1. Introduction
Nephrectomy is a surgical procedure that involves the removal of a kidney. It can be performed for various reasons, including renal tumors, chronic kidney disease, or severe kidney injury. Left radical nephrectomy (i.e.
, complete removal of the left kidney along with surrounding tissues, including the adrenal gland and nearby lymph nodes) is generally performed for the treatment of left-sided renal cell carcinoma, particularly in case of large tumors or involvement of surrounding structures. Despite many advancements that enhanced the precision and the efficacy of this surgery (laparoscopic and robotic approach), allowing for minimally invasive procedures with reduced recovery times and complication rates, several extrarenal visceral artery injuries continue to be reported since their first descriptions 50 years ago [
1,
2]. Understanding visceral artery injuries during left radical nephrectomy is crucial for several reasons. First, these injuries can lead to significant morbidity and mortality, impacting patient outcomes. Second, the visceral arteries involved, including the superior mesenteric artery and the celiac trunk, play vital roles in supplying blood to the stomach, duodenum, small bowel, proximal large bowel, liver, pancreas, and spleen. Injury to these vessels can result in ischemia, organ dysfunction, and even death. Therefore, a comprehensive understanding of the anatomy, mechanisms of injury, clinical implications, and prevention strategies is essential for any surgeon performing nephrectomies. This article aims to provide an in-depth exploration of extrarenal visceral artery injuries during left radical nephrectomy. It will cover the anatomy of the renal vasculature, mechanisms of injury, clinical implications, prevention strategies, and a review of relevant case studies. By synthesizing this information, the article seeks to enhance the understanding of this critical aspect of nephrectomy and improve surgical outcomes.
2. Renal and Visceral Vascular Anatomy
2.1. Renal Arteries (RA)
The
renal arteries (RA) are the primary blood supply to the kidneys, arising from the lateral aspect of the abdominal aorta, typically at the level of the first or second lumbar vertebra, just below the origin of the SMA [
3]. Renal arteries supply a great amount of blood to the kidneys, nearly one-third of the cardiac output. The number of RA may vary among individuals, with a double or triple RA in up to one-third of cases [
4]. Due to the mutual location in the abdominal cavity of the aorta, inferior vena cava and kidneys, the right renal artery (RRA) is normally longer than the left one. The RRA originates from the right lateral margin of the aorta, with which it forms an angle just over 60°; it passes behind the inferior vena cava, the right renal vein, the head of the pancreas and the descending portion of the duodenum, describing a curved trajectory with a posterior concavity against the psoas major muscle. The overall length of the artery is approximately 7 cm. The left renal artery (LRA) originates from the left lateral margin of the aorta, with which it forms an almost right angle, in a slightly more cranial position than the right one; it runs behind the left renal vein, the body of the pancreas, and the splenic vein, and crosses the inferior mesenteric vein. It is slightly shorter than the RRA, with a total length of approximately 5 cm. Each renal artery divides into anterior and posterior branches, which further branch into segmental arteries that supply specific regions of the kidney. Understanding this vascular anatomy is essential for surgeons to avoid inadvertent RA injuries during nephrectomy.
2.2. Superior Mesenteric Artery (SMA)
The superior mesenteric artery (SMA) it is a large arterial branch that arises from the aorta at the level of the first lumbar vertebra immediately below the celiac trunk. It serves to ensure blood supply to a large part of the intestine, from the last portion of the duodenum to the middle of the transverse colon. About 24/25 cm long, it runs behind the pancreas and then heads forward until it bypasses the third portion of the duodenum to reach the mesentery. Very important branches arise along its path. It ends by anastomosing with the ileal branch of the ileocolic artery and with the last jejunal collateral. It is responsible for the blood supply of the duodenum-pancreas (together with the celiac trunk), the small intestine and the colon up to the distal transversus, where the anastomotic arch of Riolano is located between the left branch of the middle colic artery and the ascending branch of the left colic artery (branch of the inferior mesenteric artery). From its origin, the SMA is directed inferiorly, about 3 cm, along the ventral wall of the aorta, behind the isthmus of the pancreas, then is directed at an acute angle away from the aorta on the lower margin of the pancreas, descends diagonally from uncinate process and the third portion of the duodenum, which it crosses vertically. It then penetrates the root of the mesentery, which it follows for a few centimeters and is inserted into the floating part of the same, where it ends.
2.3. Celiac Trunk (CT)
The celiac trunk or celiac artery or also celiac tripod of Haller (CT) is a voluminous arterial branch that arises from the abdominal aorta at the level of the twelfth thoracic vertebra. After about two or three centimeters from the origin, three arterial branches arise from the trunk (hence the name “tripod”): left gastric artery; common hepatic or gastrohepatic artery; lienal or splenic artery. It is responsible for the blood supply to the liver, distal esophagus and stomach, duodenum-pancreas and spleen.
2.4. Spatial Relationships
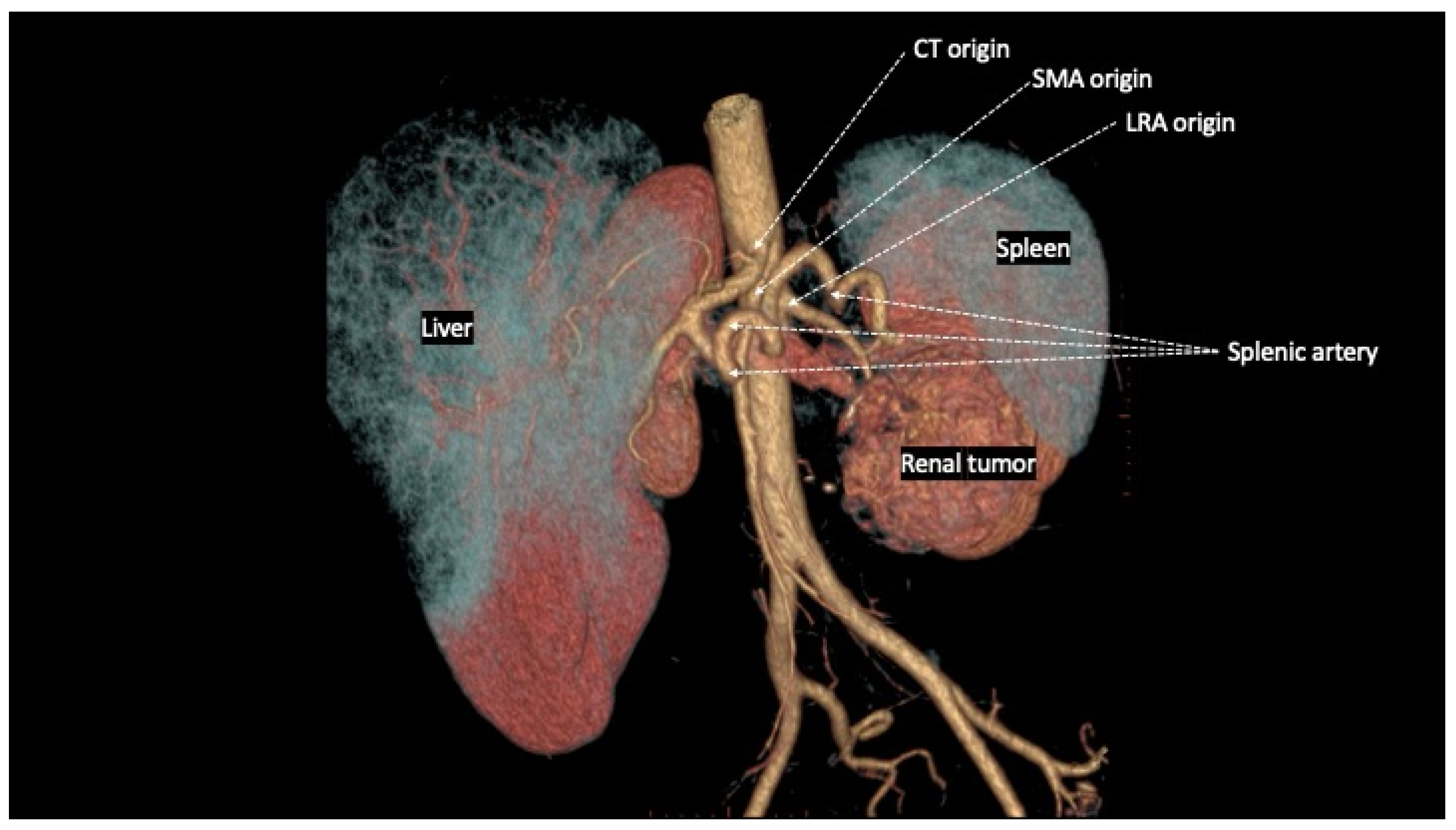
The
spatial relationships between CT, SMA and RA are extremely variable in physiological conditions, and clearly influenced by different pathological conditions. Regarding the common origin from the aorta, both CT and SMA arise, in the majority of cases, from the anterior wall, whereas the RA originate from the lateral or anterolateral wall of the aorta. In particular, the LRA originates from the anterolateral wall in 52%, from the lateral wall in 45% and from the posterior wall of the aorta in 3% of cases [
5]. Cadaveric studies have shown great variability of the distance between the ostia of origin of CT, SMA, and RA, generally not exceeding the value of 10 mm [
5,
6,
7,
8], varying around an average of 6 mm between the SMA and the LRA [
9,
10,
11] in studies based on in-vivo computed tomography scans (
Figure 1).
Moreover, the left renal vein (LRV) generally crosses the abdominal aorta anteriorly and courses posterior to the SMA in the crotch of the angle between the SMA and the aorta. The course of the LRA therefore occurs posterior to the LRV, except in 10% of cases in which the LRV has a retroaortic course [
12]. Finally, there are several collateral pathways between the visceral arteries that may compensate the effect of ischemia deriving from their injuries: the CT presents collateral circles with the SMA at the level of the superior and inferior pancreaticoduodenal arches; the SMA is in communication with the inferior mesenteric artery through the arch of Riolano and the marginal artery of Drummond [
13].
3. Extrarenal Visceral Arterial Injuries during Left Nephrectomy
3.1. Literature Review
Injuries to the SMA and/or CT during left nephrectomy/adrenalectomy probably occur more often than the small number of cases reported to date in the literature [
14,
15]. The highest incidence is that reported by Ritchey in 1992, in which a lesion of the SMA occurred in approximately 2% of children undergoing open nephrectomy for Wilms tumor with venous involvement [
16] and in 1.6% of adults undergoing laparoscopic nephrectomy for cancer [
17]. There have been several other sporadic cases of lesions of the SMA and/or CT during left nephrectomy, either through open (both laparotomic or retroperitoneal), laparoscopic or robotic approaches.
Twenty-five cases of iatrogenic extrarenal visceral arteries injuries have been reported to date (
Table 1), mostly (80%) with favorable outcomes after revascularization. Therefore, it is probable that, in addition to an
“under reporting
” phenomenon common to all iatrogenic injuries, there is also a
“publication bias
”, or the natural tendency to publish cases with a favorable outcome and not to publicize those with an unfavorable outcome. Actually, vascular complications, including those specific to the renal vessels, are the most frequently encountered during nephrectomy [
14], and the mortality rate resulting from any traumatic lesion of the proximal tract of the SMA reaches 75% of cases [
32,
33]. At the same time, although a favorable outcome is more frequently associated with early intraoperative recognition of the lesion followed by immediate revascularization, there are still cases with later recognition which, even regardless of a revascularization procedure of the SMA and/or CT, have had a favorable outcome [
19,
27,
29] due to the development of sufficient collateral circulation to maintain adequate visceral perfusion, a phenomenon also reported for post-traumatic injuries [
34].
3.2. Risk Factors
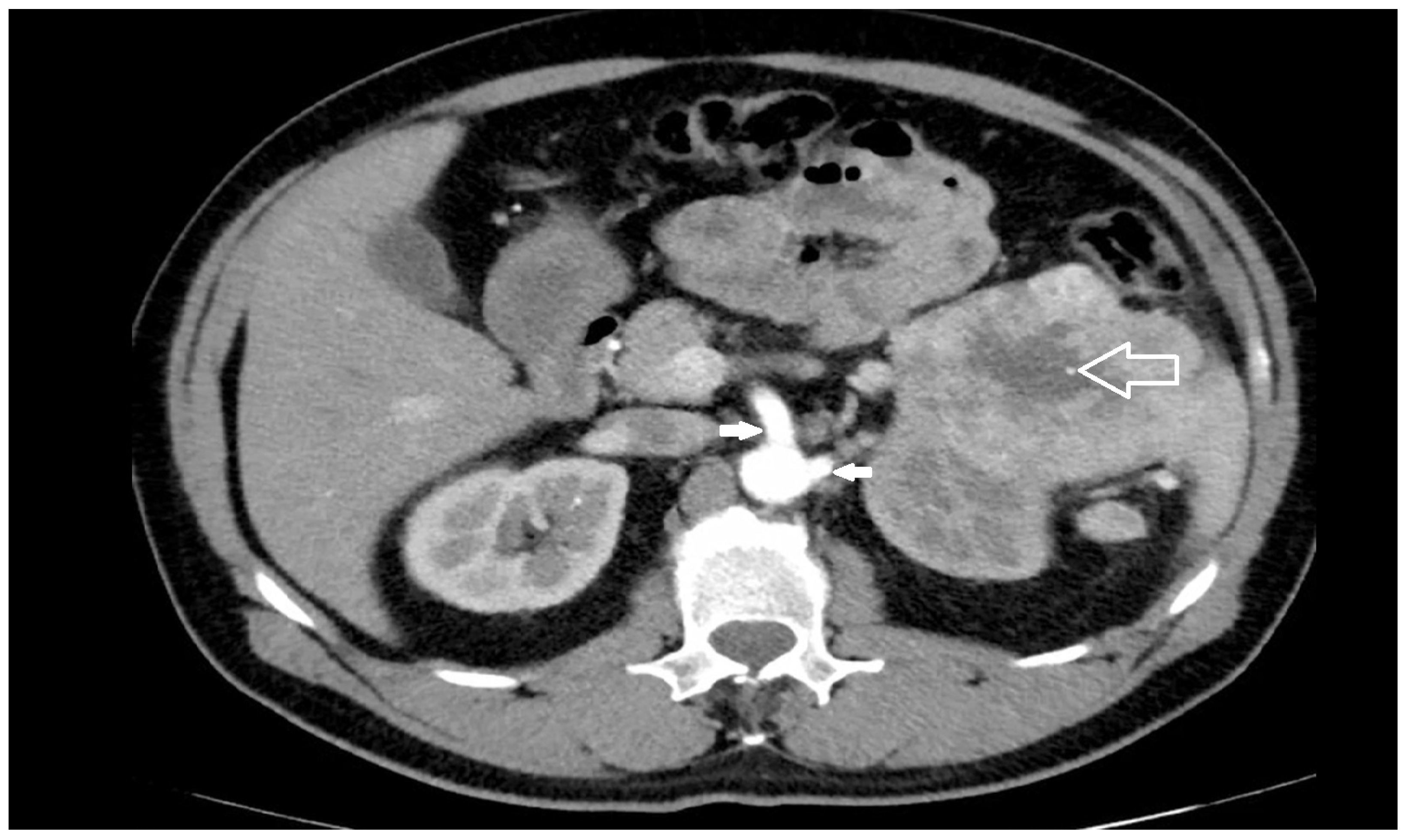
Risk factors most commonly reported for the occurrence of a iatrogenic injury of the SMA and/or CT during left nephrectomy/adrenalectomy are: the close spatial relations between renal and visceral arteries [
5,
6,
7,
8,
9,
10,
11]; surgery indicated for large neoplasms of the left upper renal pole or left adrenal gland (
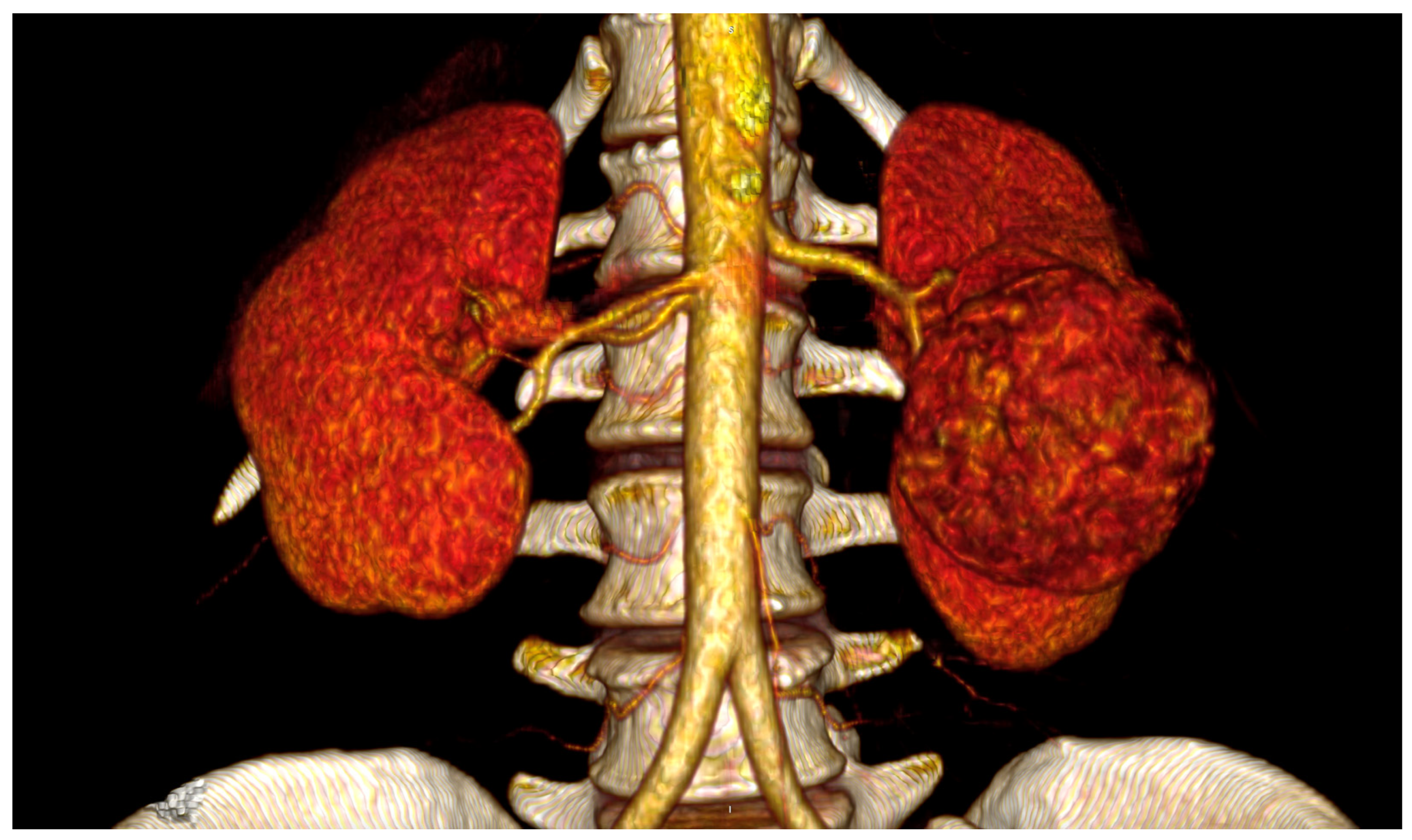
Figure 2), or with extra-renal spread or bulky hilar lymph node involvement [
15,
21]; surgery indicated for inflammatory renal diseases or completion nephrectomy after partial resection with perivisceral inflammatory adhesion to the aorta and its visceral branches [
15]; morbid obesity [
35]; lack of surgeon’s experience [
28,
36].
3.3. Pathogenesis
In the vast majority of cases, the pathogenesis of these lesions depends on a perceptive deficit of the surgeon who, convinced that he has identified the left renal artery to be divided, finds himself dealing with the SMA and/or the CT [
15,
30]. This pathogenetic mechanism is completely comparable to what occurs in the majority of “classic” iatrogenic injuries of the main bile duct during cholecystectomy [
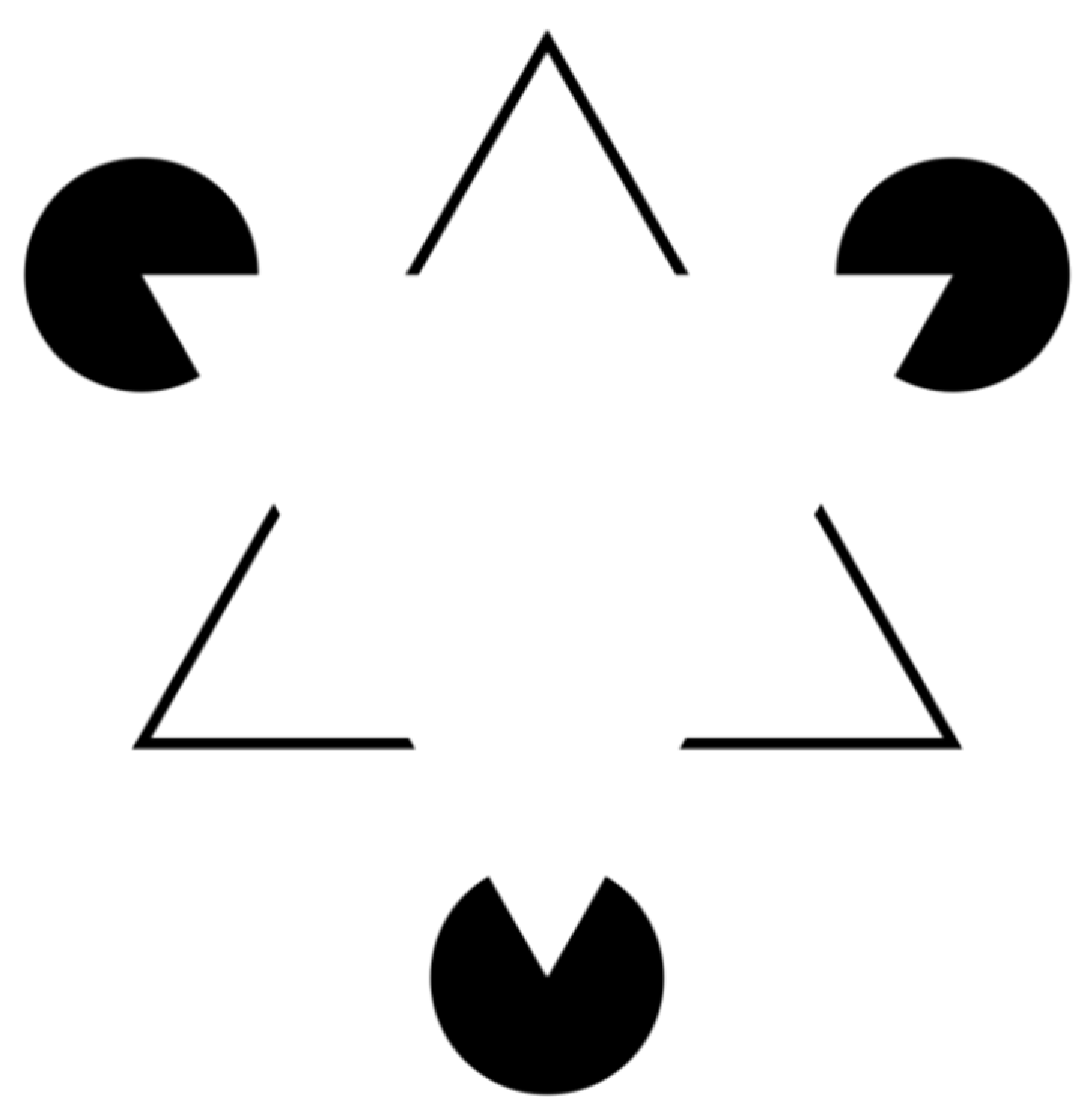
37]. The Kanizsa triangle (
Figure 3) is an optical illusion, described for the first time in 1955 by the Italian psychologist Gaetano Kanizsa [
38], which gives a perfect idea of the perceptive deficit that leads the surgeon into error.
In the figure we can “see” two white equilateral triangles, one superimposed on the other, even if neither of the two triangles is actually drawn. This effect is known as subjective or illusory profiling. Furthermore, the non-existent white triangle appears to be brighter than the surrounding area, while that area has the same brightness as the adjacent areas. This phenomenon occurs because our perceptive apparatus has an innate organizational tendency constituted by the figure/ground articulation according to which there is no figure without a background; this also happens with figures obtained with physically non-existent margins such as this triangle. This is because our perceptual evaluation needs figure/background contrast and even when this is not present, the same is created.
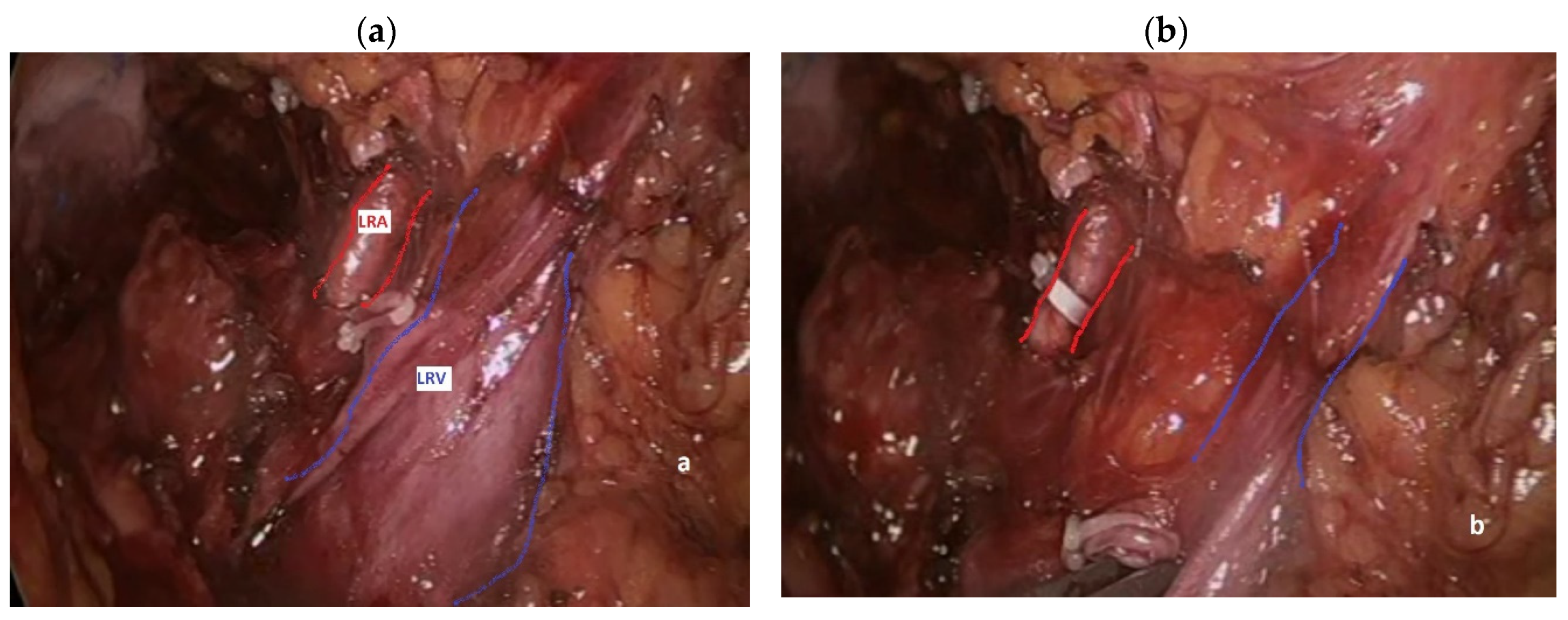
During left nephrectomy, the perceptive deficit manifests itself in a completely similar way: the surgeon determines a lesion of the SMA and/or the CT convinced that he is instead dealing with one or more LRA [
15,
30]. Actually, during transabdominal left nephrectomy, either open or mininvasive, the surgeon generally takes down the splenic flexure of the colon and directly dissects the renal hilum, finding the LRV first and the LRA immediately after (
Figure 4a), lying behind it in 90% of cases [
12]. Thereafter, an occluding clip is generally placed on the LRA first (
Figure 4b), followed by clipping and division of the left renal vein, in order to avoid renal venous engorgement.
If a single LRA was identified at preoperative imaging studies and engorgement of the LRV is noticed after LRA clip placement, the possibility that the vessel just clipped is not the LRA could arise before vessel division. This is the very first moment during nephrectomy in which the surgeon may feel a reasonable suspect that his perception of the anatomic setting is not correct. Further periaortic dissection and identification of the true LRA may allow to remove the previously placed clip and proceed to the nephrectomy. This kind of injury, when promptly recognized and treated, has little or no consequences for the patient’s outcomes, but it is very rare. Actually, only one similar case has been reported to date [
30]. Unfortunately, in the vast majority of cases the procedure continues after clipping and division of the misperceived LRA, and further intraoperative suspect of extra-renal arterial injury may arise because of: 1) venous engorgement of the renal stump of the divided left renal vein; 2) identification of a LRA anterior to the LRV; 3) atypical course of the artery (i.e.
, transversal); 4) arterial origin from the anterior aortic wall; 5) finding another artery after division of the first LRA (with preoperative imaging negative for multiple LRA); 6) impossibility of clear identification of the left aortic wall due to the disease (neoplastic and/or inflammatory). Considering the above-reported variability of origin of the RA close to the visceral vessels, therefore, the first measure for the prevention of iatrogenic arterial injuries is preoperative evaluation of the vascular anatomy of the kidneys before nephrectomy [
22,
39], using a computed tomography scan with intravenous contrast (
Figure 5), which allows the number and course of renal arteries to be identified in 99% of cases [
11,
40].
In a non-negligible number of cases, however, the diagnosis is suspected later, once the nephrectomy is completed, due to the change in color of the small intestine induced by arterial hypoperfusion, or, even later in the postoperative course, due to the appearance of abdominal pain, metabolic acidosis and/or increased serum lactates due to intestinal necrosis.
3.4. Clinical Consequences and Treatment
The clinical consequences of extra-renal visceral arteries injuries during nephrectomy depend on three main factors: 1) anatomical localization of the lesion and existence of collateral circulation, generally more developed in patients with atherosclerotic stenosis of the SMA; 2) timing of diagnosis and repair of the lesion through mesenteric revascularization procedures; 3) the body’s ability to react to the inevitable systemic inflammatory response syndrome secondary to tissue damage from ischemia/reperfusion and sepsis. In the majority of cases, in fact, the prognosis of these patients is inevitably conditioned by the onset of splanchnic ischemia, which can evolve into organ damage (intestinal, hepatic, and pancreatic necrosis) and systemic damage (ischemia/reperfusion syndrome, systemic inflammatory response syndrome), with the majority of deaths due to the unfortunate evolution of sepsis resulting from bacterial translocation from the intestinal lumen or peritonitis due to acute intestinal necrosis, leading to multi-organ failure.
The anatomical location of the SMA injury generally determines the degree of severity of the resulting intestinal ischemia, being also the most reliable predictor of related mortality based on the experience derived from traumatic lesions [
32,
33]: lesions of the zone 1 (between the aortic origin of the SMA and the emergence of the inferior pancreaticoduodenal artery) are those most frequently encountered during nephrectomy, with a mortality rate of over 75% of cases. This is due to the compromise of the collateral circulation with the CT through the upper pancreatico-duodenal arches (branch of the CT through the hepatic and gastroduodenal artery) and the lower pancreatico-duodenal arches (branch of the SMA), with residual perfusion of the territory of the SMA exclusively through the existing collateral circles with the inferior mesenteric artery (Arcade of Riolano and marginal arcade of Drummond). For the same reason, combined lesions of the SMA and CT expose to a potentially higher mortality.
When the diagnosis of the lesion occurs early during surgery, early involvement of a specialist in vascular surgery is recommended to carry out a urgent revascularization procedure of the SMA, CT, or both [
18,
20,
23,
24,
25,
30], using a direct anastomosis, an autologous or prosthetic vein interposition graft, reimplantation on the aorta or renal artery stump, or bypass with the splenic artery. Every diagnostic delay of 6 hours determines a doubling of the mortality rates [
41], but even if the diagnosis of the lesion occurs later in the postoperative course, following the appearance of intestinal ischemia, necrosis and consequent peritonitis, it is still indicated to associate a revascularization procedure with the inevitable resection of the ischemic intestinal tract [
42], as revascularized patients have an average mortality rate significantly lower than non-revascularized patients (40% vs. 60% [
43]).
Individual response to the ischemia-reperfusion injury is extremely variable. Splanchnic microvascular perfusion includes blood supply to the layers of the intestinal wall (i.e.
, mucosal, submucosal, muscular and serosal). The mucosal layer receives 60% of the microvascular perfusion due to its high metabolic demand. The mucosa constitutes the first layer of the intestinal wall to be affected by ischemia at the level of the villi, responsible for the extraction of oxygen and the recruitment of the capillary bed. As intestinal blood flow decreases, the tissue becomes ischemic and anaerobic glycolysis occurs in the mucosa and submucosa, resulting in the production of lactate, lactic acidosis, and hydrogen (H
+) and calcium (Ca
++) ions accumulation. As lactate levels increase, the liver takes longer to eliminate their excess, thus prolonging the time of lactic acidosis. The microcirculation narrows in response to the decrease in blood flow, further reducing it and therefore aggravating the ischemia. Platelets clump and small clots may form in the microvasculature in response to decreased blood flow, further impeding blood flow. The lack of oxygen and nutrients ultimately leads to tissue damage and cell death. The muscular and serosal layers are affected when the inflow-outflow of blood is interrupted for long periods, the walls of the intestine become congested and the tissue becomes edematous, friable and hemorrhagic. Extensive cell death can potentially result in ulceration and perforation of the intestinal wall, progression towards septic shock, even though the primum movens for the development of sepsis is linked to bacterial translocation which already begins with necrosis of the mucosa. When ischemic and damaged tissue is reperfused (intermittent reperfusion may be present even before surgical revascularization via slowed flow through the collateral circulation), the reflux of oxygen increases the formation of reactive oxygen and nitrogen species, which interact with other macromolecules causing oxidative stress, such as lipid peroxidation, protein carbonylation and DNA oxidation, inducing further cell damage and death [
44]. This tissue death promotes the release of proinflammatory cytokines and chemokines, such as tumor necrosis factor-α and interleukin-6, triggering a cascade of events, including increased vascular permeability, bacterial translocation, and neutrophil activation and adhesion. Proinflammatory mediators released by mast cells and macrophages further aggravate systemic inflammatory response syndrome (SIRS). Neutrophil infiltration during this acute inflammatory phase has substantial and often irreversible tissue damage effects. Despite over thirty years of experimental and clinical research in this field, there is still no effective therapy capable of preventing the SIRS-Septic Shock-Multiple Organ Failure Syndrome-Death cascade [
45].
5. Conclusions
Iatrogenic lesions of the visceral arteries during left radical nephrectomy continue to be reported over the last 50 years, representing a significant surgical challenge with potentially devastating consequences. Understanding the anatomy of the renal and visceral vasculature, the mechanisms of injury, and management strategies is crucial for improving patient outcomes. Prompt recognition and intervention are essential to mitigate the risks associated with these injuries.
Author Contributions
Conceptualization, M.C. and R.C.; methodology, M.C., L.A.M., P.C., M.B., M.M. and R.C.; validation, M.C., L.A.M., P.C., M.B., M.M. and R.C.; writing—original draft preparation, M.C. and R.C.; writing—review and editing, M.C., L.A.M., P.C., M.B., M.M. and R.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the nature of the study.
Informed Consent Statement
Patient consent was waived due to the nature of the study.
Data Availability Statement
not applicable.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Roseau, E.; Arvis, G.; Chapuis, Y. The mesenteric arterial risk during left nephrectomy. Nouv Presse Med. 1973, 2, 385–6. [Google Scholar]
- Arvis, G. The arterial risk during extended nephrectomy. J Urol Nephrol 1974, 80, 143–4. [Google Scholar]
- Stranding, S. Gray’s Anatomy: the anatomical basis of clinical practice, 42nd Ed. Elsevier 2020.
- Hazirolan, T.; Oz, M.; Turkbey, B.; Karaosmanoglu, A.D.; Oguz, B.S.; Canyigit, M. CT angiography of the renal arteries and veins: normal anatomy and variants. Diagn. Interv. Radiol. 2009, 17, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ozan H, Alemdaroglu A, Sinav A, Gümüsalan Y. Location of the ostia of the renal arteries in the aorta. Surg Radiol Anat 1997;19(4):245-247.
- Pennington, N.; Soames, R.W. The anterior visceral branches of the abdominal aorta and their relationship to the renal arteries. Surg. Radiol. Anat. 2005, 27, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Katz-Summercorn A, Bridger J. A cadaveric study of the anatomical variation of the origins of the celiac trunk and the superior mesenteric artery: a role in median arcuate ligament syndrome? Clin Anat 2013;26(8):971-974.
- Sośnik, H.; Sośnik, K. Studies on renal arteries origin from the aorta in respect to superior mesenteric artery in Polish population. Folia Morphol. 2020, 79, 86–92. [Google Scholar] [CrossRef]
- Mazzaccaro, D.; Malacrida, G.; Nano, G. Variability of Origin of Splanchnic and Renal Vessels From the Thoracoabdominal Aorta. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Niscoveanu, C.; Bordei, P.; Baz, R. Morphological characteristics of origin of superior arterial mesenteric trunk. ARS Medica Tomitana 2016, 22, 145–152. [Google Scholar] [CrossRef]
- Lawton, J.; Touma, J.; Sénémaud, J.; de Boissieu, P.; Brossier, J.; Kobeiter, H.; Desgranges, P. Computer-assisted study of the axial orientation and distances between renovisceral arteries ostia. Surg. Radiol. Anat. 2016, 39, 149–160. [Google Scholar] [CrossRef]
- Damen, N.S.; Hostiuc, S.; Jianu, A.M.; Manta, B.A.; Rusu, M.C.; Dobra, M.A. Anatomical variants of the retroaortic left renal vein. Ann. Anat. - Anat. Anz. 2024, 251, 152170. [Google Scholar] [CrossRef]
- Treffalls, R.N.; Stonko, D.P.; DeMartino, R.R.; Morrison, J.J. Acute management of mesenteric emergencies: Tailoring the solution to the problem. Semin. Vasc. Surg. 2023, 36, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.; Varkarakis, I.; Rha, K.H.; Jarrett, T.W.; Pinto, P.A.; Kavoussi, L.R. Complications of abdominal urologic laparoscopy: longitudinal five-year analysis. Urology 2004, 63, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthi V, Frota R, Turna B. Management of vascular complications. In Taneja SS (ed.). Complicatons of urologic surgery. Prevention and management, 4th Ed. 2010 Saunders, pp.237-249.
- Ritchey, M.L.; Lally, K.P.; Haase, G.M.; Shochat, S.J.; Kelalis, P.P. Superior mesenteric artery injury during nephrectomy for Wilms' tumor. J. Pediatr. Surg. 1992, 27, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Siqueira TM Jr, Kuo RL, Gardner TA, Paterson RF, Stevens LH, Lingeman JE, Koch MO, Shalhav AL. Major complications in 213 laparoscopic nephrectomy cases: the Indianapolis experience. J Urol 2002;168(4 Pt 1):1361-1365.
- Court, B.; De Catalogne, G. [Section of the superior mesenteric artery during an extended nephrectomy for cancer of the left kidney. Immediate repair by spleno-mesenteric anastomosis. J Urol Nephrol (Paris) 1976, 82, 635–40. [Google Scholar]
- Bhanot, S.C.; Beaufils, A.; Leiter, E. Importance of Collateral Circulation of Bowel in Urologic Surgery. J. Urol. 1980, 123, 893–894. [Google Scholar] [CrossRef] [PubMed]
- Blunt, L.W.; Matsumura, J.; Carter, M.F.; Gonzalez, C.M.; Smith, N.D. Repair of superior mesenteric artery ligation during left nephrectomy with a native renal vein patch. Urology 2004, 64, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mandal, A.; Acharya, N.; Thingnam, S.; Bhalla, V.; Singh, S. Superior Mesenteric Artery Injury during en bloc Excision of a Massive Left Adrenal Tumor. Urol. Int. 2007, 78, 182–184. [Google Scholar] [CrossRef]
- Nevoux, P.; Zini, L.; Villers, A.; Boleslawski, E.; Nunes, B.; Zerbib, P. Celiac Axis and Superior Mesenteric Artery: Danger Zone for Left Nephrectomy. J. Endourol. 2008, 22, 2571–2574. [Google Scholar] [CrossRef]
- Abu-Gazala, S.; Schlager, A.; Elazary, R.; Keidar, A.; Appelbaum, L.; Rivkind, A.I.; Khalaileh, A.; Abu-Gazala, M.; Merhav, H. Revascularization of the Celiac and Superior Mesenteric Arteries After Operative Injury Using Both Splenic Artery and Saphenous Graft. Ann. Vasc. Surg. 2010, 24, 693–e1. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.; Pereira, J.; Eufrásio, P.; Constantino, J.; Rebelo, P. Splenomesenteric bypass as revascularisation technique after iatrogenic injury of the superior mesenteric artery during radical nephrectomy: A case report. Int. J. Surg. Case Rep. 2019, 60, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Navariya, S.C.; Bhirud, D.P.; Ranjan, S.K.; Mittal, A.; Mammen, K.J. Revascularisation of iatrogenic superior mesenteric artery injury by end to end anastomosis during robot assisted nephrectomy. Int. J. Surg. Case Rep. 2019, 63, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Chang, P.; Yang, J.; Zheng, D.; Zhang, D.; Wen, S.; Jing, S. A Novel Approach for Repairing Superior Mesenteric Artery Injury During Left Nephrectomy—6-year Follow-up. Urology 2020, 144, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Deb, M.; Jayaram, H.; Arlikar, J. Superior mesenteric artery injury during radical nephrectomy in an infant: Delayed diagnosis and successful management. J. Indian Assoc. Pediatr. Surg. 2021, 26, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Mayor, N.; Sapre, N.; Sandford, B.; Challacombe, B. Superior Mesenteric Artery Injury During Robot-assisted Laparoscopic Nephrectomy: A Robotic Nightmare. Eur. Urol. Open Sci. 2022, 38, 44–48. [Google Scholar] [CrossRef]
- Ashraf, W.; Malik, S.A.; Hamid, A.; Wani, M.S.; Khawaja, R. “Collaterals a savior” in superior mesenteric artery injury post radical nephrectomy and retroperitoneal lymph node dissection. Urol. Case Rep. 2022, 45, 102201. [Google Scholar] [CrossRef]
- Sayegh, A.S.; Medina, L.G.; La Riva, A.; Perez, L.C.; Poncel, J.; Forsyth, E.; Cacciamani, G.E.; Challacombe, B.; Stifelman, M.; Gill, I.; et al. Superior Mesenteric Artery Injury during Robotic Radical Nephrectomy: Scenarios and Management Strategies. J. Clin. Med. 2023, 12, 427. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Q.; Mo, G.; Guo, B. Superior Mesenteric Artery Injury during Radical Nephrectomy for Massive Renal Cell Carcinoma: A Case Report and Literature Review. Asian J. Surg. 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fullen, W.D.; Hunt, J.; Altemeier, W.A. The clinical spectrum of penetrating injury to the superior mesenteric arterial circulation. J Trauma Acute Care Surg 1972;12:656–664. [CrossRef]
- Asensio JA, Britt LD, Borzotta A, et al. Multi-institutional experience with the management of superior mesenteric artery injuries. J Am Coll Surg 2001;193(4):354-65; discussion 365-366.
- Lim, K.H.; Park, J. Successful conservative treatment of acute traumatic occlusions of the celiac artery and superior mesenteric artery A case report emphasizing the importance of the visceral collateral circulations. Medicine 2018, 97, e13270. [Google Scholar] [CrossRef]
- Mendoza, D.; Newman, R.C.; Albala, D.; Cohen, M.S.; Tewari, A.; Lingeman, J.; Wong, M.; Kavoussi, L.; Adams, J.; Moore, R.; et al. Laparoscopic complications in markedly obese urologic patients (A multi-institutional review). Urology 1996, 48, 562–567. [Google Scholar] [CrossRef]
- Chinni, V. , Alhamdani, Z., Bolton, D., Lawrentschuk, N., Jack, G. Robot Assisted Laparoscopy for Renal Cancer: Transperitoneal Versus Retroperitoneal Approach. In: Goonewardene, S.S., Persad, R., Albala, D. (eds) Robotic Surgery for Renal Cancer - Management of Urology. Springer Switzerland 2022; pp.185-212.
- Way LW, Stewart L, Gantert W, Liu K, Lee CM, Whang K, Hunter JG. Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg 2003;237(4):460-469.
- Kanizsa, G. Margini quasi-percettivi in campi con stimolazione omogenea. Rivista di Psicologia 1955;4:(1):7–30.
- Hinman F, Jr. Atlas of Urologic Surgery, 2nd Ed. WB Sauders 1998.
- Ferda, J.; Hora, M.; Hes, O.; Ferdová, E.; Kreuzberg, B. Assessment of the kidney tumor vascular supply by two-phase MDCT-angiography. Eur. J. Radiol. 2007, 62, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Hsu A, Bhattacharya KR, Chan HK, Huber TC, Gardner B, Stone JR, Angle JF. Effect of timing on endovascular therapy and exploratory laparotomy outcome in acute mesenteric ischemia. Ann Gastroenterol 2019;32(6):600-604.
- Hayashi, K.; Hayashi, K.; Narita, M.; Tsunoda, A.; Kusanagi, H. Still time to perform intestinal revascularization in patients with acute mesenteric ischemia with peritonitis: An analysis of bowel viability in resections. SAGE Open Med. 2020, 8. [Google Scholar] [CrossRef]
- Chou, E.L.; Wang, L.J.; McLellan, R.M.; Feldman, Z.M.; Latz, C.A.; LaMuraglia, G.M.; Clouse, W.D.; Eagleton, M.J.; Conrad, M.F. Evolution in the Presentation, Treatment, and Outcomes of Patients with Acute Mesenteric Ischemia. Ann. Vasc. Surg. 2021, 74, 53–62. [Google Scholar] [CrossRef] [PubMed]
- de Holanda GS, Dos Santos Valença S, Carra AM, et al. Translational Application of Fluorescent Molecular Probes for the Detection of Reactive Oxygen and Nitrogen Species Associated with Intestinal Reperfusion Injury. Metabolites. 2021;11(12):802.
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Évora, P.; Castro-E-Silva, O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).