Submitted:
19 September 2024
Posted:
19 September 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
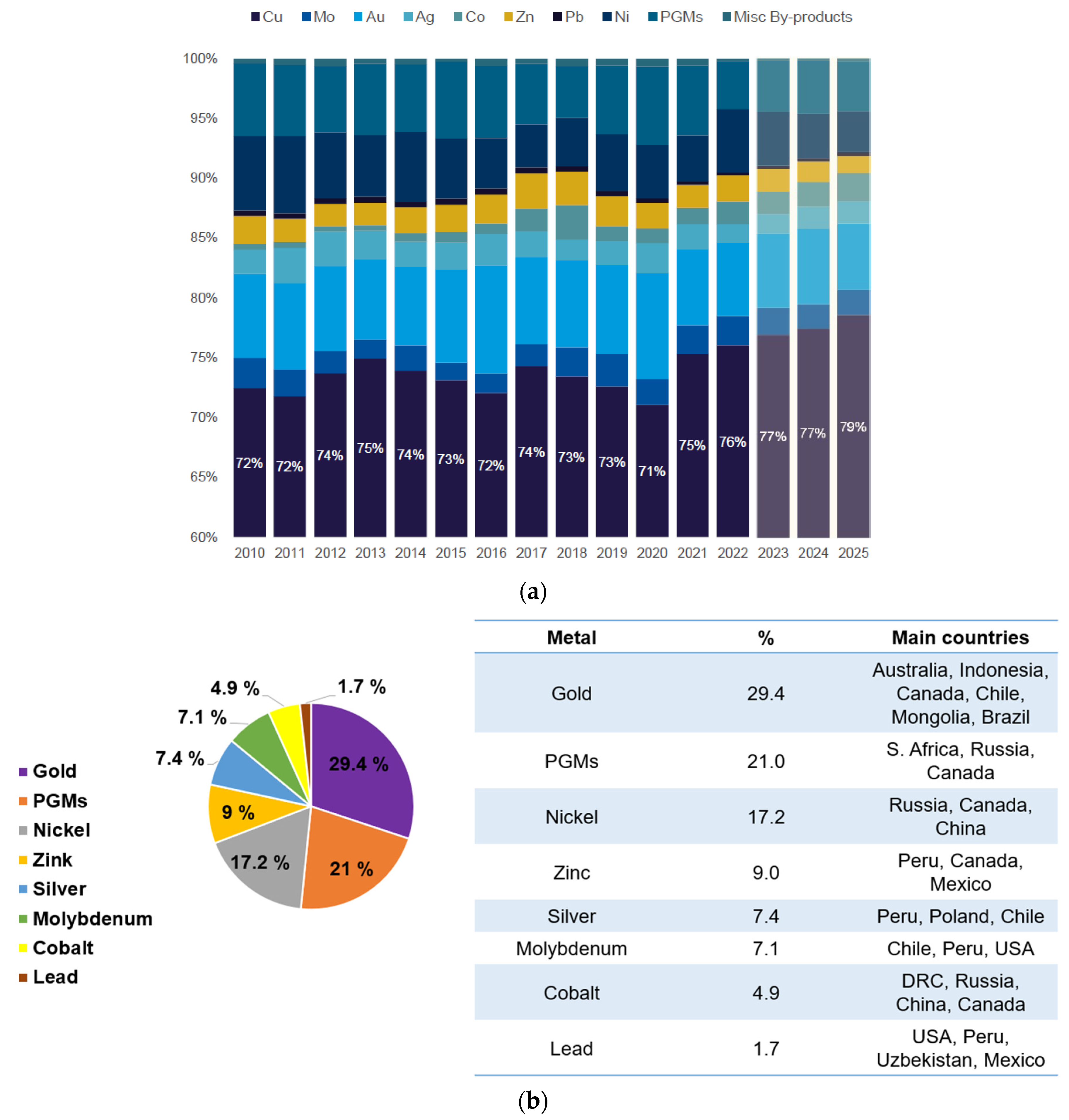
2. Overview of Global Copper Production
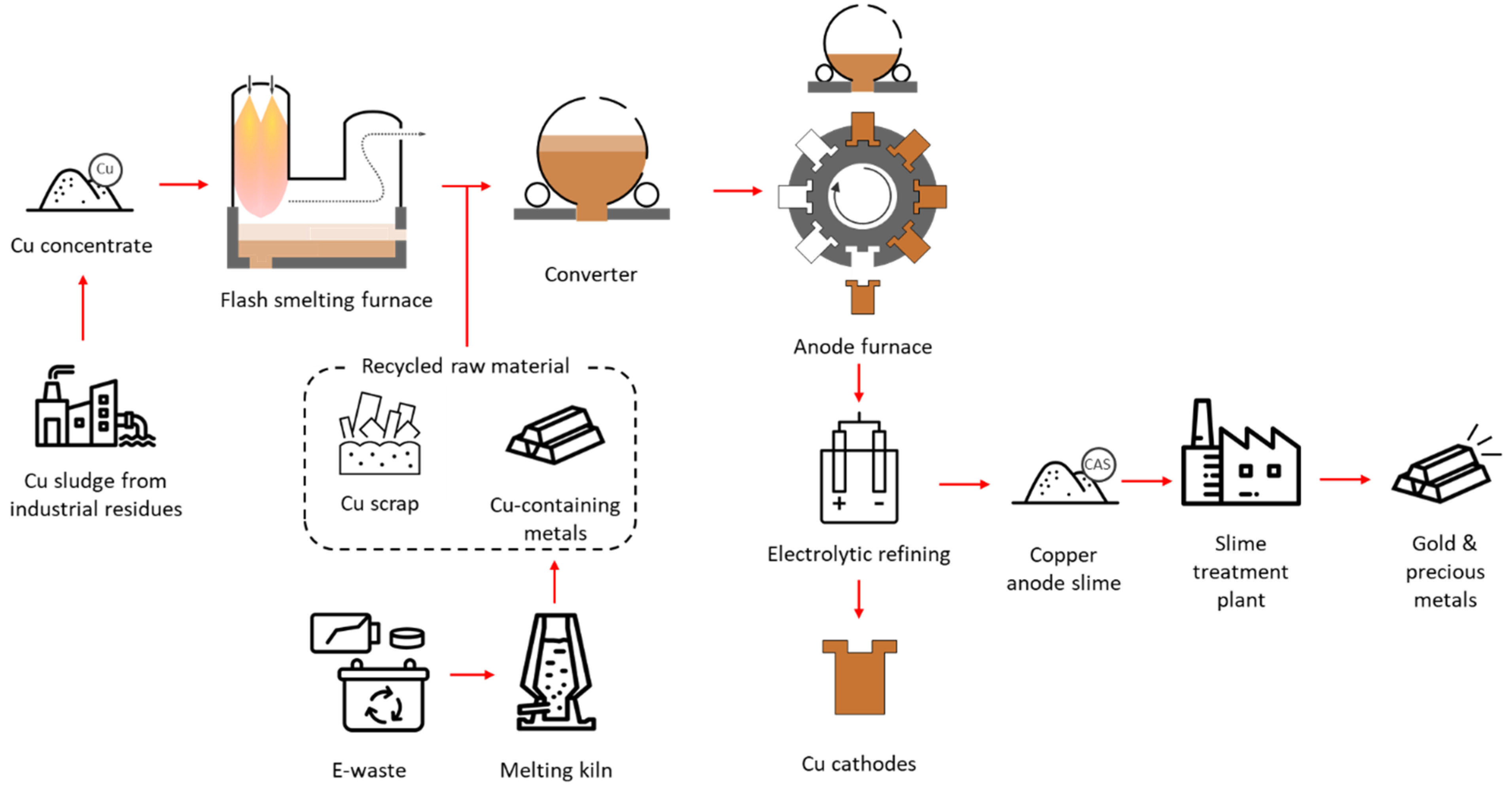
2.1. Pyrometallurgical Route of Copper Production
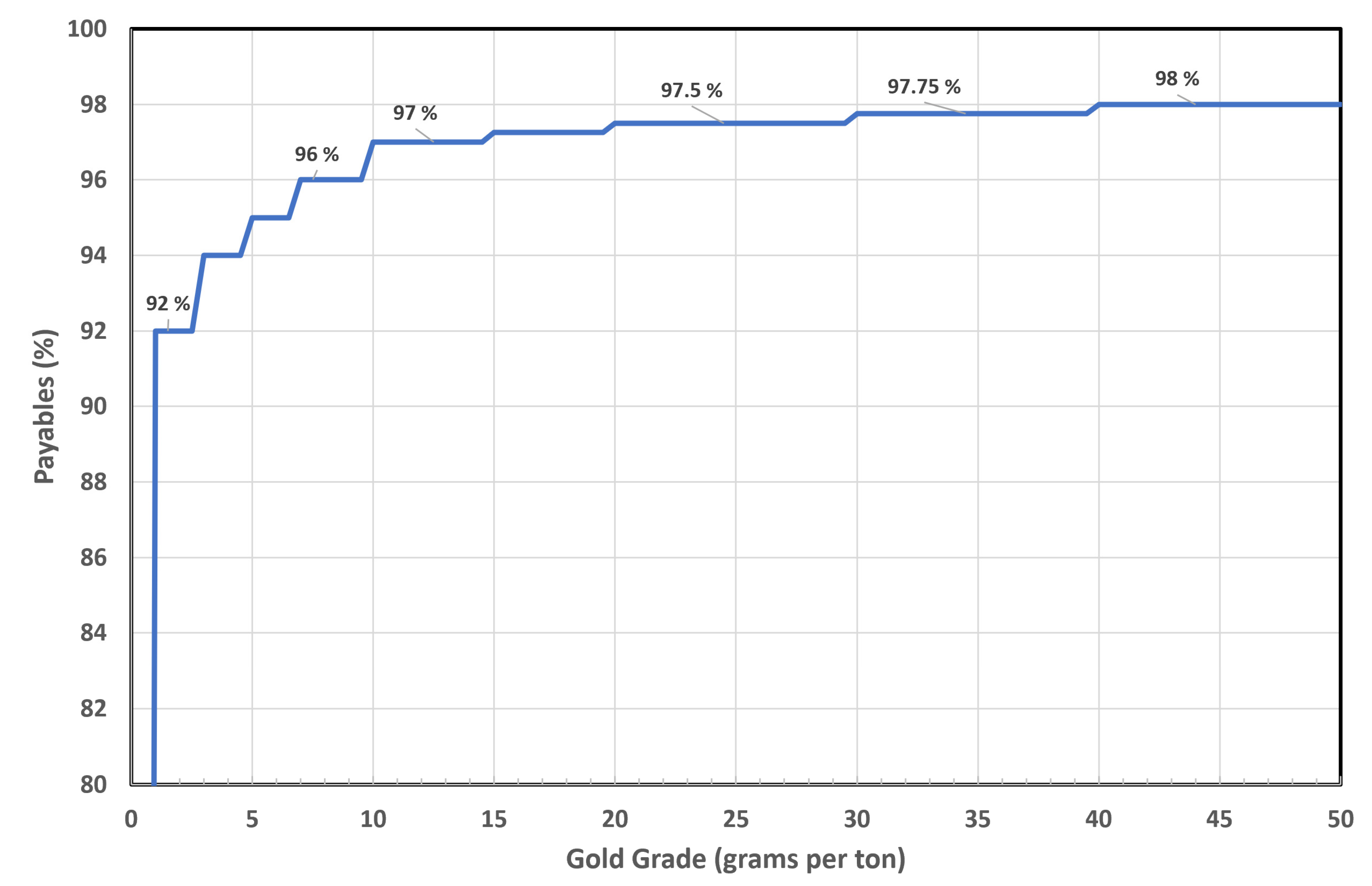
3. Gold in Copper Industry
3.1. Behavior of Gold from Copper Smelting to Refining
3.2. Chemical Loss
3.2.1. Smelting
3.2.2. Converting
3.3. Physical Loss in Slag
3.3.1. Viscosity of Slag
3.3.2. Solids in Slag
3.3.3. Density
3.3.4. Other Parameters
3.4. Loss in Electro-Refining
4. Role of Copper Processing Technologies in Gold Recovery
4.1. Smelting Technologies
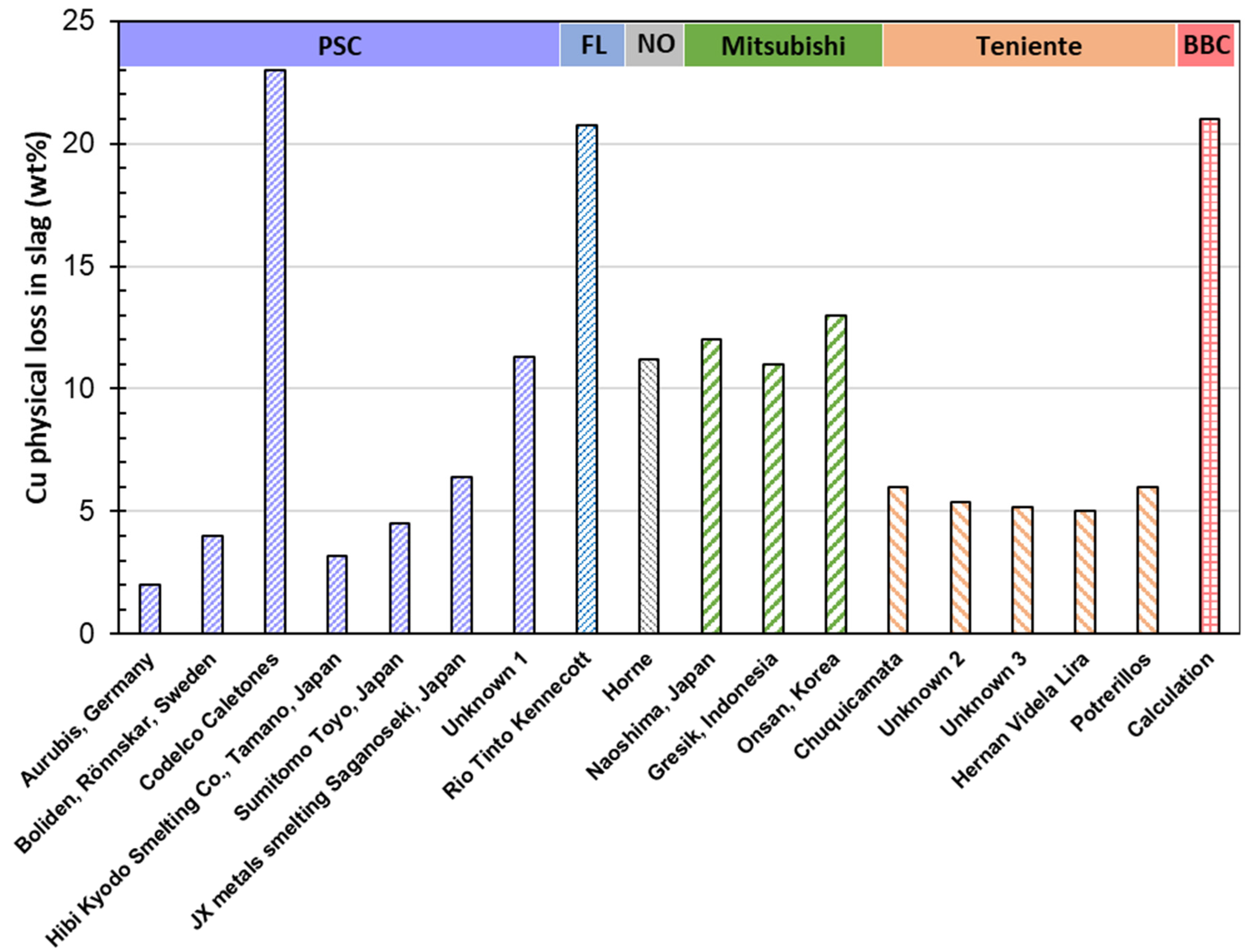
4.1.1. Metal Physical Loss
4.2. Converting Technologies
4.3. First Pass Gold Recovery
5. Conclusions
-
This study confirms that the chemical dissolution of gold in slag is negligible, with gold primarily remaining associated with copper throughout the smelting, converting, and anode refining processes:
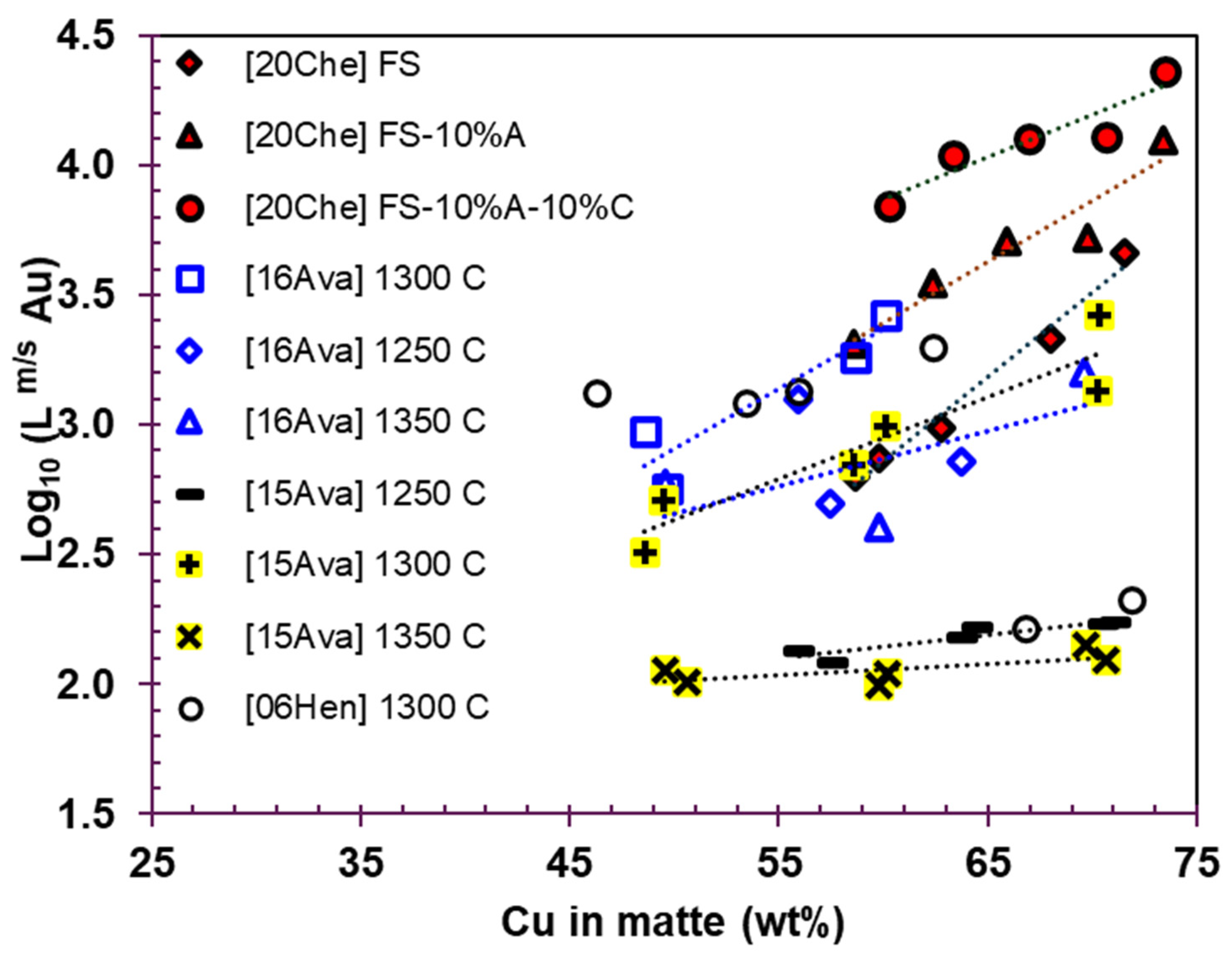
- The gold partition coefficient between matte and slag () ranges from 100 to over 2000, indicating that the majority of gold is concentrated in the matte phase.
- The gold partition coefficient between copper and slag () ranges from 104 to 106, signifying that gold predominantly reports to blister copper.
- The mass of slag in anode refining is minimal, and gold nearly completely reports to the anode.
-
Gold loss primarily occurs through the physical loss of matte or copper in slag:
-
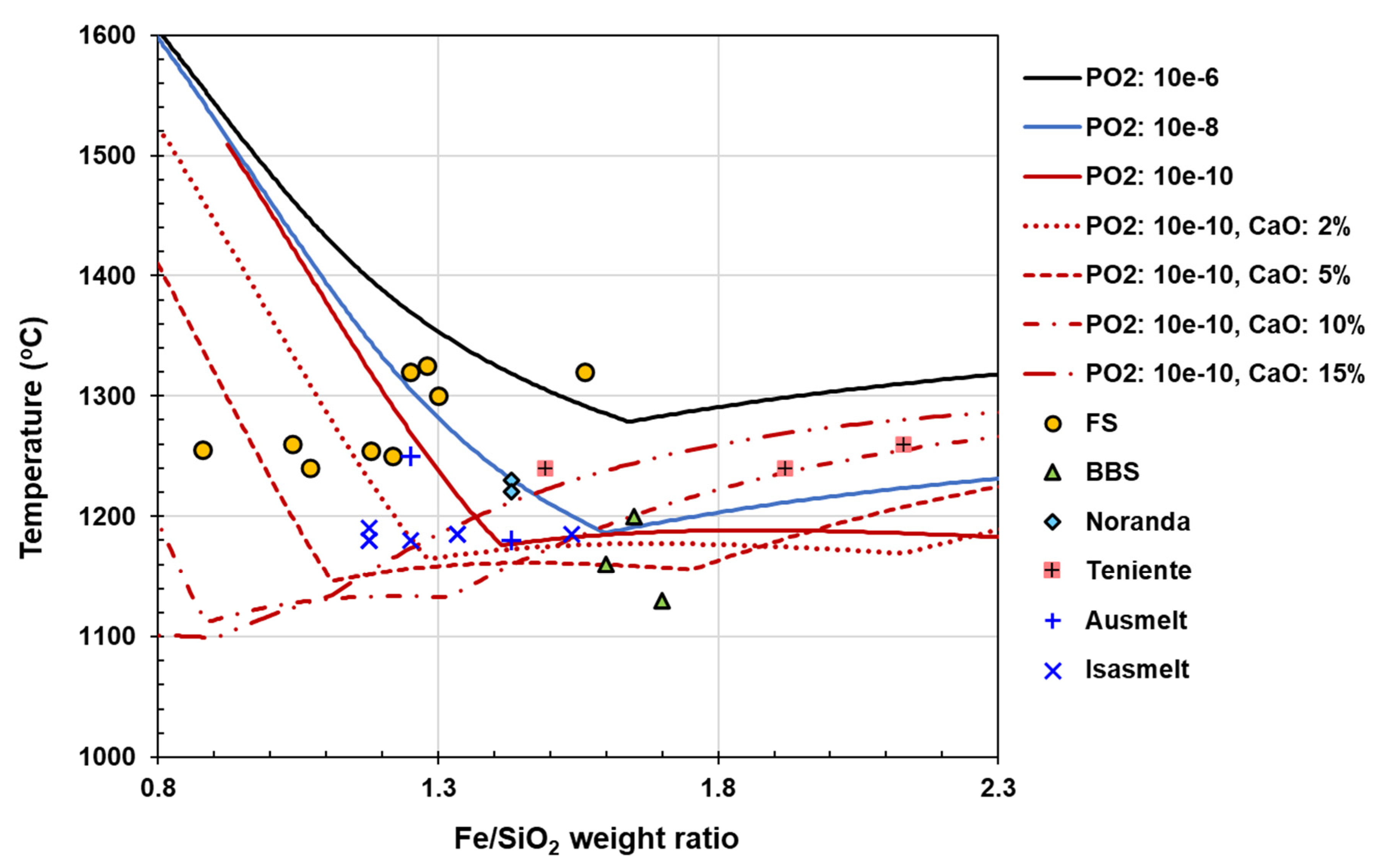
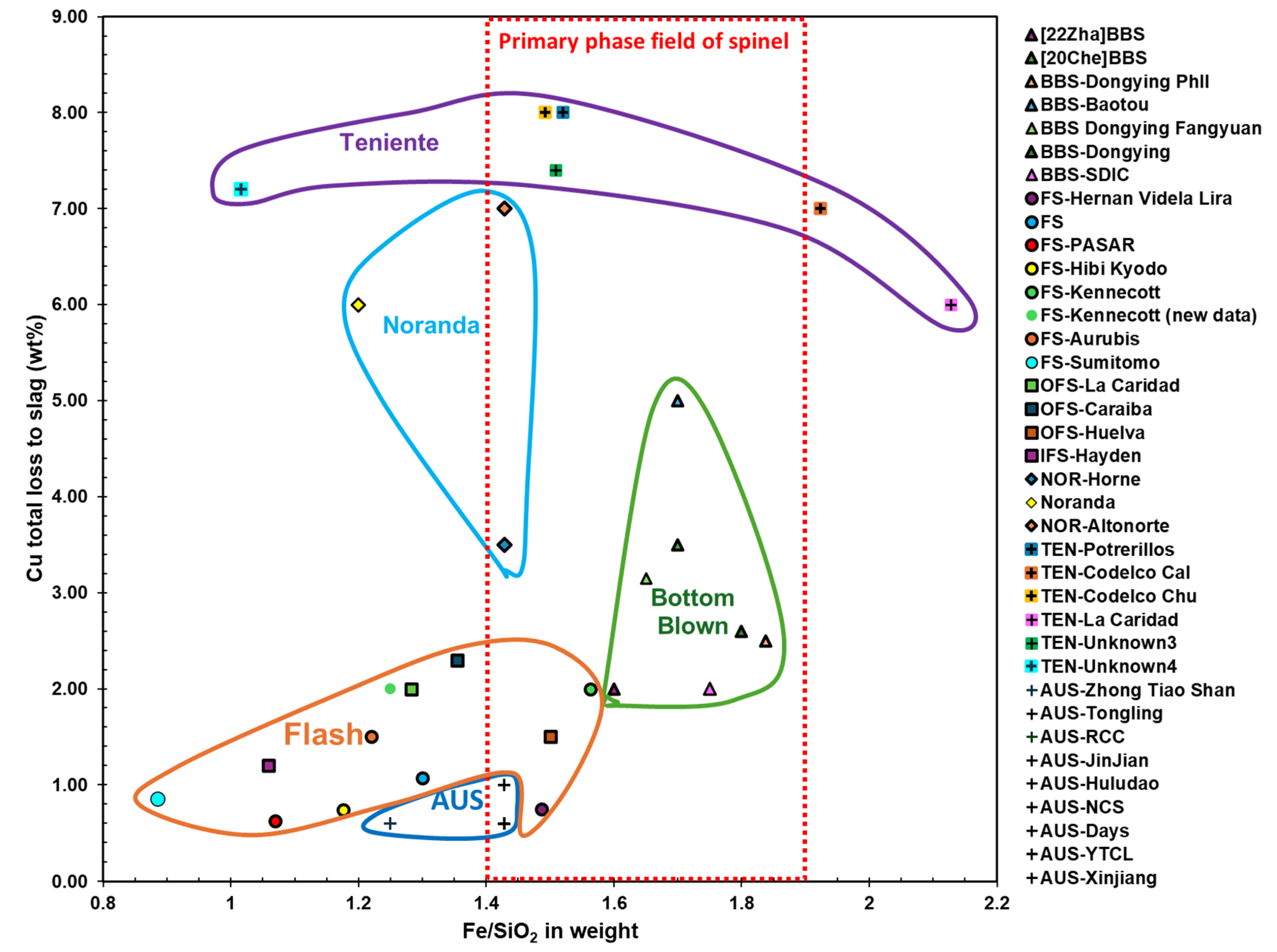
Lower slag viscosity (achieved by higher temperatures above slag liquidus temperature and rather low Fe/SiO₂ ratios) reduces physical loss.
- Higher matte grades increase the density difference between slag and matte and raise slag/matte interfacial tension, improving separation and reducing physical loss.
- Reducing slag mass for a constant droplet settling speed decreases physical loss, though slag mass should be optimized to enhance matte/metal and slag separation.
-
-
The choice of technology significantly impacts gold physical loss, which is primarily carried by copper:
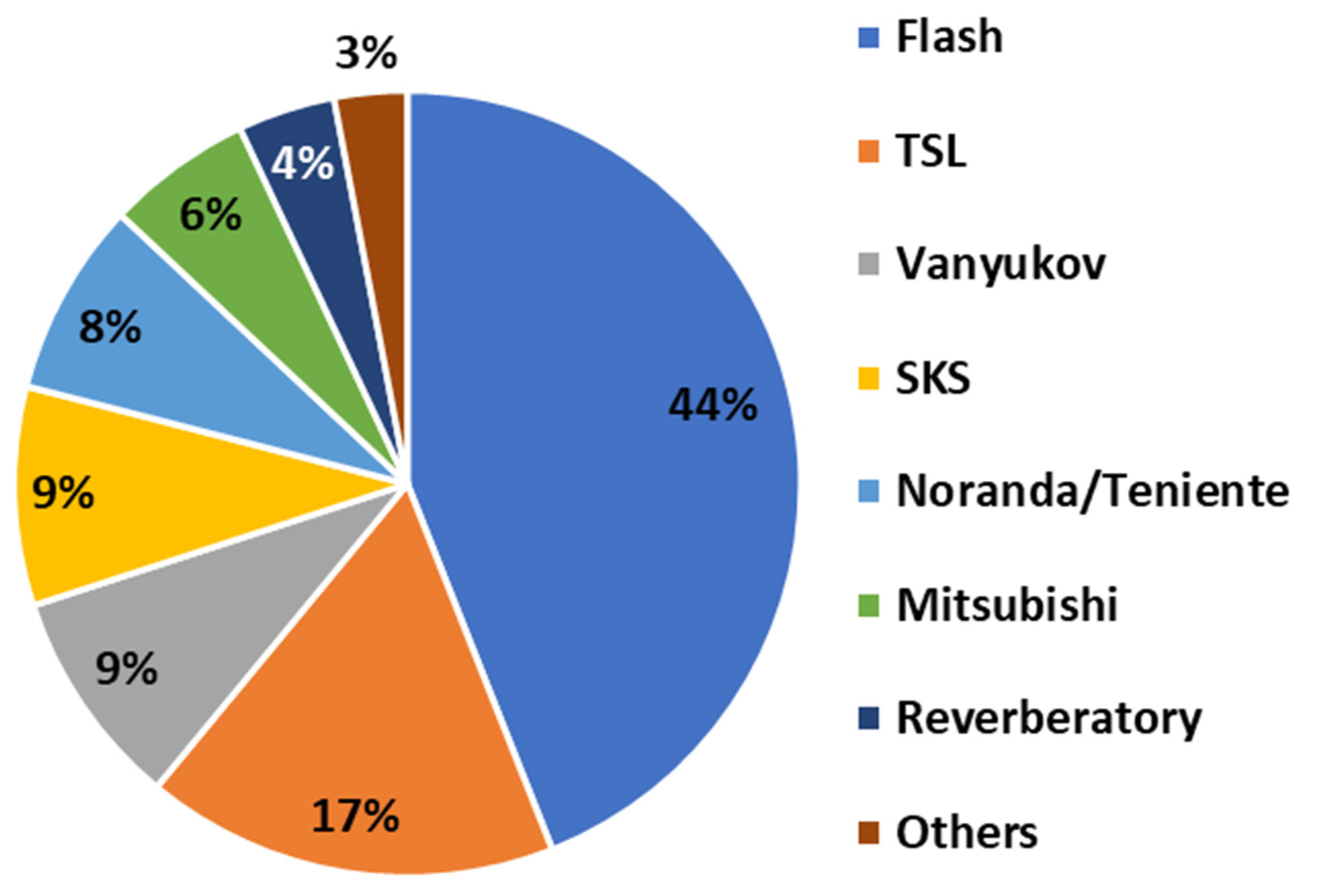
- Among smelting technologies, the Mitsubishi process achieves the highest first-pass gold recovery (99.7–99.8%), followed by flash smelting (98.3–99.9%), bottom-blown smelting (96%), Noranda furnaces (~95%), and Teniente furnaces (85.6–89%).
- Among converting technologies, the Peirce Smith Converter offers the highest gold recovery (95.2–99.3%), followed by the Mitsubishi converter (92.4–99.8%), bottom-blown converter (95.8%), Noranda furnace (93.4%), and flash converter (88.1%).
- Integrated routes show that flash smelting combined with Peirce Smith Converter provides the highest gold recovery (98.3–99.5%), followed by Mitsubishi-Mitsubishi (92.8–99.8%), bottom-blown smelting with bottom-blown converting (95.8%), Teniente with Peirce Smith Converter (95.2%), Noranda-Noranda (93.4%), and flash smelting with flash converting (88.1%).
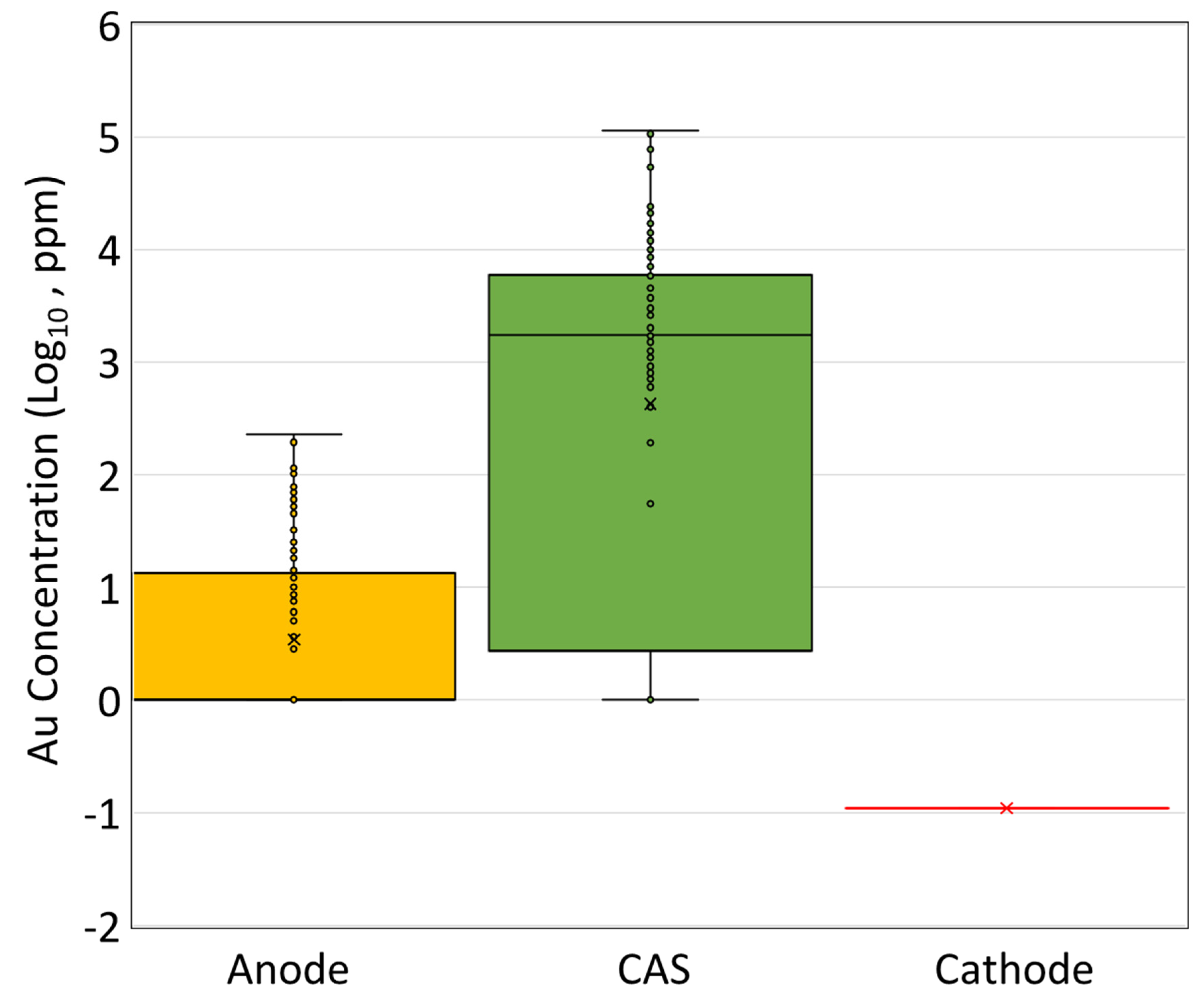
- During electro-refining, gold is found in the anode slime with << 1 ppm reported in the cathode, suggesting near 100% gold recovery during this stage.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available from: https://www.fastmarkets.com/insights/copper-concentrate-tcs-still-negative-how-and-what-does-it-mean-for-copper-prices/.
- https://www.ausimm.com/bulletin/bulletin-articles/gold-concentrate-marketing-101/.
- www.gold.org/about-gold, World Gold Council. 2021.
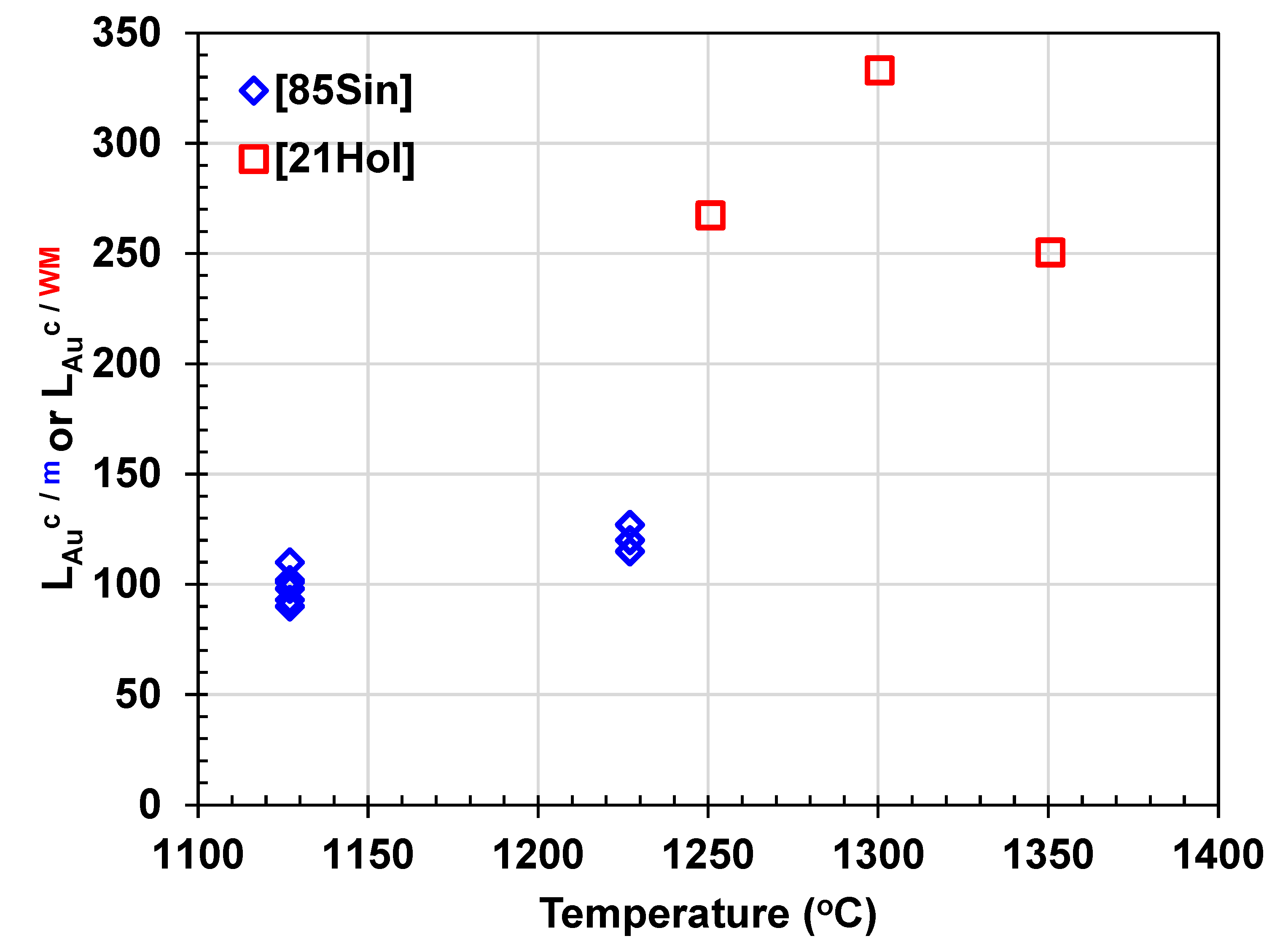
- Sinha, S.N., H.Y. Sohn, and M. Nagamori, Distribution of Gold and Silver between Copper and Matte. Metallurgical Transactions B, 1985. 16(1): p. 53-59. [CrossRef]
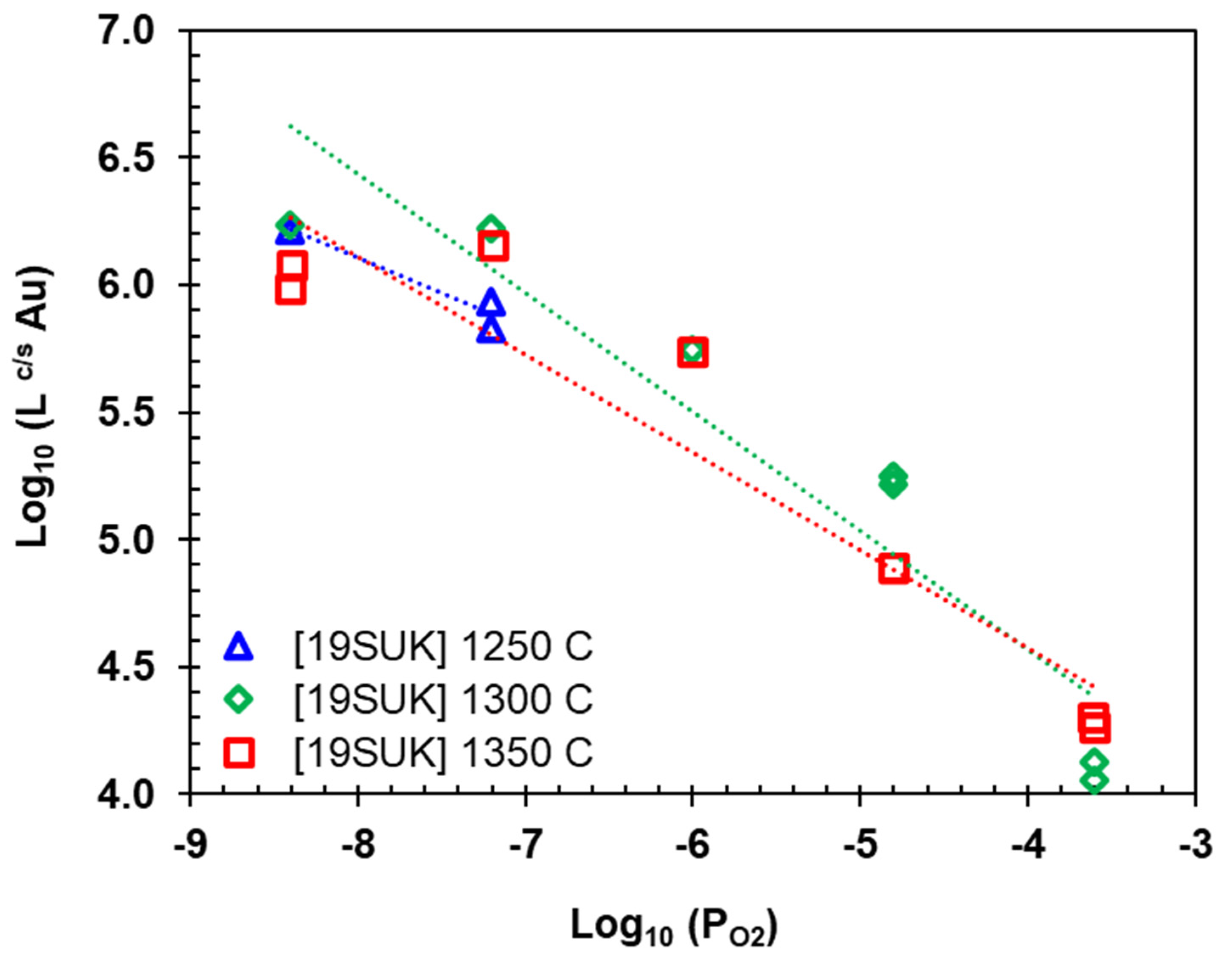
- Avarmaa, K., et al., Equilibrium Distribution of Precious Metals Between Slag and Copper Matte at 1250–1350 °C. Journal of Sustainable Metallurgy, 2015. 1. [CrossRef]
- Avarmaa, K., H. Johto, and P. Taskinen, Distribution of Precious Metals (Ag, Au, Pd, Pt, and Rh) Between Copper Matte and Iron Silicate Slag. Metallurgical and Materials Transactions B, 2016. 47(1): p. 244-255. [CrossRef]
- Sukhomlinov, D., et al., Distribution of Ni, Co, Precious, and Platinum Group Metals in Copper Making Process. Metallurgical and Materials Transactions B, 2019. 50(4): p. 1752-1765. [CrossRef]
- Chen, Y., et al., Characterization of Copper Smelting Flue Dusts from a Bottom-Blowing Bath Smelting Furnace and a Flash Smelting Furnace. Metallurgical and Materials Transactions B, 2020. 51(6): p. 2596-2608. [CrossRef]
- Reuter, M. and I. Kojo, Copper: A Key Enabler of Resource Efficiency. World of Metallurgy - ERZMETALL, 2014. 67: p. 46.
- World Copper Factbook 2023.
- Tripathi, N. and S. Mostaghel. A Review on Integrating E-Waste Recycling in Flash and Bath Copper Smelting Operations. in Proceedings of the 62nd Conference of Metallurgists, COM 2023. 2023. Cham: Springer Nature Switzerland.
- Copper 101 – Module – Concentrates & By-Products.
- https://www.reuters.com/markets/commodities/chinas-top-copper-smelters-agree-production-cut-amid-raw-material-tightness-2024-03-13/.
- https://sayari.com/, Global Trade Atlas.
- https://www.spglobal.com/commodityinsights/en/market-insights/latest-news/metals/111623-china-copper-smelters-miners-split-over-finalizing-tcrcs-for-2024.
- https://www.miningweekly.com/article/freeport-settles-six-year-high-copper-charges-for-2023-with-chinese-smelters---source-2022-11-25.
- Kaur, R., et al., KENNECOTT-OUTOTEC ‘DOUBLE FLASH’ TECHNOLOGY AFTER 16 YEARS. 2011.
- Becker, E., Modernisation of precious metals refining at Norddeutsche Affinerie AG. 2006. 59: p. 87-94.
- Hait, J., R. Jana, and S. Sanyal, Processing of copper electrorefining anode slime: a review. Mineral Processing and Extractive Metallurgy, 2009. 118(4): p. 240-252. [CrossRef]
- Furuzono, T., et al. Unique hydrometallurgical process for copper-anode slime treatment at saganoseki smelter and refinery. in Extraction 2018: Proceedings of the First Global Conference on Extractive Metallurgy. 2018. Springer.
- Barr, G., et al., The new CESL gold process. ALTA, Perth, WA, Australia, 2007.
- Zeng, Y., et al., Occurrence Behaviors of As/Sb/Bi in Copper Anode Slime and Their Separation by Compound Leaching Followed by Stepwise Precipitation. ACS Omega, 2023. 8(11): p. 10022-10029. [CrossRef]
- Sluzhenkin, S.F., Mikhov, A.B.,, Gold and silver mineralogy in the orebodies of Norilsk mining camp. Geology, Genesis anf Mining Developments of Precious Metals Orebodies, 2003: p. 326-330.
- Pannell, D., A survey of world copper smelters. World Survey of Nonferrous Smelters, 1988: p. 3-118.
- Lehner, T., et al., The 1993 survey of worldwide copper and nickel converter practices. Converting, fire refining and casting, 1994: p. 1-58.
- Schlitt, W. and K. Richards, The distribution of silver, gold, platinum and palladium in metal-matte systems. Metallurgical Transactions B, 1975. 6: p. 237-243. [CrossRef]
- Asano, N., Distribution of Gold, Silver and Selenium between Liquid Copper and Cuprous Sulfide. J. Min. Mater. Proc. Inst. Japan, 1971. 87: p. 347-352. [CrossRef]
- Ferron, C., Recovery of gold as by-product from the base-metals industries, in Gold Ore Processing. 2016, Elsevier. p. 831-856.
- Johnson, R., N. Themelis, and G. Eltringham, A survey of worldwide copper converter practices. Copper and Nickel Converters, 1979: p. 1-32.
- Moats, M., et al., Electrolytic copper refining - 2007 world tankhouse operating data. Copper - Cobre 2007 International Conference, 2007. 5: p. 195-241.
- Larouche, P., MINOR ELEMENTS IN COPPER. 2001, Faculty of Graduate Studies and Research in Partial Fulfillment of the ….
- Forsén, O., J. Aromaa, and M. Lundström, Primary copper smelter and refinery as a recycling plant—a system integrated approach to estimate secondary raw material tolerance. Recycling, 2017. 2(4): p. 19. [CrossRef]
- Wang, K., et al., A novel slag cleaning method to recover copper from molten copper converter slag. Transactions of Nonferrous Metals Society of China, 2023. 33(8): p. 2511-2522. [CrossRef]
- Nakajima, K., et al., Thermodynamic Analysis for the Controllability of Elements in the Recycling Process of Metals. Environmental Science & Technology, 2011. 45(11): p. 4929-4936. [CrossRef]
- Dosmukhamedov, N., et al., Converting of copper-lead matte: loss of gold and silver with slag. Kompleksnoe Ispolzovanie Mineralnogo Syra= Complex use of mineral resources, 2020. 314(3): p. 5-14.
- Li, X., et al., Emulsification and Flow Characteristics in Copper Oxygen-Rich Side-Blown Bath Smelting Process. Metals, 2020. 10(11): p. 1520. [CrossRef]
- Barros, K.S., et al., Chemical Composition Data of the Main Stages of Copper Production from Sulfide Minerals in Chile: A Review to Assist Circular Economy Studies. Minerals, 2022. 12(2): p. 250. [CrossRef]
- Cooper, W.C., The treatment of copper refinery anode slimes. JoM, 1990. 42(8): p. 45-49. [CrossRef]
- Mahmoudi, A., et al., Tellurium, from copper anode slime to high purity product: A review paper. Metallurgical and Materials Transactions B, 2020. 51: p. 2555-2575. [CrossRef]
- Hall, S., Gold and silver recovery from copper anode slimes at the Olympic Dam Joint venture, Roxby Downs, SA. Australasian mining and metallurgy, 1993. 19: p. 1102-1105.
- Schlesinger, M.E., et al., Extractive metallurgy of copper. 2021: Elsevier.
- Swinbourne, D.R., S. Yan, and S. Salim, The solubility of gold in metallurgical slags. Mineral Processing and Extractive Metallurgy, 2005. 114(1): p. 23-29. [CrossRef]
- Chen, M., et al., Recovery of Precious Metals (Au, Ag, Pt, and Pd) from Urban Mining Through Copper Smelting. Metallurgical and Materials Transactions B, 2020. 51(4): p. 1495-1508. [CrossRef]
- Henao, H.M., K. Yamaguchi, and S. Ueda. Distribution of precious metals (Au, Pt, Pd, Rh and Ru) between copper matte and iron-silicate slag at 1573 K. in 2006 TMS Fall Extraction and Processing Division: Sohn International Symposium. 2006.
- Rusen, A., et al., Effects of Some Additives on Copper Losses to Matte Smelting Slag. JOM, 2016. 68. [CrossRef]
- Jalkanen, H., J. Vehviläinen, and J. Poijärvi, Copper in solidified copper smelter slags. Scandinavian Journal of Metallurgy, 2003. 32(2): p. 65-70. [CrossRef]
- Yazawa, A., Thermodynamic considerations of copper smelting. Canadian Metallurgical Quarterly, 1974. 13(3): p. 443-453.
- Hyvärinen, O. and M. Hämäläinen, HydroCopper((TM)) - a new technology producing copper directly from concentrate. Hydrometallurgy, 2005. 77: p. 61-65.
- Celmer, R. and J. Toguri, Cobalt and Gold Distribution in Nickel--Copper Matte Smelting. Nickel Metallurgy., 1986. 1: p. 147-163.
- Nagamori, M. and P.J. Mackey, Thermodynamics of Copper Matte Converting: Part II. Distribution of Au, Ag, Pb, Zn, Ni, Se, Te, Bi, Sb and As Between Copper, Matte and Slag in the Noranda Process. Metallurgical Transactions B, 1978. 9: p. 567-579.
- P. Mackey, G.M., and P. Tarassoff, Minor elements in the Noranda Process. TMS-AIME, 1975.
- Mackey, P.J., The Physical Chemistry of Copper Smelting Slags—A Review. Canadian Metallurgical Quarterly, 1982. 21(3): p. 221-260.
- Toguri, J. and N. Santander, Distribution of copper between Cu-Au alloys and silica-saturated fayalite slags. Metallurgical and Materials Transactions B, 1972. 3: p. 590-592. [CrossRef]
- Holland, K., et al., Distribution of Co, Fe, Ni, and precious metals between blister copper and white metal. Mineral Processing and Extractive Metallurgy, 2021. 130(4): p. 313-323. [CrossRef]
- Klaffenbach, E., et al., Sustainable and comprehensive utilization of copper slag: a review and critical analysis. Journal of Sustainable Metallurgy, 2023. 9(2): p. 468-496. [CrossRef]
- Barnett, S., The methods and economics of slag cleaning. 1979.
- Bellemans, I., et al., Metal losses in pyrometallurgical operations-A review. Advances in Colloid and Interface Science, 2018. 255: p. 47-63. [CrossRef]
- Poggi, D., R. Minto, and W.G. Davenport, Mechanisms of Metal Entrapment in Slags. JOM - Journal of the Minerals, Metals and Materials Society, 1969. 21: p. 40-45. [CrossRef]
- Cardona, N., Martin, A., Jim_enez, F., Ríos, G., Mackey, P., & Coursol, P. , Optimizing converter aisle operation at Atlantic copper smelter, Huelva, Spain, R.P. R. Bassa, A. Luraschi, & S. Demetrio, Editor. 2013, symposium on pyrometallurgy and process engineeringProceedings of copper 2013 p. 163-183.
- Schlesinger, M., et al., Extractive Metallurgy of Copper. Extractive Metallurgy of Copper. 2011.
- Utigard, T. and A. Warczok. Density and viscosity of copper/nickel sulphide smelting and converting slags. in Proceedings of COPPER. 1995.
- Schrama, F.N.H., et al., Optimal hot metal desulphurisation slag considering iron loss and sulphur removal capacity part I: fundamentals. Ironmaking & Steelmaking, 2021. 48(1): p. 1-13. [CrossRef]
- Sundström, A., J. Eksteen, and G. Georgalli, A review of the physical properties of base metal mattes. Journal of the Southern African Institute of Mining and Metallurgy, 2008. 108(8): p. 431-448.
- Fagerlund, K. and H. Jalkanen. Some aspects on matte settling in copper smelting. in Fourth International Conference Copper 99-Cobre. 1999.
- Nakamura, T. and J. Toguri, Interfacial phenomena in copper smelting processes, in Pyrometallurgy of copper, C. Díaz and A.A.L. C. Landolt, & C. J. Newman, Editors. 1991: New York. p. 537-551.
- Isaksson, J., et al., Influence of process parameters on copper content in reduced iron silicate slag in a settling furnace. Metals, 2021. 11(6): p. 992. [CrossRef]
- Tsukahara, I., Extraction-spectrophotometric determination of traces of gold in copper in silver, lead, blister copper, copper concentrate and anode slime with 4, 4′-bis (dimethylamino)-thiobenzophenone. Talanta, 1977. 24(10): p. 633-637. [CrossRef]
- Xing, W.D. and M.S. Lee, Development of a hydrometallurgical process for the recovery of gold and silver powders from anode slime containing copper, nickel, tin, and zinc. Gold Bulletin, 2019. 52: p. 69-77. [CrossRef]
- Lee, J.-c., et al., Metallurgical process for total recovery of all constituent metals from copper anode slimes: a review of established technologies and current progress. Metals and Materials International, 2021. 27: p. 2160-2187. [CrossRef]
- MAEDA, Y., Recent Operations at Hitachi Refinery. Journal of MMIJ, 2007. 123(12): p. 605-607.
- Tripathi, R.L.a.N., Overview of Bottom Blown Technology. 2024.
- https://www.metso.com/portfolio/flash-smelting-process/.
- https://www.glencoretechnology.com/en/technologies/isasmel.
- https://www.metso.com/insights/blog/mining-and-metals/detailed-opex-comparison-of-modern-copper-smelting-technologies-using-hsc-sim/.
- Denis Shihin, N.T., Igor Babaian, Evgueni Jak, Unveiling the link between slag chemistry and arsenic flows in primary copper smelting. 2024, COM.
- Zhao, B. and J. Liao, Development of Bottom-blowing copper smelting technology: A review. Metals, 2022. 12(2): p. 190. [CrossRef]
- Chen, M., Z. Cui, and B. Zhao. Slag chemistry of bottom blown copper smelting furnace at Dongying Fangyuan. in 6th International Symposium on High-Temperature Metallurgical Processing. 2016. Springer.
- Tripathi, N., Personal communication. 2024, Rio Tinto Technical Marketing.
- Carrasco, J., Glencore, Altonorte Smelter. 2020, Personal Communication, cited in the book “Extractive Metallurgy of Copper, 6th Ed. 2021”.
- Prevost, Y., Glencore Horne Smelter. 2020, Personal communication, cited in the book “Extractive Metallurgy of Copper, 6th Ed. 2021”.
- Zapata, R., Continuous reactor Altonorte smelter, in The Carlos Diaz symposium on pyrometallurgy, C.J.N. A. E. M. Warmer, A. Vahed, D. G. George, P. J. Mackey, & A. Warczok, Editor. 2007: Montreal: CIM. p. 141e153.
- Ramachandran, V., Lehner, T., Diaz, C., Mackey, P. J., Eltringham, T., Newman, C. J., Primary copper production e a survey of operating world copper smelters, J.K. Diaz, & C. Newman, Editor. 2003, Pyrometallurgy of copper, the Hermann Schwarze symposium on copper pyrometallurgy: Montreal: CIM. p. 3e119.
- Tarasov, A.V., & Paretsky, V. M., J.K. C. Diaz, & C. Newman, Editor. 2003, Pyrometallurgy of copper (Book 1) the Hermann Schwarze symposium on copper pyrometallrgy: Montreal: CIM. p. 173e187.
- Ospanov, Y. 2020, Personal communication, cited in the book “Extractive Metallurgy of Copper, 6th Ed. 2021”.
- Burrows, A. 2011, Personal communication, cited in the book “Extractive Metallurgy of Copper, 6th Ed. 2021”.
- Metso Outotec Ausmelt. (2020). Metso Outotec Ausmelt TSL Reference List. Outotec. 2020.
- Furuta, M., et al., Analysis of copper loss in slag in Tamano type flash smelting furnace. 2006. 8: p. 123-133.
- Mariscal, L., Mexicana de Cobre. SA de CV. 2020, Personal Communication, cited in the book “Extractive Metallurgy of Copper, 6th Ed. 2021”.
- Demetrio, S., et al., Slag cleaning: The chilean copper smelter experience. JOM, 2000. 52(8): p. 20-25. [CrossRef]
- Herreros, O., et al., Copper extraction from reverberatory and flash furnace slags by chlorine leaching. Hydrometallurgy, 1998. 49(1): p. 87-101. [CrossRef]
- Busolic, D., et al., Recovery of iron from copper flash smelting slags. Mineral Processing and Extractive Metallurgy, 2011. 120(1): p. 32-36. [CrossRef]
- Nazer, A.S., O. Pavez, and F. Rojas, Use of copper slag in cement mortar. Rem: Revista Escola de Minas, 2012. 65. [CrossRef]
- Yan, J. Recent operation of the oxygen bottom-blowing copper smelting and continuous copper converting technologies. in Proceedings of the 9th International Copper Conference (Copper 2016), Kobe, Japan. 2016.
- Bin, T. and Y. Suping, Application And Development of Modern Copper Metallurgical Technologies in China’s Copper Industry. 2019, Canadian Institute of Mining, Metallurgy and Petroleum.
- Guo, X., et al. Advanced copper smelting technologies used to quadruple China copper production between 2000 and 2015. in 9th international copper conference (Copper 2016), Kobe, Japan. 2016.
- Yongcheng, Z., L. Bing, and L. Kai, Engineering and Production Practice of Double Bottom-Blowing Continuous Copper Smelting Process with “Three Connected Furnaces” Arrangement. 2019, Canadian Institute of Mining, Metallurgy and Petroleum.
- Tuominen, J., K. Pienimäki, and K. Fagerlund. Kennecott-outotec flash converting–leading the way for over 20 years. in Proc. copper. 2016.
- Marín, O., et al., Potential of Tailing Deposits in Chile for the Sequestration of Carbon Dioxide Produced by Power Plants Using Ex-Situ Mineral Carbonation. Minerals, 2021. 11(3): p. 320. [CrossRef]
- Montenegro, V., H. Sano, and T. Fujisawa, Recirculation of Chilean Copper Smelting Dust with High Arsenic Content to the Smelting Process. MATERIALS TRANSACTIONS, 2008. 49(9): p. 2112-2118. [CrossRef]
- Imris, I., et al., The Copper Losses in the Slags From the El Teniente Process. Canadian Metallurgical Quarterly, 2000. 39(3): p. 281-290.
- Cardona, N., et al., The Physical Chemistry of Copper Smelting Slags and Copper Losses at the Paipote SmelterPart 2 – Characterisation of industrial slags. Canadian Metallurgical Quarterly, 2011. 50(4): p. 330-340. [CrossRef]
- Shishin, D., et al. Thermodynamic modelling of liquid slag-matte-metal equilibria applied to the simulation of the Peirce-Smith converter. in Advances in Molten Slags, Fluxes, and Salts: Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts 2016. 2016. Springer.
- Yazawa, A., Distribution of Various Elements Between Copper, Matte and Slag. Science reports of the Research Institutes, Tohoku University. Ser. A, Physics, chemistry and metallurgy, 1981. 30: p. 154.
- Kapusta, J.P., JOM world nonferrous smelters survey, part I: copper. JOM, 2004. 56(7): p. 21-27. [CrossRef]
- Mostaghel, S., C. Samuelsson, and B. Björkman, Influence of alumina on mineralogy and environmental properties of zinc-copper smelting slags. International Journal of Minerals, Metallurgy, and Materials, 2013. 20: p. 234-245. [CrossRef]
- Lidelöw, S., et al., Leaching behaviour of copper slag, construction and demolition waste and crushed rock used in a full-scale road construction. Journal of environmental management, 2017. 204: p. 695-703. [CrossRef]
- Davenport, W.G., Jones, D. M., King, M. J., & Partelpoeg, E. H, Flash smelting, analysis, control and optimization. 2001, Warrendale, PA: TMS.
- Muravyov, M.I., et al., Leaching of copper and zinc from copper converter slag flotation tailings using H2SO4 and biologically generated Fe2 (SO4) 3. Hydrometallurgy, 2012. 119: p. 40-46. [CrossRef]
- Liao, J., K. Tan, and B. Zhao, Enhanced productivity of bottom-blowing copper-smelting process using plume eye. Metals, 2023. 13(2): p. 217. [CrossRef]
- Steinhauser, J., A. Vartiainen, and W. Wuth, Volatilization and distribution of impurities in modern pyrometallurgical copper processing from complex concentrates. JOM, 1984. 36: p. 54-61. [CrossRef]

| a) | ||||||||||||||||||||||||||
| Operational data | Flash | |||||||||||||||||||||||||
| Pasar, Philippines [41] | Aurubis, Germany [41] | JX metals smelting Saganoseki, Japan [41] | Hibi Kyodo smelting Co. Tamano, Japan [60] | Sumitomo Toyo, Japan [41] |
Rio Tinto Kennecott Utah Copper Magna, USA [60] |
|||||||||||||||||||||
| Concentrate (t/d) | 2000 | 3300 | 2204 | 2241 | 3830 | 4200 | ||||||||||||||||||||
| Concentrate Cu (wt%) | 23-29 | 29 | 28 [60] | 29.2 | 26.3 | 25.0 | ||||||||||||||||||||
| Slag (t/d) | 800-900 | 2000 | 2700 | 1326 | 2973 | 2100-3100 | ||||||||||||||||||||
| Slag Cu total (wt%) | 0.63 | 1.50 | 0.80 | 0.74 | 0.86 | 0.5-4 | ||||||||||||||||||||
| Slag Cu chemical loss (wt%) | 0.5 | 0.45 | 0.45 | 0.46 | 0.44 | 0.83 | ||||||||||||||||||||
| Slag Cu physical loss (wt%) | 0.13 | 1.05 | 0.35 | 0.28 | 0.42 | 1.42 | ||||||||||||||||||||
| SiO2/Fe weight | 0.87-1 | 0.82 | 0.96 | 0.85 | 1.13 | 0.64, 0.8 | ||||||||||||||||||||
| Fe (wt%) | ||||||||||||||||||||||||||
| SiO2 (wt%) | ||||||||||||||||||||||||||
| CaO/Fe weight | ||||||||||||||||||||||||||
| Slag T (oC) | 1240 | 1250 | 1260 | 1254 | 1255 | 1320 | ||||||||||||||||||||
| Matte Cu (wt%) | 54-56 | 63.0 | 63.0 | 63.5 | 62.0 | 66.5-74.5 | ||||||||||||||||||||
| Matte (t/d) | 1080 | 1500 | 1650 | 1129 | 1753 | 1800 | ||||||||||||||||||||
| Fe/SiO2 weight | 1.07 | 1.22 | 1.04 | 1.18 | 0.88 | 1.56, 1.25 | ||||||||||||||||||||
| b) | ||||||||||||||||||||||||||
| Operational data | Noranda | Teniente | Vanyukov | |||||||||||||||||||||||
| Noranda, Canada [41] | Altonorte, Chile [41] | Codelco Caletones, Chile [41] | Codelco Chuquicamata, Chile [60] | Balkhash smelter, Kazakhstan [41] | Norilsk Copper, Siberia*[41] | Sredneuralsky, Urals*[41] | ||||||||||||||||||||
| Concentrate (t/d) | 1900-2200 | 3000-3300 | 2000-2400 | 2200-2500 | - | - | - | |||||||||||||||||||
| Concentrate Cu (wt%) | 27.0 | 26-29 | 27-29 | 30-33 | 12-22 | 19-23 | 13-15 | |||||||||||||||||||
| Slag (t/d) | 1300-1450 | 2450 | 1400-1700 | 1550-1800 | ||||||||||||||||||||||
| Slag Cu total (wt%) | 3-4 | 7.00 | 6-8 | 6-10 | <0.9 | 1.2; 0.9; 2.0 | 0.7 | |||||||||||||||||||
| Slag Cu chemical loss (wt%) | 0.71 | 0.71 | 1.18 | 0.94 | ||||||||||||||||||||||
| Slag Cu physical loss (wt%) | 2.79 | 6.29 | 5.82 | 7.06 | ||||||||||||||||||||||
| SiO2/Fe weight | 0.6-0.8 | 0.7 | 0.45-0.59 | 0.66-0.68 | ||||||||||||||||||||||
| Fe (wt%) | 31-38 | 48,45,25 | 30 | |||||||||||||||||||||||
| SiO2 (wt%) | 29-31 | 29,29,35 | 32 | |||||||||||||||||||||||
| CaO/Fe weight | ||||||||||||||||||||||||||
| Slag T (oC) | 1230 | 1220 | 1240 | 1240 | 1250-1300 | 1320; 1320; 1250 | 1280 | |||||||||||||||||||
| Matte Cu (wt%) | 71-73 | 72-73 | 72-75 | 72-74 | 45-55 | 65,55,74 | 45 | |||||||||||||||||||
| Matte (t/d) | 650-900 | 1350.0 | 800-950 | 990-1150 | ||||||||||||||||||||||
| Fe/SiO2 weight | 1.43 | 1.43 | 1.92 | 1.49 | ||||||||||||||||||||||
| c) | ||||||||||||||||||||||||||
| Operational data | Bottom Blowing | Mitsubishi | ||||||||||||||||||||||||
| [43] | [76] | [76] | [77] | Dongying [78] | Baotou [78] | Calculation [75] | Mitsubishi Materials Corp., Naoshima, Japan [60] | Gresik, Indonesia [60] | Onsan, Korea [60] | |||||||||||||||||
| Concentrate (t/d) | 90-100 | 50 | 70 | 230 t/h | 2300 | 2000-2300 | 2109 | |||||||||||||||||||
| Concentrate Cu (wt%) | 20 | 22 | 20-22 | 18 | 25 | 34 | 31.7 | 33.2 | ||||||||||||||||||
| Slag (t/d) | 153.4 t/h | 1300 | 1450 | 1331 | ||||||||||||||||||||||
| Slag Cu total (wt%) | 3.0-4.0 | 1.0-3.0 | 2.6 | 2.5-3.8 | 2.6 | 5 | 7.6 | 0.7 | 0.7 | 0.8 | ||||||||||||||||
| Slag Cu chemical loss (wt%) | 0.48 | 0.48 | 0.58 | 1.18 | 0.58 | 0.71 | 1.18 | 0.50 | 0.50 | 0.58 | ||||||||||||||||
| Slag Cu physical loss (wt%) | 3.02 | 1.52 | 2.02 | 1.97 | 2.02 | 4.29 | 6.42 | 0.20 | 0.20 | 0.22 | ||||||||||||||||
| SiO2/Fe weight | 0.62-0.56 | 0.59-0.67 | 0.56 | 0.53-0.71 | 0.56 | 0.59 | 0.63 | 0.9 | 0.8 | 0.9 | ||||||||||||||||
| Fe (wt%) | ||||||||||||||||||||||||||
| SiO2 (wt%) | ||||||||||||||||||||||||||
| CaO/Fe weight | ||||||||||||||||||||||||||
| Slag T (oC) | 1080-1180 | 1150-1170 | 1190-1210 | 1150-1180 | 1180 | 1200 | 1250 | |||||||||||||||||||
| Matte Cu (wt%) | 52-60 | 50.0 | 70 | 75.0 | 70.0 | 72.0 | 74.0 | 68 | 68 | 68.8 | ||||||||||||||||
| Matte (t/d) | 80.1 t/h | 1400 | 1260 | 1240 | ||||||||||||||||||||||
| Fe/SiO2 weight | 1.69 | 1.59 | 1.79 | 1.61 | 1.60 | 1.11 | 1.25 | 1.11 | ||||||||||||||||||
| d) | ||||||||||||||||||||||||||
| Operational data | Isasmelt [60] | |||||||||||||||||||||||||
| Mount Isa, Australia | Kunming, China | Miami, USA | Ilo, Peru | Sterlite, India | Mufulira, Zambia | Ust-Kamenogorsk, Kazakhstan | ||||||||||||||||||||
| Concentrate (t/d) | ||||||||||||||||||||||||||
| Concentrate Cu (wt%) | 22-26 | 18-22 | 25-29 | 25-29 | 26-31 | 28-32 | 22-26 | |||||||||||||||||||
| Slag (t/d) | ||||||||||||||||||||||||||
| Slag Cu total (wt%) | ||||||||||||||||||||||||||
| Slag Cu chemical loss (wt%) | ||||||||||||||||||||||||||
| Slag Cu physical loss (wt%) | ||||||||||||||||||||||||||
| SiO2/Fe weight | 0.85 | 0.85 | 0.65 | 0.75 | 0.75 | 0.85 | 0.8 | |||||||||||||||||||
| Fe (wt%) | ||||||||||||||||||||||||||
| SiO2 (wt%) | ||||||||||||||||||||||||||
| CaO/Fe weight | ||||||||||||||||||||||||||
| Slag T (oC) | 1190 | 1180 | 1185 | 1185 | 1185 | - | 1180 | |||||||||||||||||||
| Matte Cu (wt%) | 60-63 | 52-55 | 55-60 | 60-65 | 60-65 | 60-65 | 55-60 | |||||||||||||||||||
| Matte (t/d) | ||||||||||||||||||||||||||
| Fe/SiO2 weight | 1.18 | 1.18 | 1.54 | 1.33 | 1.33 | 1.18 | 1.25 | |||||||||||||||||||
| e) | ||||||||||||||||||||||||||
| Operational data | Ausmelt [60] | |||||||||||||||||||||||||
| Zhong Tiao Shan, China | Tongling, China | RCC, Russia | JinJian, China | Huludao, China | NCS, Namibia | Days, China | YTCL, China | Xinjiang, Wuxin China | ||||||||||||||||||
| Concentrate (t/d) | ||||||||||||||||||||||||||
| Concentrate Cu (wt%) | 17-22 | 25 | 14-23 | 26 | 22 | 25 | 20 | 22 | 19 | |||||||||||||||||
| Slag (t/d) | ||||||||||||||||||||||||||
| Slag Cu total (wt%) | 0.6 | 0.6 | 1 | 0.6 | 0.6 | 1 | 0.6 | 0.6 | 0.6 | |||||||||||||||||
| Slag Cu chemical loss (wt%) | ||||||||||||||||||||||||||
| Slag Cu physical loss (wt%) | ||||||||||||||||||||||||||
| SiO2/Fe weight | 0.8 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | |||||||||||||||||
| Fe (wt%) | ||||||||||||||||||||||||||
| SiO2 (wt%) | ||||||||||||||||||||||||||
| CaO/Fe weight | ||||||||||||||||||||||||||
| Slag T (oC) | 1200-1300 | 1180 | 1180 | 1180 | 1180 | 1180 | 1180 | 1180 | 1180 | |||||||||||||||||
| Matte Cu (wt%) | 60 | 50 | 40 | 50 | 50 | 50 | 55 | 60 | 56 | |||||||||||||||||
| Matte (t/d) | ||||||||||||||||||||||||||
| Fe/SiO2 weight | 1.25 | 1.43 | 1.43 | 1.43 | 1.43 | 1.43 | 1.43 | 1.43 | 1.43 | |||||||||||||||||
| a) | ||||||||||||||||
| Operational data | Peirce Smith | |||||||||||||||
| Pasar, Philippines [41] | Aurubis, Germany [41] | Boliden, Rönnskar, Sweden [41] | Codelco Caletones [41] | Hibi Kyodo Smelting Co., Tamano, Japan [41] | Sumitomo Toyo, Japan [41] | JX metals smelting Saganoseki, Japan [41] | Unknown 1 [98] | |||||||||
| Matte (t/converter, t/d or t/h) | 300 | 350 | 200 | 205 | 215 | - | ||||||||||
| Matte Cu (wt%) | 63 | 58 | 74.3 | 64 [60] | 62 [60] | 63 | ||||||||||
| Slag (t/converter, t/d, or t/h) | 70 | 140-160 | 30 | 65 | 54 | |||||||||||
| Slag Cu total (wt%) | 10-15 | 4 | 6 | 25 | 5.2 | 6.5 | 8.4 | 13.3 | ||||||||
| Slag Cu chemical loss (wt%) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||||||||
| Slag Cu physical loss (wt%) | 10.5 | 2 | 4 | 23 | 3.2 | 4.5 | 6.4 | 11.3 | ||||||||
| SiO2/Fe weight ratio | 0.55 | 0.75 | - | 0.41 | 0.45 | 0.46 | 0.46 | |||||||||
| Fe (wt%) | 41.1 | |||||||||||||||
| SiO2 (wt%) | 18.9 | |||||||||||||||
| CaO/Fe weight | ||||||||||||||||
| Slag T (oC) | ||||||||||||||||
| Blister Cu (t/converter, t/d or t/h) | 260-300 | 270-310 | 145 | 214 | 208 | - | ||||||||||
| Fe/SiO2 (Fe/CaO) weight | 1.82 | 1.33 | 2.44 | 2.22 | 2.17 | 2.17 | ||||||||||
| b) | ||||||||||||||||
| Operational data | Flash | Noranda | Mitsubishi | Teniente | Bottom Blowing | |||||||||||
|
Rio Tinto Kennecott [41] |
Horne [41] | Naoshima, Japan [41] | Gresik, Indonesia [41] |
Onsan, Korea [60] |
Chuquicamata [99] |
Unknown 2 [89] |
Unknown 3 [100] | Hernan Videla Lira [101] | Potrerillos [101] |
Calculation [75] |
||||||
| Matte (t/converter, t/d or t/h) | 1200-1800 | 679 | 1080 | 1080-1290 | 1018 | 80.1 | ||||||||||
| Matte Cu (wt%) | 67-70 | 65 | 67 | 68.8 | 74 | |||||||||||
| Slag (t/converter, t/d, or t/h) | 200-400 | 297 | 290 | 576 | 360 | 11.5 | ||||||||||
| Slag Cu total (wt%) | 17-26 | 13.2 | 14 | 13 | 15 | 8 | 7.4 | 7.2 | 7.03 | 8 | 23 | |||||
| Slag Cu chemical loss (wt%) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |||||
| Slag Cu physical loss (wt%) | 19.5 | 11.2 | 12 | 11 | 13 | 6 | 5.4 | 5.2 | 5.03 | 6 | 21 | |||||
| SiO2/Fe weight ratio | 1.49 | 0.66 | 0.98 | 0.66 | 1 | |||||||||||
| Fe (wt%) | 39.4 | 38.11 | 38 | |||||||||||||
| SiO2 (wt%) | 26.1 | 37.5 | 24.19 | 25 | ||||||||||||
| CaO/Fe weight | 0.24-0.36 | 0.4 | 0.4 | 0.34 | ||||||||||||
| Slag T (oC) | 1260 | 1250 | ||||||||||||||
| Blister Cu (t/converter, t/d or t/h) | 1100-1500 | 640 | 790 | 850-1000 | 820 | 57.2 | ||||||||||
| Fe/SiO2 (Fe/CaO) weight | (2.63-5) | 0.67 | (2.5) | (2.5) | (2.94) | 1.5 | 1.0 | 1.5 | 1.0 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





