1. Introduction
Currently, bacterial infections account for one in every eight deaths worldwide, and this figure continues to rise [
1]. Moreover, this problem is aggravated by the emergence of bacteria that are multidrug-resistant to antimicrobials [
2]. In the absence of effective preventive measures, projections suggest that this type of bacteria could become the leading cause of death in the coming years [
3]. Consequently, experts have emphasized the urgent need for immediate intervention to mitigate this escalating crisis [
4,
5].
Among the particularly dangerous multidrug-resistant bacteria, considered as priority pathogens by the World Health Organization,
Salmonella stands out for its high morbidity rate (6). Worldwide, between 200 million to 1 billion infections caused by
Salmonella are reported annually, leading to approximately 420,000 deaths [
7,
8,
9].
In addition, growing evidence suggests that
Salmonella is implicated in the development of different types of cancer in the gastrointestinal tract [
10,
11,
12,
13]. Although many aspects of
Salmonella’s in promoting precancerous lesions and tumorogénesis remain unclear, it is known that the oncogenic potential exhibited by certain
Salmonella strains is directly attributed to the ability to synthesize certain effector proteins, including AvrA, SopB, CdtB, PltA, and PltB [
14,
15,
16,
17]. These proteins contribute chronic inflammation, DNA damage, and manipulation of host signaling pathways [
18,
19].
This underscores the pressing need for more effective antimicrobial agents to reinforce public health strategies against these types of bacteria. In this context, there is renewed interest in the use of bacteriophages, also called phages, viruses that infect and lyse bacteria, as therapeutic agents [
20,
21,
22]. These viruses represent one of the most promising alternatives against resistant bacteria due to their highly specific, which enables the selective targeting and destruction of pathogenic bacteria without harming beneficial ones, a crucial advantage in precision medicine [
23]. Furthermore, bacteriophages are generally recognized as safe, as they do not directly affect human or animal health [
24]. Their low production costs also make them an attractive option for biopharmaceutical application [
25].
However, not all bacteriophages are suitable as therapeutic agents, as some may carry genes encoding pathogenicity factors, which could increase the virulence of the bacteria. Others may contain proteins with allergenic potential, be unstable during storage, or fail to exert their therapeutic effect at the intended site of action [
26,
27,
28]. Therefore, it is essential to select bacteriophages through comprehensive characterization to assess both their therapeutic potential and biosafety.
Therefore, the main aim of this research is to isolate and characterize a bacteriophage with preclinical potential as an alternative for the treatment for multidrug-resistant Salmonella strains, particularly those encoding virulence factors associated with the induction of precancerous lesions.
2. Materials and Methods
2.1. Isolation of Salmonella Strains and Identification of Genes with Oncogenic Potential
Salmonella strains were isolated from 54 urban wastewater samples collected in August-September 2023 from the central zone of the State of Sinaloa, Mexico. Samples were obtained from the sewage system in the municipalities of Culiacan and Navolato. The ultrafiltration method described by Liu et al. (2021) was employed due to its high efficiency to concentrate pathogenic bacteria present in environmental samples, enabling a more effective recovery of bacteria such as
Salmonella [
29]. Subsequently, the bacteria were isolated by inoculating the eluate onto Hektoen enteric agar. The presence of the
invA gene was confirmed using polymerase chain reaction (PCR), ensuring accurate identification of each bacterial strain [
30]. In addition, PCR analysis also was utilized to detect genes associated with the promotion of precancerous lesions, which are encoded by specific
Salmonella strains. The genes targeted in this study included
avrA,
sopB,
cdtB,
pltA, and
pltB. For this purpose, the oligonucleotides and protocols described by lshaheeb et al., 2023, Hawwas et al., 2022, and Mezal et al., 2014 were used for analysis (
Table 1) [
31,
32,
33].
2.2. Antibiotic Susceptibility Tests
The disk diffusion method was used to evaluate the
in vitro susceptibility of the isolated bacterial strains to 12 antibiotics, representing six distinct families and five mechanisms of action. The antibiotics included beta-lactams (ampicillin, amoxicillin/clavulanic acid, cephalothin, imipenem), which inhibit cell wall synthesis; quinolones (ciprofloxacin, nalidixic acid), targeting DNA synthesis; aminoglycosides (gentamicin, amikacin), phenicols (chloramphenicol), and tetracyclines (tetracycline), which inhibit protein synthesis; polymyxins (colistin), which disrupt the cell membrane; and sulfonamides (sulfamethoxazole/trimethoprim), inhibiting folic acid synthesis. This selection covers key biochemical pathways to provide a comprehensive profile of antimicrobial resistance. Antibiotics were chosen based on their clinical relevance in treating
Salmonella infections and their usage in the local agricultural sector, the predominant activity in the sampling area. For this purpose, 6 mm discs impregnated with standard antibiotic concentrations, as recommended by the International Committee for Laboratory Standards (CLSI), were used. The bacterial inoculums were adjusted to a turbidity equivalent to 0.5 on the McFarland scale, and the plates were incubated at 37°C for 24 h. After incubation, the diameter of the inhibition zones was measured with a calibrated Bernier [
34].
Salmonella ATCC 14028 was used as the reference strain.
2.3. Bacteriophage Isolation and Purification
For bacteriophage isolation, urban wastewater samples were filtered through aluminum oxide nanofibers (NanoCeram-Argonide Corp), due to their high efficiency in retaining viral particles, using a MasterFlex peristaltic pump (Cole-Palmer, USA) at a constant flow rate of 500 ml/min [
35]. The eluate was recovered, and the double agar layer technique was used to determine the presence of bacteriophages with lytic activity on
Salmonella. Briefly 1 ml of the overnight bacterial culture grown in trypticasein soy broth (TSB) was mixed with 200 µl of the eluate and 3 ml of 0.4% TSB-agarose, and the mixture was poured onto Petri dishes containing trypticasein soy agar (TSA). The plates were incubated at 37 °C for 24 h, after which lysis plaques were identified, excised with a Pasteur pipette, and transferred to microcentrifuge tubes containing sterile distilled water. An aliquot of 100 µl was taken, and the double agar layer technique was repeated. Plates were again incubated for 24 h at 37 °C [
36]. This process was repeated five times to ensure the recovery of a single phage type. The bacteriophage was propagated following the protocol described by [
37]. Purification of the bacteriophage suspension was achieved via dialysis using the 20,000 MWCO Slide-A-Lyzer system (Thermo Fisher, USA), followed by endotoxin removal with the EndoTrap HD system (Lionex). Subsequently, the suspension was filtered through a polyvinylidene difluoride membrane with 0.22 µm pores.
2.4. Transmission Electron Microscopy
For electron microscopy, 30 µl of the purified bacteriophage suspension were placed on a Formvar-coated copper grid with 400-mesh carbon baking and allowed to absorb for 10 min [
38]. Subsequently, the grid was placed in a vacuum evaporator (JEE400, JEOL Ltd. Tokyo, Japan), stained with 2% (w/v) phosphotungstic acid (pH 7.2) for 1 min, and air-dried in a dust-free environment. The samples were examined using a transmission electron microscope (JEM-1011, JEOL Ltd. Tokyo, Japan) operating with an accelerating voltage of 80 kV.
2.5. One-Step Replication Curve
To evaluate the replication kinetics of bacteriophage, a colony of the host strain was resuspended in 50 ml of TSB medium and incubated at 37 °C with shaking at 100 rpm. When the optical density at 600 nm reached 0.1, the bacteriophage was added at a multiplicity of infection (MOI) of 0.1. Every five minutes, two aliquots were collected from de culture, one treated with chloroform and the other untreated. The aliquots were centrifuged at 10,000 ×
g for 1 min, and the supernatant was used to determine the bacteriophage concentration through serial decimal dilutions and the double agar layer method by [
37]. This procedure was performed in triplicate for each time point to ensure reproducibility and accuracy of the results.
2.6. Host Range
The host range of the bacteriophage was evaluated by the agar double-layer method using a purified suspension of the phage at a concentration of 1 × 103 PFU/ml. The assay included 37 Salmonella strains, 8 Escherichia coli strains, and 5 probiotic bacterial strains known to be part of human gut microbiota and considered beneficial, including Bacillus clausii, Bacillus coagulans, Lactobacillus paracasei, Lactobacillus rhamnosus and Limosilactobacillus reuteri. Double-layer agar plates were prepared for each strains according to the method described in section 2.3. The plates were inspected for the formation of lysis plaques on the agar, which indicated the lytic activity of the bacteriophage against the tested strains.
2.7. Stability of the Bacteriophage
Accelerated stability test. The accelerated stability test of the phage was conducted according to the protocol described by Xu et al. (2023), with modifications outline below [
39]. A purified suspension of bacteriophage was incubated at 60, 65, and 70 ◦C, its concentration was continuously monitored at 1 or 2 h intervals. The data on viral concentration were analyzed using three reaction kinetic models: zero-order (kt = M
0 - M), first-order (kt = In (M
0 / M)), and second-order (kt = 1 / M - M
0), respectively. The kinetic model that exhibited the lowest coefficient of determination (
r2) was selected for subsequent calculations, indicating the best fit to the experimental data. The thermodynamic temperature (T) and rate constant (
k) obtained from the selected kinetic model were applied to the Arrhenius equation (-In(
k) = [Ea/RT] - In[A]), where
Ea is the activation energy and R is the gas constant. The values of Ea and A were determined experimentally by a nonlinear fit of the viral concentration data. By combining the Arrhenius equation with the selected reaction kinetics model, was used to calculate the relationship between time (t) and the actual phage concentration (M), which was the model for predicting phage lifetime. To validate the model´s applicability under realistic conditions, it was tested at 28 °C, with bacteriophage concentration measured monthly during six months.
Stability in simulated gastrointestinal environment. The simulated gastrointestinal system was composed of three phases: oral phase, gastric phase and intestinal phase. The composition of each phase is detailed in
Table 2. Electrolyte solutions were sterilized at 121 °C for 15 min at 1 atm pressure. After sterilization, the corresponding enzymes and bile solution, previously filtered with 0.45 μm filters, were added. The bacteriophage was then inoculated into the oral phase at a concentration of approximately 1 × 10⁶ CFU/ml, with final pH adjusted to 7. The mixture was incubated at 37 °C with agitation at 100 rpm for 2 min. Subsequently, the contents of the oral phase were mixed in a 1:1 ratio with the gastric phase, adjusting the final pH of 3, and incubated under the same conditions for 2 h. Finally, the contents of the gastric phase and the intestinal phase were mixed in a 1:1 ratio, adjusting the final pH to 7, and the solution was incubated for 2 h at the described conditions [
40]. At each phase of the experiment, the bacteriophage concentration was determined through serial decimal dilutions and the agar double-layer method. Results were expressed as means ± standard deviation of the bacteriophage concentration, and values of p < 0.01 were considered significant.
2.8. Bacteriolytic Activity of the Bacteriophage
In culture medium. The
Salmonella strain exhibiting the highest antibiotic resistance and coding for all five evaluated virulence genes was cultured in TSB medium at 37°C for 24 hours. Afterward, 1 ml of this culture was added to four separate flasks, each containing 200 ml of TSB, and incubated at 37°C in a shaker at 100 rpm. Bacterial growth was monitored by measuring the optical density at 600 nm (OD
600). When the OD
600 reached 0.5 (~2 × 10
8 CFU/ml), varying concentrations of purified bacteriophage suspension were added: 100 µl to the first flask to final concentration of 1 × 10
7 PFU/ml, 1 × 10
6 PFU/ml to the second, and 1 × 10
5 PFU/ml to the third. The fourth flask served as a control without bacteriophage. All cultures were incubated under the same conditions, and optical density measurements were taken hourly [
41].
In simulated gastrointestinal system. The bacteriolytic capacity of the bacteriophage was evaluated using the
in vitro system described in section 2.7, with modifications to incorporate a food model similar to that proposed by Akritidou et al. (2023), designed to promote bacterial survival, was provided [
42] . To simulate a microbiome, the five bacterial species described in section 2.6 were used. All strains were mixed in an equal proportion and used immediately [
43]. In the simulated intestinal fluid, each of the following treatments were inoculated: (1) beneficial bacteria consortium (1×10
6 CFU/ml) + 50 µl of sterile water, (2)
Salmonella (1×10
6 CFU/ml) + 50 µl of sterile water, (3) beneficial bacteria consortium (1×10
6 CFU/ml) + 50 µl of purified bacteriophage suspension (1×10
4 PFU/ml), (4)
Salmonella (1×10
6 CFU/ml) + 50 µl of the purified bacteriophage suspension (1×10
4 CFU/ml), (5)
Salmonella (1×10
6 CFU/ml) + ciprofloxacin 0. 5 mg/ml and (6) beneficial bacteria consortium (1×10
6 CFU/ml) + 0.5 mg/ml. The bacterial concentration of each treatment was quantified by plaque extension. Results were presented as the mean concentration ± standard deviation, with p-values < 0.01 considered statistically significant.
2.9. Genomic Sequencing and Bioinformatic Analysis
Bacteriophage genetic material was extracted according to the proteinase K/SDS protocol [
44]. Genomic libraries were generated using the MGIEasy DNA library Prep Universal System, and nucleotide sequencing was conducted on the MGISEQ-2000 platform utilizing the DNBSEQTM (nanoballs DNA) system. Assembly and bioinformatics analysis was performed according to the guidelines proposed by Philipson et al. (2018) and Turner et al. (2021) [
45,
46]. Random sampling of reads (50,000 to 100,000) was performed using the Seqtk Toolkit tool until a coverage depth of ~100× was achieved. After analyzing the quality of reads with FastQC, low quality adapters and sequences (Phred index < 30) were removed using Trimmomatic.
De novo assembly of reads was performed with SPAdes, employing k-mer of 21, 33 and 55. Open reading frames (ORFs) were identified using Glimmer, Genemark, Genemark.hmm, Genemark S, Prodigal, RAST and MetaGene. Promoters were identified with PhagePromoter and PHIRE, while FindTerm and RNAold were used to identify Rho-independent terminators. The tRNAs were determined by ARAGORN and tRNAscan-SE. The ability of the bacteriophage to establish lysogenic cycles was evaluated using PhageAI and PHACTS. The presence of genes associated with antibiotic resistance was analyzed in the CARD platform (
https://card.mcmaster.ca) and AMRFinderPlus, while virulence factors were identified through VFDB (
www.mgc.ac.cn/VFs/search_VFs.htm) and VirulentPred (
http://203.92.44.117/virulent/submit.html).
2.10. Statistical Analysis
All statistical analyses were conducted using Minitab 19. Normality assumptions were verified with the Shapiro-Wilk test, and homogeneity of variance was assessed with the Levene's test. Differences in bacteriophage stability under simulated gastrointestinal conditions and bacteriolytic activity were analyzed by repeated measures ANOVA and two-factor ANOVA, respectively, both followed by Tukey's post hoc test. Significance was set at p ≤ 0.01. All assays were performed in triplicate.
3. Results
3.1. Isolation of Salmonella and Identification of Genes Associated to Cancer Induction
A total of 37
Salmonella strains were isolated from the wastewater samples, with an average isolation rate of 43%. This high prevalence of this bacterium suggests a significant occurrence of
Salmonella in urban wastewater, underscoring the importance of monitoring its presence due to its potential impact on public health risks. These findings are congruent with previous studies that have documented the presence of
Salmonella in urban wastewater systems as an indicator of fecal contamination and insufficient water treatment efforts [
47,
48].
Notably, 17 of the 37 isolates harbored five virulence genes associated with potential cancer induction, specifically
avrA,
sopB,
cdtB,
pltA, and
pltB (
Table 3). According to numerous studies,
Salmonella strains that encode these genes produce effector proteins implicated in chronic inflammation and the induction of precancerous lesions. Although several aspects remain unclear, it is known that the AvrA protein modulates the β-catenin signaling pathway, potentially influencing the regulation, differentiation, and proliferation of intestinal epithelial cells [
49]. In contrast, SopB promotes intracellular invasion of
Salmoenlla and inhibits apoptosis [
50,
51]. Additionally, the cytolethal distending toxin, composed of CdtB, PltA, and PltB proteins, induces DNA damage, which results in genomic instability and a proinflammatory environment associated with precancerous lesions [
52]. Consequently, future research will aim to evaluate the pathogenic potential of these strains in cancer promotion.
The detection of Salmonella strains harboring genes associated with cancer induction in wastewater highlights the urgent need for stricter water treatment policies to mitigate the spread of these pathogens and their long-term public health implications
3.2. Antibiotic Sensitivity Tests
The antimicrobial sensitivity of the 37
Salmonella strains was evaluated against 12 antibiotics commonly used for treating bacterial infections. The results revealed that four (sal01, sal08, sal27, and sal28) of the strains exhibited resistance or intermediate sensitivity to at least three different classes of antibiotics, categorizing them as multidrug-resistant (
Table 3). A significantly higher incidence of resistance was observed for colistin (11 strains), followed by nalidixic acid (9 strains), tetracycline (7 strains), and ampicillin (6 strains). The resistance to ciprofloxacin and colistin, two critical antibiotics used the treating severe
Salmonella infections, and other bacterial infections, is particularly alarming. These findings are consistent with recent reports from other Latin America regions, which also indicate a rising prevalence of antibiotic-resistant bacterial strains [
53,
54]. The identification of multidrug-resistant
Salmonella strains highlights the need for new strategies to combat antimicrobial resistant. In this context, research into therapeutic alternatives, such as the use of bacteriophages, may offer a promising approach for addressing this growing public health challenge [
22].
3.3. Isolation of the Bacteriophage
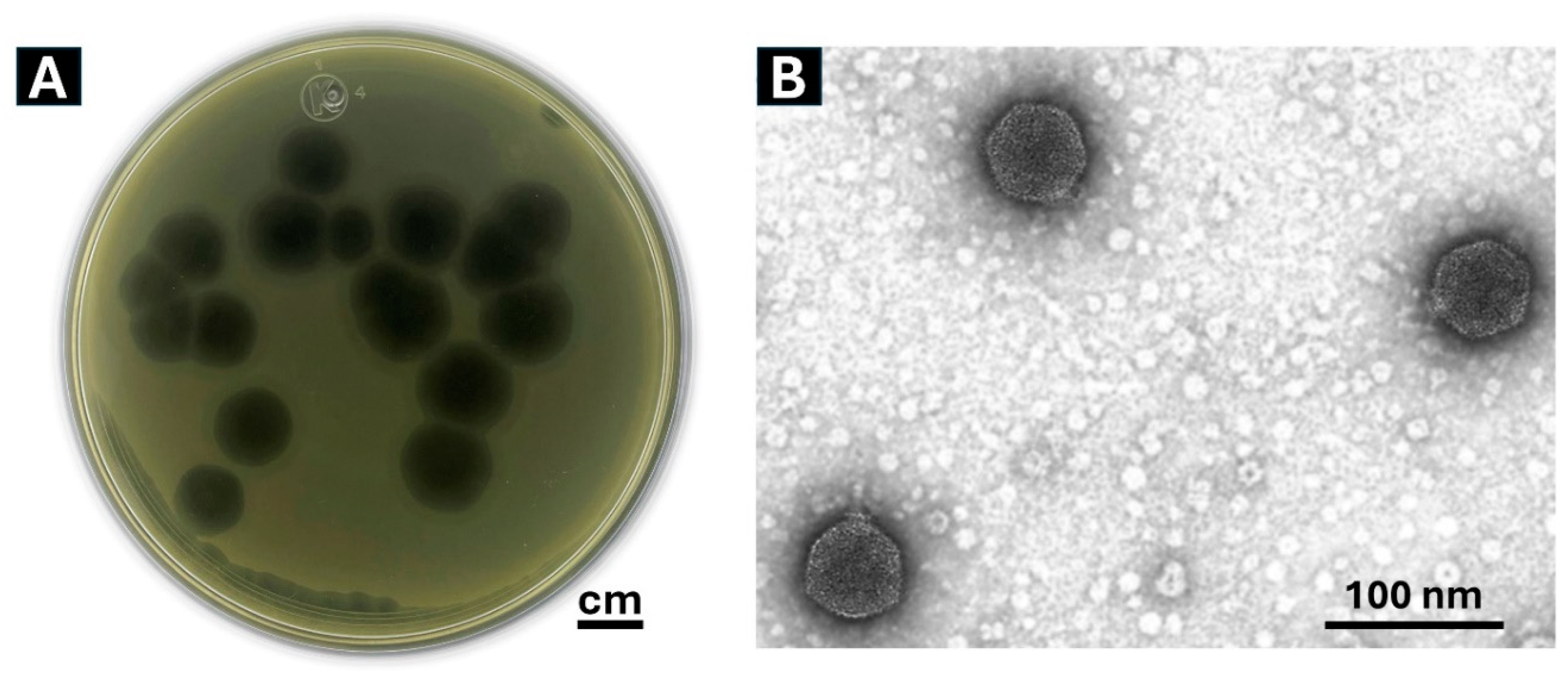
A bacteriophage, designated Phylax-28 (from the Greek “guard” or “protector), was successfully isolated, producing clear and well-defined lysis plaques, with a diameter of approximately 1.2 cm (
Figure 1A). The plaque size generated by Phylax-28 is notably larger than typical
Salmonella phages, which are generally reported to form plaques ranging between 0.5- 3.5 mm in diameter [
55,
56,
57,
58].
In addition, Phylax-28 induces the formation of an inhibition halo, a semitransparent zone surrounding the lysis plaques. This phenomenon is consistent with findings by Jurczak-Kurek and coworkers, who noted that phages that produce clear plaques are often associated with a strong lytic activity against bacteria. The appearance of the halo is attributed to the activity of some enzymes, encoded by certain bacteriophage, that degrade the cell wall, thereby enhancing the phage’s potential for bacterial elimination [
59].
3.4. Bacteriophage Morphology
Analysis of transmission electron micrographs of 20 virions of the Phylax-28 revealed that it has an icosahedral-isometric capsid 28 ± 0.2 nm in diameter, along with a thin, short, non-contractile and rigid tail 8.2 ± 0.1 nm in length (
Figure 1B). Based on these morphological features and the criteria established by the International Committee on Viral Taxonomy, the bacteriophage Phylax-28 is classified as a new member of the family
Autographiviridae.
Usually, bacteriophages with short tails or absent tails, such as Phylax-28, are typically associated with increased resistance to harsh environmental conditions [
60,
61]. These morphological traits suggest that Phylax-28 may maintain stability under aggressive bio-physicochemical conditions, such as those prevailing in the gastrointestinal system. However, further experimental data are required to confirm this hypothesis. Subsequent sections will detail the experimental results of phage stability under these conditions.
3.5. Bacteriophage Replication Curve
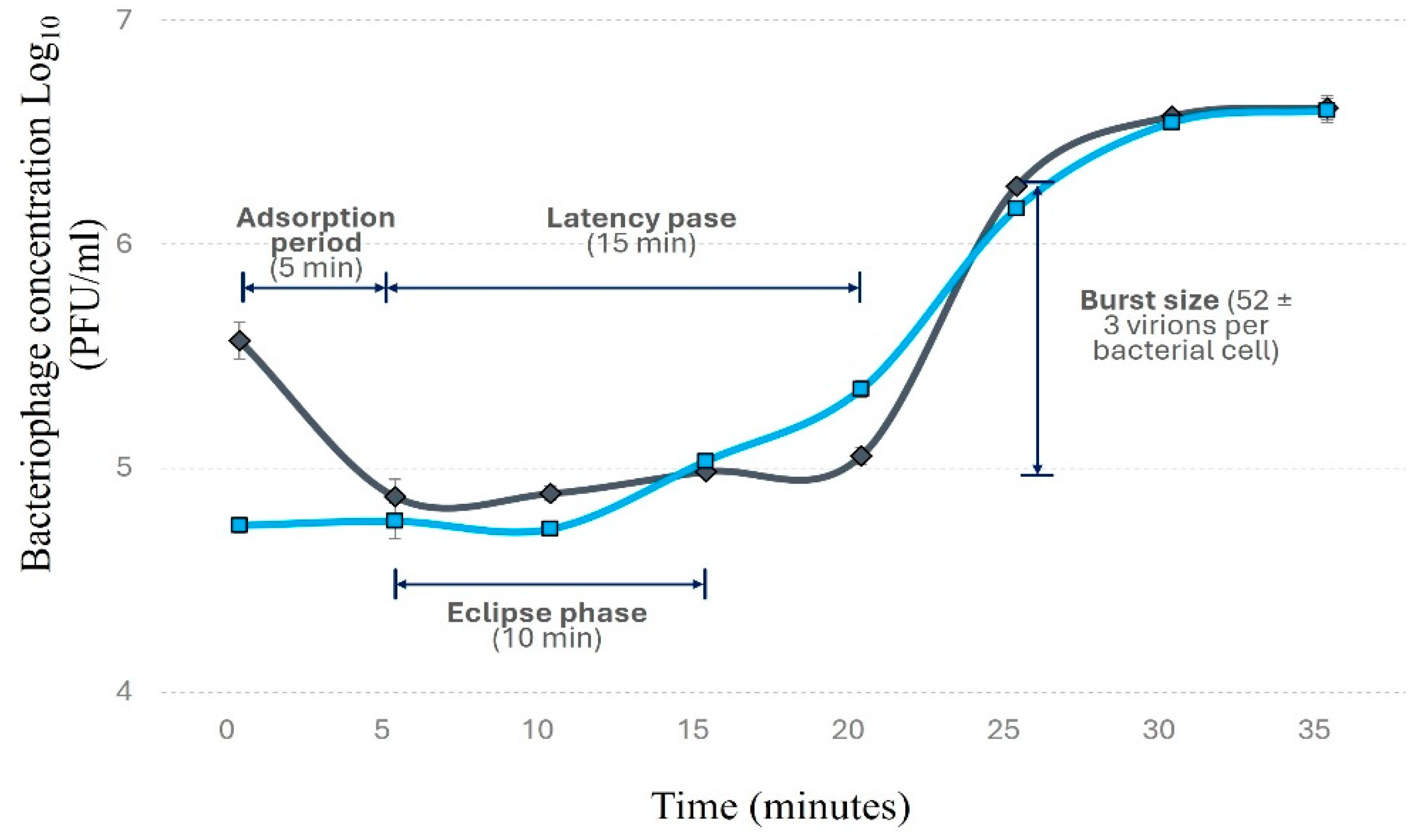
Within 5 minutes of exposure, approximately 80% of Phylax-28 virions were adsorbed to the bacterial surface (
Figure 2). Phylax-28 presents a latency period of 15 min, with bacterial lysis occurring 20 min post-infection, releasing 52 ± 3 virions per bacterial cell. This burst size observed in Phylax-28 is relatively large compared to other
Salmonella- phages, which typically produce between 34 and 37 virions per cell [
62,
63]. Some
Salmonella-infecting phages, however, have been reported to produce larger burst size but with longer latency periods [
64,
65]. The short latency period of Phylax-28 suggest a competitive advantage over other phages, as they can produce enough virions to lyse the bacterium in a short period of time, making it a promising candidate for
Salmonella control.
3.6. Host Range
Phylax-28 showed the ability to lyse 21 of the 37
Salmonella strains and 3 strains of
E. coli. Bacteriophages capable of lysing two or more bacterial species or diverse strains within a bacterial species, like Phylax-28, are considered to have a broad host range [
66]. These bacteriophages are more likely to be enhances as therapeutic agents [
67]. However, the therapeutic efficacy of a phage also depends on its specificity. It is essential that phages selectively target pathogenic bacteria without harming bacteria that perform beneficial functions [
68]. Notably, Phylax-28 did not exhibit lytic activity against probiotic strains such as
Bacillus clausii,
Bacillus coagulans,
Lactobacillus paracasei,
Lactobacillus rhamnosus and
Limosilactobacillus reuteri, an attribute that could prove advantageous foro control the population of pathogenic bacteria during infection without affecting beneficial bacteria.
3.7. Stability of the Bacteriophage
3.7.1. Storage stability. An essential criterion for selecting bacteriophages as therapeutic is their stability during storage and under the biophysicochemical conditions prevailing at the site of action [
39]. For this reason, the storage stability of the Phylax-28 phage was assessed through an accelerated stability assay and validated by monitoring the decrease in phage concentration at 28 °C over a six-months.
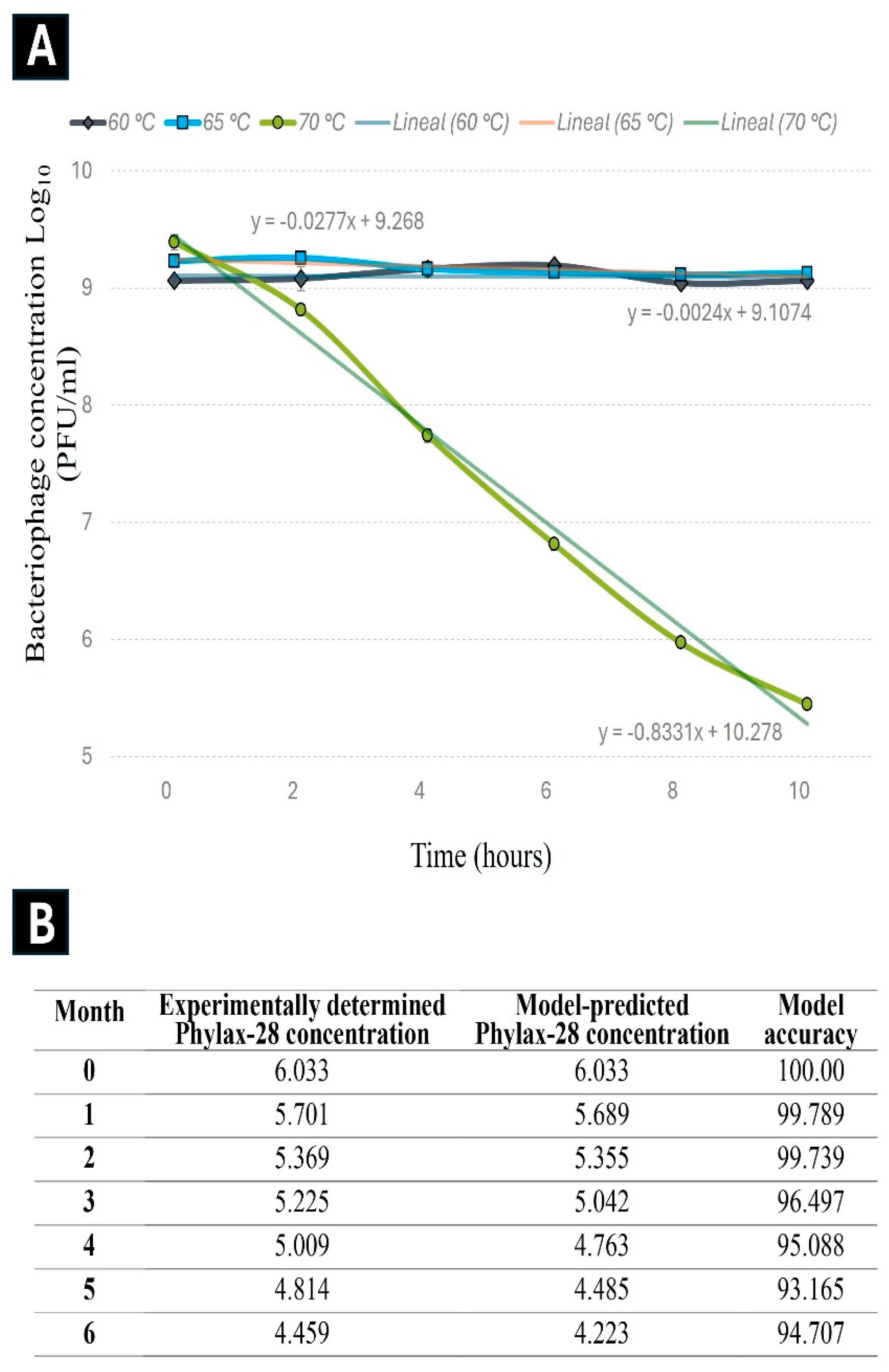
The experimental data from the accelerated stability revealed that the reduction in Phylax-28 concentration follows first-order kinetics, with a correlation coefficient (
r) of 0.991 at 70 °C (
Figure 3). The coefficient of determination (
r²) for the first-order kinetics was 2.80, higher than the values obtained for the zero-order (2.40) and second-order (2.63) reactions, confirming that the first-order model best describe the changes in Phylax-28 concentration. The degradation constant (
k), calculated from the experimental data obtained for the stability of Phylax-28 at a temperature of 28 °C over six-months, was estimated to be 0.045 days
-1, while the activation energy (
Ea) was 158.6 kJ/mol.
The predictive model suggests that the bacteriophage suspension will retain its stable concentration for approximately 92 days at 28 °C before undergoing a 1-logarithm reduction. This prediction is based on the application of the first-order kinetic model together with the Arrhenius equation.
Notably, experimental results support the accuracy of this model: after three months of storage at 28°C, only a 1 logarithm reduction in phage concentration was observed, consistent with model predictions at 94% accuracy. According to Xu et al. (2023), this level of accuracy is considered highly. However, the full validation of the model remains ongoing.
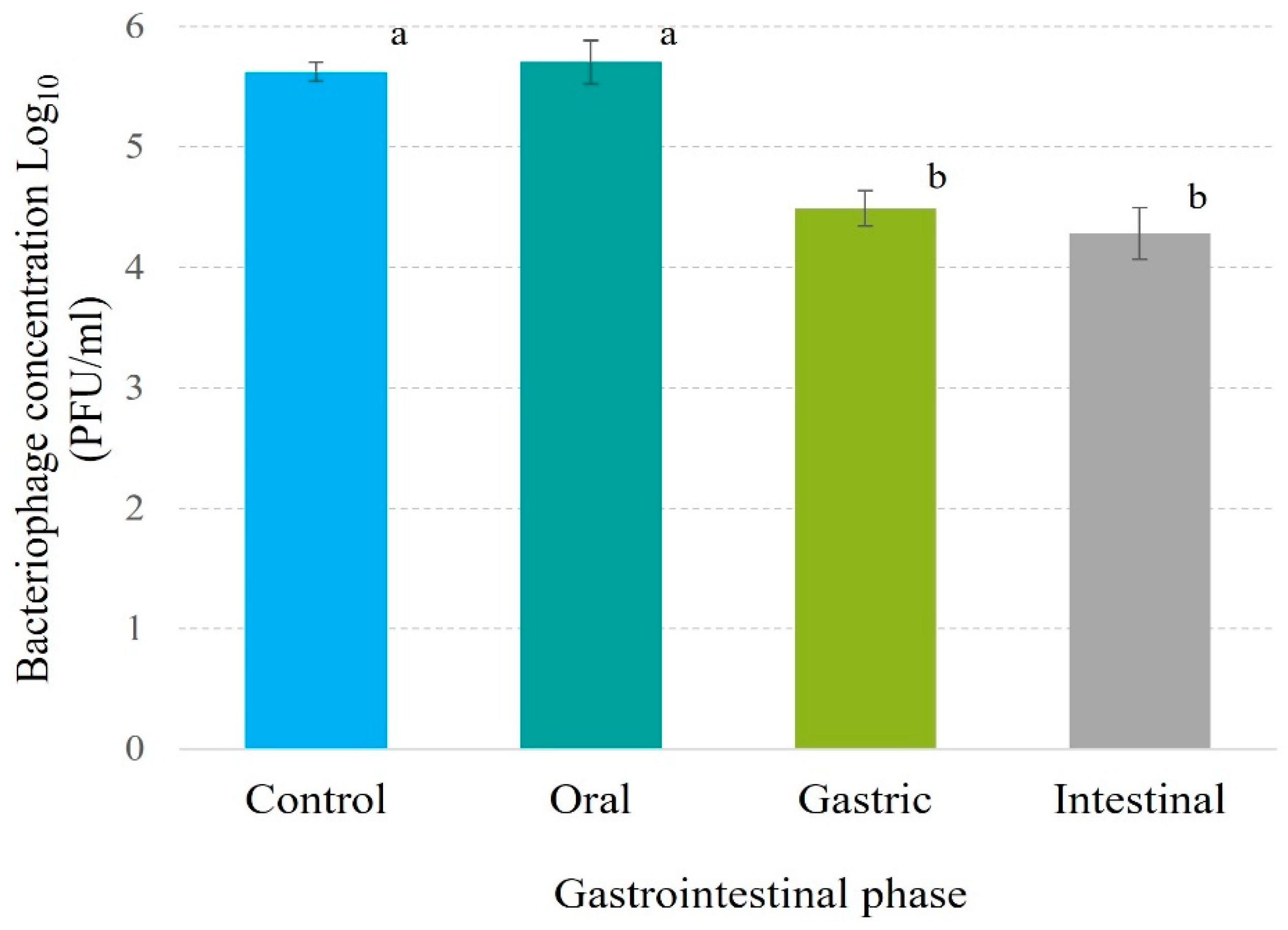
3.7.2. Stability in simulated gastrointestinal system. Phage Phylax-28 exhibits a remarkable stability under simulated gastrointestinal conditions. In the oral phase, no significant reduction in viral concentration was observed, and at the gastric level, the decrease was limited to 1.1 logarithms. Furthermore, the concentration remained stable at the intestinal level in relation to the gastric phase (Figure 4). These finding stand in contrast to those report for coliphage Ace, which experienced a 4 logarithms reduction compared to the initial dose under similar conditions [69]. Additionally, when five Salmonella phages, Dlamini et al. (2023) documented reductions ranging from 4.86 to 5.55 logarithms, concluding that these phages would require encapsulation in CaCO3 for therapeutic viability [70]. Similarly, bacteriophage ZCEC5 showed comparable reductions, with authors recommending microencapsulation in chitosan-alginate for stability [71]. In comparison, Phylax-28 demonstrates superior stability under gastrointestinal conditions, suggesting its potential as a more robust therapeutic agent.
3.8. Capacity of the Bacteriophage to Control Salmonella
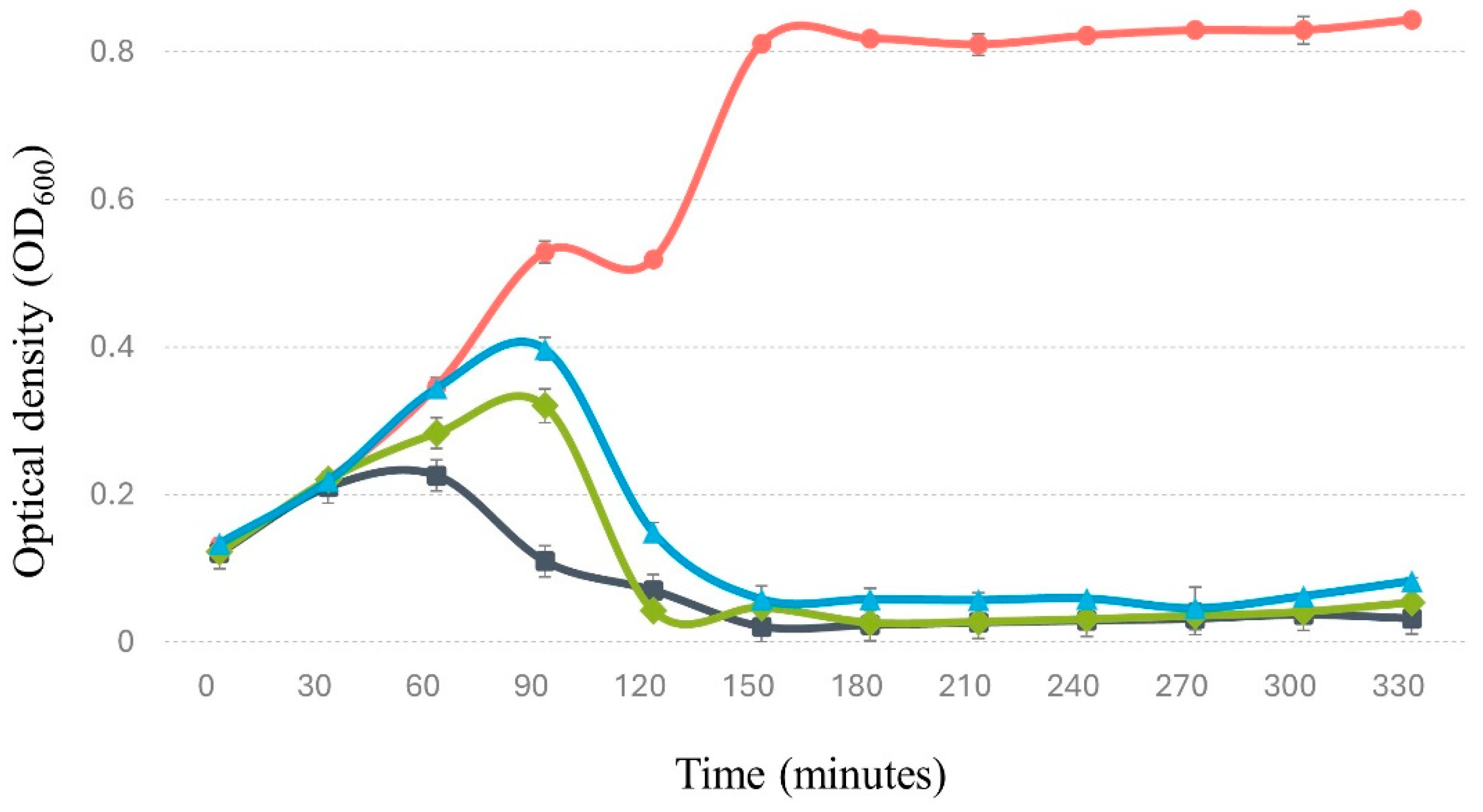
3.8.1. In culture medium. Under these conditions, Phylax-28 exhibits bacteriolytic activity against
Salmonella, achieving a significant reduction in bacterial concentration within 60 minutes of inoculation compared to normal bacterial growth (p < 0.01). This effect was observed even at low multiplicities of infection (MOI) of 0.01 and 0.001 (
Figure 5). These findings are noteworthy, as many phages demonstrate rapid bacteriolytic activity only at higher MOIs. For instance, [
72] reported a significant reduction in bacterial load only when using MOIs of 10 and 100. Phylax-28's bacteriolytic activity at low MOIs presents a significant advantage for therapeutic applications, as it suggests that lower phage doses may effectively control
Salmonella populations. This reduces the potential risk of side effects commonly associated with higher phage doses, offering a safer and more efficient approach to phage therapy.
3.8.2. In simulated gastrointestinal system
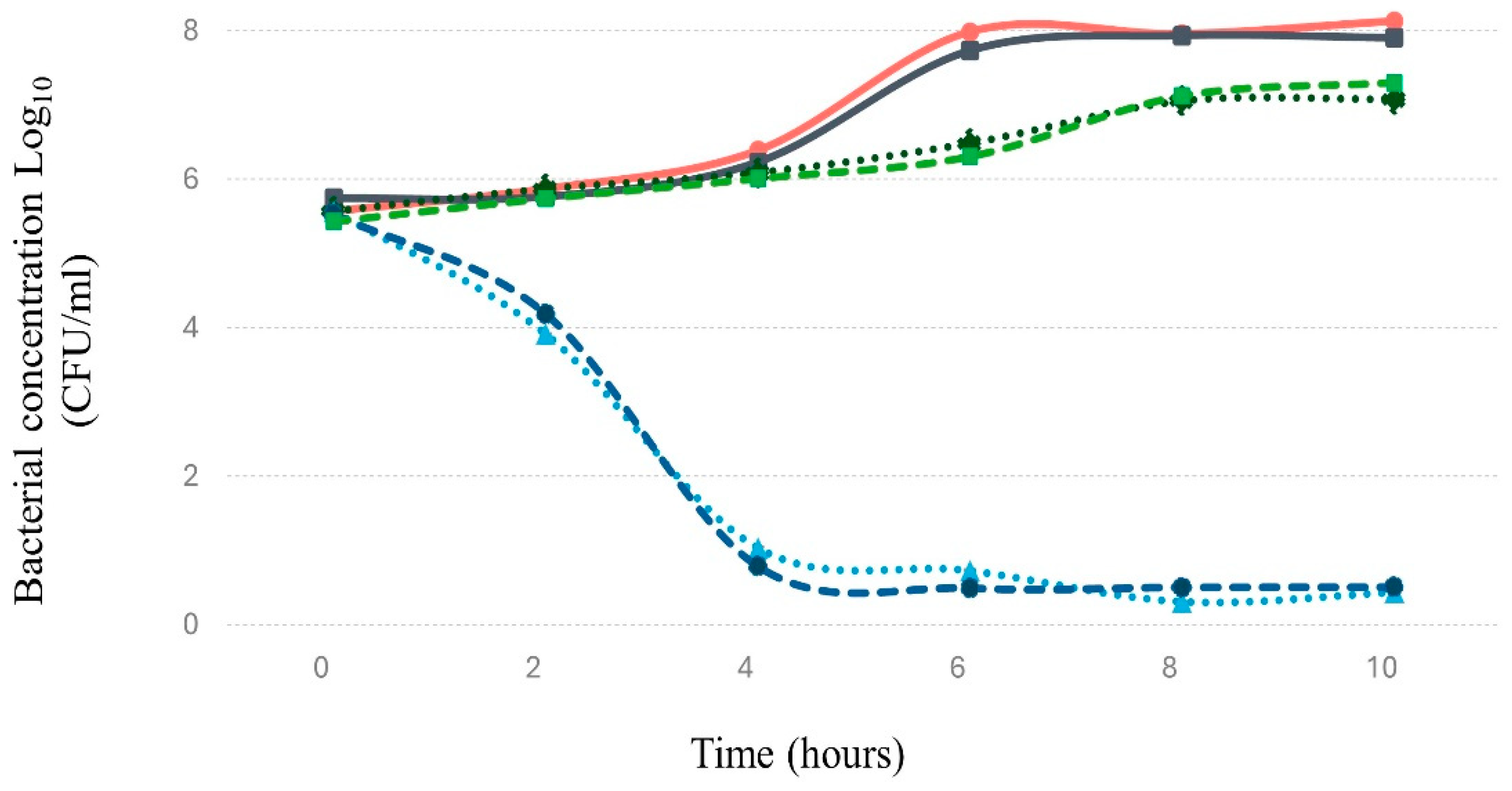
. Salmonella tends to colonize and invade the human intestine [
73], making it crucial to evaluate the bacteriophage’s ability to control this pathogen under such conditions. In this regard, the results indicate that Phylax-28 significantly reduces
Salmonella concentration in simulated intestinal environment (p ≤ 0.01). Moreover, no significant changes were observed in the population of beneficial bacteria due to phage activity (
Figure 6). In contrast, in the treatment with ciprofloxacin did not reduce
Salmonella concentration compared to the control, and there was a marked reduction in the population of beneficial bacteria caused by the antibiotic. While in the beneficial bacteria there is a marked reduction in the concentration due to the action of the antibiotic. These findings are highly relevant, as they suggest that Phylax-28 could potentially control
Salmonella without adversely affecting the microbiome, positioning it as a promising tool in precision medicine. However, further studies are necessary to validate these results.
3.9. Bacteriophage Genomic Analysis
The genome of the bacteriophage Phylax-28 consists of linear double-stranded DNA, with a molecular size of 40,989 bp and GC content of 57.81%. It encodes 50 genes, none of which are associated with tRNA. Gene functions were annotated using the NCBI BLAST database. Gene functions were identified with an e-value of <10⁻⁵, sequence coverage exceeding 50%, and identity greater than 85%. The genome is organized into distinct functional modules: a morphogenesis module, containing ten genes responsible for the synthesis of virion structural proteins; a packaging module, comprising four genes involved in DNA encapsulation within the capsid; a DNA processing module with 13 genes regulating the replication and transcription of genetic material; and a lysis module, consisting of three genes responsible for cell wall degradation and bacterial lysis; the remaining genes encode hypothetical proteins. Critically, for biosafety considerations, no genes related to virulence factors, antimicrobial resistance, or allergenic responses were identified. Moreover, Phylax-28 was classified as strictly lytic, a desirable trait for bacteriophages intended for therapeutic applications [
74]. The complete genome sequence of phage Phylax-28 was deposited in GenBank/EMBL/DDBJ under the accession number PQ306468.
5. Conclusions
Phylax-28 exhibits promising potential as a therapeutic agent against multidrug-resistant Salmonella strains that encode virulence factors associated with the development of precancerous lesions. Although further research is necessary to confirm its safety and efficacy in clinical settings, preliminary data suggest that this bacteriophage could become a viable biopharmaceutical option in the near future.
Author Contributions
LA: LL, and MEZ contributed to the experimental design, data collection, and analysis. LA, LL and RLC provided technical expertise in bacteriophage characterization and bioinformatics analysis. FP and JP were involved in the microbiological assays and data validation. ELA participated in the experimental setup and contributed to the drafting of the manuscript. MEA was responsible for overseeing the research project, conceptualization, supervision of the study, and manuscript preparation. All authors read and approved the final version of the manuscript.
Funding
This research was funded by CONAHCYT Mexico through the “Ciencia de Frontera 2019” program, under project with ID: 1043168.
Acknowledgments
We gratefully acknowledge the financial support from CONAHCYT (Project ID: 1043168), which was crucial to the successful completion of this research. We also wish to extend our sincere appreciation to Armado Favela Olivas, Jesús Antonio Núñez, and Zendy Mariela Martínez for their invaluable technical assistance and contributions throughout the experimental phases. Their expertise and unwavering commitment were instrumental to the progress and outcome of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ikuta KS, Swetschinski LR, Robles Aguilar G, Sharara F, Mestrovic T, Gray AP, et al. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2022 Dec;400(10369):2221–48. [CrossRef]
- Loftus MJ, Everts RJ, Cheng AC, Eti P, Fakasiieiki T, Isaia L, et al. Antimicrobial susceptibility of bacterial isolates from clinical specimens in four Pacific Island countries, 2017-2021. 2022. [CrossRef]
- Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 2022 Feb 12;399(10325):629–55. [CrossRef]
- Karvanen M, Cars O. The language of antimicrobial and antibiotic resistance is blocking global collective action. Vol. 56, Infectious Diseases. Taylor and Francis Ltd.; 2024. p. 487–95. [CrossRef]
- Heinzel M, Koenig-Archibugi M. National action on antimicrobial resistance and the political economy of health care. J Eur Public Policy. 2024. [CrossRef]
- WHO. WHO Bacterial Priority Pathogens List, 2024: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. Geneva: World Health Organization 12-13., editor. 2024.
- Soubeiga AP, Kpoda DS, Compaoré MKA, Somda-Belemlougri A, Kaseko N, Rouamba SS, et al. Molecular characterization and the antimicrobial resistance profile of Salmonella spp. isolated from ready-to-eat foods in Ouagadougou, Burkina Faso. Int J Microbiol. 2022.
- Wang Z, Zhou H, Liu Y, Huang C, Chen J, Siddique A, et al. Nationwide trends and features of human salmonellosis outbreaks in China. Emerg Microbes Infect. 2024;13(1). [CrossRef]
- Lamichhane B, Mawad AMM, Saleh M, Kelley WG, Harrington PJ, Lovestad CW, et al. Salmonellosis: an overview of epidemiology, pathogenesis, and innovative approaches to mitigate the antimicrobial resistant infections. Vol. 13, Antibiotics. Multidisciplinary Digital Publishing Institute (MDPI); 2024. [CrossRef]
- Scanu T, Spaapen RM, Bakker JM, Pratap CB, Wu L en, Hofland I, et al. Salmonella manipulation of host signaling pathways provokes cellular transformation associated with gallbladder carcinoma. Cell Host Microbe. 2015 Jun 1;17(6):763–74. [CrossRef]
- Lu R, Wu S, Zhang Y guo, Xia Y, Zhou Z, Kato I, et al. Salmonella protein AvrA activates the STAT3 signaling pathway in colon cancer. Neoplasia (United States). 2016. 1;18(5):307–16. [CrossRef]
- Lohman E de S, Duijster J, Koerkamp BG, van der Post R, Franz E, Gras LM, et al. Severe Salmonella spp. or Campylobacter spp. infection and the risk of biliary tract cancer: A population-based study. Cancers (Basel). 2020;12(11):1–12. [CrossRef]
- Wang DN, Ni JJ, Li JH, Gao YQ, Ni FJ, Zhang ZZ, et al. Bacterial infection promotes tumorigenesis of colorectal cancer via regulating CDC42 acetylation. PLoS Pathog. 2023 Feb 1;19(2). [CrossRef]
- Lopez Chiloeches M, Bergonzini A, Martin OCB, Bergstein N, Erttmann SF, Aung KM, et al. Genotoxin-producing Salmonella enterica induces tissue-specific types of DNA damage and DNA damage response outcomes. Front Immunol. 2023;14. [CrossRef]
- Lu R, Bosland M, Xia Y, Zhang YG, Kato I, Sun J. Oncotarget 55104 www.impactjournals.com/oncotarget Presence of Salmonella AvrA in colorectal tumor and its precursor lesions in mouse intestine and human specimens. Vol. 8, Oncotarget. 2017. [CrossRef]
- Zhao S, Xu Q, Cui Y, Yao S, Jin S, Zhang Q, et al. Salmonella effector SopB reorganizes cytoskeletal vimentin to maintain replication vacuoles for efficient infection. Nat Commun. 2023 Dec 1;14(1). [CrossRef]
- ElGhazaly M, Collins MO, Ibler AEM, Humphreys D. Typhoid toxin hijacks Wnt5a to establish host senescence and Salmonella infection. Cell Rep. 2023 Oct 31;42(10). [CrossRef]
- Shukla R, Shukla P, Behari A, Khetan D, Chaudhary RK, Tsuchiya Y, et al. Roles of Salmonella typhi and Salmonella paratyphi in gallbladder cancer development. Asian Pacific Journal of Cancer Prevention. 2021;22(2):509–16. [CrossRef]
- Mughini-Gras L, Schaapveld M, Kramers J, Mooij S, Neefjes-Borst EA, Van Pelt W, et al. Increased colon cancer risk after severe Salmonella infection. PLoS One. 2018 Jan 1;13(1). [CrossRef]
- Onallah H, Hazan R, Nir-Paz R, Yerushalmy O, Rimon A, Braunstein R, et al. Compassionate use of bacteriophages for failed persistent infections during the first 5 years of the Israeli Phage Therapy Center. Open Forum Infect Dis. 2023 May 1;10(5). [CrossRef]
- Zaki BM, Fahmy NA, Aziz RK, Samir R, El-Shibiny A. Characterization and comprehensive genome analysis of novel bacteriophage, vB_Kpn_ZCKp20p, with lytic and anti-biofilm potential against clinical multidrug-resistant Klebsiella pneumoniae. Front Cell Infect Microbiol. 2023 Jan 23;13. [CrossRef]
- Ferreira R, Sousa C, Gonçalves RFS, Pinheiro AC, Oleastro M, Wagemans J, et al. Characterization and genomic analysis of a new phage infecting Helicobacter pylori. Int J Mol Sci. 2022 Jul 1;23(14). [CrossRef]
- Principi N, Silvestri E, Esposito S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Vol. 10, Frontiers in Pharmacology. Frontiers Media S.A.; 2019. [CrossRef]
- Gindin M, Febvre HP, Rao S, Wallace TC, Weir TL. bacteriophage for gastrointestinal health (PHAGE) study: evaluating the safety and tolerability of supplemental bacteriophage consumption. J Am Coll Nutr. 2019 Jan 2;38(1):68–75. [CrossRef]
- Krysiak-Baltyn K, Martin GJO, Gras SL. Computational modelling of large scale phage production using a two-stage batch process. Pharmaceuticals. 2018 Jun 1;11(2). [CrossRef]
- Castillo D, Kauffman K, Hussain F, Kalatzis P, Rørbo N, Polz MF, et al. Widespread distribution of prophage-encoded virulence factors in marine Vibrio communities. Sci Rep. 2018 Dec 1;8(1). [CrossRef]
- Flint R, Laucirica DR, Chan HK, Chang BJ, Stick SM, Kicic A. Stability considerations for bacteriophages in liquid formulations designed for nebulization. Vol. 12, Cells. Multidisciplinary Digital Publishing Institute (MDPI); 2023. [CrossRef]
- Berkson JD, Wate CE, Allen GB, Schubert AM, Dunbar KE, Coryell MP, et al. Phage-specific immunity impairs efficacy of bacteriophage targeting vancomycin resistant Enterococcus in a murine model. Nat Commun. 2024 Dec 1;15(1). [CrossRef]
- Liu P, Ibaraki M, Kapoor R, Amin N, Das A, Miah R, et al. development of moore swab and ultrafiltration concentration and detection methods for Salmonella typhi and Salmonella paratyphi A in wastewater and application in Kolkata, India and Dhaka, Bangladesh. Front Microbiol. 2021 Jul 15;12. [CrossRef]
- Yanestria SM, Rahmaniar RP, Wibisono FJ, Effendi MH. Detection of invA gene of Salmonella from milkfish (Chanos chanos) at Sidoarjo wet fish market, Indonesia, using polymerase chain reaction technique. Vet World. 2019;12(1):170–5. [CrossRef]
- Alshaheeb ZA, Thabit ZA, Oraibi AG, Baioumy AA, Abedelmaksoud TG. Salmonella enterica species isolated from local foodstuff and patients suffering from foodborne illness: surveillance, antimicrobial resistance and molecular detection. Theory and Practice of Meat Processing. 2023;8(2):112–23. [CrossRef]
- Hawwas HAEH, Aboueisha AKM, Fadel HM, El-Mahallawy HS. Salmonella serovars in sheep and goats and their probable zoonotic potential to humans in Suez Canal Area, Egypt. Acta Vet Scand. 2022 Dec 1;64(1). [CrossRef]
- Mezal EH, Bae D, Khan AA. Detection and functionality of the CdtB, PltA, and PltB from Salmonella enterica serovar Javiana. Pathog Dis. 2014 Nov 1;72(2):95–103. [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests. 14th ed. CLSI standard M02. Clinical and Laboratory Standards Institute; 2024. 2024.
- Ikner LA, Soto-Beltran M, Bright KR. New method using a positively charged microporous filter and ultrafiltration for concentration of viruses from tap water. Appl Environ Microbiol. 2011 May;77(10):3500–6. [CrossRef]
- Li C, Yuan X, Li N, Wang J, Yu S, Zeng H, et al. Isolation and characterization of Bacillus cereus phage vb_bcep-dlc1 reveals the largest member of the Φ29-like phages. Microorganisms. 2020 Nov 1;8(11):1–22. [CrossRef]
- Le Guellec S, Pardessus J, Bodier-Montagutelli E, L’Hostis G, Dalloneau E, Piel D, et al. Administration of bacteriophages via nebulization during mechanical ventilation: in vitro study and lung deposition in macaques. Viruses. 2023 Mar 1;15(3). [CrossRef]
- Kabwe M, Brown T, Speirs L, Ku H, Leach M, Chan HT, et al. Novel bacteriophages capable of disrupting biofilms from clinical strains of Aeromonas hydrophila. Front Microbiol. 2020 Feb 14;11. [CrossRef]
- Xu Z, Ding Z, Zhang Y, Liu X, Wang Q, Shao S, et al. Shelf-life prediction and storage stability of Aeromonas bacteriophage vB_AsM_ZHF. Virus Res. 2023 Jan 2;323. [CrossRef]
- Mulet-Cabero AI, Egger L, Portmann R, Ménard O, Marze S, Minekus M, et al. A standardised semi-dynamic: in vitro digestion method suitable for food-an international consensus. Food Funct. 2020 Feb 1;11(2):1702–20. [CrossRef]
- Sonalika J, Srujana ;, Akhila ;, Juliet ;, Santhosh KS. Application of bacteriophages to control Salmonella enteritidis in raw eggs. Vol. 21. 2020.
- Akritidou T, Akkermans S, Smet C, Delens V, Van Impe JFM. Effect of food structure and buffering capacity on pathogen survival during in vitro digestion. Food Research International. 2023 Feb 1;164. [CrossRef]
- Cieplak T, Soffer N, Sulakvelidze A, Nielsen DS. A bacteriophage cocktail targeting Escherichia coli reduces E. coli in simulated gut conditions, while preserving a non-targeted representative commensal normal microbiota. Gut Microbes. 2018 Sep 3;9(5):391–9. [CrossRef]
- Sambrook J, Russell DW. Extraction of Bacteriophage λ DNA from Large-scale Cultures Using Proteinase K and SDS. CSH protocols. Vol. 1. 2006. [CrossRef]
- Philipson CW, Voegtly LJ, Lueder MR, Long KA, Rice GK, Frey KG, et al. Characterizing phage genomes for therapeutic applications. Viruses. 2018 Apr 10;10(4). [CrossRef]
- Turner D, Adriaenssens EM, Tolstoy I, Kropinski AM. Phage annotation guide: guidelines for assembly and high-quality annotation. PHAGE: Therapy, Applications, and Research. 2021 Dec 1;2(4):170–82. [CrossRef]
- Diemert S, Yan T. Municipal wastewater surveillance revealed a high community disease burden of a rarely reported and possibly subclinical Salmonella enterica serovar derby strain. 2020; Available from: https://doi.org/10.1128/AEM. [CrossRef]
- Martone-Rocha S, Dropa M, da Cruz BMC, Leite DBMO, dos Santos TP, Razzolini MTP. Antimicrobial profile of non-typhoidal Salmonella isolated from raw sewage in the Metropolitan Region of São Paulo, Brazil. J Infect Dev Ctries. 2023 Jan 1;17(1):86–92. [CrossRef]
- Dougherty MW, Jobin C. Intestinal bacteria and colorectal cancer: etiology and treatment. Vol. 15, Gut Microbes. Taylor and Francis Ltd.; 2023. [CrossRef]
- Zhao S, Xu Q, Cui Y, Yao S, Jin S, Zhang Q, et al. Salmonella effector SopB reorganizes cytoskeletal vimentin to maintain replication vacuoles for efficient infection. Nat Commun. 2023 Dec 1;14(1). [CrossRef]
- Ruan H, Zhang Z, Tian L, Wang S, Hu S, Qiao JJ. The Salmonella effector SopB prevents ROS-induced apoptosis of epithelial cells by retarding TRAF6 recruitment to mitochondria. Biochem Biophys Res Commun. 2016 Sep 16;478(2):618–23. [CrossRef]
- Sepe LP, Hartl K, Iftekhar A, Berger H, Kumar N, Goosmann C, et al. Genotoxic effect of Salmonella paratyphi a infection on human primary gallbladder cells. mBio. 2020 Sep 1;11(5):1–39. [CrossRef]
- Ricardo Castellanos L, van der Graaf-Van Bloois L, Donado-Godoy P, Veldman K, Duarte F, Acuña MT, et al. Antimicrobial resistance in Salmonella enterica serovar paratyphi B variant Java in poultry from Europe and Latin America. Emerg Infect Dis. 2020 Jun 1;26(6):1164–73. [CrossRef]
- Amancha G, Celis Y, Irazabal J, Falconi M, Villacis K, Thekkur P, et al. High levels of antimicrobial resistance in Escherichia coli and Salmonella from poultry in Ecuador. Revista Panamericana de Salud Publica/Pan American Journal of Public Health. 2023;47. [CrossRef]
- Zhang Y, Chu M, Liao Y Te, Salvador A, Wu VCH. Characterization of two novel Salmonella phages having biocontrol potential against Salmonella spp. in gastrointestinal conditions. Sci Rep. 2024 Dec 1;14(1). [CrossRef]
- Ge H, Xu Y, Hu M, Zhang K, Zhang S, Jiao X, et al. Isolation, characterization, and application in poultry products of a Salmonella-specific bacteriophage, S55. J Food Prot. 2021 Jul 1;84(7):1202–12. [CrossRef]
- Islam MS, Hu Y, Mizan MFR, Yan T, Nime I, Zhou Y, et al. Characterization of Salmonella phage LPST153 that effectively targets most prevalent Salmonella serovars. Microorganisms. 2020 Jul 1;8(7):1–18. [CrossRef]
- Teklemariam AD, Alharbi MG, Al-Hindi RR, Alotibi I, Aljaddawi AA, Azhari SA, et al. Isolation and characterization of Chi-like Salmonella bacteriophages infecting two Salmonella enterica Serovars, Typhimurium and Enteritidis. Pathogens. 2022 Dec 1;11(12). [CrossRef]
- Jurczak-Kurek A, Gasior T, Nejman-Faleńczyk B, Bloch S, Dydecka A, Topka G, et al. Biodiversity of bacteriophages: Morphological and biological properties of a large group of phages isolated from urban sewage. Sci Rep. 2016 Oct 4;6. [CrossRef]
- Elsayed MM, Elkenany RM, EL-Khateeb AY, Nabil NM, Tawakol MM, Hassan HM. Isolation and encapsulation of bacteriophage with chitosan nanoparticles for biocontrol of multidrug-resistant methicillin-resistant Staphylococcus aureus isolated from broiler poultry farms. Sci Rep. 2024 Dec 1;14(1). [CrossRef]
- Wójcicki M, Średnicka P, Błażejak S, Gientka I, Kowalczyk M, Emanowicz P, et al. Characterization and genome study of novel lytic bacteriophages against prevailing saprophytic bacterial microflora of minimally processed plant-based food products. Int J Mol Sci. 2021 Nov 1;22(22). [CrossRef]
- Unverdi A, Erol HB, Kaskatepe B, Babacan O. Characterization of Salmonella phages isolated from poultry coops and its effect with nisin on food bio-control. Food Sci Nutr. 2024 Apr 1;12(4):2760–71. [CrossRef]
- Jeon G, Ahn J. Evaluation of phage adsorption to Salmonella Typhimurium exposed to different levels of pH and antibiotic. Microb Pathog. 2021 Jan 1;150. [CrossRef]
- Shang Y, Sun Q, Chen H, Wu Q, Chen M, Yang S, et al. Isolation and characterization of a novel Salmonella phage vB_SalP_TR2. Front Microbiol. 2021 Jun 21;12. [CrossRef]
- Khan MAS, Islam Z, Barua C, Sarkar MMH, Ahmed MF, Rahman SR. Phenotypic characterization and genomic analysis of a Salmonella phage L223 for biocontrol of Salmonella spp. in poultry. Sci Rep. 2024 Dec 1;14(1). [CrossRef]
- Holtappels D, Alfenas-Zerbini P, Koskella B. Drivers and consequences of bacteriophage host range. Vol. 47, FEMS Microbiology Reviews. Oxford University Press; 2023. [CrossRef]
- Whittle MJ, Bilverstone TW, Van Esveld RJ, Lücke AC, Lister MM, Kuehne SA, et al. A novel bacteriophage with broad host range against Clostridioides difficile ribotype 078 supports slpa as the likely phage receptor. 2022. Available from: https://journals.asm.org/journal/spectrum. [CrossRef]
- Suleman M, Clark JR, Bull S, Jones JD. Ethical argument for establishing good manufacturing practice for phage therapy in the UK. J Med Ethics. 2024.
- Pinto G, Shetty SA, Zoetendal EG, Gonçalves RFS, Pinheiro AC, Almeida C, et al. An in vitro fermentation model to study the impact of bacteriophages targeting Shiga toxin-encoding Escherichia coli on the colonic microbiota. NPJ Biofilms Microbiomes. 2022 Dec 1;8(1). [CrossRef]
- Dlamini SB, Gigante AM, Hooton SPT, Atterbury RJ. Efficacy of different encapsulation techniques on the viability and stability of diverse phage under simulated gastric conditions. Microorganisms. 2023 Oct 1;11(10). [CrossRef]
- Abdelsattar AS, Abdelrahman F, Dawoud A, Connerton IF, El-Shibiny A. Encapsulation of E. coli phage ZCEC5 in chitosan–alginate beads as a delivery system in phage therapy. AMB Express. 2019 Dec 1;9(1). [CrossRef]
- Chen C, Tao Z, Li T, Chen H, Zhao Y, Sun X. Isolation and characterization of novel bacteriophage vB_KpP_HS106 for Klebsiella pneumonia K2 and applications in foods. Front Microbiol. 2023;14. [CrossRef]
- Harrell JE, Hahn MM, D’Souza SJ, Vasicek EM, Sandala JL, Gunn JS, et al. Salmonella biofilm formation, chronic infection, and immunity within the intestine and hepatobiliary tract. Vol. 10, Frontiers in Cellular and Infection Microbiology. Frontiers Media S.A.; 2021. [CrossRef]
- Fernández L, Gutiérrez D, García P, Rodríguez A. The perfect bacteriophage for therapeutic applications—A quick guide. Vol. 8, Antibiotics. MDPI AG; 2019. [CrossRef]
Figure 1.
(A) Morphology of lysis plaques and (B) structural features of Phylax-28 bacteriophage virions. The clarity and size of the plaques reflect the lytic activity and efficacy of the bacteriophage against Salmonella. The virions exhibit an icosahedral structure, characterized by a capsid composed of 20 equidistant triangular faces.
Figure 1.
(A) Morphology of lysis plaques and (B) structural features of Phylax-28 bacteriophage virions. The clarity and size of the plaques reflect the lytic activity and efficacy of the bacteriophage against Salmonella. The virions exhibit an icosahedral structure, characterized by a capsid composed of 20 equidistant triangular faces.
Figure 2.
Replication curve of bacteriophage Phylax-28 using Salmonella as the host bacterium. The replication dynamics are depicted under two conditions: with chloroform (blue line), which indicates the formation of intracellular virions, and without chloroform (black line), which shows the release of virions into the extracellular medium.
Figure 2.
Replication curve of bacteriophage Phylax-28 using Salmonella as the host bacterium. The replication dynamics are depicted under two conditions: with chloroform (blue line), which indicates the formation of intracellular virions, and without chloroform (black line), which shows the release of virions into the extracellular medium.
Figure 3.
Kinetics of Phylax-28 bacteriophage concentration reduction at various temperatures. (A) No significant reduction was observed at 60 °C (black line) and 65 °C (blue line). However, at 70 °C (green line), the reduction follows first-order kinetics with an r² value of 0.991. (B) Experimental results on the stability of Phylax-28 at 28 °C, measured monthly over six months, were compared with the model’s predicted stability. The model's accuracy was expressed as a percentage.
Figure 3.
Kinetics of Phylax-28 bacteriophage concentration reduction at various temperatures. (A) No significant reduction was observed at 60 °C (black line) and 65 °C (blue line). However, at 70 °C (green line), the reduction follows first-order kinetics with an r² value of 0.991. (B) Experimental results on the stability of Phylax-28 at 28 °C, measured monthly over six months, were compared with the model’s predicted stability. The model's accuracy was expressed as a percentage.
Figure 4.
Stability of Phylax-28 bacteriophage under simulated gastrointestinal conditions. In the oral cavity, no significant reduction in phage concentration was observed, indicating high stability in this phase. A slight loss of viability was noted in the gastric environment, but the phage retained functionality. In the intestinal phase, the phage concentration remained stable with no significant changes. Error bars represent the standard deviation of the measurements, reflecting variability across replicate experiments.
Figure 4.
Stability of Phylax-28 bacteriophage under simulated gastrointestinal conditions. In the oral cavity, no significant reduction in phage concentration was observed, indicating high stability in this phase. A slight loss of viability was noted in the gastric environment, but the phage retained functionality. In the intestinal phase, the phage concentration remained stable with no significant changes. Error bars represent the standard deviation of the measurements, reflecting variability across replicate experiments.
Figure 5.
The figure demonstrates the lytic activity of bacteriophage Phylax-28 against Salmonella in trypticase soy broth (TSB). The red line indicates the normal growth of Salmonella without bacteriophage treatment, serving as the control. The black, green, and blue lines represent treatments with Phylax-28 at multiplicities of infection (MOI) of 0.1, 0.01, and 0.001, respectively. A significant reduction in bacterial growth is observed across all MOI levels, with Phylax-28 showing pronounced efficacy in reducing Salmonella concentrations, even at the lowest MOI.
Figure 5.
The figure demonstrates the lytic activity of bacteriophage Phylax-28 against Salmonella in trypticase soy broth (TSB). The red line indicates the normal growth of Salmonella without bacteriophage treatment, serving as the control. The black, green, and blue lines represent treatments with Phylax-28 at multiplicities of infection (MOI) of 0.1, 0.01, and 0.001, respectively. A significant reduction in bacterial growth is observed across all MOI levels, with Phylax-28 showing pronounced efficacy in reducing Salmonella concentrations, even at the lowest MOI.
Figure 6.
Effect of bacteriophage and ciprofloxacin on the concentration of Salmonella and probiotic bacteria in a simulated intestinal environment. The graph displays Salmonella growth (red line) in the absence of antibiotic, Salmonella in the presence of antibiotic (black line), probiotic bacteria without antibiotic (dashed green line), probiotic bacteria with antibiotic (dashed dark blue line), Salmonella in the presence of bacteriophage (dotted light blue line), and probiotic bacteria with bacteriophage (dotted dark green line). The magnitude of error bars, represented as standard deviation, is not discernible in the graph.
Figure 6.
Effect of bacteriophage and ciprofloxacin on the concentration of Salmonella and probiotic bacteria in a simulated intestinal environment. The graph displays Salmonella growth (red line) in the absence of antibiotic, Salmonella in the presence of antibiotic (black line), probiotic bacteria without antibiotic (dashed green line), probiotic bacteria with antibiotic (dashed dark blue line), Salmonella in the presence of bacteriophage (dotted light blue line), and probiotic bacteria with bacteriophage (dotted dark green line). The magnitude of error bars, represented as standard deviation, is not discernible in the graph.
Table 1.
List of oligonucleotide sequences used for the amplification of genes in Salmonella strains encoding effector proteins linked to the promotion of precancerous lesions and tumorigenesis.
Table 1.
List of oligonucleotide sequences used for the amplification of genes in Salmonella strains encoding effector proteins linked to the promotion of precancerous lesions and tumorigenesis.
| Oligonucleotide |
Sequence |
Gene |
Fragment size (bp) |
| AvrA-F |
CCTGTATTGTTGAGCGTCTGG |
avrA |
422 |
| AvrA-R |
AGAAGAGCTTCGTTGAATGTCC |
| SopB-F |
TCAGAAGRCGTCTAACCACTC |
sopB |
517 |
| SopB-R |
TACCGTCCTCATGCACACTC |
| CdtB-F |
GAAACAAGTCAGGCATTGCC |
cdtB |
819 |
| CdtB-R |
GAATGGCTCATAAACACGCC |
| PltA-F |
GTGGGACTATCATCGTGCAG |
pltA |
729 |
| PltA-R |
AGGGTGATCAACGTAACCAC |
| PltB-F |
GCCGGAAGTACCTGTGTTAT |
pltB |
414 |
| PltB-R |
AGTAGTGAAAACCCATCGCG |
Table 2.
Composition of fluids in each phase of the simulated gastrointestinal tract.
Table 2.
Composition of fluids in each phase of the simulated gastrointestinal tract.
| Reagent |
Oral phase (mmol/l) |
Gastric phase (mmol/l) |
Intestinal phase (mmol/l) |
| KCl |
15.1 |
6.9 |
6.8 |
| KH2PO4 |
3.7 |
0.9 |
0.8 |
| NaHCO3 |
13.6 |
25 |
85 |
| NaCl |
- |
47.2 |
38.4 |
| MgCl2(H2O)6 |
0.15 |
0.12 |
0.33 |
| (NH4)2CO3 |
0.06 |
0.5 |
- |
| CaCl2(H2O)2 |
1.5 |
0.15 |
0.6 |
| Enzymes |
|
| α-amylase |
150 U/ml |
- |
- |
| Pepsin |
- |
4,000 U/ml |
- |
| Lipase |
- |
120 U/ml |
- |
| Pancreatin |
- |
- |
200 U/ml (based on trypsin activity) |
| Bile salts |
- |
- |
10 mM |
Table 3.
Antimicrobial resistance profiles and virulence factors of Salmonella strains isolated from urban wastewater.
Table 3.
Antimicrobial resistance profiles and virulence factors of Salmonella strains isolated from urban wastewater.
| |
Antimicrobials and average inhibition diameter (mm) |
Virulence genes associated with the potential to induce precancerous lesions |
| Bacterial strain |
IMP |
SXT |
GEN |
AMP |
CIP |
NAL |
CHL |
TET |
COL |
AMC |
CFP |
AMK |
avrA |
sopB |
cdtB |
pltA |
pltB |
|
Salmonella sal01
|
26 |
21 |
16 |
15 |
16 |
14 |
24 |
19 |
8 |
21 |
24 |
17 |
- |
+ |
+ |
- |
+ |
|
Salmonella sal02
|
26 |
24 |
18 |
19 |
25 |
21 |
25 |
22 |
9 |
10 |
24 |
18 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal03
|
22 |
18 |
19 |
16 |
22 |
21 |
17 |
19 |
8 |
17 |
22 |
19 |
+ |
- |
+ |
+ |
+ |
|
Salmonella sal04
|
25 |
24 |
18 |
14 |
22 |
17 |
22 |
18 |
7 |
18 |
21 |
18 |
+ |
- |
+ |
- |
+ |
|
Salmonella sal05
|
29 |
26 |
21 |
22 |
31 |
21 |
23 |
20 |
8 |
22 |
24 |
19 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal06
|
26 |
22 |
18 |
21 |
30 |
21 |
23 |
20 |
9 |
22 |
17 |
17 |
+ |
- |
- |
- |
+ |
|
Salmonella sal07
|
27 |
25 |
18 |
21 |
31 |
21 |
31 |
19 |
20 |
17 |
8 |
16 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal08
|
15 |
18 |
17 |
15 |
14 |
12 |
21 |
27 |
8 |
22 |
19 |
19 |
+ |
- |
+ |
+ |
+ |
|
Salmonella sal09
|
29 |
25 |
23 |
31 |
22 |
24 |
29 |
9 |
24 |
19 |
25 |
19 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal10
|
26 |
24 |
18 |
23 |
30 |
22 |
26 |
22 |
9 |
22 |
24 |
19 |
+ |
+ |
+ |
- |
- |
|
Salmonella sal11
|
26 |
25 |
16 |
21 |
29 |
20 |
24 |
19 |
9 |
22 |
26 |
19 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal12
|
26 |
24 |
18 |
21 |
25 |
21 |
25 |
22 |
9 |
23 |
24 |
18 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal13
|
22 |
22 |
19 |
21 |
30 |
21 |
24 |
19 |
9 |
21 |
22 |
19 |
+ |
- |
+ |
- |
+ |
|
Salmonella sal14
|
25 |
23 |
18 |
22 |
32 |
22 |
22 |
18 |
9 |
22 |
28 |
18 |
+ |
- |
- |
+ |
+ |
|
Salmonella sal15
|
29 |
20 |
21 |
22 |
31 |
21 |
23 |
20 |
8 |
22 |
24 |
19 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal16
|
26 |
24 |
18 |
21 |
30 |
21 |
23 |
20 |
9 |
22 |
23 |
17 |
+ |
+ |
+ |
+ |
- |
|
Salmonella sal17
|
21 |
19 |
12 |
21 |
22 |
21 |
31 |
21 |
25 |
18 |
8 |
22 |
+ |
- |
+ |
+ |
+ |
|
Salmonella sal19
|
25 |
26 |
17 |
22 |
32 |
20 |
21 |
27 |
8 |
22 |
23 |
19 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal20
|
22 |
18 |
16 |
21 |
19 |
20 |
18 |
19 |
10 |
18 |
26 |
20 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal21
|
26 |
24 |
18 |
21 |
25 |
21 |
24 |
22 |
9 |
23 |
24 |
18 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal21
|
22 |
24 |
19 |
21 |
30 |
21 |
22 |
19 |
9 |
21 |
22 |
19 |
+ |
- |
+ |
+ |
- |
|
Salmonella sal23
|
25 |
24 |
18 |
22 |
32 |
22 |
22 |
18 |
9 |
22 |
28 |
18 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal24
|
29 |
26 |
21 |
22 |
31 |
21 |
23 |
20 |
8 |
22 |
24 |
19 |
+ |
- |
+ |
+ |
+ |
|
Salmonella sal25
|
26 |
24 |
18 |
21 |
30 |
21 |
23 |
20 |
9 |
22 |
23 |
17 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal26
|
21 |
19 |
18 |
21 |
15 |
21 |
31 |
21 |
15 |
18 |
8 |
21 |
+ |
- |
+ |
+ |
+ |
|
Salmonella sal27
|
22 |
26 |
17 |
18 |
17 |
12 |
21 |
27 |
8 |
22 |
23 |
19 |
+ |
- |
+ |
+ |
+ |
|
Salmonella sal28
|
12 |
14 |
13 |
14 |
9 |
12 |
20 |
9 |
8 |
10 |
14 |
13 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal29
|
26 |
24 |
18 |
23 |
30 |
22 |
26 |
22 |
9 |
22 |
24 |
19 |
+ |
- |
+ |
- |
+ |
|
Salmonella sal30
|
26 |
25 |
16 |
21 |
29 |
20 |
24 |
19 |
9 |
22 |
26 |
18 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal31
|
19 |
20 |
18 |
17 |
14 |
19 |
17 |
22 |
9 |
19 |
16 |
18 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal32
|
18 |
16 |
17 |
18 |
18 |
16 |
20 |
19 |
9 |
19 |
22 |
19 |
+ |
- |
+ |
+ |
+ |
|
Salmonella sal33
|
24 |
24 |
18 |
22 |
32 |
22 |
22 |
18 |
9 |
22 |
28 |
18 |
+ |
- |
+ |
+ |
+ |
|
Salmonella sal34
|
26 |
22 |
21 |
22 |
31 |
21 |
23 |
20 |
8 |
22 |
24 |
19 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal35
|
26 |
24 |
18 |
21 |
30 |
21 |
23 |
20 |
9 |
22 |
23 |
17 |
+ |
+ |
+ |
- |
+ |
|
Salmonella sal36
|
21 |
25 |
18 |
21 |
31 |
21 |
31 |
21 |
25 |
18 |
8 |
22 |
+ |
+ |
+ |
+ |
+ |
|
Salmonella sal37
|
25 |
18 |
17 |
22 |
32 |
14 |
18 |
16 |
8 |
22 |
23 |
12 |
+ |
+ |
+ |
- |
+ |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).