1. Introduction
Endometriosis is a condition characterized by the presence of endometrial tissue outside of the uterus and affects around 10% of women of reproductive age [1-4]. It is often debilitating presenting with dysmenorrhea, pelvic pain, and infertility and treated with hormonal suppression, surgery, or both [
3]. Surgical management includes laparoscopic ablation of endometrial implants or excision surgery (OES) with suspected lesions sent to pathology. This technique allows for histological confirmation of endometriosis [
5]. Historical data shows that 40-60% of patients undergo repeat surgery by ablation within 1-2 years of initial procedure [
6,
7] but there are few studies that compare the recurrence rate between the two surgeries. A 2008 Cochrane review suggests that OES is more favorable than ablation of endometrioma in terms of lesion recurrence, pain recurrence, and pregnancy in women who were previously sub fertile [
8]. A RCT by Carmona et al. suggests that laparoscopic laser ablation of ovarian endometrioma has a higher rate of recurrence than cystectomy at 12 months but similar results at 24 months. The ablative group also demonstrated a shorter time to recurrence [
9,
10]. To our understanding, there are no studies comparing the recurrence rate of endometriosis in non-specific areas of the female reproductive system between ablation and OES.
Alternatively, hormonal suppression therapy targets the overproduction of estrogen, progesterone resistance, and chronic inflammation and are normally offered prior to surgical consideration. Therapeutic agents include GnRH agonists and antagonists which lead to hypoestrogenism and hence regression of endometriotic implants, as well as progesterone containing contraceptives [
11]. Studies show that pre-surgical hormone suppression is effective at reducing endometrial cyst size and facilitating laparoscopic resection [12-14]. We aimed to further explore the effect of hormonal suppression on endometrial progression using the ASRM classification system.
2. Materials and Methods
Consecutive patients seen for pelvic pain at the Center of Endometriosis at Saint Louis University (SLU), a referral center for the surgical management of endometriosis, were recruited for the study during the time frame 2012-2019. Patients included in this study were those who demonstrated histological confirmation of endometriosis upon laparoscopic excisional surgery. All patients underwent optimal or complete excision of all visible manifestations of endometriosis. Patients were also classified by stage of endometriosis through a point system created by the American Society for Reproductive Medicine (ASRM) and is divided into four stages: minimal (Stage I, 1-5 points), mild (Stage II, 6-15 points), moderate (Stage III, 16-40 points) and severe (Stage IV, >40 points).
All surgeries were performed by a single surgeon specializing in optimal excision. Optimal excision was defined as completely excising all areas of abnormal peritoneum, wherever found in the pelvis, having looked systematically with near contact laparoscopy. Preoperative data, operative data, and follow-up data were collected prospectively as part of an on-going database approved by the SLU Institutional Review Board (IRB). Follow-up data was collected from annual questionnaires sent postoperatively to patients. Patients were not specifically recommended to take hormonal suppression after surgery.
Continuous variables initially were expressed as medians and ranges due to lack of normality of the distributions. Categorical variables were expressed as numbers and percentages. Differences in operative data and outcomes between women receiving optimal endometriosis excision surgery and women not receiving optimal endometriosis excision surgery were assessed using chi-square and Fisher’s Exact test for categorical variables. Continuous variables were assessed using the Mann-Whitney U test. Differences in categorical variables for hormonal suppression before endometriosis excision surgery by stage of endometriosis were assessed using chi-square and Fisher’s Exact test for pairwise comparisons and chi-square for 4-group comparisons. Differences in length of hormonal suppression before endometriosis excision surgery were assessed using the Mann-Whitney U test for pairwise comparisons and the Kruskal-Wallis test for 4-group comparisons. A p-value of <0.05 was used to denote statistical significance. All analyses were performed using SPSS version 24.0 for Windows (IBM Corporation, Armonk, New York). Accession numbers will be provided during the review process.
3. Results
3.1. Optimal Excision Surgery Results
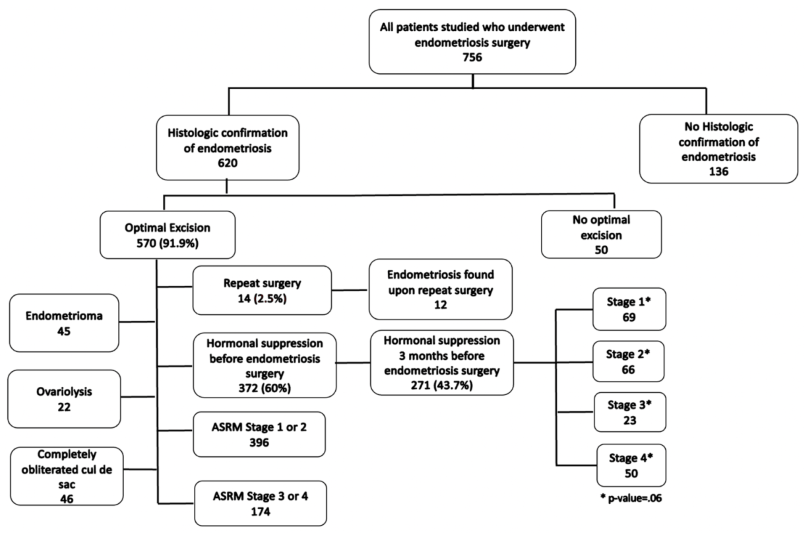
3.1.1. Figure 1: Repeat Surgery Rate, ASRM Staging & Extraneous Findings
Flow of participants through the study detailing results of optimal excision surgery and hormonal suppression according to ASRM staging.
-
620 of the 756 patients in the database who underwent surgery for endometriosis from 2012-2019 had histological confirmation of endometriosis in any location excised (
Table 1).
91.9% received optimal endometriosis excision surgery.
Of the 620 patients with tissue-proven endometriosis, 570 women had OES for endometriosis (
Table 2).
Of the 570 who underwent OES, only 14 patients underwent repeat surgery (2.5%).
Endometriosis was found in 12 of these 14 women. 69.5% of the women who underwent OES were found to be in ASRM stages 1 or 2, and 30.6% in ASRM stages 3 or 4.
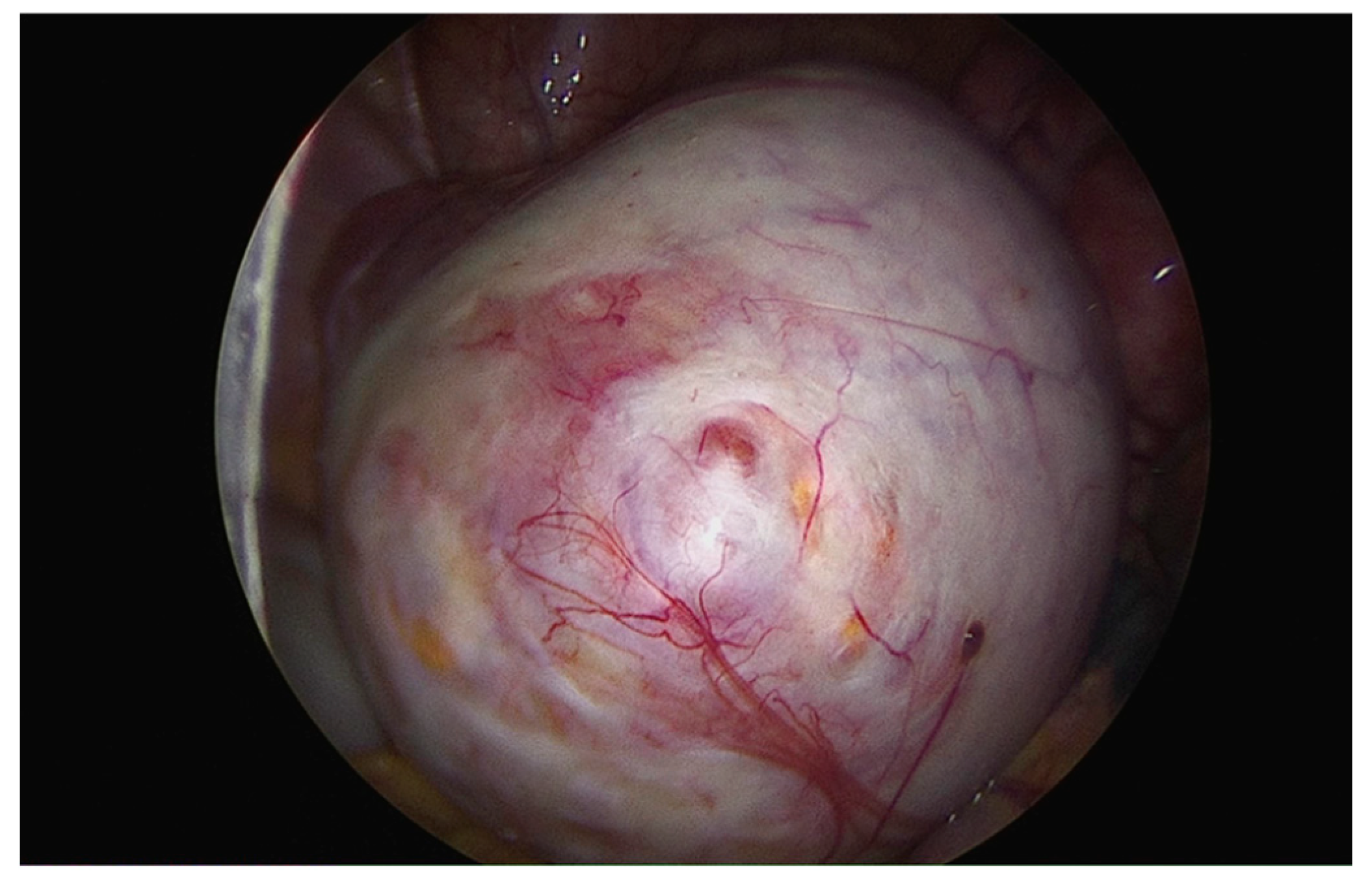
Endometrioma was found in 45 of these women (23.1%), and ovariolysis was performed in 22 (53.7%). (
Figure 2)
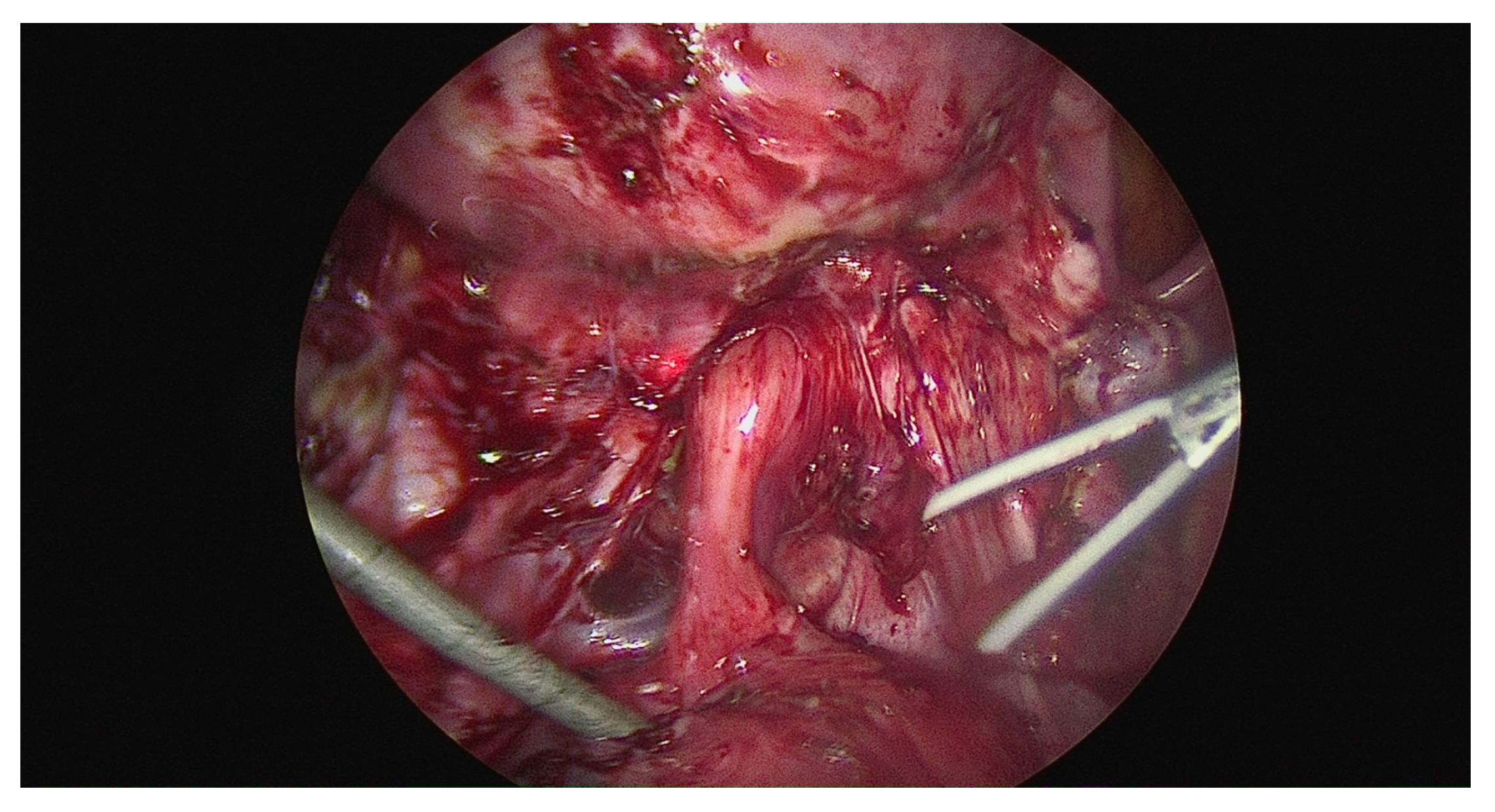
46 had a completely obliterated cul-de-sac (8.9%); 33 had partial (6.4%); and 440 had an absent cul-de-sac (84.8%). (
Figure 3)
Hormonal suppression before endometriosis excision surgery was unknown for four women; hormonal contraceptives were unknown for nine women who reported using hormonal suppression before endometriosis excision surgery; gonadotropin-releasing agonists/antagonists/chemical menopause and progesterone-secreting IUD were unknown for 10 women who reported using hormonal suppression before endometriosis excision surgery; length of hormonal suppression before endometriosis excision surgery was unknown for 40 women who reported using hormonal suppression before endometriosis excision surgery; used hormonal suppression in the three months before endometriosis excision surgery was unknown for 103 women who reported using hormonal suppression before endometriosis excision surgery; number of ablation surgeries was unknown for 15 women; endometrioma was unknown for 415 women; ovariolysis was unknown for six women who had an endometrioma; obliterated posterior cul-de-sac was unknown for 52 women; endometriosis stage was unknown for 229 women.
* All known and suspected endometriosis is removed.
† Calculated for 366 women reporting using hormonal suppression before endometriosis excision surgery.
3.1.2. Effects of Use of Hormonal Suppression
Of the 620 women who underwent endometriosis excision surgery, approximately 60% reported use of hormonal suppression before their surgery (
Table 1).
Hormonal contraceptives were reported by 96.4% of the women, gonadotropin-releasing agonists/antagonists/chemical menopause were reported by 29.5% of the women, and a progesterone-secreting intrauterine device (IUD) was reported by 14.6% of the women.
The median length of hormonal suppression before endometriosis excision surgery was 36 months with a range of 1-336 months.
The response rate of follow-up post operatively was 49% (304/620 patients).
Use of hormonal suppression in the three months before endometriosis excision surgery was reported by 43.7% of the women.
While no significant overall difference in the use of hormonal suppression before endometriosis excision surgery was found between women receiving OES and women not receiving OES (60.2% versus 50.0%, p=0.21,
Table 2), a significantly higher proportion of women receiving OES used hormonal contraceptives (97.0% versus 87.0%, p<0.05).
Of the 387 women with a known stage of endometriosis and known value for hormonal suppression prior to surgery, 179 reported that they did not take any hormonal suppression prior to excision surgery (
Table 3).
Of those who were known to be in stage 1, 61.6% took hormonal suppression and 38.4% did not; in stage 2, 50.4% took hormonal suppression and 49.6% did not; in stage 3, 50% took hormonal suppression and 50% did not; and in stage 4, 51% took hormonal suppression and 49% did not.
Use of progesterone-secreting IUD before endometriosis excision surgery between women with either ASRM stage 1 (21.2%) or stage 3 (27.3%) and stage 4 (8.3%) approached statistical significance (p=0.06).
No significant overall association was found for any hormonal suppression before endometriosis excision surgery variable with stage of endometriosis when all four endometriosis stages were examined simultaneously.
* All known and suspected endometriosis is removed. † Hormonal suppression before endometriosis excision surgery was unknown for four women; hormonal contraceptives were unknown for seven women who reported using hormonal suppression before endometriosis excision surgery; gonadotropin-releasing agonists/antagonists/chemical menopause and progesterone-secreting IUD were unknown for eight women who reported using hormonal suppression before endometriosis excision surgery; length of hormonal suppression before endometriosis excision surgery was unknown for 36 women; used hormonal suppression in the three months before endometriosis excision surgery was unknown for 87 women; number of ablation surgeries was unknown for 14 women; endometrioma found was unknown for 375 women; ovariolysis was unknown for four women who had an endometrioma; obliterated posterior cul-de-sac was unknown for 51 women; endometriosis stage was unknown for three women; follow-up was unknown for 294 women.
‡ Hormonal contraceptives, gonadotropin-releasing agonists/antagonists/chemical menopause, and progesterone-secreting IUD were unknown for two women who reported using hormonal suppression before endometriosis excision surgery; length of hormonal suppression before endometriosis excision surgery was unknown for four women; used hormonal suppression in the three months before endometriosis excision surgery was unknown for 16 women; number of ablation surgeries and obliterated cul-de-sac were unknown for one woman; endometrioma found was unknown for 40 women; ovariolysis was unknown for two women who had an endometrioma; follow-up was unknown for 22 women.
§ Calculated for 366 women reporting using hormonal suppression before endometriosis excision surgery.
* Hormonal contraceptives were unknown for six women who reported using hormonal suppression before endometriosis excision surgery; gonadotropin-releasing agonists/antagonists/CM and progesterone -secreting IUD were unknown for seven women who reported using hormonal suppression before endometriosis excision surgery; length of hormonal suppression before endometriosis excision surgery was unknown for 26 women who reported using hormonal suppression before endometriosis excision surgery; used hormonal suppression in the three months before endometriosis excision surgery was unknown for 74 women who reported using hormonal suppression before endometriosis excision surgery.
† Hormonal contraceptives, gonadotropin-releasing agonists/antagonists/CM, and progesterone-secreting IUD were unknown for three women who reported using hormonal suppression before endometriosis excision surgery; length of hormonal suppression before endometriosis excision surgery was unknown for 14 women who reported using hormonal suppression before endometriosis excision surgery; used hormonal suppression in the three months before endometriosis excision surgery was unknown for 29 women who reported using hormonal suppression before endometriosis excision surgery.
4. Discussion
There have been two primary methods to approach endometriosis surgery in attempts to reduce symptoms: ablation and excisional surgery, both of which result in various surgical outcomes. According to a study conducted by Healey, excisional surgery techniques were found to result in a significant reduction in all VAS pain scores over a 5-year follow-up period particularly for dyspareunia [
15]. The study also reports that more women in the ablation group continued to receive medical treatment of endometriosis at 5 years. When compared to excisional techniques, ablative procedures are theoretically more likely to be incomplete due to failure of this technique to appreciate the depth of invasion and significant risks of heat damage to surrounding structures. With excisional techniques the resection of the endometriosis tissue can be extended to the surrounding normal peritoneum, thus making excision potentially more complete than ablation. With ablative procedures a greater amount of necrotic tissue can be left behind, with a potentially greater inflammatory reaction and increased risk of adhesions and pain [
16].
The rate of repeat surgery is an important clinical factor when looking at surgical outcomes. The data collected in this retrospective study was obtained prospectively via annual questionnaires. The use of patient answered questionnaires introduces a potential of response bias as well as non-response to surveys. Since the data is derived from patients who decide to respond to the survey and are responding based on their memory of events passed, there is a possibility of recall bias which could lead to inaccurate or incomplete data. Non-response is also a potential factor for this study as the response rate of follow-up post operatively was 49% meaning the data could potentially be skewed based on the remaining percentage of responses if theoretically collected. Historically, a response rate of approximately 60% is ideal for general research purposes meaning the response rate of this study at 49% is comparable to research standards [
17].
The rate of repeat surgery after optimal excision surgery for endometriosis was remarkably low (2.5%) as compared to historical rates of repeat surgery by ablation. According to CJ Sutton, the historical rate of repeat surgery via ablation is 40-60% in 1-2 years following the initial procedure [
7]. Often outcomes such as pain and fertility are analyzed but a clinically vital outcome that directly affects the patient experience is the rate of repeat surgery. This study confirms the hypothesis that optimal excision surgery yields lower rates of repeat surgery as compared to historical data for ablation surgery for endometriosis. With this study demonstrating a repeat surgery rate of 2.5%, less unnecessary repeat surgeries will be performed. This will lead to less postoperative complications as compared to the increased rate of repeat surgery utilizing the ablation technique. Due to the results of this study, we recommend performing optimal excision surgeries over ablation surgeries in regard to surgical treatment of endometriosis.
Additionally, the lack of significant evidence between hormonal suppression use and progression of stage of endometriosis provides implicit evidence that hormonal suppression does not prevent progression of the disease. These results are comparable to those of other studies that have also analyzed the impact of hormonal suppression prior to endometriosis surgery including a study that found the efficacy of medical therapy for endometriosis prior to surgery inconclusive [
2]. Another factor to evaluate would be timing of hormonal suppression to see if this affects the progression of endometriosis prior to surgery.
National organizations and experts have long advised endometriosis patients to use oral contraceptives prior to resorting to surgical management due to the belief that hormonal suppression can delay the progression or recurrence of endometriosis. A recent Cochrane review examined the use of combined oral contraceptives or GnRH analogues and no significant difference in dysmenorrhea between the two groups when observed at 6 months after stopping treatment was found (OR, .48; 95% CI, .08-2.90) [
18]. After analyzing the results of this study combined with the findings of the 2004 Cochran review which showed perioperative use of hormonal suppression did not demonstrate long-term benefit, it has been implied that hormonal suppression does not prevent the progression of endometriosis [
19]. Further studies would be needed.
Limitations of this study include results that may not be applicable to other surgeons who do not use optimal excisional surgery techniques including generalists or even specialists who do not exclusively use excisional techniques. Additionally, the questionnaire used in this study has inherent limitations as it relies on patient responsiveness for rate calculations which may envelope a degree of response bias since acquisition of the most accurate data requires patients to remember and respond accurately which cannot always be guaranteed. Relapse rate of endometriosis necessitating repeat surgery for endometriosis has been related to the diagnosis of particular diseases, such as endometrioid ovarian cancer. While such an association would be interesting to examine, this information was lacking in our data set and was a limitation of our study [
20].
There were a multitude of strengths in this study including that all analyzed patient surgeries were conducted by a single surgeon at a tertiary referral center. Additionally, the long-term (2012-2019) timeline of this study presents itself as a strength as there is a paucity of data on outcomes following surgical management of endometriosis past two years of analysis. Consistent surgical technique was used, specifically optimal excision which is defined as complete excision of all areas of abnormal peritoneum, wherever found in the pelvis, having looked systematically with near contact laparoscopy. For the entirety of the study, a commitment was made to not rely on postoperative hormonal suppression for treating residual disease as well as to prevent postoperative pelvic adhesions.
Our findings on the efficacy of optimal endometriosis excision surgery for reducing repeat surgery may have special relevance for particular subgroups of women, such as young women and adolescents where endometriosis appears to be progressive over time [
21,
22].
Author Contributions
Conceptualization, A.M. and P.Y.; methodology P.Y.; formal analysis, J.G.; investigation, P.Y.; resources, P.Y..; data curation, A.M.; writing—original draft preparation, A.M.; writing—review and editing, A.M. and P.Y.; visualization, A.M.; supervision, P.Y.; funding acquisition, P.Y. All authors have read and agreed to the published version of the manuscript. .
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Saint Louis University (protocol code 20900, date of approval- 2/25/2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Special thanks to Felicia Lee, medical student at Saint Louis University School of Medicine, for assistance in preparation of the manuscript and to Shohreh Jamalabadi-Majdi for administrative assistance during the research process. Shohreh Majdi is from the Division of Research, Department of Obstetrics, Gynecology, and Women’s Health, 299 Saint Louis University School of Medicine.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Nirgianakis K, Ma L, McKinnon B, Mueller MD. Recurrence Patterns after Surgery in Patients with Different Endometriosis Subtypes: A Long-Term Hospital-Based Cohort Study. Journal of Clinical Medicine. 2020, 9. [Google Scholar] [CrossRef]
- Chen I, Veth VB, Choudhry AJ, Murji A, Zakhari A, Black AY, Agarpao C, Maas JW. Pre- and postsurgical medical therapy for endometriosis surgery. Cochrane Database Syst Rev. 2020, 11, CD003678. [Google Scholar] [CrossRef]
- Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction. 2016, 152, R63–R78. [Google Scholar] [CrossRef]
- Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev 2008, CD004992. Published 2008 Apr 16. [CrossRef]
- Yeung P Jr, Tu F, Bajzak K, et al. A pilot feasibility multicenter study of patients after excision of endometriosis. JSLS. 2013, 17, 88–94. [Google Scholar] [CrossRef]
- Takenaka M, Yano R, Hiraku Y, et al. Exploratory study of pre-surgical medications with dienogest or leuprorelin in laparoscopic cystectomy of endometrial cysts. J Obstet Gynaecol Res. 2015, 41, 1234–1239. [Google Scholar] [CrossRef]
- Sutton CJ, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994, 62, 696–700. [Google Scholar] [CrossRef]
- Alkatout I, Mettler L, Beteta C, et al. Combined surgical and hormone therapy for endometriosis is the most effective treatment: prospective, randomized, controlled trial. J Minim Invasive Gynecol. 2013, 20, 473–481. [Google Scholar] [CrossRef]
- Bozdag, G. Recurrence of endometriosis: risk factors, mechanisms and biomarkers. Womens Health (Lond). 2015, 11, 693–699. [Google Scholar] [CrossRef]
- Carmona F, Martínez-Zamora MA, Rabanal A, Martínez-Román S, Balasch J. Ovarian cystectomy versus laser vaporization in the treatment of ovarian endometriomas: a randomized clinical trial with a five-year follow-up. Fertil Steril. 2011, 96, 251–254. [Google Scholar] [CrossRef]
- Rafique S, Decherney AH. Medical Management of Endometriosis. Clin Obstet Gynecol. 2017, 60, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Sørensen SS, Colov NP, Vejerslev LO. Pre- and postoperative therapy with GnRH agonist for endometrial resection. A prospective, randomized study. Acta Obstet Gynecol Scand. 1997, 76, 340–344. [Google Scholar] [CrossRef]
- Rana N, Thomas S, Rotman C, Dmowski WP. Decrease in the size of ovarian endometriomas during ovarian suppression in stage IV endometriosis. Role of preoperative medical treatment. J Reprod Med. 1996, 41, 384–392. [Google Scholar]
- Healey M, Cheng C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. J Minim Invasive Gynecol. 2014, 21, 999–1004. [Google Scholar] [CrossRef]
- Bignardi T, Khong SY, Lam A. Excisional versus ablative surgery for peritoneal endometriosis. Cochrane Database Syst Rev. 2019, 2019, CD008979, Published 2019 Jul 29. [Google Scholar] [CrossRef]
- Fincham, JE. Response rates and responsiveness for surveys, standards, and the Journal. Am J Pharm Educ. 2008, 72, 43. [Google Scholar] [CrossRef]
- Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011, 17, 327–346. [Google Scholar] [CrossRef]
- Yap C, Furness S, Farquhar C. Pre and post operative medical therapy for endometriosis surgery. Cochrane Database Syst Rev. 2004, 2004, CD003678. [Google Scholar] [CrossRef]
- Pecorino B, Lagana AS, Chiantera V, Ferrara M, Di Stefano AB, Di Donna MC, Sorrentino F, Nappi L, Mikus M, Scollo P. Progression Free Survival, Overall Survival, and Relapse Rate in Endometrioid Ovarian Cancer and Synchronous Endometrial-Ovarian Endometrioid Cancer (SEO-EC): Results from a Large Retrospective Analysis. Medicina (Kaunas). 2022, 58, 1706. [Google Scholar] [CrossRef]
- Martire FG, Russo C, Selntigia A, Nocita E, Soreca G, Lazzeri L, Zupi E, Exacoustos C. Early noninvasive diagnosis of endometriosis: dysmenorrhea and specific ultrasound findings are important indicators in young women. Fertil Steril. 2023, 119, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Millischer AE, Santulli P, Da Costa S, Bordonne C, Cazaubon E, Marcellin L, Chapron C. Adolescent endometriosis: prevalence increases with age on magnetic resonance imaging scan. Fertil Steril. 2023, 119, 626–633. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).