Submitted:
18 September 2024
Posted:
19 September 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Animal Ethics Statement
Animals and Husbandry
Experimental Design
-
Treatment MBM:
- ○
- Diet based on corn, soybean meal, and meat and bone meal administered from 1 to 90 weeks.
-
Treatment BAC:
- ○
- Diet based on corn, soybean meal, and meat and bone meal, with the addition of 0.05% zinc bacitracin administered from 1 to 90 weeks.
-
Treatment SIMC:
- ○
- Diet based on corn, soybean meal, and meat and bone meal, supplemented with 0.1% symbiotic, administered from 1 to 90 weeks.
-
Treatment SIMR:
- ○
- From 1 to 5 weeks: Diet based on corn, soybean meal, and meat and bone meal.
- ○
- From 6 to 90 weeks: Diet based on corn, soybean meal, and meat and bone meal, supplemented with 0.1% symbiotic.
-
Treatment SIMP:
- ○
- From 1 to 16 weeks: Diet based on corn, soybean meal, and meat and bone meal.
- ○
- From 17 to 90 weeks: Diet based on corn, soybean meal, and meat and bone meal, supplemented with 0.1% symbiotic.
Dietary Treatments
Blood Collection and Biochemical Analysis
Organ Collection
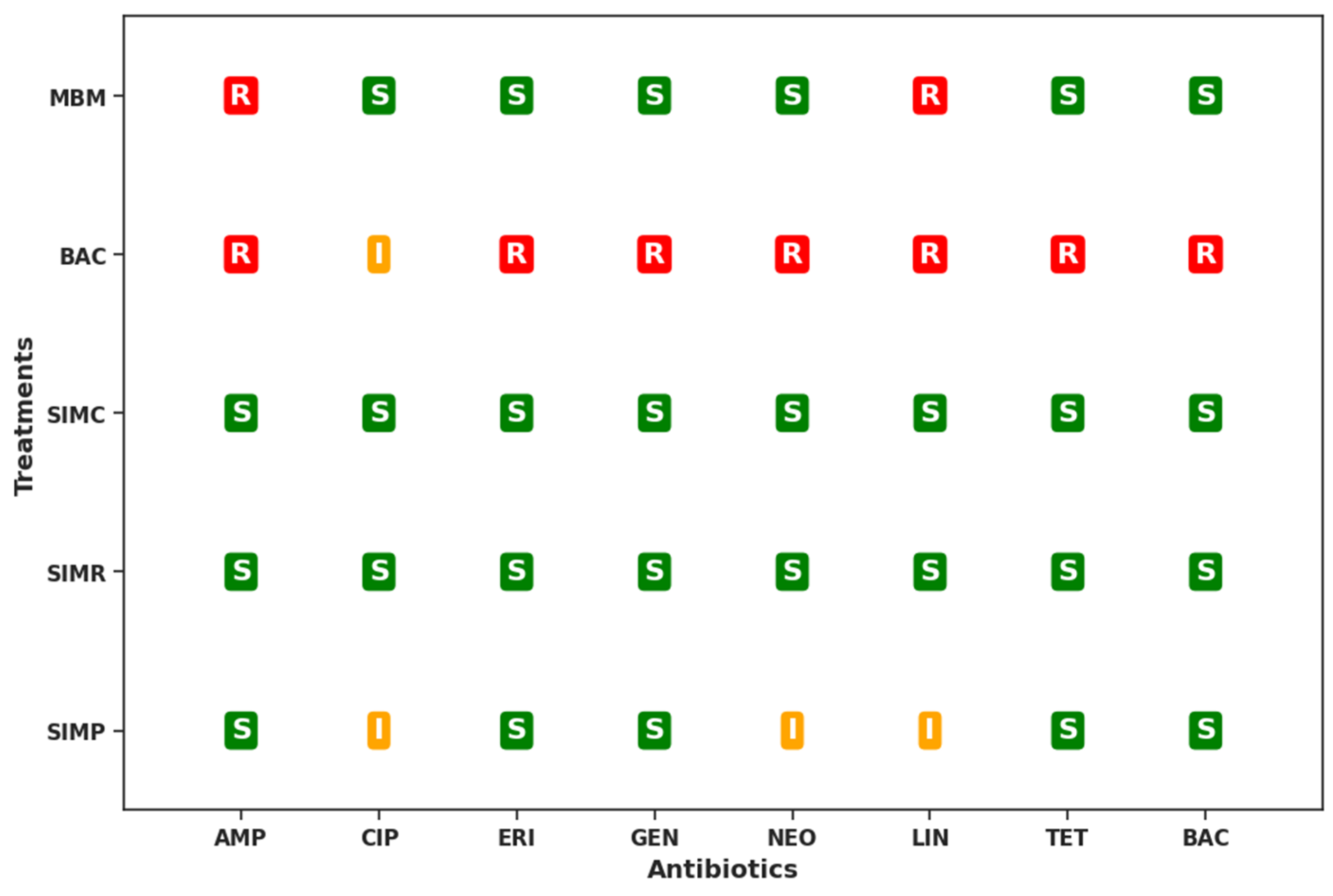
Isolation of C. perfringens and Antimicrobial Resistance
Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hester, P.Y.; Enneking, S.A.; Jefferson-Moore, K.; Einstein, M.; Cheng, H.W.; Rubin, D.A. The effect of perches in cages during pullet rearing and egg laying on hen performance, foot health, and plumage. Poultry Science 2013, 92, 310–320. [CrossRef]
- Jing, M.; Munyaka, P.M.; Tactacan, G.B.; Rodriguez-Lecompte, J.C.; O, K.; House, J.D. Performance, serum biochemical responses, and gene expression of intestinal folate transporters of young and older laying hens in response to dietary folic acid supplementation and challenge with Escherichia coli lipopolysaccharide. Poultry Science 2014, 93, 122–131. [CrossRef] [PubMed]
- Adetunji, C.O.; Adejumo, I.O. Potency of agricultural wastes in mushroom (Pleurotus sajor-caju) biotechnology for feeding broiler chicks (Arbor acre). International Journal of Recycling of Organic Waste in Agriculture 2018, 8, 37–45. [CrossRef]
- FAO. Outputs and activities of FAO Project FMM/RAS/298/MUL and summary of FAO’s recent work on antimicrobial resistance in aquaculture. FAO 2020.
- Hamasalim, H.J. Synbiotic as feed additives relating to animal health and performance. Advances in Microbiology 2016, 6, 288–302. [CrossRef]
- Wanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nature Reviews Gastroenterology & Hepatology 2020, 17, 687–701.
- Mountzouris, K.C.; Tsitrsikos, P.; Palamidi, I.; Arvaniti, A.; Mohnl, M.; Schatzmayr, G.; Fegeros, K. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poultry Science 2010, 89, 58–67. [CrossRef]
- Sjofjan, O.; Natsir, M.H.; Adli, D.N.; Adelina, D.D.; Triana, L.M. Effect of symbiotic flour (Lactobacillus Sp. and FOS) to the egg quality and performance of laying hens. IOP Conference Series: Earth and Environmental Science 2020, 465, 012033.
- Lv, J.; Guo, L.; Chen, B.; Hao, K.; Ma, H.; Liu, Y.; Min, Y. Effects of different probiotic fermented feeds on production performance and intestinal health of laying hens. Poultry Science 2022, 101, 101570. [CrossRef]
- Khattab, A.A.; Basuini, M.F.M.E.; El-Ratel, I.T.; Fouda, S.F. Dietary probiotics as a strategy for improving growth performance, intestinal efficacy, immunity, and antioxidant capacity of white Pekin ducks fed with different levels of CP. Poultry Science 2021, 100, 100898. [CrossRef]
- Deng, Q.; Shi, H.; Luo, Y.; Liu, N.; Deng, X. Dietary lactic acid bacteria modulate yolk components and cholesterol metabolism by Hmgr pathway in laying hens. Brazilian Journal of Poultry Science 2020, 22, 1–8. [CrossRef]
- Adriani, L.; Latipudin, D.; Joni, I.M.; Panatarani, C.; Sania, G. Hematological status and egg production of laying hen with probiotic powder as feed supplements. IOP Conference Series: Earth and Environmental Science 2021, 902, 012032.
- Mookiah, S.; Sieo, C.C.; Ramasamy, K.; Abdullah, N.; Ho, Y.W. Effects of dietary prebiotics, probiotic, and synbiotics on performance, caecal bacterial populations, and caecal fermentation concentrations of broiler chickens. Journal of the Science of Food and Agriculture 2013, 94, 341–348. [CrossRef] [PubMed]
- Iji, P.A.; Tivey, D.R. Natural and synthetic oligosaccharides in broiler chicken diets. World’s Poultry Science Journal 1998, 54, 129–143.
- Yan, F.F.; Mohammed, A.A.; Murugesan, G.R.; Cheng, H.W. Effects of a dietary synbiotic inclusion on bone health in broilers subjected to cyclic heat stress episodes. Poultry Science 2019, 98, 1083–1089. [CrossRef]
- Dai, D.; Qi, G.; Wang, J.; Zhang, H.; Qiu, K.; Wu, S. Intestinal microbiota of layer hens and its association with egg quality and safety. Poultry Science 2022, 101, 102008. [CrossRef]
- Biggs, P.; Parsons, C.M.; Fahey, G.C. The effects of several oligosaccharides on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks. Poultry Science 2007, 86, 2327–2336. [CrossRef] [PubMed]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śbikowski, A.; Szeleszczuk, P. The effect of synbiotic preparations on the intestinal microbiota and her metabolism in broiler chickens. Scientific Reports 2020, 10, 1–13. [CrossRef] [PubMed]
- Lamora, Z.V.D.; Nuño, K.; Vázquez-Paulino, O.; Avalos, H.; Castro-Rosas, J.; Gómez-Aldapa, C.; Angulo, C.; Ascencio, F.; Villarruel-López, A. Effect of a synbiotic mix on intestinal structural changes and Salmonella Typhimurium and Clostridium Perfringens colonization in broiler chickens. Animals 2019, 9, 777. [CrossRef] [PubMed]
- Kiu, R.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium Perfringens. Emerging Microbes & Infections 2018, 7, 1–15.
- Latorre, J.D.; Adhikari, B.; Park, S.H.; Teague, K.D.; Graham, L.E.; Mahaffey, B.D.; Baxter, M.F.A.; Hernandez-Velasco, X.; Kwon, Y.M.; Ricke, S.C. Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Frontiers in Veterinary Science 2018, 5, 1–11. [CrossRef] [PubMed]
- Bouassi, T.; Libanio, D.; Mesa, M.D.; Oke, O.E.; Gil, A.H.; Tona, K.; Ameyapoh, Y. Supplementation with liquid whey and ACIDAL® ML in drinking water affect gut pH and microflora and productive performance in laying hens. British Poultry Science 2020, 62, 138–146. [CrossRef] [PubMed]
- Kimminau, E.A.; Karnezos, T.P.; Berghaus, R.D.; Jones, M.K.; Baxter, J.A.; Hofacre, C.L. Combination of probiotic and prebiotic impacts Salmonella Enteritidis infection in layer hens. Journal of Applied Poultry Research 2021, 30, 100200. [CrossRef]
- Yaqoob, M.U.; El-Hack, M.E.; Hassan, F.; El-Saadony, M.T.; Khafaga, A.F.; Batiha, G.E.; Yehia, N.; Elnesr, S.S.; Alagawany, M.; El-Tarabilly, K.A. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poultry Science 2021, 100, 101143. [CrossRef]
- Rostagno, H.S.; Albino, L.F.T.; Hannas, M.I.; et al. Tabelas Brasileiras para Aves e Suínos, 4th ed.; UFV/Departamento de Zootecnia: Viçosa, Brazil, 2017.
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-first Informational Supplement; CLSI: Wayne, PA, USA, 2014.
- Li, X.; Liu, L.; Li, K.; Hao, K.; Xu, C. Effect of fructooligosaccharides and antibiotics on laying performance of chickens and cholesterol content of egg yolk. British Poultry Science 2007, 48, 185–189. [CrossRef]
- Sugiharto, S.; Isroli, I.; Yudiarti, T.; Widiastuti, E. The effect of supplementation of multistrain probiotic preparation in combination with vitamins and minerals to the basal diet on the growth performance, carcass traits, and physiological response of broilers. Veterinary World 2018, 11, 240–247. [CrossRef] [PubMed]
- Chauhan, S.S.; Sharma, R.K.; Singh, D.V.; Shukla, S.K.; Palod, J.; Singh, M.K. Studies on serum mineral profile and kidney function of broiler chickens fed diets containing different supplements. Indian Journal of Animal Research 2020, 189–192. [CrossRef]
- Hatab, M.H.; Elsayed, M.A.; Ibrahim, N.S. Effect of some biological supplementation on productive performance, physiological, and immunological response of layer chicks. Journal of Radiation Research and Applied Sciences 2016, 9, 185–192. [CrossRef]
- Melillo, A. Applications of serum protein electrophoresis in exotic pet medicine. Veterinary Clinics of North America: Exotic Animal Practice 2013, 16, 211–225.
- Rashidi, N.; Khatibjoo, A.; Taherpour, K.; Akbari-Gharaei, M.; Shirzadi, H. Effects of licorice extract, probiotic, toxin binder, and poultry litter biochar on performance, immune function, blood indices, and liver histopathology of broilers exposed to aflatoxin-B1. Poultry Science 2020, 99, 5896–5906. [CrossRef]
- Xu, Q.; Azzam, M.M.M.; Zou, X.; Dong, X. Effects of chitooligosaccharide supplementation on laying performance, egg quality, blood biochemistry, antioxidant capacity, and immunity of laying hens during the late laying period. Italian Journal of Animal Science 2020, 19, 1180–1187. [CrossRef]
- Khabirov, A.; Khaziakhmetov, F.; Kuznetsov, V.; Tagirov, H.; Rebezov, M.; Andreyeva, A.; Basharov, A.; Yessimbekov, Z.; Ayaz, M. Effect of Normosil probiotic supplementation on the growth performance and blood parameters of broiler chickens. Indian Journal of Pharmaceutical Education and Research 2020, 54, 1046–1055. [CrossRef]
- Ndrepepa, G. Aspartate aminotransferase and cardiovascular disease—a narrative review. Journal of Laboratory and Precision Medicine 2021, 6, 6. [CrossRef]
- Thrall, A.M. Hematologia e Bioquímica Clínica Veterinária, 2nd ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2015.
- González, F.; Silva, S.C. Perfil Bioquímico Sanguíneo: Introdução à Bioquímica Clínica Veterinária; [n.p.]: Rio Grande do Sul, Brazil, 2006.
- Tang, S.G.H.; Sieo, C.C.; Ramasamy, K.; Saad, W.Z.; Wong, H.K.; Ho, Y.W. Performance, biochemical, and haematological responses, and relative organ weights of laying hens fed diets supplemented with prebiotic, probiotic, and synbiotic. BMC Veterinary Research 2017, 13, 1–12. [CrossRef]
- Attia, Y.; Hamed, R.; El-Hamid, A.; Al-Harthi, M.; Shahba, H.; Bovera, F. Performance, blood profile, carcass and meat traits, and tissue morphology in growing rabbits fed mannanoligosaccharides and zinc-bacitracin continuously or intermittently. Animal Science Papers and Reports 2015, 33, 85–101.
- Michalska, K.; Gesek, M.; Sokoł, R.; Murawska, D.; Mikiewicz, M.; Chłodowska, A. Effective microorganisms (EM) improve internal organ morphology, intestinal morphometry, and serum biochemical activity in Japanese quails under Clostridium Perfringens challenge. Molecules 2021, 26, 2786. [CrossRef]
- Alvarenga, R.R.; Zangeronimo, M.G.; Pereira, L.J.; Rodrigues, P.B.; Gomide, E.M. Lipoprotein metabolism in poultry. World’s Poultry Science Journal 2011, 67, 431–440.
- Gilliland, S.E.; Nelson, C.R.; Maxwell, C. Assimilation of cholesterol by Lactobacillus acidophilus. Applied and Environmental Microbiology 1985, 49, 377–381. [CrossRef]
- Fukushima, M.; Nakano, M. The effect of a probiotic on faecal and liver lipid classes in rats. British Journal of Nutrition 1995, 73, 701–710. [CrossRef] [PubMed]
- Ooi, L.G.; Liong, M.T. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. International Journal of Molecular Sciences 2010, 11, 2499–2522. [CrossRef]
- Ogboko, B. Lipid profile of broilers fed zinc bacitracin on plant and animal protein diets. International Journal of Poultry Science 2011, 10, 567–573. [CrossRef]
- Reuben, R.C.; Sarkar, S.L.; Ibnat, H.; Setu, M.A.; Roy, P.C.; Jahid, I.K. Novel multi-strain probiotics reduces Pasteurella multocida induced fowl cholera mortality in broilers. Scientific Reports 2021, 11, 1–16. [CrossRef]
- Wang, W.; Yang, H.; Wang, Z.; Han, J.; Zhang, D.; Sun, H.; Zhang, F. Effects of prebiotic supplementation on growth performance, slaughter performance, growth of internal organs and small intestine, and serum biochemical parameters of broilers. Journal of Applied Animal Research 2014, 43, 33–38. [CrossRef]
- Yu, W.; Hao, X.; Zhiyue, W.; Haiming, Y.; Lei, X. Evaluation of the effect of Bacillus subtilis and Pediococcus acidilactici mix on serum biochemistry growth, promotion of body, and visceral organs in Lohmann Brown chicks. Brazilian Journal of Poultry Science 2020, 22, 1–8. [CrossRef]
- Sharma, P.; Bhardwaj, P.; Singh, R. Administration of Lactobacillus casei and Bifidobacterium bifidum ameliorated hyperglycemia, dyslipidemia, and oxidative stress in diabetic rats. International Journal of Preventive Medicine 2016, 7, 102.
- Abdelqader, A.; Al-Fataftah, A.R.; Daş, G. Effects of dietary Bacillus subtilis and inulin supplementation on performance, eggshell quality, intestinal morphology, and microflora composition of laying hens in the late phase of production. Animal Feed Science and Technology 2013, 179, 103–111. [CrossRef]
- Cummings, J.H.; Macfarlane, G.T. Gastrointestinal effects of prebiotics. British Journal of Nutrition 2002, 87, S145–S151. [CrossRef]
- Lone, A.; Mottawea, W.; Chait, Y.A.; Hammami, R. Dual inhibition of Salmonella enterica and Clostridium perfringens by new probiotic candidates isolated from chicken intestinal mucosa. Microorganisms 2021, 9, 166. [CrossRef]
- Pal, C.; Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. The structure and diversity of human, animal, and environmental resistomes. Microbiome 2016, 4, 1–15. [CrossRef] [PubMed]
- Roth, N.; Hofacre, C.; Zitz, U.; Mathis, G.F.; Moder, K.; Doupovec, B.; Berghouse, R.; Domig, K.J. Prevalence of antibiotic-resistant E. coli in broilers challenged with a multi-resistant E. coli strain and received ampicillin, an organic acid-based feed additive, or a synbiotic preparation. Poultry Science 2019, 98, 2598–2607.
- Achakzai, R.; Taj, M.K.; Achakzai, K.B. Microbiological studies on Clostridium perfringens isolated from commercial poultry of Balochistan. Asian Journal of Biological and Life Sciences 2020, 9, 204–208. [CrossRef]
- Li, J.; Zhou, Y.; Yang, D.; Zhang, S.; Sun, Z.; Wang, Y.; Wang, S.; Wu, C. Prevalence and antimicrobial susceptibility of Clostridium perfringens in chickens and pigs from Beijing and Shanxi, China. Veterinary Microbiology 2021, 252, 108932. [CrossRef]
- Xu, W.; Wang, H.; Liu, L.; Miao, Z.; Huo, Y.; Zhong, Z. Prevalence and characterization of Clostridium perfringens isolated from different chicken farms in China. Anaerobe 2021, 72, 102467. [CrossRef]
- Sáenz, J.S.; Marques, T.V.; Barone, R.S.C.; Cyrino, J.E.P.; Kublik, S.; Nesme, J.; Schloter, M.; Rath, S.; Vestergaard, G. Oral administration of antibiotics increased the potential mobility of bacterial resistance genes in the gut of the fish Piaractus mesopotamicus. Microbiome 2019, 7, 1–14. [CrossRef] [PubMed]
- Van Immerseel, F.; De Buck, J.; Pasmans, F.; Huyghebaert, G.; Haesebrouck, F.; Ducatelle, R. Clostridium perfringens in poultry: An emerging threat for animal and public health. Avian Pathology 2004, 33, 537–549. [CrossRef]
- Khademi, F.; Sahebkar, A. The prevalence of antibiotic-resistant Clostridium species in Iran: A meta-analysis. Pathogens and Global Health 2019, 113, 58–66. [CrossRef]
- Mwangi, S.; Timmons, J.; Fitz-Coy, S.; Parveen, S. Characterization of Clostridium perfringens recovered from broiler chicken affected by necrotic enteritis. Poultry Science 2019, 98, 128–135. [CrossRef]
- Jang, Y.-S.; Kim, D.-H.; Bae, D.; Kim, S.-H.; Kim, H.; Moon, J.-S.; Song, K.-Y.; Chon, J.-W.; Seo, K.-H. Prevalence, toxin-typing, and antimicrobial susceptibility of Clostridium perfringens from retail meats in Seoul, Korea. Anaerobe 2020, 64, 102235. [CrossRef] [PubMed]
- Silva, R.O.S.; Salvarani, F.M.; Assis, R.A.; Martins, N.R.S.; Pires, P.S.; Lobato, F.C.F. Antimicrobial susceptibility of Clostridium perfringens strains isolated from broiler chickens. Brazilian Journal of Microbiology 2009, 40, 262–264. [CrossRef] [PubMed]
- Adams, V.; Han, X.; Lyras, D.; Rood, J.I. Antibiotic resistance plasmids and mobile genetic elements of Clostridium perfringens. Plasmid 2018, 99, 32–39. [CrossRef] [PubMed]
- Han, X.; Du, X.-D.; Southey, L.; Bulach, D.M.; Seemann, T.; Yan, X.-X.; Bannam, T.L.; Rood, J.I. Functional analysis of a bacitracin resistance determinant located on ICE Cp1, a novel Tn916-like element from a conjugative plasmid in Clostridium perfringens. Antimicrobial Agents and Chemotherapy 2015, 59, 6855–6865. [CrossRef]

| Ingredients | Starter | Grower I | Grower II | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MBM | BAC | SIMC | MBM | BAC | SIMR | MBM | BAC | SIMR | |
| Corn | 59.4 | 59.4 | 59.4 | 63.83 | 63.83 | 63.83 | 65.06 | 65.06 | 65.06 |
| Soybean Meal, 46% | 33.8 | 33.8 | 33.8 | 27.04 | 27.04 | 27.04 | 23.85 | 23.85 | 23.85 |
| Meat and Bone Meal | 2.51 | 2.51 | 2.51 | 2.51 | 2.51 | 2.51 | 2.01 | 2.01 | 2.01 |
| Soybean Oil | 0.54 | 0.54 | 0.54 | ---- | ---- | ---- | ---- | ---- | ---- |
| Limestone | 0.80 | 0.80 | 0.80 | 0.81 | 0.81 | 0.81 | 1.15 | 1.15 | 1.15 |
| Salt | 0.17 | 0.17 | 0.17 | 0.15 | 0.15 | 0.15 | 0.13 | 0.13 | 0.13 |
| Sodium bicarbonate | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin Premix1 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Mineral Premix2 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Zinc Bacitracin | ---- | 0.05 | ---- | ---- | 0.05 | ---- | ---- | 0.05 | ---- |
| Symbiotic | ---- | ---- | 1,0 | ---- | ---- | 1,0 | ---- | ---- | 1.0 |
| DL-Methionine 99% | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.089 | 0.089 | 0.089 |
| L-lysine HCl, 78.8% | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.02 | 0.02 | 0.02 |
| L-Threonine 98.5% | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | ---- | ---- | ---- |
| Phytase | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 |
| Inert | 2 | 2 | 2 | 4.85 | 4.85 | 4.85 | 9.24 | 9.24 | 9.24 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Calculated Nutritional Composition (%) | |||||||||
| ME (kcal/kg) | 2950 | 2950 | 2950 | 2900 | 2900 | 2900 | 2800 | 2800 | 2800 |
| Crude Protein | 21.4 | 21.4 | 21.4 | 18.7 | 18.7 | 18.7 | 16.0 | 16.0 | 16.0 |
| Calcium | 0.97 | 0.97 | 0.97 | 0.95 | 0.95 | 0.95 | 1.0 | 1.0 | 1.0 |
| Available Phosphorus | 0.45 | 0.45 | 0.45 | 0.44 | 0.44 | 0.44 | 0.40 | 0.40 | 0.40 |
| Sodium | 0.18 | 0.18 | 0.18 | 0.17 | 0.17 | 0.17 | 0.16 | 0.16 | 0.16 |
| Chlorine | 0.19 | 0.19 | 0.19 | 0.17 | 0.17 | 0.17 | 0.16 | 0.16 | 0.16 |
| Potassium | 0.82 | 0.82 | 0.82 | 0.71 | 0.71 | 0.71 | 0.62 | 0.62 | 0.62 |
| Digestible amino acids (%) | |||||||||
| Methionine + Cystine | 0.86 | 0.86 | 0.86 | 0.80 | 0.80 | 0.80 | 0.58 | 0.58 | 0.58 |
| Methionine | 0.54 | 0.54 | 0.54 | 0.50 | 0.50 | 0.50 | 0.32 | 0.32 | 0.32 |
| Lysine | 1.16 | 1.16 | 1.16 | 1.00 | 1.00 | 1.00 | 0.73 | 0.73 | 0.73 |
| Threonine | 0.78 | 0.78 | 0.78 | 0.68 | 0.68 | 0.68 | 0,58 | 0,58 | 0,58 |
| Tryptophan | 0.26 | 0.26 | 0.26 | 0.23 | 0.23 | 0.23 | 0.19 | 0.19 | 0.19 |
| Ingredients | Pre-laying phase | Peak phase | Post-peak phase | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MBM | BAC | SIMP | MBM | BAC | SIMP | MBM | BAC | SIMP | |
| Corn | 65.17 | 65.17 | 65.17 | 60.2 | 60.2 | 60.2 | 60.1 | 60.1 | 60.1 |
| Soybean Meal, 46% | 21.99 | 21.99 | 21.99 | 24.5 | 24.5 | 24.5 | 22.9 | 22.9 | 22.9 |
| Meat and Bone Meal | 2.54 | 2.54 | 2.54 | 1.41 | 1.41 | 1.41 | 1.49 | 1.49 | 1.49 |
| Soybean Oil | 2.34 | 2.34 | 2.34 | 1.0 | 1.0 | 1.0 | 1.05 | 1.05 | 1.05 |
| Limestone | 4.14 | 4.14 | 4.14 | 10.4 | 10.4 | 10.4 | 10.6 | 10.6 | 10.6 |
| Salt | 0.15 | 0.15 | 0.15 | 0.26 | 0.26 | 0.26 | 0.25 | 0.25 | 0.25 |
| Sodium Bicarbonate | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin Premix | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Mineral Premix | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Zinc Bacitracin | ---- | 0.05 | 0.05 | ---- | 0.05 | ---- | ---- | 0.05 | ---- |
| Symbiotic | ---- | ---- | 1.0 | ---- | ---- | 1.0 | ---- | ---- | 1.0 |
| DL-Methionine 99% | 0.19 | 0.19 | 0.19 | 0.27 | 0.27 | 0.27 | 0.26 | 0.26 | 0.26 |
| L-lysine HCl, 78.8% | 0.15 | 0.15 | 0.15 | 0.04 | 0.04 | 0.04 | 0.05 | 0.05 | 0.05 |
| L-Threonine 98.5% | 0.014 | 0.014 | 0.014 | ---- | ---- | ---- | ---- | ---- | ---- |
| Phytase | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 |
| Inert | 2.92 | 2.92 | 2.92 | 1.62 | 1.62 | 1.62 | 2.73 | 2.73 | 2.73 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Calculated Nutritional Composition (%) | |||||||||
| ME (kcal/kg) | 2799 | 2799 | 2799 | 2780 | 2780 | 2780 | 2750 | 2750 | 2750 |
| Crude Protein | 16.50 | 16.50 | 16.50 | 16.7 | 16.7 | 16.7 | 15.89 | 15.89 | 15.89 |
| Calcium | 2.2 | 2.2 | 2.2 | 4.4 | 4.4 | 4.4 | 4.5 | 4.5 | 4.5 |
| Available Phosphorus | 0.44 | 0.44 | 0.44 | 0.37 | 0.37 | 0.37 | 0.37 | 0.37 | 0.37 |
| Sodium | 0.18 | 0.18 | 0.18 | 0.21 | 0.21 | 0.21 | 0.20 | 0.20 | 0.20 |
| Chlorine | 0.15 | 0.15 | 0.15 | 0.23 | 0.23 | 0.23 | 0.22 | 0.22 | 0.22 |
| Potassium | 1.04 | 1.04 | 1.04 | 0.65 | 0.65 | 0.65 | 0.62 | 0.62 | 0.62 |
| Digestible amino acids (%) | |||||||||
| Methionine + Cystine | 0.68 | 0.68 | 0.68 | 0.77 | 0.77 | 0.77 | 0.74 | 0.74 | 0.74 |
| Methionine | 0.41 | 0.41 | 0.41 | 0.50 | 0.50 | 0.50 | 0.48 | 0.48 | 0.48 |
| Lysine | 0.83 | 0.83 | 0.83 | 0.79 | 0.79 | 0.79 | 0.76 | 0.76 | 0.76 |
| Threonine | 0.57 | 0.57 | 0.57 | 0.61 | 0.61 | 0.61 | 0.59 | 0.59 | 0.59 |
| Tryptophan | 0.18 | 0.18 | 0.18 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Treatments | Liv (%) | URI | TP | CK | AST | ALT | LDH | Phos |
| (mg/dL) | (mg/dL) | (UI/L) | (UI/L) | (UI/L) | (UI/L) | (mg/dL) | ||
| MBM | 2.64ab | 5.45a | 6.21ab | 1195 | 197b | 18b | 1154a | 6.95b |
| BAC | 2.30b | 6.54a | 6.77a | 1154 | 253a | 37a | 873b | 11.0a |
| SIMC | 2.51ab | 3.68b | 5.40bc | 1259 | 171ab | 14c | 764b | 5.52b |
| SIMR | 2.88a | 3.59b | 5.32bc | 1194 | 173ab | 11d | 749b | 5.18b |
| SIMP | 2.77ab | 2.77b | 4.93c | 1106 | 147b | 11d | 643b | 6.22b |
| Mean | 2.62 | 4.41 | 5.72 | 1181 | 188 | 18 | 836 | 7.06b |
| P-value | 0.049 | 0.001 | 0.001 | 0.953 | 0.001 | 0.001 | 0.009 | 0.017 |
| SEM | 0.10 | 0.69 | 0.33 | 25 | 18 | 4.85 | 87 | 1.14 |
| Treatments | HDL | LDL | TRI | COL |
|---|---|---|---|---|
| (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | |
| MBM | 32.3c | 18.5 | 1004a | 119 |
| BAC | 24.2d | 35.8 | 989ab | 171 |
| SIMC | 46.9b | 7.96 | 977b | 144 |
| SIMR | 45.7b | 11.9 | 989ab | 137 |
| SIMP | 61.6a | 11.1 | 984b | 173 |
| Média | 42 | 17 | 989 | 149 |
| P-value | <0.001 | 0.649 | 0.031 | 0.121 |
| SEM | 6.46 | 4.99 | 4.62 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





