Submitted:
18 September 2024
Posted:
19 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Method
2.1. Preparation of Fe3O4/ATO Hybrid Nanofluid
2.2. Optical Property Measurement Method
2.3. Experimental Setup for Photothermal Conversion
3. Results and Discussion
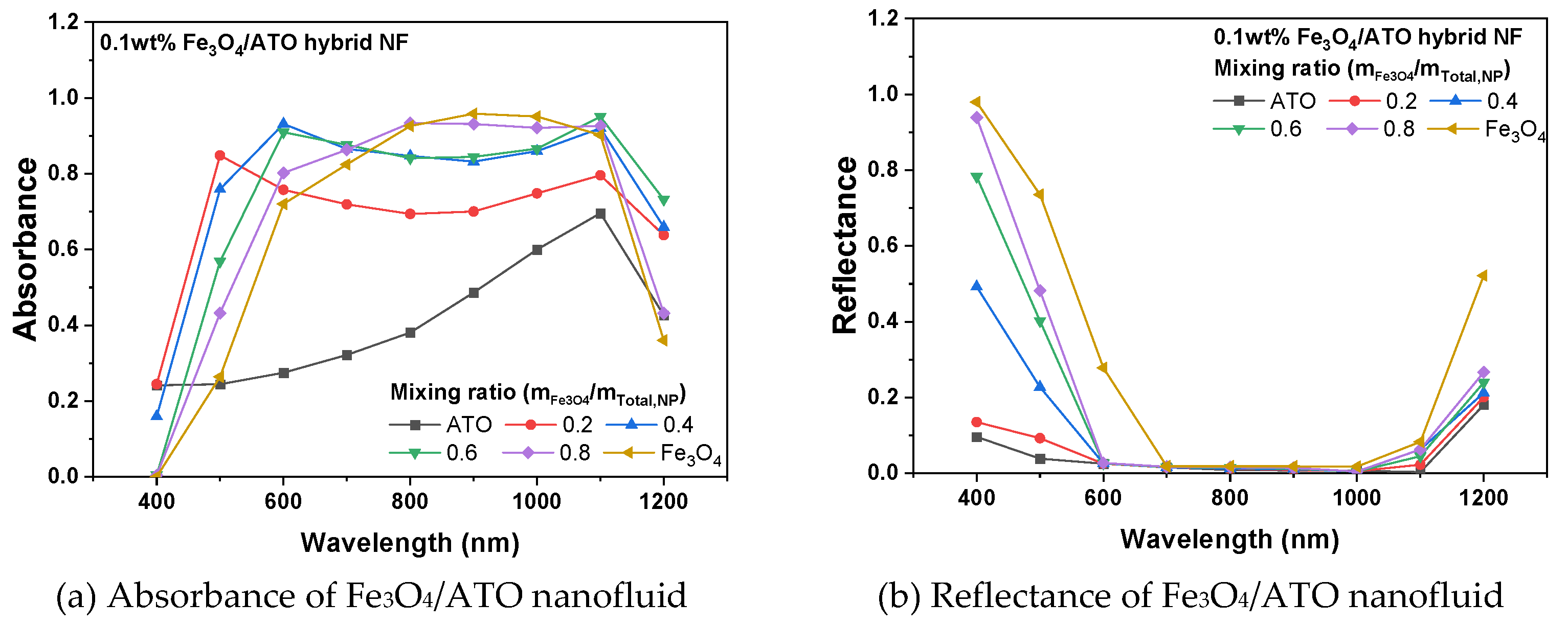
3.1. Optical Characteristics of Fe3O4, ATO and Fe3O4/ATO Hybrid Nanofluid
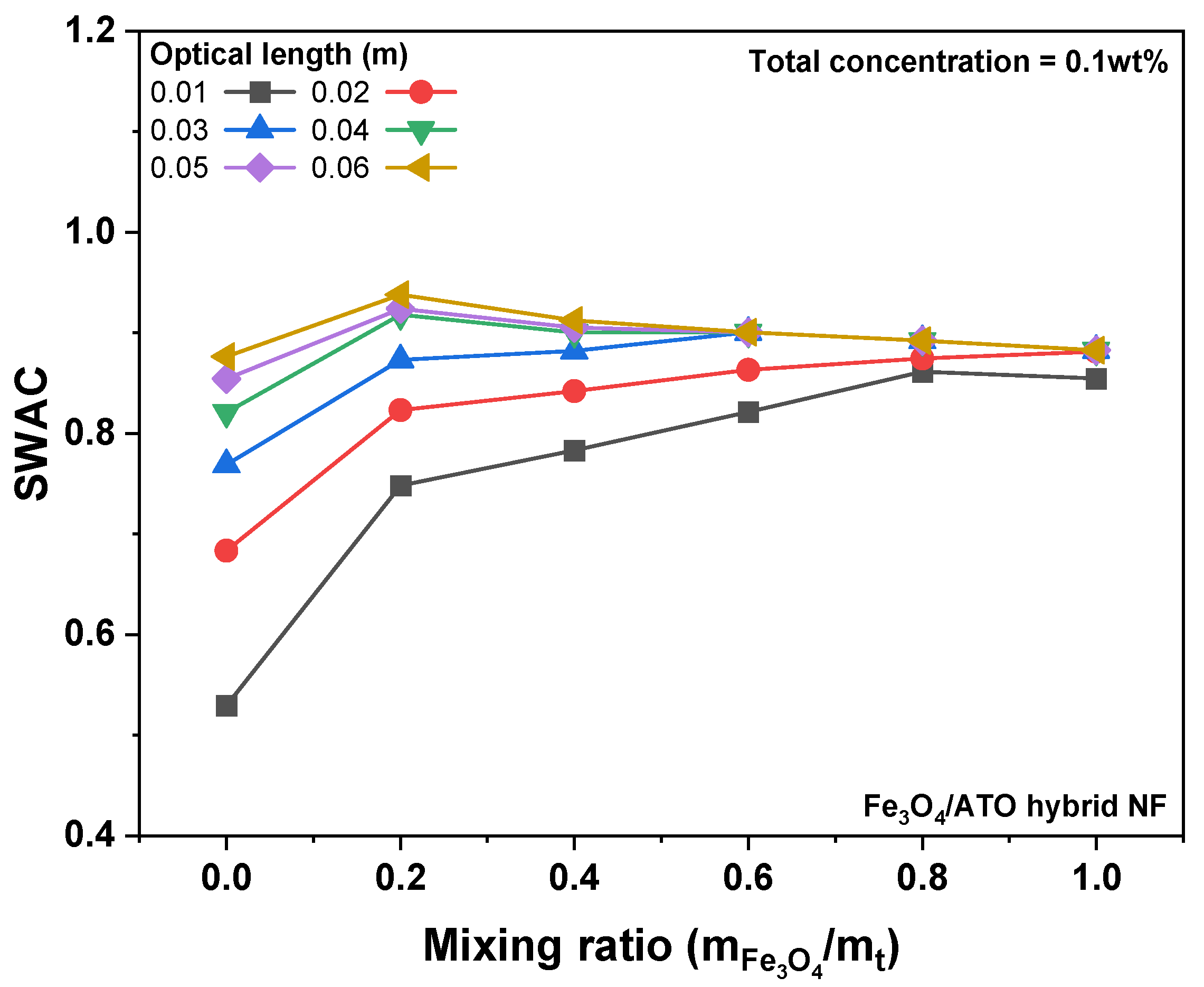
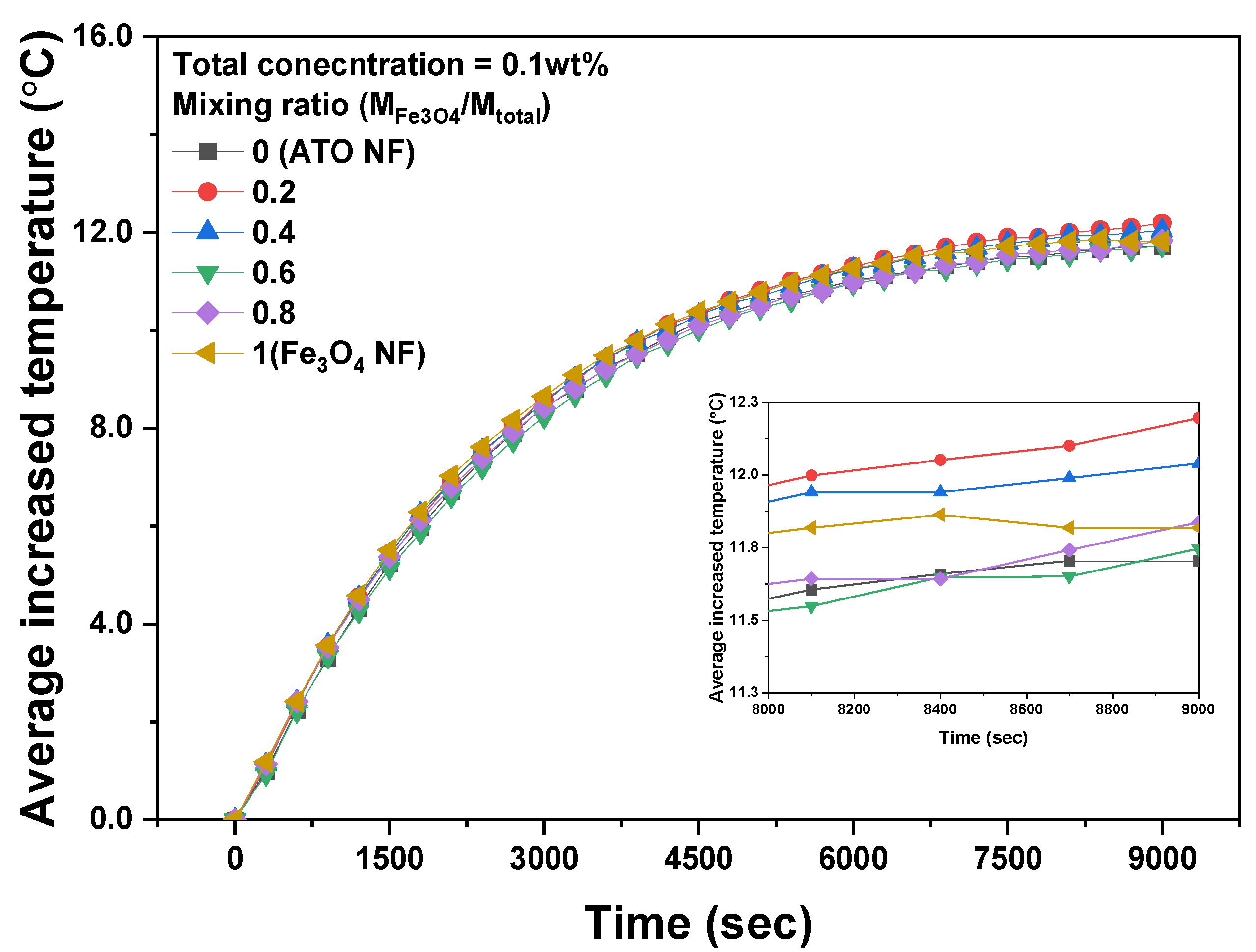
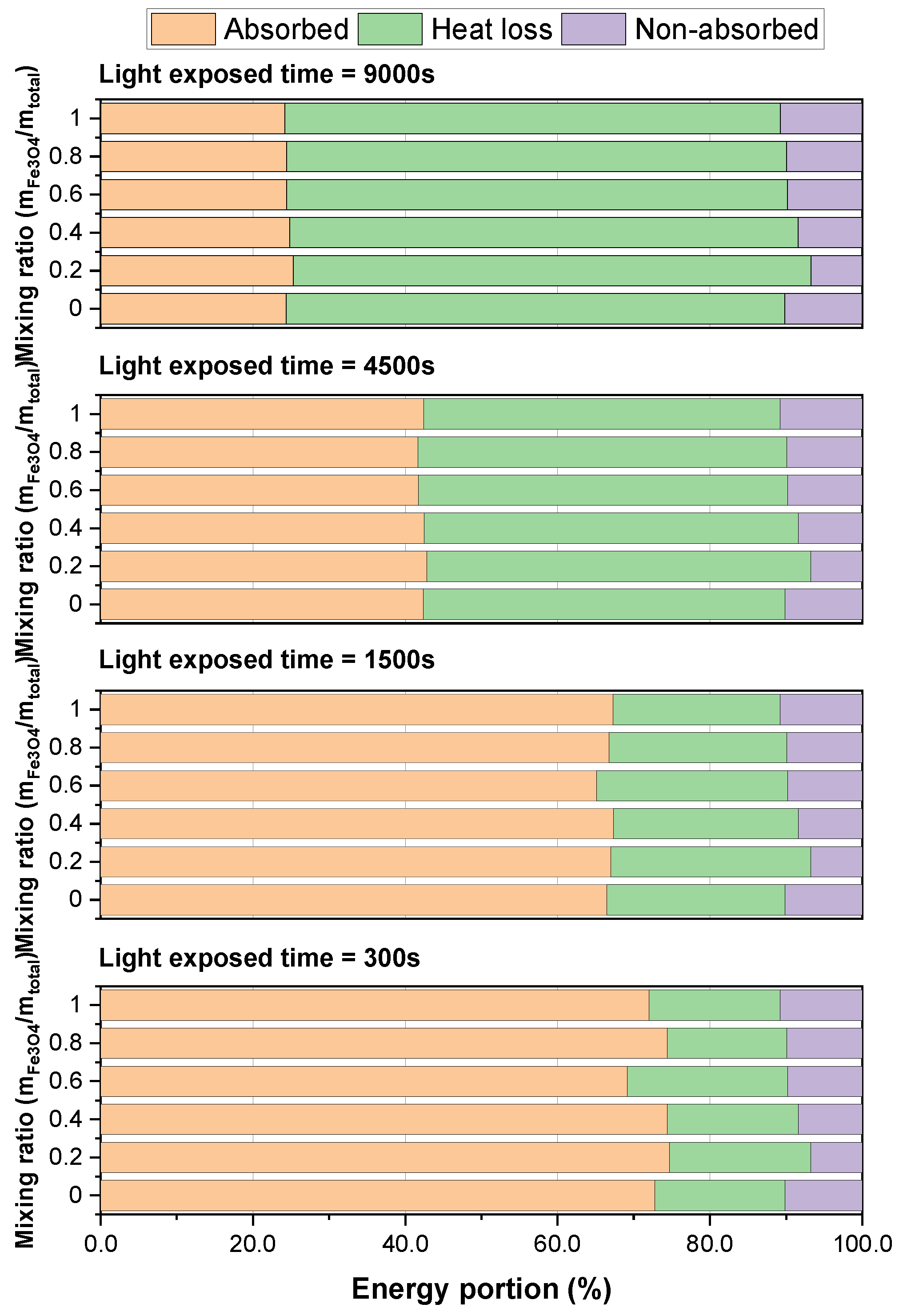
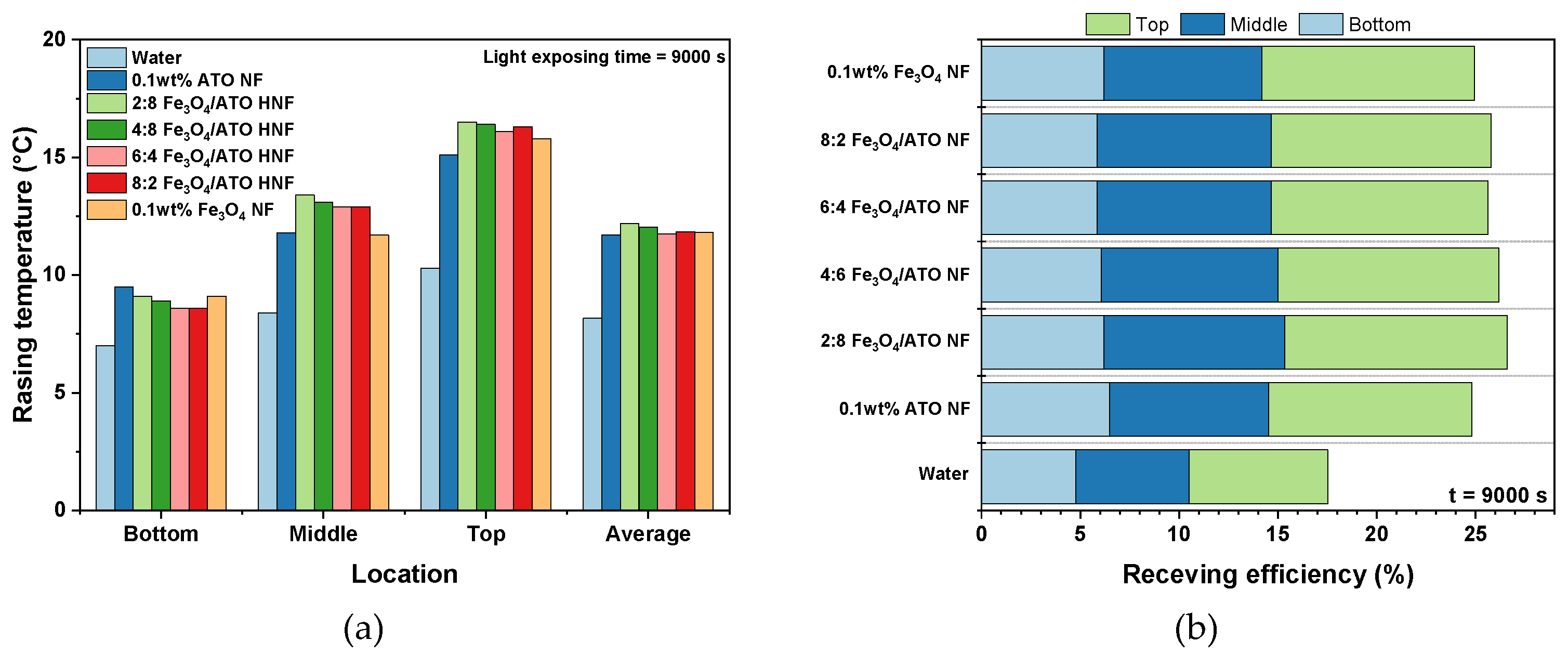
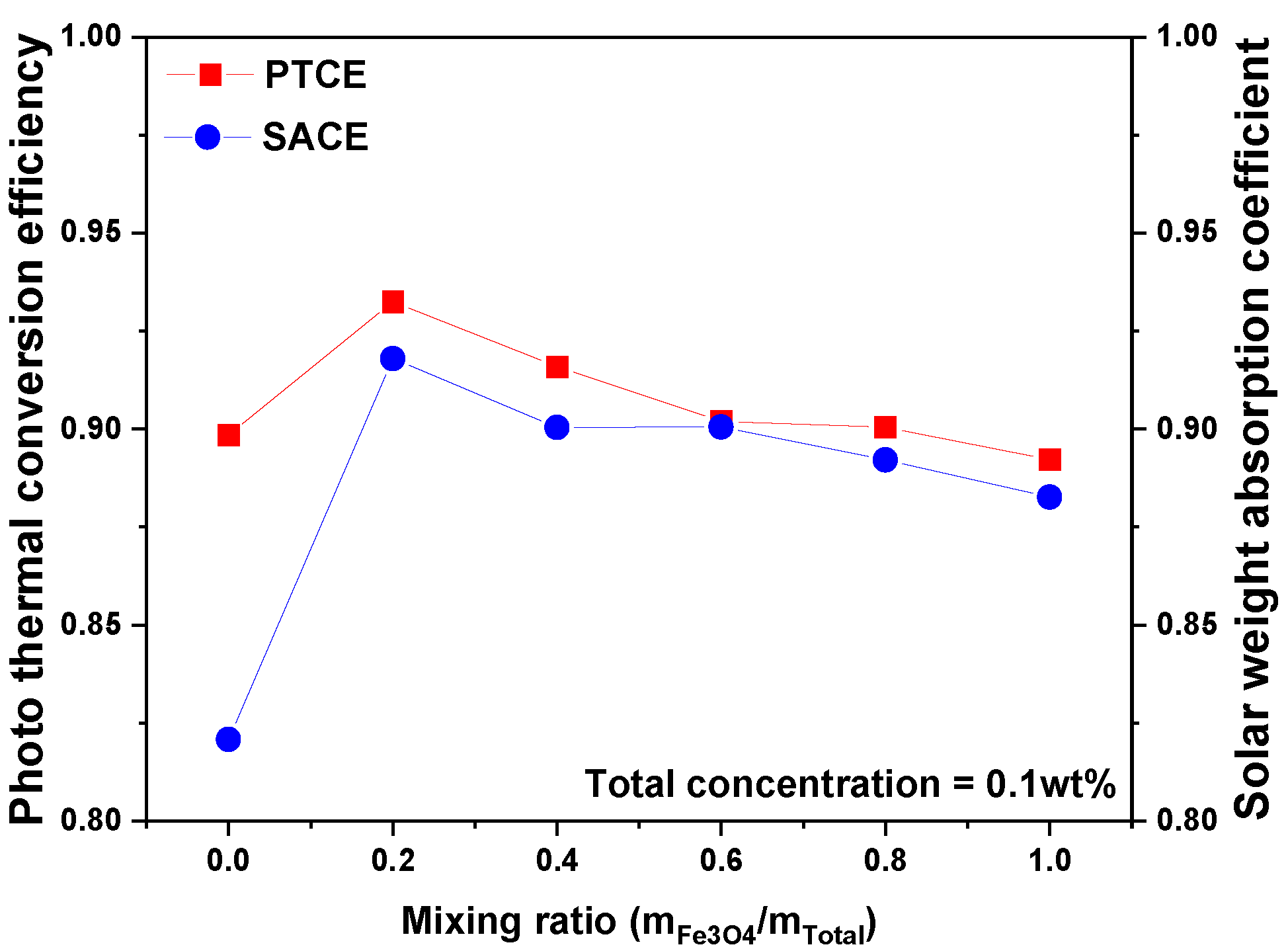
3.2. Photothermal Conversion Performance of Fe3O4/ATO Hybrid Nanofluid
4. Conclusions
Abbreviation
| Nomenclature | Greek symbols | |||
| Area (m2) | Efficiency | |||
| Absorbance | Wavelength (nm) | |||
| ATO | Antimony-doped tin oxide | Interface reflectance | ||
| Heat dissipation rate (1/s) | ||||
| Specific heat (J/kg·℃) | Subscript | |||
| Incident intensity (W/m2) | Ambient | |||
| Extinction coefficient (1/m) | AM1.5 | Am 1.5 global | ||
| Optical path length (m) | Bottom | |||
| Mass (kg) | Equilibrium stage | |||
| Nanofluid | Middle | |||
| Nanoparticle | Photothermal conversion | |||
| Reflectance | Top | |||
| Solar weight absorption coefficient | Receiving | |||
| Temperature () | ||||
| Transmittance | ||||
| Time (s) | ||||
Author Contributions
Funding
Conflicts of Interest
References
- Aissa, A.; Qasem, N.A.A.; Mourad, A.; Laidoudi, H.; Younis, O.; Guedri, K.; Alazzam, A. A Review of the Enhancement of Solar Thermal Collectors Using Nanofluids and Turbulators. Appl Therm Eng 2023, 220, 119663. [Google Scholar] [CrossRef]
- Miglioli, A.; Aste, N.; Del Pero, C.; Leonforte, F. Photovoltaic-Thermal Solar-Assisted Heat Pump Systems for Building Applications: Integration and Design Methods. Energy and Built Environment 2023, 4, 39–56. [Google Scholar] [CrossRef]
- Saini, P.; Ghasemi, M.; Arpagaus, C.; Bless, F.; Bertsch, S.; Zhang, X. Techno-Economic Comparative Analysis of Solar Thermal Collectors and High-Temperature Heat Pumps for Industrial Steam Generation. Energy Convers Manag 2023, 277, 116623. [Google Scholar] [CrossRef]
- Vahidhosseini, S.M.; Rashidi, S.; Hsu, S.H.; Yan, W.M.; Rashidi, A. Integration of Solar Thermal Collectors and Heat Pumps with Thermal Energy Storage Systems for Building Energy Demand Reduction: A Comprehensive Review. J Energy Storage 2024, 95, 112568. [Google Scholar] [CrossRef]
- Qin, C.; Kim, J.B.; Lee, B.J. Performance Analysis of a Direct-Absorption Parabolic-Trough Solar Collector Using Plasmonic Nanofluids. Renew Energy 2019, 143, 24–33. [Google Scholar] [CrossRef]
- Howe, M.L.; Paul, T.C.; Khan, J.A. Radiative Properties of Al2O3 Nanoparticles Enhanced Ionic Liquids (NEILs) for Direct Absorption Solar Collectors. Solar Energy Materials and Solar Cells 2021, 232, 111327. [Google Scholar] [CrossRef]
- Vital, C.V.P.; Farooq, S.; de Araujo, R.E.; Rativa, D.; Gómez-Malagón, L.A. Numerical Assessment of Transition Metal Nitrides Nanofluids for Improved Performance of Direct Absorption Solar Collectors. Appl Therm Eng 2021, 190, 116799. [Google Scholar] [CrossRef]
- Sreekumar, S.; Joseph, A.; Kumar, C.S.S.; Thomas, S.; Sujith Kumar, C.S.; Thomas, S. Investigation on Influence of Antimony Tin Oxide/Silver Nanofluid on Direct Absorption Parabolic Solar Collector. J Clean Prod 2020, 249, 119378. [Google Scholar] [CrossRef]
- Gupta, H.K.; Agrawal, G. Das; Mathur, J. An Experimental Investigation of a Low Temperature Al2O3-H2O Nanofluid Based Direct Absorption Solar Collector. Solar Energy 2015, 118, 390–396. [Google Scholar] [CrossRef]
- Kalidoss, P.; Venkatachalapathy, S.G.; Suresh, S. Photothermal Performance of Hybrid Nanofluids with Different Base Fluids for Solar Energy Applications. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2021; 1–16. [Google Scholar]
- Vallejo, J.P.; Sani, E.; Żyła, G.; Lugo, L. Tailored Silver/Graphene Nanoplatelet Hybrid Nanofluids for Solar Applications. J Mol Liq 2019, 296, 112007. [Google Scholar] [CrossRef]
- Huminic, G.; Vărdaru, A.; Huminic, A.; Fleacă, C.; Dumitrache, F. Broad-Band Absorption and Photo-Thermal Conversion Characteristics of RGO-Ag Hybrid Nanofluids. J Mol Liq 2024, 408, 125347. [Google Scholar] [CrossRef]
- Fang, J.; Xuan, Y. Investigation of Optical Absorption and Photothermal Conversion Characteristics of Binary CuO/ZnO Nanofluids. RSC Adv 2017, 7, 56023–56033. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, L.; Zhou, X.; Wu, X. Stability and Photothermal Properties of Fe3O4-H2O Magnetic Nanofluids. Nanomaterials 2023, 13, 1962. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.; Shin, Y.; Cho, H. Comparison of Thermal Performance between a Surface and a Volumetric Absorption Solar Collector Using Water and Fe3O4 Nanofluid. Energy 2022, 239, 122282. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Yan, S.; Yang, X.; Tian, R.; Zhao, X. Stability, Thermal Conductivity and Photothermal Conversion Performance of Water-Based ZnO Nanofluids. Journal of Thermal Science 2021, 30, 1581–1595. [Google Scholar] [CrossRef]
- Shin, Y.; Ham, J.; Boldoo, T.; Cho, H. Magnetic Effect on the Enhancement of Photo-Thermal Energy Conversion Efficiency of MWCNT/Fe3O4 Hybrid Nanofluid. Solar Energy Materials and Solar Cells 2020, 215, 110635. [Google Scholar] [CrossRef]
- Li, X.; Zeng, G.; Lei, X. The Stability, Optical Properties and Solar-Thermal Conversion Performance of SiC-MWCNTs Hybrid Nanofluids for the Direct Absorption Solar Collector (DASC) Application. Solar Energy Materials and Solar Cells 2020, 206, 110323. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, R.; Shang, L.; Wang, Z. Graphene Oxide/Multi-walled Carbon Nanotube—Therminol® 66 Hybrid Nanofluids for Low-to-medium Temperature Volumetric Solar Collectors. Int J Energy Res 2020, 44, 7216–7228. [Google Scholar] [CrossRef]
- Chen, L.; Xu, C.; Liu, J.; Fang, X.; Zhang, Z. Optical Absorption Property and Photo-Thermal Conversion Performance of Graphene Oxide/Water Nanofluids with Excellent Dispersion Stability. Solar Energy 2017, 148, 17–24. [Google Scholar] [CrossRef]
- Zuo, X.; Yang, W.; Shi, M.; Yan, H.; Guan, C.; Wu, S.; Zhang, Z.; Li, X.; Li, Z. Experimental Investigation on Photothermal Conversion Properties of Lampblack Ink Nanofluids. Solar Energy 2021, 218, 1–10. [Google Scholar] [CrossRef]
- Ni, Z.; Cao, X.; Wang, X.; Zhou, S.; Zhang, C.; Xu, B.; Ni, Y. Facile Synthesis of Copper (I) Oxide Nanochains and the Photo-Thermal Conversion Performance of Its Nanofluids. Coatings 2021, 11, 749. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, K.; Wang, L.; Xie, H.; Yu, W. Mesoporous CuO with Full Spectrum Absorption for Photothermal Conversion in Direct Absorption Solar Collectors. Solar Energy 2020, 201, 628–637. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Luo, B.; Wei, K.; Zeng, G. The MXene/Water Nanofluids with High Stability and Photo-Thermal Conversion for Direct Absorption Solar Collectors: A Comparative Study. Energy 2021, 227, 120483. [Google Scholar] [CrossRef]
- Tong, Y.; Ham, J.; Cho, H. Investigation of Thermo-Optical Properties and Photothermal Conversion Performance of MWCNT, Fe3O4, and ATO Nanofluid for Volumetric Absorption Solar Collector. Appl Therm Eng 2024, 246, 123005. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, M.; Yan, H.; Zhou, P.; Chen, X.Y. Enhanced Solar Thermal Conversion Performance of Plasmonic Gold Dimer Nanofluids. Appl Therm Eng 2020, 178, 115561. [Google Scholar] [CrossRef]
- Shang, L.; Qu, J.; Wang, Z.; Zhang, M.; Li, C. Optical Absorption Property and Photo-Thermal Conversion Performance of Ag@Al2O3 Plasmonic Nanofluids with Al2O3 Nano-Shell Fabricated by Atomic Layer Deposition. J Mol Liq 2021, 326, 115388. [Google Scholar] [CrossRef]
- Joseph, A.; Sreekumar, S.; Kumar, C.S.S.; Thomas, S. Optimisation of Thermo-Optical Properties of SiO2/Ag–CuO Nanofluid for Direct Absorption Solar Collectors. J Mol Liq 2019, 296, 111986. [Google Scholar] [CrossRef]
- Hazra, S.K.; Michael, M.; Nandi, T.K. Investigations on Optical and Photo-Thermal Conversion Characteristics of BN-EG and BN/CB-EG Hybrid Nanofluids for Applications in Direct Absorption Solar Collectors. Solar Energy Materials and Solar Cells 2021, 230, 111245. [Google Scholar] [CrossRef]
- Tong, Y.; Boldoo, T.; Ham, J.; Cho, H. Improvement of Photo-Thermal Energy Conversion Performance of MWCNT/Fe3O4 Hybrid Nanofluid Compared to Fe3O4 Nanofluid. Energy 2020, 196, 117086. [Google Scholar] [CrossRef]
- Wang, R.; Xing, L.; Ha, Y.; Zhong, P.; Wang, Z.; Cao, Y.; Li, Z. Photothermal Conversion and Thermal Management of Magnetic Plasmonic Fe3O4@ Au Nanofluids. Solar RRL 2023. [Google Scholar] [CrossRef]
- Qu, D.; Cheng, L.; Bao, Y.; Gao, Y.; Zheng, X.; Qin, G. Enhanced Optical Absorption and Solar Steam Generation of CB-ATO Hybrid Nanofluids. Renew Energy 2022, 199, 509–516. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, W.; Yu, L.; Chen, M.; Zheng, X.; Qin, G. Tunable Optical Properties of ATO-CuO Hybrid Nanofluids and the Application as Spectral Beam Splitters. Energy 2023, 129964. [Google Scholar] [CrossRef]
- ASTM G. 173-03Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37° Tilted Surface, American Society for Testing and Materials, 2012.

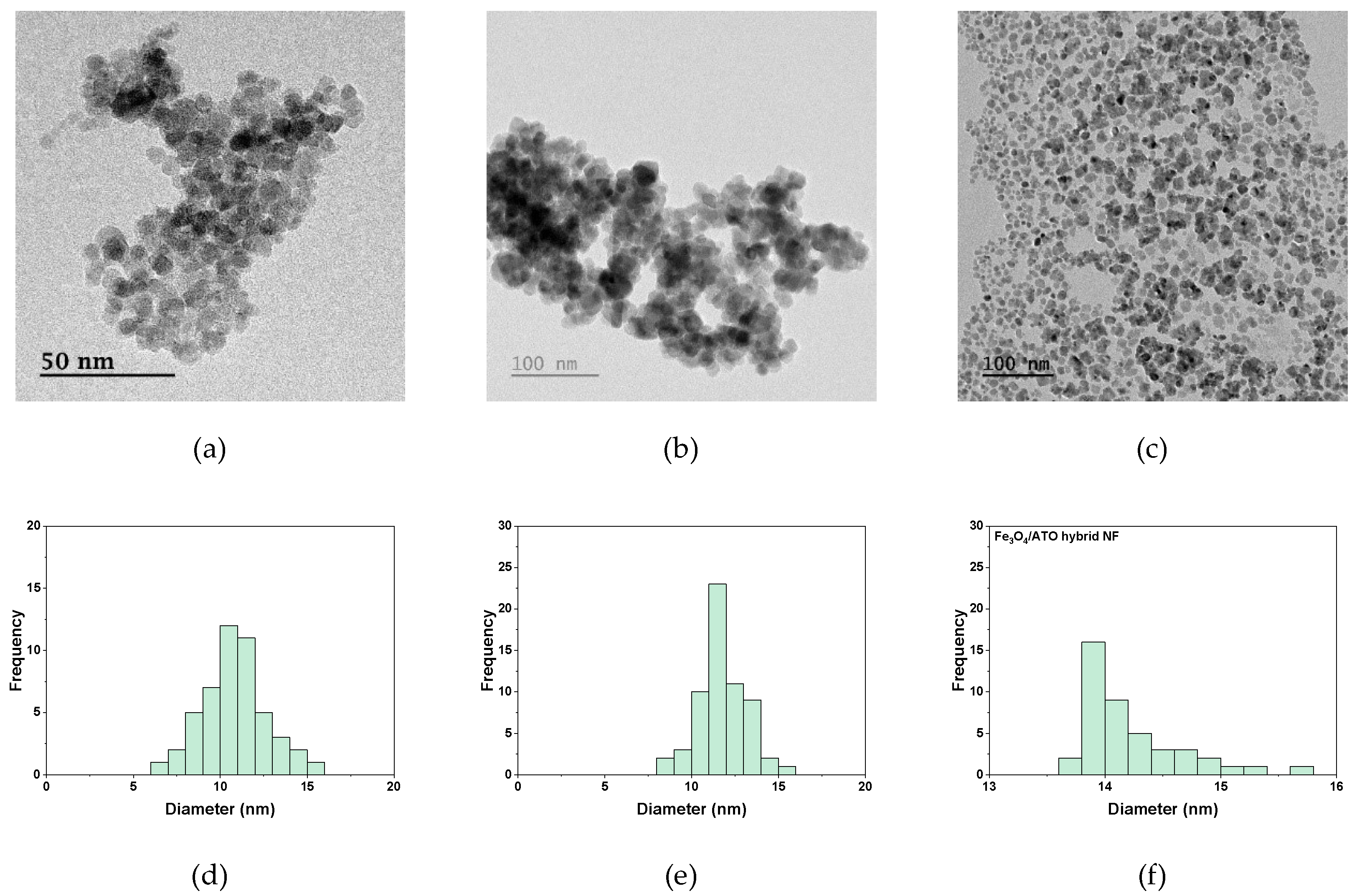
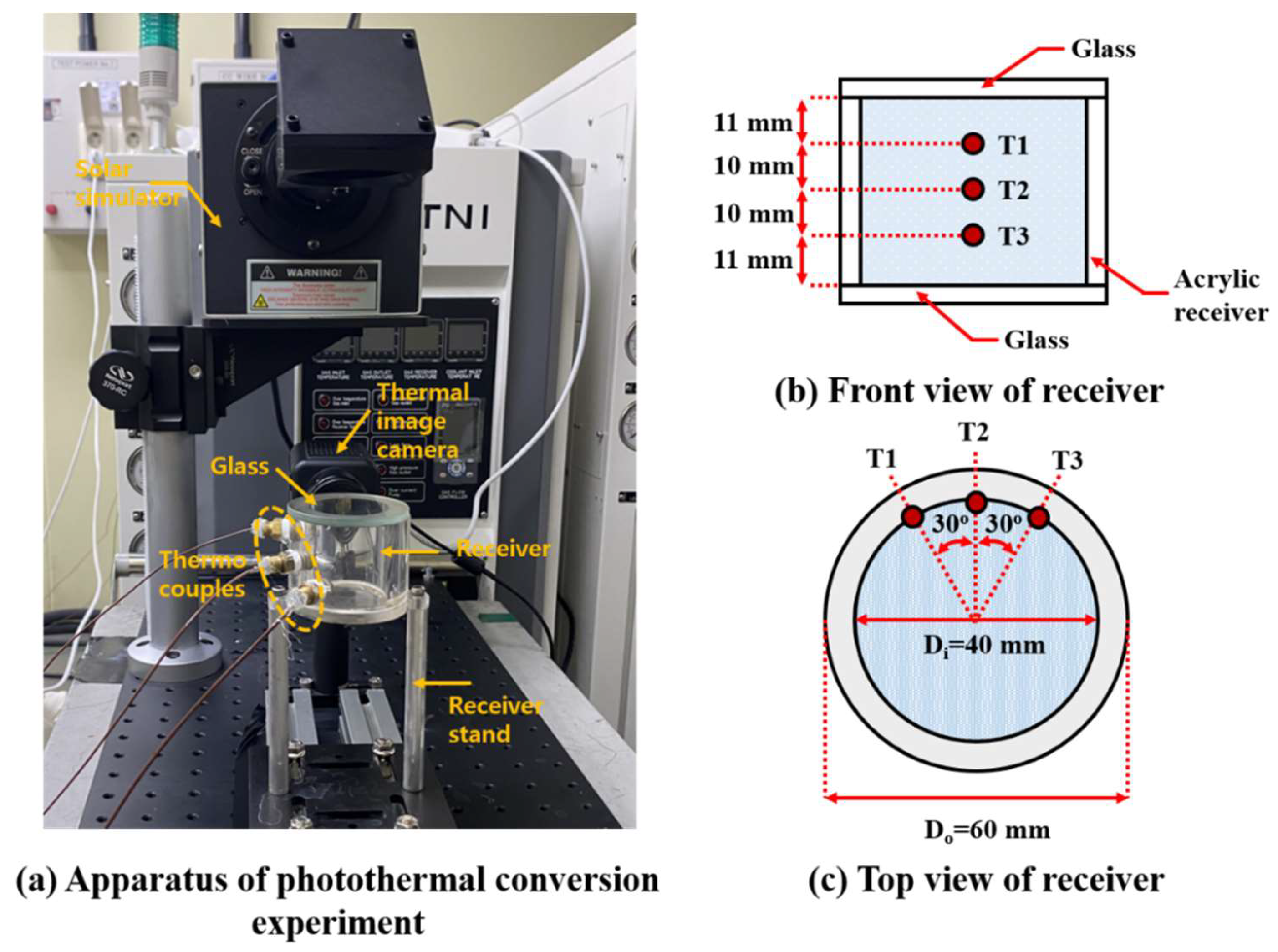
| NP | Fe3O4 | ATO |
|---|---|---|
| Purity | 99% | |
| Color | Dark brown | Blue |
| Outer diameter | 5–20 nm | nm |
| Thermal conductivity | 80 W/mK | 4.4 W/mK |
| True density | 5.1 g/cm3 | 6.8 g/cm3 |
| Manufacturing method | Co-precipitation | 50% aqua solution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





