (N=66).
Chronic subepithelial inflammation was found in 13.8% of cases (N=11). Two cases presented vasculitis (2,5%) and one case presented with thrombotic microangiopathy and chronic inflammation (1,2%), all cases in the covid group (
Table 2).
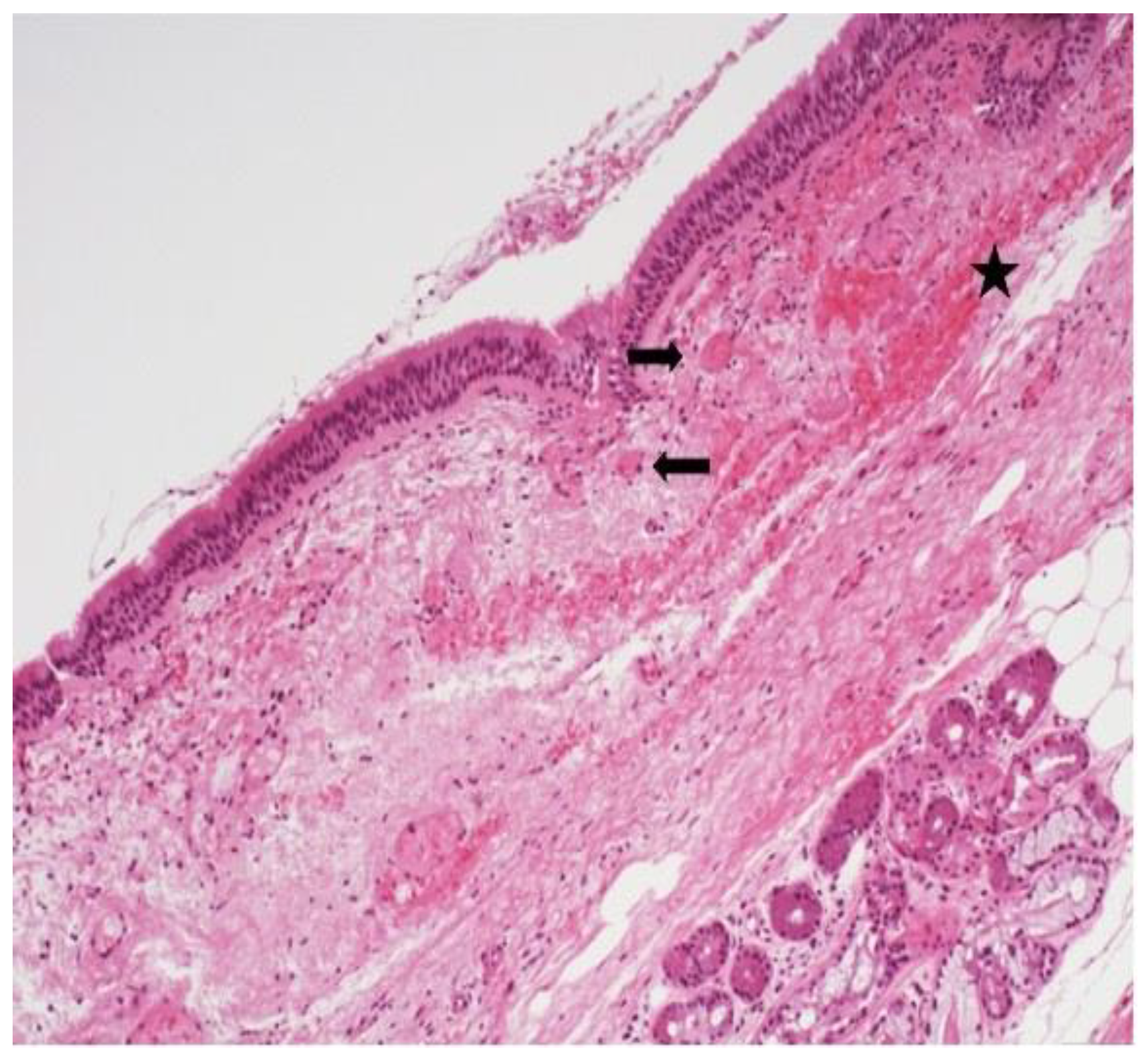
The most prominent histological finding was subepithelial chronic inflammation (
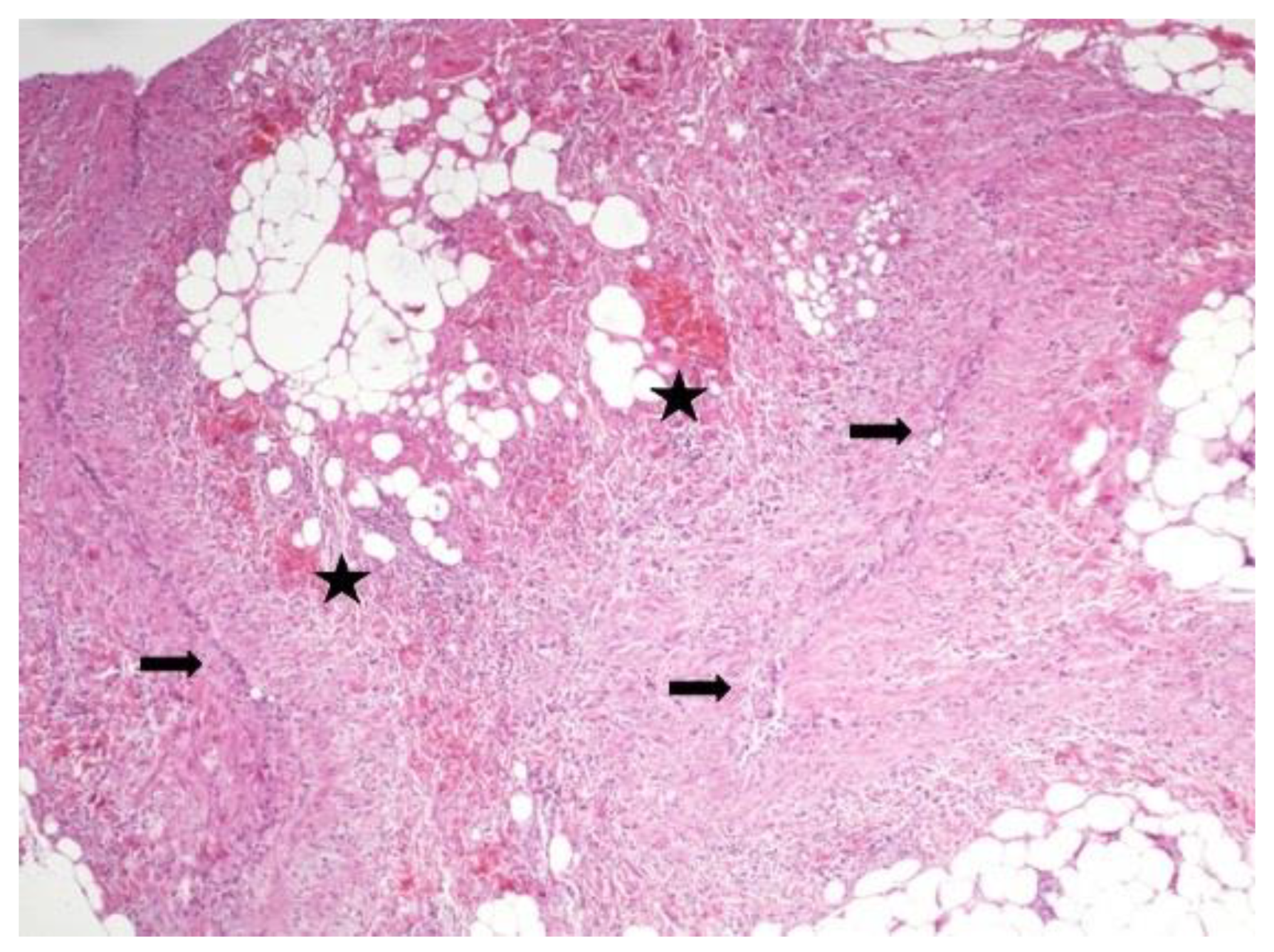
Figure 2) present in 13,8% of samples. Thrombotic microangiopathy (
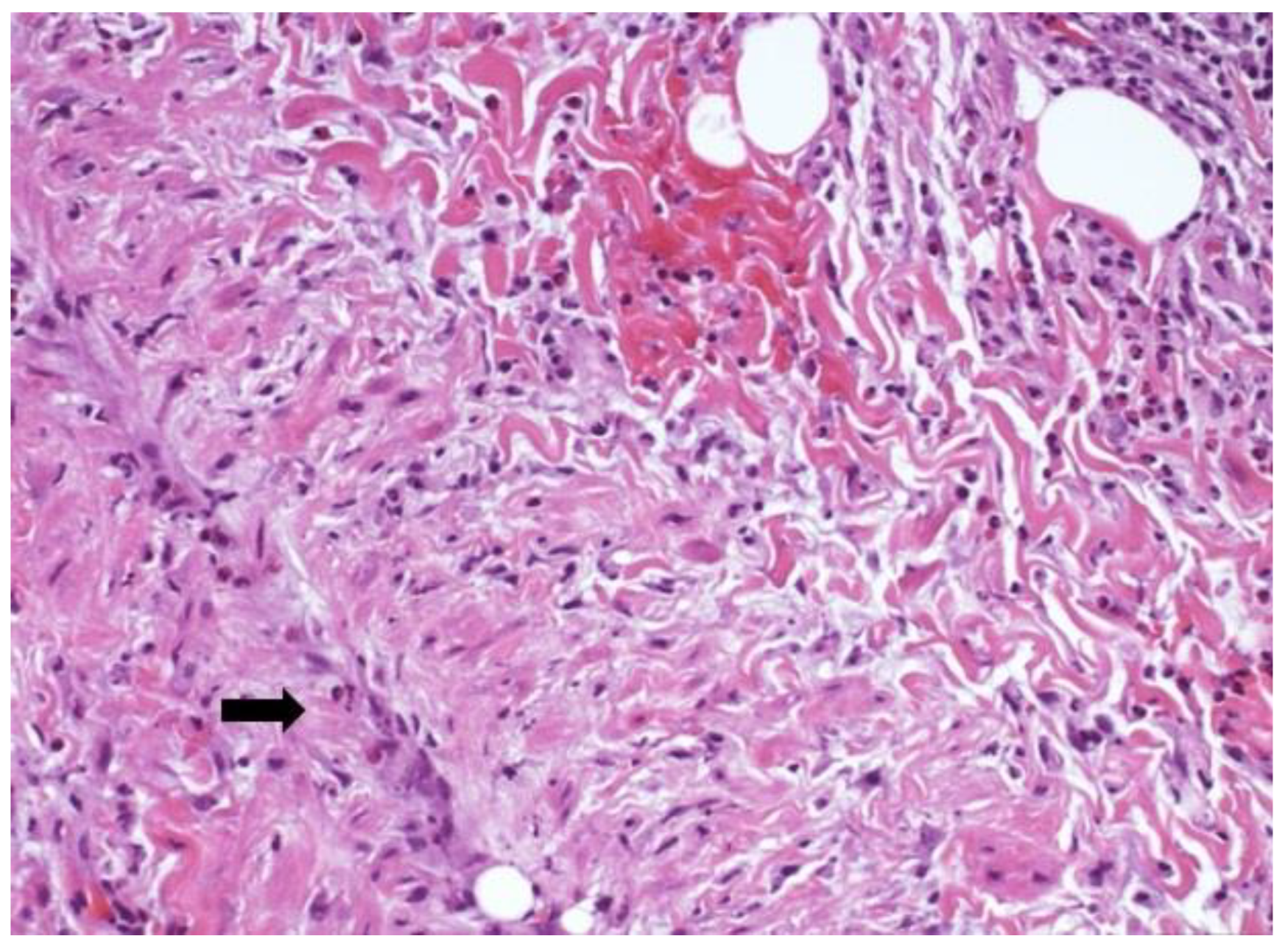
Figure 3) was documented in one ring and areas of hematic extravasation and vasculitis were present in one case (
Figure 4).
We found no statistically significant association between relevant histologic findings and SARS-CoV-2 infection (X2 = 0.779, p = 0.377; Fisher’s Exact Test: p= 0.378; Cramer V=0.132, 95%CI= 0.000 - 0.351).
In addition, the risk indices obtained contemplated a value of 1 in the estimated 95% confidence intervals (
RR = 1.8, 95% CI = 0.66 - 4.9;
OR=2.032, 95% CI = 0. 614 - 6.716;
PAR= 44%, 95% CI = -51.2 - 79.6). Therefore, although the risk of suffering alterations in the positive SARS-CoV-2 group seemed to be descriptively higher than in the negative SARS-CoV-2 group, we could not affirm that the SARS-CoV-2 infection represented a significantly higher risk (
Table 3).
On the other hand, none of the variables mentioned in the previous section seem to be associated with the appearance of relevant histological findings (
Table 4), except for PAFI at the day of tracheostomy (p<0.048, r=0.024). The group with no relevant histological findings had a mean PAFI at the day of tracheostomy score of 193.0 (45.5), significantly higher than the group with an altered histology (160.2 (50.2)).
4. Discussion
In the Community of Madrid, Spain, the first wave of SARS-CoV-2 infection occurred in March 2020. The first 19 tracheotomies performed on patients with COVID-19 pneumonia and prolonged intubation were excluded, as they were conducted in an emergency phase with personal protective equipment and using the standard tracheostomy technique, where the resected tracheal ring was not sent for pathological examination [
10]. The second challenge of the study was to collect enough patients who underwent tracheostomy due to prolonged intubation but with a diagnosis other than SARS-CoV-2 pneumonia, to compare both groups.
The study was therefore conducted in an exceptional epidemiological situation, without information on the risks associated with performing the tracheotomy and the expected findings in the trachea.
Now, we know that our study expands the literature by documenting 6 cases of altered histology in tracheal samples obtained
in vivo during tracheostomy performed on patients with COVID-19 disease and oral prolonged intubation. Since ulceration and edema of the subglottis extending beyond the third tracheal ring have been described in COVID-19 cases, rendering extubation impossible [
10,
11], we aimed to determine whether there were any pathological findings in the resected ring during tracheostomy.
We found a case report in the literature that describes histopathology histopathological findings from a surgical specimen after tracheal resection for post-tracheostomy stenosis in a COVID-19 patient. Histologic findings of the tracheal sample included lymphomonocytic perivascular inflammatory infiltrate and giant cell granulomas, coagulative necrosis of the submucosal tissue and neoangiogenesis [
12]. Similarly, in our study, we report the presence of lymphocytic and plasma cells inflammatory infiltrate, hematic extravasation and fine-caliber vessels with lumens occupied by fibrin, as well as venulitis in the tracheal ring of tracheostomized Covid-19 patients.
These inflammatory findings may provide a histological explanation for the macroscopic changes observed by the surgeon during tracheostomy. Our clinical observations align with studies suggesting that airway vasculitis phenomena may contribute to the increased incidence of tracheal injuries [
13]. Additionally, inflammation was also noted in another study that evaluated, by histology and immunohistochemistry, the fibrotic tissue resected after a subglottic stenosis post-tracheostomy in a severe ill Covid-19 patient, which revealed a high localized density of immunoglobulin G4 (IgG4)-secreting plasma cells [
14].
The interest of our study is twofold: first, to document the morphological changes associated with SARS-CoV-2-related tracheitis in "in vivo" samples, avoiding post-mortem detachment of epithelial cells, and secondly, to explain the relationship between oxygenation and the presence of histological findings in patients with COVID-19 who underwent intubation and tracheotomy.
In 2022, Ward et al. conducted a study comparing tracheal rings in COVID-19 and non-COVID-19 tracheostomized patients, evaluating 38 tracheal rings excised at tracheostomy from long-term intubated COVID-19 patients and 5 from long-term intubated non-COVID-19 patients. They also underwent histological examination on four tracheal autopsy samples from COVID-19 patients who died without undergoing prolonged mechanical ventilation. Histological findings were similar between mechanically ventilated COVID-19 positive and negative patients [
15].
Similarly, our study concludes that there is no increased risk of presenting histological abnormalities in the presence of SARS-CoV-2 infection. Findings associated with chronic inflammation may also be present in patients undergoing tracheostomy due to prolonged intubation without SARS-CoV-2 infection.
Analyzing in depth the characteristics of the groups in our study, we observed differences between them in terms of comorbidity, severity of the disease and oxygenation.
The number of comorbidities in the SARS-COV-2 negative group was significantly higher than the number of comorbidities in the SARS-COV-2 positive group, suggesting that COVID-19 affected healthier patients with fewer underlying diseases, which is consistent with the presentation of severe COVID-19 in previously healthy people who develop severe pneumonia.
PEEP levels at the time of intubation were higher in the COVID-19 group suggesting more severe respiratory involvement. An elevated PEEP could generate changes in the tracheal structure due to prolonged ventilatory support and pressure on the tissues, but no differences were found between the groups with/without findings.
The PaO2/FiO2 ratio (PAFI) was lower in the COVID-19 group at the time of intu-bation, indicating the compromise in the oxygenation of these patients, but these differences evened out by the time of tracheostomy indicating the time of best oxygenation and stabilization of the patient to perform the tracheostomy.
PAFI was higher at the time of tracheostomy in those without histological findings, suggesting that better oxygenation might be linked to the absence of significant tracheal pathology.
5. Conclusions
The histological examination of the tracheal ring of tracheostomized positive SARS-CoV-2 patients present subepithelial chronic inflammation, thrombotic microangiopathy and vasculitis phenomena. There is no statistically significant association between relevant histologic findings and SARS-CoV-2 infection. There is no evidence of an increased risk of histopathological findings in the resected tracheal ring of tracheostomized COVID-19 patients. The oxygenation status at the time of the tracheostomy is linked to the presence or absence of pathological findings in the tracheal ring.
Author Contributions
N. Mata-Castro performed conceptualization, methodology and final writing; L. Sanz López provided resources and reviewing and editing, data curation and final rewieving; R. Castañeda-Vozmediano provided statistical analysis; C. Perna and C. Prada Puentes contributed histological details and images. All authors discussed the results and implications and commented on the manuscript at all stages.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study has the approval of the institutional review board and the ethics committee of the Torrevieja and Elche-Vinalopó University Hospitals. This article does not contain any studies with animals performed by any of the author.
Informed Consent Statement
Informed consent was obtained from all patients.
Data Availability Statement
None data associated with our study has been deposited into a publicly available repository.
Acknowledgments
Thank you to the following surgeons of the ENT Department who also participated in the surgeries and sample collection: L. Carles Espinosa, R. Gomez-Blasi Camacho, I. Fernández-Carrera González, J. Lora Díaz, G. Narciso Martínez and P. Pinacho Martínez.
Conflicts of Interest
Authors declare that they have no conflict of interest. Authors have not received research grants from any company.
Abbreviations
The following abbreviations are used in this manuscript: COVID-19 Infection SARS-CoV-2.
References
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bradley, B.T.; Maioli, H.; Johnston, R.; Chaudhry, I.; Fink, S.L.; Xu, H.; et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 2020, 396, 320–332, Erratum in: Lancet. 2020 Aug 1;396(10247):312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vasquez-Bonilla, W.O.; Orozco, R.; Argueta, V.; Sierra, M.; Zambrano, L.I.; Muñoz-Lara, F.; et al. A review of the main histopathological findings in coronavirus disease 2019. Hum Pathol. 2020, 105, 74–83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polak, S.B.; Van Gool, I.C.; Cohen, D.; von der Thüsen, J.H.; van Paassen, J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020, 33, 2128–2138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deshmukh, V.; Motwani, R.; Kumar, A.; Kumari, C.; Raza, K. Histopathological observations in COVID-19: a systematic review. J Clin Pathol. 2020, Epub ahead of print. jclinpath-2020-206995. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, I.M.; Padera, R.F.; Solomon, I.H.; Kanjilal, S.; Hammer, M.M.; Hornick, J.L.; et al. In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. 2020 Nov;33(11):2104-2114. Mod Pathol. 2020, 33, 2104–2114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borczuk, A.C.; Salvatore, S.P.; Seshan, S.V.; Patel, S.S.; Bussel, J.B.; Mostyka, M.; et al. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020, 33, 2156–2168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; https://www.R-project.
- Mata-Castro, N.; Sanz López, L.; Pinacho-Martínez, P.; Varillas-Delgado, V.; Miró-Murillo, M.; Martín-Delgado, M.C. Tracheostomy in patients with SARS-CoV-2 reduces time on mechanical ventilation but not intensive care unit stay. Am J Otolaryngol. 2021, 42, 102867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oliver, C.M.; Campbell, M.; Dulan, O.; Hamilton, N.; Birchall, M. Appearance and management of COVID-19 laryngo-tracheitis: two case reports. F1000Res. 2020, 9, 310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lucchi, M.; Ambrogi, M.; Aprile, V.; Ribechini, A.; Fontanini, G. Laryngotracheal resection for a post-tracheotomy stenosis in a patient with coronavirus disease 2019 (COVID-19). JTCVS Tech. 2020, 4, 360–364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bassi, M.; Anile, M.; Pecoraro, Y.; Ruberto, F.; Martelli, S.; Piazzolla, M.; et al. Bedside transcervical transtracheal post-intubation injury repair in a Covid-19 patient. Ann Thorac Surg.
- Roncati, L.; Bergonzini, G.; Lusenti, B.; Nasillo, V.; Paolini, A.; Zanelli, G.; et al. High density of IgG4-secreting plasma cells in the fibrotic tissue from a surgically resected tracheal ring impaired by complex subglottic stenosis post-tracheostomy as immune expression of a Th2 response due to severe COVID-19. Ann Hematol. 2021, 100, 2659–2660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ward, P.A.; Collier, J.M.; Weir, J.; Osborn, M.; Osborn, B.; Smellie, W.J.B. Chelwest COVID-19 Tracheostomy Group. Histological findings of tracheal samples from COVID-19 positive critically ill mechanically ventilated patients. Clin Otolaryngol. 2022, 47, 131–137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).